Abstract
Objective
To evaluate the safety and survival in women treated with adjuvant pelvic radiation “sandwiched” between six cycles of paclitaxel and carboplatin chemotherapy with completely resected UPSC.
Methods
Surgically staged women with UPSC (FIGO stage 1-4) and no visible residual disease were enrolled. Treatment involved paclitaxel (175 mg/m2) and carboplatin (AUC=6.0-7.5) every 21 days for 3 doses, followed by radiation therapy (RT), followed by an additional 3 cycles of paclitaxel and carboplatin (AUC=5-6). Survival analysis, using Kaplan-Meier methods, was performed on patients who completed at least 3 cycles of chemotherapy and RT.
Results
A total of 81 patients were enrolled, of which 72 patients completed the first 3 cycles of chemotherapy followed by prescribed RT. Median age was 67 years (range: 43–82 years). 59/72 (82%) had disease confined to the uterus and 13/72 (18%) had completely resected extra-uterine disease (stage 3&4). 65 (83%) completed the protocol. Overall PFS and OS for combined stage 1&2 patients was 65.5±3.6 months and 76.5±4.3 months, respectively. PFS and OS for combined stage 3&4 patients was 25.8±3.0 and 35.9±5.3 months, respectively. Three-year % survival probability for stage 1&2 patients was 84% and for stage 3&4 patients was 50%. Of the 435 chemotherapy cycles administered, there were 11(2.5%) G3/G4 non-hematologic toxicities. 26(6.0%) cycles had dose reductions and 37(8.5%) had dose delays.
Conclusions
Compared to prior studies of single modality adjuvant therapy, RT “sandwiched” between paclitaxel and carboplatin chemotherapy is well-tolerated and highly efficacious in women with completely resected UPSC.
Introduction
Among the other non-endometrioid subtypes of endometrial cancer, uterine papillary serous carcinoma (UPSC) is the most common. Although UPSC constitutes approximately 10% of endometrial cancers, it accounts for a disproportionate amount of all endometrial cancer deaths.[1] It is an aggressive epithelial endometrial cancer subtype and it is often diagnosed at an advanced stage. Recurrence of UPSC, particularly extra-pelvic recurrence, is common and difficult to control. Pelvic radiation decreases the risk of pelvic recurrence in UPSC, but has not been shown to improve overall survival secondary to extra-pelvic recurrences and distant metastasis.[2]
Due to the systemic nature of UPSC, there have been a number of studies sequentially combining pelvic radiation therapy (RT) for local control, in addition to chemotherapy for systemic control. Combining whole abdominal radiation (WAR) with platinum containing regimens has shown promise with improved survival in patients who had measurable disease following primary surgery.[3] This was followed by a number of reports of sequential use of paclitaxel and carboplatin followed by pelvic radiation in patients with advanced endometrial cancer (stage 3&4 with measurable disease after surgery), including some patients with UPSC.[4-7] In these studies, in a treatment and not adjuvant setting, the toxicity profile was acceptable with minimal pelvic recurrences and improved survival.
In a multi-institutional retrospective analysis of 356 patients with stages 3&4 endometrial cancer, of which 86 women had UPSC, investigators compared outcomes in patients who received either RT alone, chemotherapy alone, and differing combinations of chemotherapy and RT. In this analysis, patients receiving chemotherapy alone had poorer 3-year OS and PFS compared to either RT alone or combination therapy. Also, adjuvant chemotherapy and RT was associated with improved survival in patients with advanced stage disease compared to either modality alone, with little difference in the toxicity profile of multimodal therapies.[8] These findings were confirmed in another retrospective study that included patients treated with various therapies, including sequential chemotherapy and radiation therapy. In this analysis, patients who had stage II UPSC who were treated with chemotherapy had a favorable PFS when compared to patients who did not receive chemotherapy.[9]
Because of the success of combined sequential chemotherapy and RT treatments for advanced uterine cancers, including UPSC, with measurable disease after primary surgery, a number of groups have evaluated the recurrence and survival patterns of patients undergoing adjuvant treatment for UPSC with no visible disease after surgery. A phase II GOG study by Sutton et al. evaluated the role of adjuvant WAR in 21 patients with stages I and II UPSC and clear cell uterine carcinomas.[10] In this study the authors concluded that other adjuvant approaches such as chemotherapy in combination with RT should be considered. Obermair and colleagues conducted a multi-center, prospective, non-randomized phase II clinical trial in women with early stage UPSC and clear cell carcinoma using 4 cycles of paclitaxel plus carboplatin followed by pelvic RT to evaluate the tolerability and safety of sequential therapy in these patients. The 2-year overall survival was 86% with stage 1 & 2 disease and 69% with stage 3 & 4 disease.[7]
Our group performed and reported our pilot data of adjuvant sequential chemotherapy with radiation therapy in a “sandwich” fashion.[11] The “sandwich” strategy hypothetically allows for control of systemic disease with chemotherapy while treating micro-metastasis in the pelvis with RT. Furthermore, the sequential delivery of chemotherapy and RT limits the overall toxicity and allows for maximum therapeutic dosing for both the chemotherapy and the RT. In our pilot study, we found improved survival, particularly in advanced stage patients, with acceptable toxicity in the first 30 women who received the “sandwich” therapy. As a result of these pilot data, we performed a non-randomized phase II prospective trial of radiation “sandwiched” between paclitaxel/platinum chemotherapy for patients with completely resected UPSC.[11]
Materials and methods
After IRB approval, eligible patients were recruited from 1999-2009 to this registered phase II trial (clinicaltrials.gov identifier: NCT00231868). In addition to newly-recruited patients, this analysis also includes subjects that were treated with carboplatin and paclitaxel with RT in the same fashion with updated follow-up from our previously reported pilot data.[11] During this pilot phase we optimized the carboplatin dosing, specifically due to the additional toxicity often seen after RT with the higher carboplatin doses. After optimization, our standard dosing for the trial led to a carboplatin dose at an AUC of 6 before and an AUC of 5 after RT.
After IRB approval and clinical trials registration, eligible patients were recruited over a 10 year period at Montefiore Medical Center and Albert Einstein College of Medicine. Eligible patients included histologically documented UPSC (updated FIGO stage 1-4) with no visible residual disease after surgery. All FIGO stage 1 patients had residual disease in the hysterectomy specimen. All eligible subjects underwent surgical staging, which included a hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node sampling and peritoneal washings. Infracolic omentectomy was preferred, especially for the stage IV recruited subjects who had omental metastases, but this was not required in patients without visible omental disease. All eligible subjects had an ECOG performance status of 0 or 1, adequate hematologic function (hematocrit ≥ 30%, WBC≥ 300/mm3, platelet count ≥ 100,000/mm3), BUN≤25 mg%, creatinine≤2 mg%, total bilirubin ≤ 1.5 mg/dl, aminotransferases ≤ 2.5 times the institutional upper limit of normal. Patients with significant concurrent medical conditions limiting their life expectancy to ≤ 3 months or those who received prior chemotherapy and/or RT for pelvic malignancy, were excluded.
At screening, all patients had protocol-required lab testing, including tumor markers, EKG, chest X-ray, and CT scan of the chest, abdomen, and pelvis. In addition to a physical examination, complete blood counts, serum electrolytes, and markers were performed prior to each cycle of chemotherapy and after completion of chemotherapy, every 3 months for 24 months, and then every 6 months thereafter. Imaging was repeated after treatment and during follow-up as clinically indicated. Adverse events were monitored for each cycle during therapy and during follow-up and graded using the National Cancer Institute Common Toxicity Criteria, version 3.0 as this study began prior to instituting NCI CTC v4.0.
Treatment
Chemotherapy
Registration and screening for the protocol was initiated within 6 weeks after surgery. Paclitaxel was administered at a dose of 175 mg/m2 over 3 hours. During the pilot phase and the beginning of the registration trial, the carboplatin doses were adjusted to minimize hematologic toxicity, while maintaining therapeutic doses. Chemotherapy was administrated every 21 days for 3 cycles prior to RT; then an additional 3 cycles every 21 days were administered after RT. Over half of the patients (52.2%) were administered the 4th cycle (1st cycle after RT) the same week as the brachytherapy. Standard premedications to minimize hypersensitivity and nausea for paclitaxel and carboplatin were administered.
Prior to each subsequent cycle of therapy, patients were required to have recovered to an ANC ≥1500/mm3 or WBC ≥3000/mm3, platelets ≥ 100,000/mm3 and renal and hepatic parameters the same as for screening. Treatment modifications for hematologic toxicities included cycle delay until recovery with subsequent dose reduction, in addition to the addition of G-CSF and/or erythropoietin according to provider preference. All toxicities, dose delays, and dose reductions were recorded.
Radiation Therapy
Radiation therapy began one week after the 3rd cycle of chemotherapy. The total dose of external beam pelvic radiation therapy (EBRT) was 45 Gy over 5 weeks. Patients were treated once per day, 5 days per week, with a daily fraction size of 1.8 Gy. A four-field conformal radiation therapy technique (AP-PA opposed and lateral opposed fields) or intensity modulated radiation therapy (IMRT) was used with a megavoltage beam of ≥ 6MV. The fields were extended to include the para-aortic nodal region in the case of ≥ 2 positive pelvic nodes or documented para-aortic lymph node disease, with positive lymph nodes marked with hemoclips at the time of surgery.
In 63 of the 72 patients who had chemotherapy and RT, high dose rate (HDR) brachytherapy via vaginal cylinder was used to boost the dose to the proximal 1/2-2/3rds of the vagina. Segmented cylinders of the largest size that would be accommodated by the vagina were used. Three fractions of 5 Gy each prescribed to 0.5 cm depth from the vaginal surface were given once per week using the Nucletron microSelectron HDR Ir-192 remote afterloading technique.
Statistical considerations
The accrual of additional evaluable subjects in addition to the original pilot patients was to further evaluate toxicity, extend survival analysis, and improve precision. Toxicity and survival analysis was performed on the 72 patients who had at least 3 cycles of chemotherapy and had any amount of prescribed RT.
Response was evaluated using an end point of 3-year progression-free survival. Disease-free survival (DFS) was calculated from the date of study registration to date of recurrence. Overall survival (OS) was calculated from the date of registration until the date of death or the date of last visit. Disease-free survival and overall survival curves were constructed using the Kaplan– Meier (KM) method. Site and date of recurrence were recorded, and confirmed by histology and/or cytology when possible. For added power, stage 1&2, or early-stage patients, were combined as well as stage 3&4 patients with completely resected metastatic disease. Frequencies for toxicity and adverse events were also recorded and tabulated.
Results
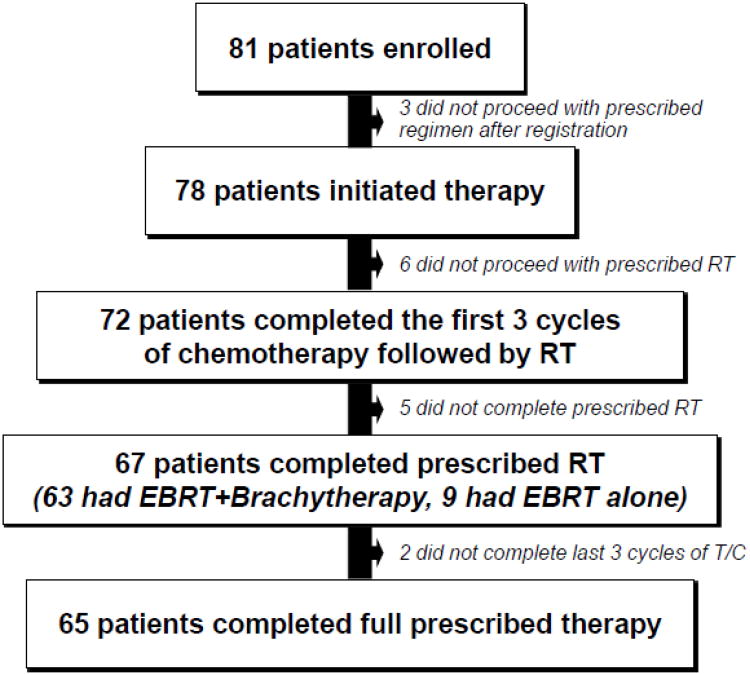
A total of 81 patients were enrolled and 72 evaluable patients were included in this analysis (see Figure 1). Three subjects did not proceed with the prescribed regimen after registration. Of the 78 subjects who proceeded with the protocol-prescribed regimen, 72 patients completed the first 3 cycles of chemotherapy followed by RT. Of the 72, 67 patients completed the prescribed RT (63 had EBRT + brachytherapy and 9 had EBRT alone). Of the 67 patients who completed the first 3 cycles of chemotherapy and prescribed RT, 65 (97%) completed the last 3 cycles of paclitaxel and carboplatin. The carboplatin doses for the 72 subjects who received carboplatin before RT was as follows: carboplatin at an AUC of 7.5 (n=20), 6.5 (n=20), 6 (n=24), under 6 (n=14). Of the 65 who received chemotherapy after RT, the carboplatin doses were 6.5 (n=14), 6.0 (n=14), 5.0 (n=37), under 5 (n=2).
Figure 1.
Treatment numbers on protocol. Toxicity analysis performed on the 78 patients who received at least one dose of chemotherapy. Survival analysis was performed in all patients who received the first 3 cycles of chemotherapy followed by RT
The patient demographics and stage are in Table 1. This is an ethnically and racially diverse referral population from Bronx, NY and the surrounding communities. The mean age of the patients was 67±7.9 (mean±SD). The mean BMI of the patients was 30.7±6.35 (mean±SD). All of the advanced staged patients underwent omental sampling (omentectomy or biopsy) and 37/59 (63%) of the early stage patients underwent omental sampling.
Table 1. Patient Characteristics.
| Number | Percent | |
|---|---|---|
| Race | ||
| Black | 42 | 54 |
| White | 32 | 41 |
| Asian | 2 | 2.5 |
| Other | 2 | 2.5 |
| Ethnicity | ||
| Hispanic | 15 | 19 |
| Non-Hispanic | 63 | 81 |
| FIGO Stage | ||
| Total Stage I | 56 | 72 |
| IA | 45 | 58 |
| IB | 11 | 14 |
| Total Stage II | 8 | 10 |
| Total Stage III | 11 | 14 |
| IIIA | 1 | 1 |
| IIIB | 0 | 0 |
| IIIC1 | 6 | 8 |
| IIIC2 | 4 | 5 |
| Total Stage IV | 3 | 4 |
| IVA | 0 | 0 |
| IVB | 3 | 4 |
Survival
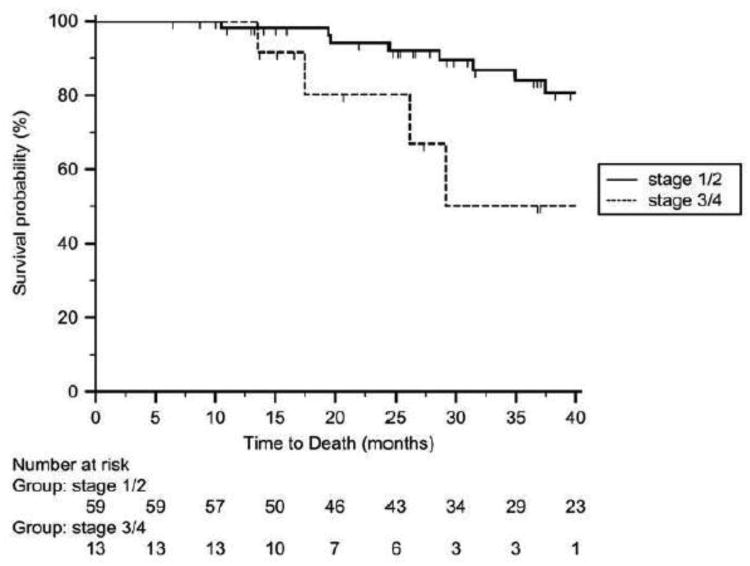
All evaluable patients had a minimum follow-up of 9 months after completion of therapy. Four patients were lost to follow-up after at least 16 months from initiation of treatment and their survival status was censored after the date of last follow-up. Figure 2 shows the overall survival probability dichotomized to early stage (stage 1&2) or advanced stage (stage 3&4) patients. 13/59 (22.0%) early stage patients recurred and 7/13 (53.8%) advanced stage patients recurred during this study period. In the 72 patients who received at least 3 cycles of chemotherapy and EBRT, the overall PFS and OS for early stage patients was 65.5±3.6 (mean±standard error) months and 76.5±4.3 months, respectively. PFS and OS for patients with advanced stage was 25.8±3.0 and 35.9±5.3 months, respectively. Three-year % survival probability for early stage was 84% and advanced stage was 50%, respectively. Of the 65 patients who completed all 3 cycles of chemotherapy followed by EBRT and brachytherapy followed by 3 more cycles of chemotherapy, The three-year % survival probability for early stage patients was 85% and advanced stage patients was 62%, respectively.
Figure 2.
Kaplan-Meier overall survival curve of early stage (stage 1&2) and late stage (stage 3&4) UPSC patients who were prescribed radiation “sandwiched” between three cycles of paclitaxel/platinum chemotherapy before and after RT. In the 72 patients who received at least 3 cycles of chemotherapy and EBRT, overall PFS and OS for early stage patients was 65.5±3.6 (mean±standard error) months and 76.5±4.3 months, respectively. PFS and OS for advanced stage patients was 25.8±3.0 and 35.9±5.3 months, respectively. Three-year % survival probability for early stage patients was 84% and for advanced stage patients was 50%.
There were no patterns or preferential region for recurrence. Of the 20 patients who recurred, 4/20 (20%) recurred in the radiated field and 16/20 (80%) recurred outside the radiated field. Three are still alive with disease getting additional treatment. Of those who died, all but one died of their recurrent cancer. The mean time from disease recurrence to death was 29±11.7 months. There were no treatment related deaths.
Toxicity
Table 2 is a summary of the hematologic and non-hematologic toxicities. A total of 435 cycles of paclitaxel/carboplatin were administered on protocol from which frequencies of hematologic and non-hematologic toxicities were tabulated. There were no significant differences in the frequencies of toxicities of the higher doses of carboplatin used in the minority of subjects during the pilot phase, so the toxicities were combined for analysis. Of note, 71/118 (60.2%) grade 3 and grade 4 toxicities occurred after RT resulting in the majority of dose reductions and/or dose delays (44/62 or 71%; see Table 3). The majority were self-limiting hematologic toxicities and in most cases it was the provider preference to administer growth factor support in subsequent cycles after a grade 3 or 4 hematologic toxicity. There were no patterns of increased long term toxicity attributable to radiation therapy with this regimen.
Table 2. Summary of Grade 3 and 4 Heamatologic and Non-Hematologic Toxicities (n=435 total cycles).
| Grade 3 | Grade 4 | |
|---|---|---|
| Hematologic Toxicities | ||
| Neutropenia | 27 | 44 |
| Anemia | 11 | 2 |
| Thrombocytopenia | 24 | 10 |
| Total | 62 (14.3%) | 56 (12.9%) |
| Non-Hematologic Toxicities | ||
| Infection (e.g. urinary) | 5 | 0 |
| Deep vein thrombosis | 2 | |
| Neuropathy | 2 | 2 |
| Electolyte abnormality | 1 | 0 |
| Dehydration | 1 | |
| Hypertension | 0 | 1 |
| Small bowel obstruction | 0 | 1 |
| Total | 12 (2.8 %) | 4 (0.9%) |
Table 3. Number of dose reduction and delays by cycle (n=435 total cycles).
| Cycle Number | # of Reductions | # of Delays |
|---|---|---|
| 1 | 0 | 2 |
| 2 | 4 | 5 |
| 4 | 4 | 4 |
| 4 | 3 | 5 |
| 5 | 5 | 9 |
| 6 | 10 | 12 |
| Total | 26 (6.0%) | 37 (8.5%) |
Discussion
In this large prospective study of women with completely resected UPSC, RT “sandwiched” between paclitaxel/carboplatin chemotherapy is well-tolerated and highly efficacious, when compared to prior studies of single modality adjuvant treatment for UPSC, including in treated patients with advanced stage disease. The toxicities were acceptable and predictably more common following the RT with the vast majority being self-limiting and managed conservatively. These results show additional long term survival and considerably more prospectively tracked efficacy and toxicity data from our prior pilot report.[11] Patients with both early and advanced stage UPSC who are treated with this “sandwich” protocol have excellent survival, with a 3 year survival probability of 84% in early stage patients and 50% in advanced stage patients. Even better survival was noted among patients who completed all of the prescribed therapy, 85% in early stage patients and 62% in advanced stage patients. These results compare favorably with prior reports in treatments of UPSC.
There is a lack of consensus regarding the standard management of women with UPSC. Most treatments for UPSC appear to involve adjuvant therapy that includes chemotherapy, RT, or both.[4-6,8,12] However most of the prior reports lack the size, power, or prospective data collection to effectively evaluate the true effect size. To our knowledge, this is the largest prospective trial for this rare tumor, and our survival exceeds most prior reports and obviates the added toxicity of this combination regimen. This protocol was well tolerated with generally self-limiting toxicities and limited dose reductions and dose delays.
Despite the fact that this was a prospective trial, there are limitations. The minor differences in the carboplatin dosing regimens during the pilot phase may raise criticism. The carboplatin dose was optimized during the pilot phase, but all doses were within the therapeutic window for carboplatin. After further optimization during the early part of the pilot and registration phase, the prescribed dose regimen remained consistent. Homogeneity between the groups was confirmed by Cox-regression matched analysis regarding toxic events, recurrence, and survival. Also, this is a single institution trial, with the potential for all biases attributed to any single institution study. However, we believe this is a strength of this study, as well, as most of the investigators were actively working together during this whole trial period and became comfortable with the treatment and managing the toxicity. Also, all subjects who met criteria were approached to register. Only 63% of the early stage patients underwent omental sampling. However, if any of the remaining early stage patients had been misclassified as being understaged as a result of lack of an omentectomy, this would only improve our efficacy results in misclassified early stage patients as they would have been upstaged to stage 4, which would improve the advanced stage survival even more. Additionally, we accrued a limited number (n=14) advanced stage patients to this protocol. This is primarily due to the fact that most patients with metastatic UPSC have measurable disease even after staging surgery. The survival in the patients on this protocol cannot be extrapolated to stage 3&4 UPSC patients with measurable disease after surgery.
While the sequencing of the chemotherapy and radiation therapy in this protocol was 3 cycles prior to RT, then 3 cycles after RT, this might not be the optimal sequence for a “sandwich” therapy. It should be noted that the vast majority of patients (65/67) who received 3 doses of chemotherapy followed by EBRT, were able to complete the remaining three cycles of chemotherapy. The additional three cycles of chemotherapy after RT is well-tolerated. Also, in the subset of 65 patients who completed all cycles of chemotherapy and RT there was a slightly higher survival, though not statistically significant. Another “sandwich” method of sequential adjuvant chemotherapy and radiation was further investigated by Geller et al in advanced stage endometrial cancer.[4] This was a retrospective analysis of 23 patients with advanced stage III or IV endometrial cancer, including 12 (52%) of the patients with UPSC, who received adjuvant chemotherapy consisting of carboplatin and a taxane, with total number of chemotherapy cycles prescribed ranging from 4-6, with at least 2 cycles post-radiation. Of the 23 patients, 5 progressed and 3 of these patients died during follow-up. The estimated 1, 3, and 5 year PFS was 100%, 80%, and 74% respectively. This is comparable to the study by Obermair and colleagues who used 4 cycles of paclitaxel plus carboplatin followed by pelvic RT.[7] Further studies on the optimal sequencing in an adjuvant setting in patients with UPSC are warranted. Until other sequences are tested, through, this “sandwich” therapy should be considered and potentially confirmed as a treatment arm in future cooperative group trials.
Research highlights.
Radiation therapy “sandwiched” between paclitaxel/carboplatin chemotherapy is highly efficacious in women with completely resected uterine papillary serous cancer
Toxicities were acceptable and mostly following radiation therapy
Acknowledgments
Funding: This project was funded, in part, by the Albert Einstein Cancer Center NIH/NCI P30CA013330
Footnotes
A portion of this data was presented at the Society of Gynecologic Oncologists (SGO) 2011 Annual Meeting in Orlando, FL
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006:642–6. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton CA, Kapp DS, Chan JK. Clinical aspects of uterine papillary serous carcinoma. Curr Opin Obstet Gynecol. 2008;20:26–33. doi: 10.1097/GCO.0b013e3282f2b10d. [DOI] [PubMed] [Google Scholar]
- 3.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 4.Geller MA, Ivy J, Dusenbery KE, Ghebre R, Isaksson Vogel R, Argenta PA. A single institution experience using sequential multi-modality adjuvant chemotherapy and radiation in the “sandwich” method for high risk endometrial carcinoma. Gynecol Oncol. 2010;118:19–23. doi: 10.1016/j.ygyno.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Lupe K, D'Souza DP, Kwon JS, Radwan JS, Harle IA, Hammond JA, et al. Adjuvant carboplatin and paclitaxel chemotherapy interposed with involved field radiation for advanced endometrial cancer. Gynecol Oncol. 2009;114:94–8. doi: 10.1016/j.ygyno.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Lupe K, Kwon J, D'Souza D, Gawlik C, Stitt L, Whiston F, et al. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys. 2007;67:110–6. doi: 10.1016/j.ijrobp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Obermair A, Mileshkin L, Bolz K, Kondalsamy-Chennakesavan S, Cheuk R, Vasey P, et al. Prospective, non-randomized phase 2 clinical trial of carboplatin plus paclitaxel with sequential radical pelvic radiotherapy for uterine papillary serous carcinoma. Gynecol Oncol. 2011;120(2):179–84. doi: 10.1016/j.ygyno.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Secord AA, Havrilesky LJ, O'Malley DM, Bae-Jump V, Fleming ND, Broadwater G, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009;114:442–7. doi: 10.1016/j.ygyno.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Fader AN, Nagel C, Axtell AE, Zanotti KM, Kelley JL, Moore KN, et al. Stage II uterine papillary serous carcinoma: Carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol Oncol. 2009;112:558–62. doi: 10.1016/j.ygyno.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley H, Lee RB, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;100:349–54. doi: 10.1016/j.ygyno.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2008;108:201–6. doi: 10.1016/j.ygyno.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Geller MA, Ivy JJ, Ghebre R, Downs LS, Jr, Judson PL, Carson LF, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “Sandwich” method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol. 2011;121:112–7. doi: 10.1016/j.ygyno.2010.12.338. [DOI] [PMC free article] [PubMed] [Google Scholar]