The monothiol glutaredoxin Grx5 is defined as a core member of mitochondrial Fe/S protein biogenesis. Grx5 undergoes a highly specific protein interaction with the dedicated Hsp70 chaperone Ssq1. The simultaneous presence of the scaffold protein Isu1 and Grx5 on Ssq1 facilitates the transfer of newly synthesized Fe/S clusters from Isu1 to Grx5.
Abstract
The mitochondrial Hsp70 chaperone Ssq1 plays a dedicated role in the maturation of iron–sulfur (Fe/S) proteins, an essential process of mitochondria. Similar to its bacterial orthologue HscA, Ssq1 binds to the scaffold protein Isu1, thereby facilitating dissociation of the newly synthesized Fe/S cluster on Isu1 and its transfer to target apoproteins. Here we use in vivo and in vitro approaches to show that Ssq1 also interacts with the monothiol glutaredoxin 5 (Grx5) at a binding site different from that of Isu1. Grx5 binding does not stimulate the ATPase activity of Ssq1 and is most pronounced for the ADP-bound form of Ssq1, which interacts with Isu1 most tightly. The vicinity of Isu1 and Grx5 on the Hsp70 chaperone facilitates rapid Fe/S cluster transfer from Isu1 to Grx5. Grx5 and its bound Fe/S cluster are required for maturation of all cellular Fe/S proteins, regardless of the type of bound Fe/S cofactor and subcellular localization. Hence Grx5 functions as a late-acting component of the core Fe/S cluster (ISC) assembly machinery linking the Fe/S cluster synthesis reaction on Isu1 with late assembly steps involving Fe/S cluster targeting to dedicated apoproteins.
INTRODUCTION
Proteins with iron–sulfur (Fe/S) cofactors play important roles in fundamental cellular processes such as redox reactions, catalysis, and the sensing of environmental conditions (Beinert, 2000; Fontecave, 2006; Py and Barras, 2010). In eukaryotes, mitochondria perform a central function in the biosynthesis of cellular Fe/S proteins (Lill, 2009; Sheftel and Lill, 2009; Lill et al., 2012; Balk and Pilon, 2010; Ye and Rouault, 2010). They harbor the Fe/S cluster (ISC) assembly system that is essential for maturation of all cellular Fe/S proteins, whether located in mitochondria, cytosol, or nucleus. The ISC assembly system has been inherited from bacteria, which encode proteins of similar structure and function in the isc operon, which is widely distributed throughout the bacterial kingdom (Johnson et al., 2005; Ayala-Castro et al., 2008; Py and Barras, 2010). Biosynthesis of cytosolic and nuclear Fe/S proteins additionally requires the mitochondrial ISC export machinery and the cytosolic Fe/S protein assembly system (Lill, 2009; Sheftel and Lill, 2009; Sharma et al., 2010). The components involved in the biogenesis of Fe/S cofactors in eukaryotes are highly conserved, and the majority are essential for cell viability, underscoring the importance of Fe/S proteins for a eukaryotic cell.
In the mitochondrial ISC assembly system the de novo synthesis of Fe/S clusters is accomplished on the scaffold protein Isu1 (related to bacterial IscU; Johnson et al., 2005; Lill and Muhlenhoff, 2006, 2008; Fontecave and Ollagnier-de-Choudens, 2008; Ye and Rouault, 2010). Isu1 binds to the cysteine desulfurase complex Nfs1-Isd11, which releases sulfur from cysteine and transfers it to Isu1 as a persulfide (Gerber et al., 2003; Schmucker et al., 2011). De novo Fe/S cluster synthesis on Isu1 further involves the electron transfer chain consisting of the ferredoxin reductase Arh1 (Li et al., 2001) and the ferredoxin Yah1 (Lange et al., 2000; Sheftel et al., 2010; Shi et al., 2012), as well as frataxin (yeast Yfh1) as a putative iron donor and/or regulator of the desulfurase activity (Stemmler et al., 2010; Tsai and Barondeau, 2010).
Fe/S cluster transfer from the Isu1/IscU scaffold protein to target apoproteins requires a dedicated Hsp70 chaperone, its J-type cochaperone, and the monothiol glutaredoxin 5 (Grx5; Rodriguez-Manzaneque et al., 2002; Muhlenhoff et al., 2003; Vickery and Cupp-Vickery, 2007; Kampinga and Craig, 2010; Craig and Marszalek, 2011). Most eukaryotes use the multifunctional Hsp70 of the mitochondrial matrix together with the specialized cochaperone Jac1, which specifically recruits Hsp70 for Fe/S protein maturation (Schilke et al., 2006; Kampinga and Craig, 2010; Pukszta et al., 2010). In contrast, several fungi, including Saccharomyces cerevisiae, and the isc operon–containing bacteria use a specialized Hsp70 chaperone (yeast Ssq1 and bacterial HscA) for Fe/S protein biogenesis (Ciesielski et al., 2012). Unlike multifunctional Hsp70s that bind to a large variety of hydrophobic substrates, Ssq1/HscA selectively recognizes and interacts with the conserved LPPVK peptide loop of Isu1/IscU (Hoff et al., 2002, 2003; Dutkiewicz et al., 2004; Tapley and Vickery, 2004; Knieszner et al., 2005). This interaction is regulated by the cochaperone Jac1 (bacterial HscB), which recruits the scaffold protein Isu1/IscU via its conserved recognition domain and guides it to the chaperone (Silberg et al., 2004; Andrew et al., 2006; Fuzery et al., 2011). Jac1 and Isu1 synergistically stimulate the ATPase activity of the Hsp70 chaperone, as expected for cochaperones and client proteins (Dutkiewicz et al., 2003; Mayer and Bukau, 2005; Vickery and Cupp-Vickery, 2007). Depletion of Jac1 or Ssq1 results in the accumulation of Fe/S clusters on Isu1 because they are not actively transferred to target apoproteins (Dutkiewicz et al., 2003, 2006; Muhlenhoff et al., 2003). Consistently, in vitro experiments with bacterial ISC assembly proteins have documented an accelerated transfer of Fe/S clusters from IscU to acceptor apoproteins in the presence of HscA and HscB (Chandramouli and Johnson, 2006; Bonomi et al., 2008, 2011; Shakamuri et al., 2012). ATP hydrolysis by Ssq1/HscA leads to tight binding of Isu1/IscU and is accompanied by a conformational change that decreases the affinity for the bound Fe/S cluster and thus facilitates its dissociation. The fate of the Fe/S cluster after its release from Isu1/IscU is unknown.
Deficiency of the mitochondrial monothiol glutaredoxin Grx5 diminishes the activities of Fe/S proteins and impairs heme biosynthesis in yeast, zebrafish, and humans (Rodriguez-Manzaneque et al., 2002; Wingert et al., 2005; Camaschella et al., 2007; Ye et al., 2010). Depletion of yeast Grx5 results in the accumulation of Fe/S clusters on Isu1, suggesting that Grx5, similar to Ssq1 and Jac1, is also involved in Fe/S cluster transfer from Isu1 to apoproteins (Muhlenhoff et al., 2003). Purified monothiol glutaredoxins are capable of binding a bridging [2Fe-2S] cluster that is coordinated by two active-site cysteine residues and two non–covalently bound glutathione molecules (GSH; Herrero and de la Torre-Ruiz, 2007; Picciocchi et al., 2007; Lillig et al., 2008; Johansson et al., 2011). The Grx5-bound Fe/S cluster is labile and can be readily transferred to recipient apoproteins in vitro, consistent with an ISC transfer role of Grx5 (Bandyopadhyay et al., 2008; Iwema et al., 2009; Rouhier et al., 2010). However, the relevance of these in vitro observations for its physiological role is unclear.
In this work we investigate the roles of the glutaredoxin Grx5 and the Ssq1-Jac1 chaperones in transferring the Fe/S cluster from its site of synthesis on Isu1 to target apoproteins. We search for interaction partners of Grx5 in the ISC assembly system, ask whether Grx5 binds an Fe/S cluster in vivo, and address its Fe/S target protein specificity. Taken together, our findings suggest a molecular model for how Grx5 cooperates with the Ssq1-Jac1 chaperone system to mechanistically connect the early and late steps of mitochondrial Fe/S protein assembly.
RESULTS
Grx5 specifically interacts with the dedicated Hsp70 chaperone Ssq1
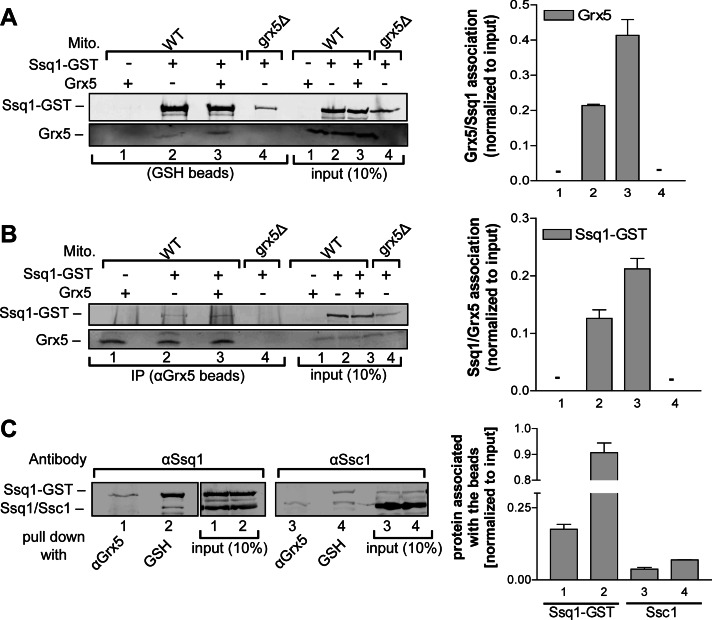
Yeast SSQ1 and GRX5 genetically interact (Rodriguez-Manzaneque et al., 2002), and both gene products participate in the same step of Fe/S protein biogenesis (Muhlenhoff et al., 2003). This prompted us to analyze a potential physical interaction between the two proteins. A yeast expression vector encoding an Ssq1–glutathione S-transferase (GST) fusion protein (Gerber et al., 2003) was cotransformed with a vector overproducing Grx5 into wild-type and grx5Δ yeast cells. Mitochondria were purified and lysed in detergent-containing buffer, and extracts were subjected to GST-affinity purification (Figure 1A) or immunoprecipitation of Grx5 with specific antibodies (Figure 1B; Gerber et al., 2003). In both cases, Grx5 and Ssq1-GST were coisolated as identified by immunostaining and quantitative densitometry (Figure 1, A and B, lanes 2 and 3). This interaction was specific, because no Grx5-specific signal was detected in wild-type mitochondria that overproduced Grx5 but lacked Ssq1-GST (Figure 1, A and B, lane 1) or in mitochondria isolated from grx5Δ cells that expressed only Ssq1-GST (lane 4). Furthermore, the amount of copurified Grx5 increased twofold upon overproduction of Grx5, indicating a dosage-specific interaction with Ssq1-GST (Figure 1, A and B, lanes 2 and 3). Conversely, no Ssq1-GST–specific signal was observed in an anti-Grx5 immunoprecipitation using mitochondria from cells that did not express Ssq1-GST or did not contain Grx5 (Figure 1B, lanes 1, 2, and 4).
FIGURE 1:
Grx5 specifically interacts with the dedicated Hsp70 chaperone Ssq1 in vivo. (A, B) Mitochondria (Mito) from wild-type (WT) and grx5Δ yeast cells overproducing Ssq1-GST, Grx5, or both were lysed by detergent (0.5% Triton X-100) in buffer A and subjected to affinity purification with (A) glutathione (GSH)–Sepharose or (B) antibodies against Grx5 bound to protein A–Sepharose. The purified proteins Ssq1-GST and Grx5 were analyzed by SDS–PAGE and immunostaining (left; by α-Ssq1 and α-Grx5 antibodies) and quantified by densitometry (right). Data were normalized to the protein levels in the respective extracts. Numbers on the right correspond to lane numbers of the immunoblots. +, presence, and –, absence, of the indicated overproduced protein. (C) Mitochondrial lysates from WT cells with overproduced Ssq1-GST and Grx5 were subjected to immunoprecipitations with GSH–Sepharose or antibodies against Grx5. Analysis for the presence of Ssq1 or Ssc1 was as described. The immunoblots for the two proteins were performed on the same gel in parallel. The α-Ssq1 and α-Ssc1 antibodies show slight cross-reactivity due to similarities between Ssq1 and Ssc1 proteins. Error bars, SEM (n = 3).
We asked whether the observed Grx5-Ssq1 interaction is specific or related to a general Hsp70 chaperone function of Ssq1. Previously, it was found that Ssq1 does not cooperate with the cochaperone Mdj1 and thus should not be involved in protein folding in yeast mitochondria (Dutkiewicz et al., 2003). On the other hand, purified Ssq1 has been shown to be capable of protecting denatured rhodanese from aggregation in vitro (Dutkiewicz et al., 2006). A similar situation is observed in Escherichia coli, in which the ISC-specialized Hsp70 HscA is not able to promote protein folding in vivo but can prevent protein aggregation in vitro (Silberg et al., 1998). Hence it was important to analyze the relative Grx5-binding efficiencies of Ssq1 and Ssc1. Extracts from wild-type mitochondria containing overproduced Ssq1-GST and Grx5 were subjected to GST-affinity purification or anti-Grx5 immunoprecipitation, and bound Ssq1-GST or Ssc1 was analyzed by immunostaining and quantified (Figure 1C). As expected for a protein-folding chaperone, both GSH and anti-Grx5 beads copurified a small amount of Ssc1 (Figure 1C, lanes 3 and 4). However, the amount of Ssq1-GST binding to anti-Grx5 immunobeads was at least four times higher than that of Ssc1 (lanes 1–3) documenting the preferential interaction of Grx5 with Ssq1 rather than with Ssc1. This result is even more significant, since Ssc1 is at least 500 times more abundant than Ssq1 in yeast mitochondria (Schilke et al., 1996; Voisine et al., 2000). Hence, if the Grx5-Ssq1 interaction were due to a general Hsp70 chaperone function, Grx5 would be expected to bind almost exclusively to Ssc1. Collectively our in vivo coprecipitation results suggest a specific interaction between Ssq1 and Grx5. Of note, we could not detect any significant interaction of Ssq1 with canonical Fe/S proteins, in neither the holo nor in the apo form (see Supplemental Figure S1 for aconitase; and data not shown).
ISC components and ATP modulate interaction of Grx5 and Ssq1
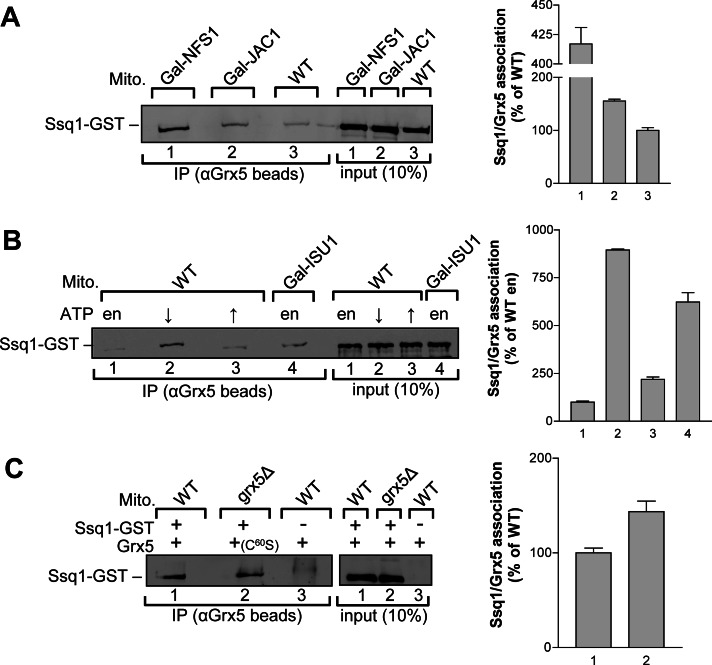
We next examined which parameters influence Grx5-Ssq1 association. First, we investigated whether this interaction is modulated by the availability of other ISC components. Regulatable GAL1-10 promoter-exchange strains of ISC components overproducing both Ssq1-GST and Grx5 were grown in glucose-containing minimal medium until the regulated ISC proteins were depleted to critical levels. Mitochondrial extracts were subjected to immunoprecipitation with anti-Grx5 antibodies, and the amount of coimmunoprecipitated Ssq1-GST was quantified by densitometry. When compared with the wild-type situation, mitochondria from Gal-NFS1 cells showed a fourfold increase in Ssq1-GST association with Grx5 (Figure 2A). Because no Fe/S clusters are formed on the scaffold protein Isu1 in the absence of the cysteine desulfurase Nfs1, this increase indicated that Grx5 can efficiently associate with Ssq1 before the chaperone binds Fe/S cluster-loaded Isu1. A 1.5-fold increase in Grx5-Ssq1 association was observed in mitochondria depleted for Jac1, the J-type protein known to deliver Isu1 to Ssq1 (Figure 2A). The relatively low increase in Grx5-Ssq1 complex formation was likely due to the less efficient depletion of this ISC protein. Mitochondria from depleted Gal-ISU1/isu2Δ cells showed a fivefold increase in Ssq1-GST association with Grx5 (Figure 2B). These data strongly suggest that Grx5 binding to Ssq1 can occur independently of both Fe/S cluster formation on Isu1 and complex formation between Ssq1 and holo-Isu1.
FIGURE 2:
ISC components and ATP/ADP levels modulate the interaction of Grx5 and Ssq1. (A) Mitochondrial detergent lysates (Mito) from Gal-NFS1, Gal-JAC1, and wild-type (WT) cells (grown on SD medium) with overproduced Ssq1-GST and Grx5 were subjected to immunoprecipitations (IPs) with antibodies against Grx5. Immunobeads were analyzed for the presence of Ssq1-GST by SDS–PAGE, immunostaining (left), and quantitation by densitometry (right) as in Figure 1. (B) Mitochondrial lysates from WT or Gal-ISU1/isu2Δ cells (grown on SD medium) overproducing Ssq1-GST and Grx5 were subjected to IPs with antibodies against Grx5 in buffer A without ATP supplementation (endogenous [en]) or with 1 mM ATP (↑). Another sample was first depleted for ATP in the presence of hexokinase and glucose-6-phosphate (↓). The amount of coimmunopurified Ssq1-GST was determined by immunostaining and densitometry as in Figure 1. (C) Mitochondrial lysates from WT and grx5Δ cells with overproduced Ssq1-GST, Grx5, or the site-directed mutant protein Grx5C60S in the indicated combinations were subjected to IPs with antibodies against Grx5. +, presence, and –, absence, of the indicated protein. The amount of coimmunopurified Ssq1-GST was determined by immunostaining and densitometry as in Figure 1. Error bars, SEM (n = 3).
Because Ssq1 is an ATP-dependent Hsp70 chaperone, the nucleotide status of Ssq1 might influence the Grx5-Ssq1 interaction. To test this hypothesis, we incubated wild-type mitochondria with overproduced Ssq1-GST and Grx5 in buffer without and with 1 mM ATP. In addition, ATP was depleted in the organelles by treatment with hexokinase and glucose-6-phosphate. Mitochondrial extracts were subjected to immunoprecipitation with antibodies against Grx5. Under conditions of ATP supplementation, a twofold increase in Ssq1-GST association with Grx5 was observed compared with endogenous ATP levels (Figure 2B, lanes 1 and 3). This increase suggests that Grx5 is able to bind to Ssq1 in its ATP-bound state and is consistent with a Grx5-Ssq1 interaction before the recruitment of holo-Isu1. Strikingly, under ATP-depleting conditions, an eightfold increase in Ssq1-Grx5 association was observed (Figure 2B, lane 2). This indicates that Grx5 binding to Ssq1 is not restricted to one of the nucleotide-binding states, yet is most stably bound to Ssq1-ADP. Consistent with this conclusion, we found a similar efficiency of Grx5 and Ssq1-GST coimmunoprecipitation in the presence of the nonhydrolyzable ATP analogue AMP-PNP as with endogenous ATP levels (Supplemental Figure S2). Taken together, these data indicate that the ADP-bound state of Ssq1 has the highest affinity for Grx5.
Previous in vivo studies showed that the active-site cysteine residue of the monothiol glutaredoxin Grx5 is essential for protein function (Belli et al., 2002; Lillig et al., 2008). To test whether binding of Grx5 to Ssq1 was dependent on this active-site residue, we created a point mutation in which Cys-60 was exchanged for serine. The resulting Grx5C60S protein was overproduced together with Ssq1-GST in grx5Δ cells. Mitochondrial extracts were subjected to immunoprecipitation with antibodies against Grx5, and the Ssq1-GST association with Grx5C60S was determined. Ssq1-GST bound to wild-type and mutant Grx5 with similar efficiency (Figure 2C). The independence of the Grx5-Ssq1 interaction on the active-site cysteine of Grx5 suggests that the Grx5-Ssq1 complex formation does not directly involve the thiol-related function of Grx5. The active-site cysteine residue therefore may play a role in either the stimulation of Ssq1 ATPase activity, Fe/S cluster binding, and/or Fe/S cluster delivery from Isu1 to client apoproteins, as previously suggested (Bandyopadhyay et al., 2008).
Grx5 does not stimulate the ATPase activity of Ssq1, distinguishing it from Isu1
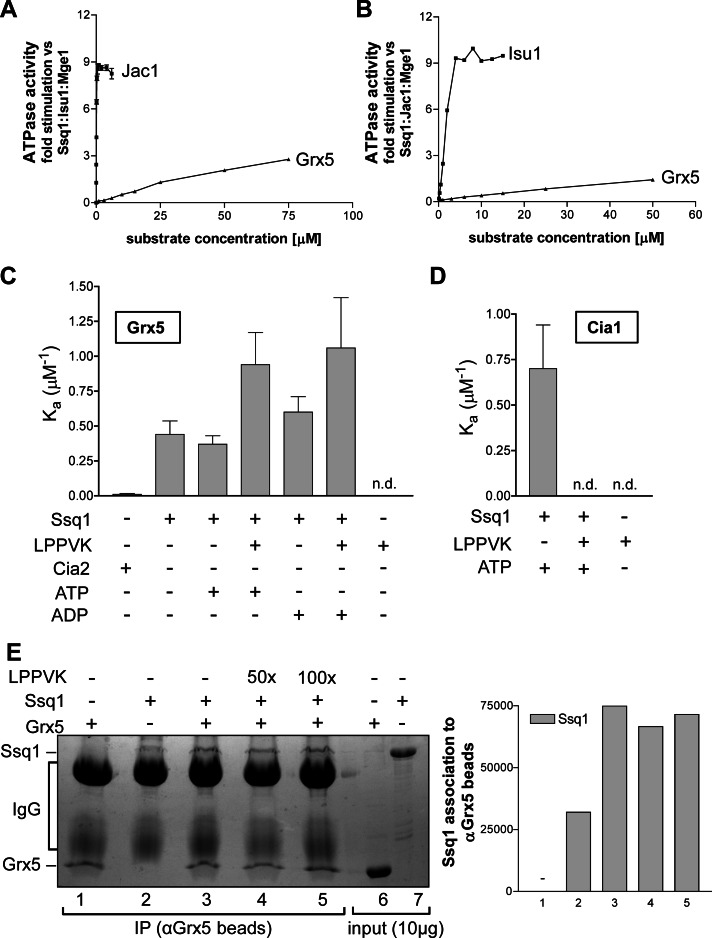
Client proteins interacting with the peptide-binding domain of Hsp70 chaperones stimulate their ATPase activity (Mayer and Bukau, 2005; Craig and Marszalek, 2011). The increased ATPase activity of Hsp70 is considered to be a hallmark of productive interaction with partner proteins. The effect of Grx5 on the Ssq1 ATPase activity was tested in vitro using purified proteins, and the results were compared with those for added Jac1 and Isu1 (Dutkiewicz et al., 2003). The Ssq1 ATPase activity was measured in presence of Mge1 and either Isu1 (Figure 3A) or Jac1 (Figure 3B) under steady-state conditions. Under these conditions, the overall rate of ADP formation depends on the rates of three subreactions: ATP binding, ATP hydrolysis, and dissociation of the reaction products ADP and phosphate. Hardly any stimulation of the ATPase activity of Ssq1 was detected upon addition of up to 10 μM Grx5 in the presence of Mge1 and Isu1 (Figure 3A) or Jac1 (Figure 3B), whereas increasing levels of Jac1 and Isu1, respectively, were highly effective. The slight increase in ATP hydrolysis at higher concentrations of Grx5 might be due to contaminants present in the protein samples. Taken together, these data suggest that Grx5 differs from Isu1 and Jac1, the specific chaperone cycle interaction partners of Ssq1, in that Grx5 is not able to stimulate the ATPase activity of Ssq1. Grx5 is thus unlikely to interact at the canonical peptide-binding site of Ssq1 (see later discussion).
FIGURE 3:
Grx5 interacts with Ssq1 in vitro but does not stimulate its ATPase activity. (A, B) The ATPase activity of Ssq1 (0.5 μM) was measured in the presence of various amounts of Grx5 as a potential substrate. Samples contained (A) 0.5 μM Mge1 and 10 μM Isu1 or (B) Mge1 and Jac1 (0.5 μM each). The ATPase stimulation by increasing amounts of (A) Jac1 or (B) Isu1 as a substrate is included for comparison. The ATPase activity in the absence of titrated proteins was set to zero. Reactions (15 μl) were started by the addition of [γ-32P]ATP (1 μCi) to a final concentration of 120 μM. Incubation was carried out at 25°C, and the reaction was terminated after 15 min by the addition of 100 μl of 1 M perchloric acid and 1 mM sodium phosphate. The release of radioactive Pi from [γ-32P]ATP was measured as described (Dutkiewicz et al., 2003). (C, D) NT-647 dye–labeled Grx5 (C) or Cia1 (D) at 200 nM each was titrated with serial (1:2) dilutions of Cia2 (ranging from 116 μM to 3.5 nM), Ssq1 (87 μM to 2.65 nM), or the Isu1-derived peptide AKELSLPPVKLHC (LPPVK; 130 μM to 3.9 nM) in the indicated combinations in the presence of 1 mM ATP or ADP as indicated. MST assays were performed, and apparent affinity constants (Ka) were calculated by theoretical fitting (see Supplemental Figure S3). Error bars, SD (n = 3). n.d., not determinable, meaning that due to the lack of significant protein interactions, MST data cannot satisfactorily be used to determine Ka values by fitting. (E) Purified Grx5 and/or Ssq1 proteins (4 μM each) were incubated in buffer H containing 2 mM ADP and, where indicated, the Isu1 peptide (LPPVK) at 200 μM (50×) or 400 μM (100×) concentration. Immunoprecipitations were carried out with antibodies against Grx5. The presence of (co-) immunoprecipitated Grx5 and/or Ssq1 was analyzed by SDS–PAGE and Coomassie staining (left), and Ssq1 was quantified by densitometry (right). Numbers on the right graph correspond to lanes of the left. C–E: +, presence, and –, absence, of the indicated component.
To investigate whether the physical interaction between Grx5 and Ssq1 can be observed with the isolated proteins, we carried out an in vitro association study by microscale thermophoresis (MST). MST is the directed movement of particles in a microscopic temperature gradient. Changes in the structure, hydration, and/or conformation of biomolecules result in a change of their thermophoretic behavior. This effect can be used to determine binding affinities, binding kinetics, and activity kinetics (Wienken et al., 2010; Zillner et al., 2012). To observe the interaction between purified Grx5 and Ssq1, we fluorescently labeled Grx5 and subjected it to microscale thermophoresis in the presence of increasing amounts of Ssq1. The presence of Ssq1 changed the thermophoretic behavior of Grx5, suggesting a binary association (Figure 3C and Supplemental Figure S5A). From a theoretical fitting, we calculated a binding constant Ka = 0.44 (± 0.1) μM−1. This interaction was specific, because no significant binding of Grx5 to the cytosolic protein Cia2 was observed under the same experimental setup (Figure 3C, bar 1). Remarkably, the Grx5-Ssq1 interaction did not significantly increase upon addition of ATP or ADP (bars 3 and 5). This might suggest that Ssq1 did not function as a canonical Hsp70 under these conditions and possibly bound Grx5 outside of its substrate-binding site.
To investigate this hypothesis, we performed Grx5-Ssq1 binding studies in the presence of the Isu1-derived peptide LPPVK, which specifically and tightly interacts with the peptide-binding pocket of Ssq1 and stimulates its ATPase activity (Hoff et al., 2003; Dutkiewicz et al., 2004; Vickery and Cupp-Vickery, 2007). If Isu1 and Grx5 were using overlapping binding sites on Ssq1, the LPPVK peptide would be expected to compete with Grx5 for Ssq1 binding. However, in the presence of both ATP and ADP the Grx5-Ssq1 interaction increased twofold rather than decreased upon addition of excess LPPVK peptide (Figure 3C, bars 3, 4, and 6, and Supplemental Figure S3A). Because no direct interaction of Grx5 with the LPPVK peptide was detected (Figure 3C, bar 7), these observations indicate that the Ssq1-binding sites for Grx5 and Isu1 do not overlap. This conclusion is consistent with the observation described previously that Grx5 is not a client protein of Ssq1, as it failed to stimulate the chaperone ATPase activity of Ssq1. We note that the interaction between Grx5 and Ssq1 was strongest in the presence of nucleotide and the LPPVK peptide. Because ATP may hydrolyze upon binding of the peptide, this finding was qualitatively reminiscent of the strong Grx5-Ssq1 interaction seen upon ATP depletion in organello (Figure 2B).
As a control for the specificity of the binding of the LPPVK-containing peptide to the substrate-binding site of Ssq1, we analyzed the interaction of Ssq1 with the cytosolic protein Cia1, unlikely to functionally engage with Ssq1 in Fe/S cluster assembly, and also measured the influence of the LPPVK peptide (Lill, 2009). In the presence of ATP and Ssq1, changes in the thermophoretic behavior of fluorescently labeled Cia1 were observed that indicated binding of Cia1 to Ssq1, likely serving as a folding Hsp70 chaperone in this case, as previously observed for rhodanese (Dutkiewicz et al., 2006; Figure 3D, bar 1, and Supplemental Figure S3B). Strikingly, upon addition of excess LPPVK peptide the interaction between Cia1 and Ssq1 disappeared (Figure 3D, bar 2). Because Cia1 did not interact with the peptide (lane 3), this observation indicates that the LPPVK peptide successfully competes with Cia1 for the substrate-binding site of Ssq1, clearly indicating that Cia1 is treated as a client protein of the Ssq1 chaperone under these in vitro conditions. The reverse binding behavior of Grx5 to Ssq1 in the presence or absence of the LPPVK peptide (Figure 3, C and D) further supports the idea that Grx5 binds outside the substrate-binding site of Ssq1.
The Grx5-Ssq1 interaction was further characterized by in vitro binding studies using protein A–Sepharose coupled with antibodies against Grx5. When Ssq1 was incubated alone with anti-Grx5 beads, only weak binding to the beads was observed (Figure 3E, lane 2). In the presence of Grx5, this binding was considerably increased, verifying the Grx5-Ssq1 interaction under in vitro conditions (lane 3). When binding experiments were carried out in the presence of the LPPVK-containing peptide the Grx5-Ssq1 interaction remained unchanged, even in the presence of a 100-fold excess of the peptide (Figure 3E, lanes 4 and 5). Taken together, the results from these different interaction studies indicate that the Grx5- and Isu1-binding sites on Ssq1 do not overlap.
Finally, we investigated whether Grx5 modulated the known interactions of Isu1 with Jac1 and Ssq1. We subjected purified mitochondria from wild-type and grx5Δ cells overproducing both Isu1 and Jac1 to coimmunoprecipitation experiments with specific anti-Isu1 and anti-Jac1 immunobeads. No significant difference in the binding of Isu1 to Jac1 was observed between wild-type and grx5Δ cells (Supplemental Figure S4A). For studying the Isu1-Ssq1 association, mitochondrial extracts from wild-type and grx5Δ cells overproducing Ssq1-GST were subjected to affinity purification with glutathione–Sepharose. In both cells, Isu1 was bound to glutathione–Sepharose (Supplemental Figure S4B). However, in grx5Δ cells the overproduction of Ssq1-GST was inefficient and could be achieved only by omitting lysine and glutamate from the medium (Supplemental Figure S4C). As a consequence, the amount of copurified Isu1 during Ssq1-GST affinity purification was low. When the amount of copurified Isu1 per Ssq1-GST was quantified by densitometry, no difference in relative binding was detectable between wild-type and grx5Δ cells (Supplemental Figure S4D). Taken together, the results indicate that Grx5 specifically binds to Ssq1 at a site different from that of Isu1. This interaction does not stimulate the chaperone ATPase activity of Ssq1 and does not influence the interactions of Ssq1 with its other partners, Isu1 and Jac1.
Grx5 transiently binds a [2Fe-2S] cluster and is involved in maturation of both [2Fe-2S] and [4Fe-4S] proteins
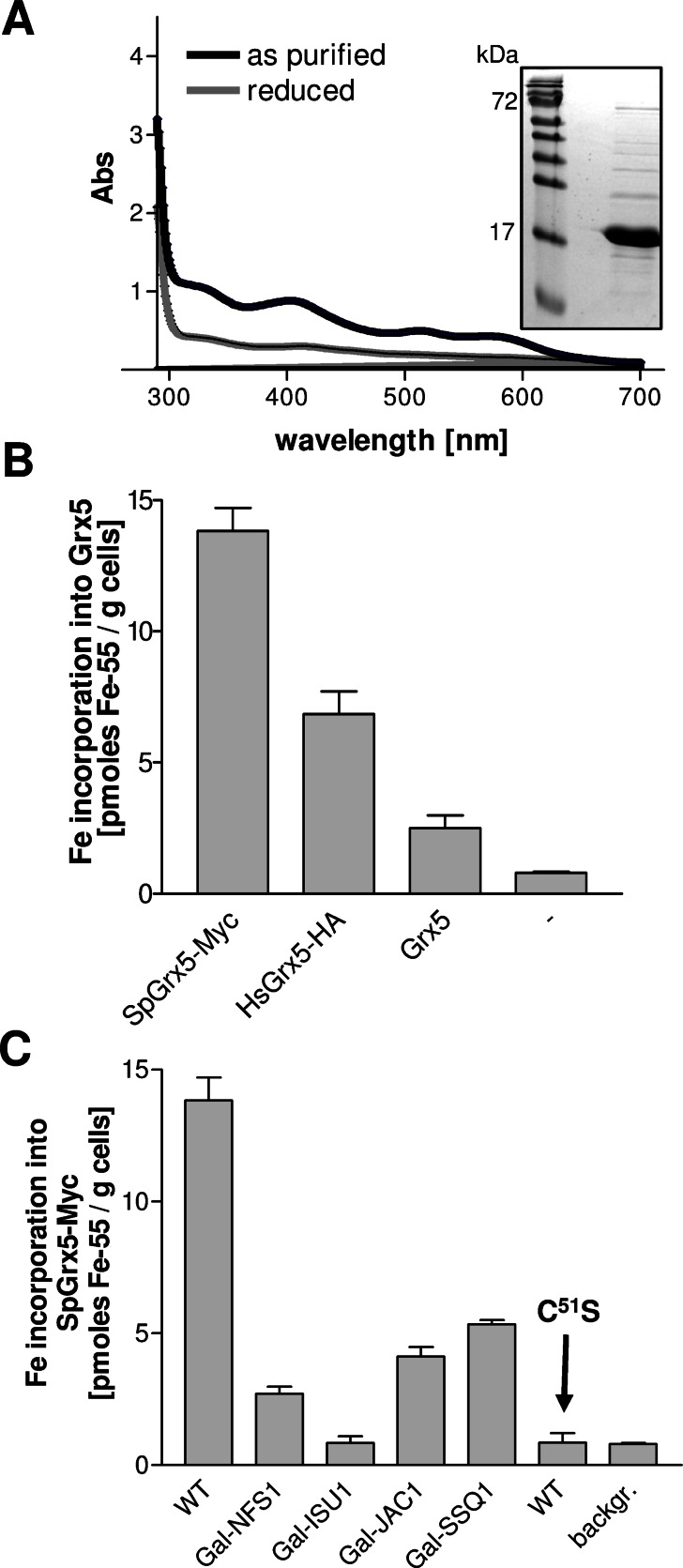
Monothiol glutaredoxins have been shown in vitro to bind a labile, GSH-coordinated Fe/S cofactor that can be transferred to recipient apoproteins, suggesting a role as an Fe/S scaffold or transfer protein (Bandyopadhyay et al., 2008; Iwema et al., 2009; Rouhier et al., 2010). When Grx5 from S. cerevisiae was synthesized and purified from E. coli, the protein carried a [2Fe-2S] cluster as judged by ultraviolet–visible spectroscopy (Figure 4A). However, the physiological relevance of Fe/S cluster binding to mitochondrial Grx5 has not been confirmed by in vivo approaches. We therefore investigated this question in yeast. Cells overproducing S. cerevisiae Grx5 were radiolabeled with 55Fe, Grx5 was immunoprecipitated with specific antibodies, and the amount of coimmunoprecipitated 55Fe was quantified by scintillation counting. The amount of 55Fe specifically coimmunoprecipitated with Grx5 was low, yet the signal was significantly above background (Figure 4B). Given that we previously observed largely increased Fe/S cluster binding to ectopically expressed cytosolic monothiol glutaredoxins Grx3-Grx4 (Hoffmann et al., 2011), we analyzed whether Grx5 homologues from other species would bind iron more efficiently. We used a Myc-tagged Grx5 from Schizosaccharomyces pombe (Sp) and a hemagglutinin (HA)-tagged version of human Grx5 (Hs). Both foreign Grx5 proteins were targeted to mitochondria and were functional, as they rescued the growth defects of grx5Δ cells (Supplemental Figure S5). Remarkably, upon 55Fe radiolabeling, both SpGrx5 and HsGrx5 bound much higher levels of 55Fe than S. cerevisiae Grx5 (Figure 4B). Subsequent 55Fe-binding studies were therefore performed with SpGrx5.
FIGURE 4:
Grx5 binds a Fe/S cluster in vivo and in vitro. (A) Ultraviolet–visible spectra of recombinant Grx5 from S. cerevisiae were recorded for 1 mg of protein, as purified or after reduction by 2 mM sodium dithionite. The inset shows a Coomassie staining of the purified protein analyzed by SDS–PAGE. (B) Wild-type cells overproducing Grx5 from S. pombe (SpGrx5-Myc), Homo sapiens (HsGrx5-HA), or S. cerevisiae (Grx5) or harboring the empty vector p424-TDH3 (–) were radiolabeled with 10 μCi of 55Fe for 2 h. The overproduced proteins were immunoprecipitated from cell extracts with antibodies against Myc (SpGrx5-Myc), HA (HsGrx5-HA), or Grx5, and the amount of coprecipitated 55Fe was quantified by scintillation counting. (C) The indicated yeast strains overproducing SpGrx5-Myc and wild-type (WT) cells overproducing SpGrx5C51S-Myc (C51S) were grown in iron-poor SD medium. The 55Fe binding to SpGrx5-Myc proteins was determined by radiolabeling and immunoprecipitation as described. Gal strains were depleted to critical levels before experiments. Error bars, SEM (n > 4).
We first determined whether the 55Fe is coordinated by the active-site cysteine residue of SpGrx5. When this amino acid was exchanged to serine (mutant C51S), 55Fe binding to SpGrx5 was completely abolished, indicating the specificity of 55Fe binding (Figure 4C). To analyze whether the bound iron is part of a Fe/S cluster, we determined the dependence of 55Fe binding on components of the mitochondrial ISC assembly machinery such as the cysteine desulfurase Nfs1, the scaffold protein Isu1, and the chaperones Jac1 and Ssq1. The ISC proteins were depleted to critical levels by cultivation of the corresponding GAL promoter–regulated strains in presence of glucose, and 55Fe binding was measured by the described radiolabeling-immunoprecipitation assay. On depletion of the four ISC proteins, the amounts of 55Fe coimmunoprecipitated with SpGrx5 were severely decreased (Figure 4C). These results suggest that Grx5 binds an Fe/S cluster under physiological conditions. The dependence on the active-site cysteine supports the physiological relevance of the [2Fe-2S] cluster assembled on Grx5 under in vitro conditions (Picciocchi et al., 2007; Figure 4, A and C). Of importance, the fact that 55Fe binding to Grx5 was dependent on Isu1 made a scaffold function of Grx5 in the de novo synthesis of Fe/S clusters unlikely. Instead, our findings indicate that Grx5 receives its Fe/S cluster from Isu1 and hence might function subsequently to Isu1. This scenario fits perfectly with our previous in vivo observation that depletion of Grx5 leads to an accumulation of Fe/S clusters on Isu1 (Muhlenhoff et al., 2003). We therefore suggest a role of Grx5 in mediating the transfer of Fe/S clusters from Isu1 to target apoproteins, possibly by acting as a Fe/S cluster transfer protein, as recently proposed (Rouhier et al., 2010).
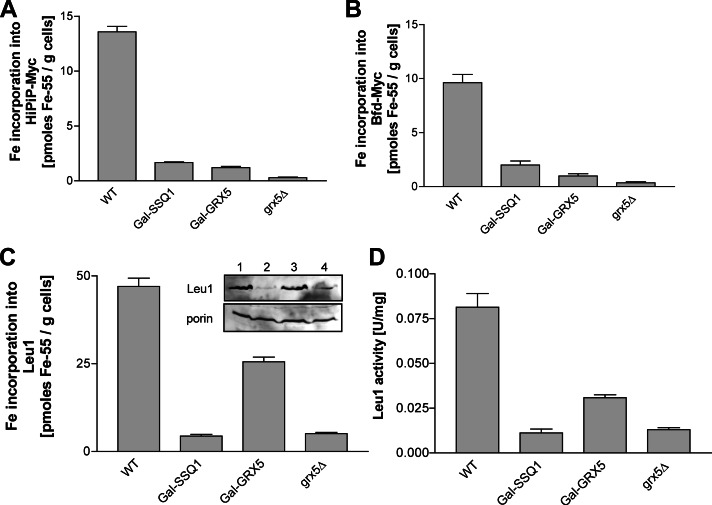
We next asked whether Grx5 was required for the maturation of both [2Fe-2S] and [4Fe-4S] cluster–containing proteins or whether its function was specific for one type of Fe/S cluster, as was found, for example, for the Isa1-Isa2 or Iba57 proteins, which specifically assist the maturation of mitochondrial [4Fe-4S] proteins but are dispensable for [2Fe-2S] protein maturation (Gelling et al., 2008; Muhlenhoff et al., 2011; Sheftel et al., 2012). The previously observed genetic interaction of GRX5 and ISA2 and the fact that grx5Δ cells display a glutamate auxotrophy, similar to ISA- and IBA57-defective cells, might suggest a specificity for [4Fe-4S] proteins (Rodriguez-Manzaneque et al., 2002; Kim et al., 2010). To clarify the Fe/S cluster specificity of Grx5, we used the 55Fe radiolabeling assay to study the maturation of two bacterial reporter Fe/S proteins: the HiPIP ferredoxin, which harbors a [4Fe-4S] cluster, and the small [2Fe-2S] protein Bfd from E. coli (Garg et al., 1996; Muhlenhoff et al., 2011). For both proteins, the amount of 55Fe binding declined to background levels in grx5Δ cells and was strongly diminished in Grx5-depleted Gal-GRX5 cells similar to the extent found for cells depleted of Ssq1 (Figure 5, A and B). This observation clearly distinguishes Grx5 from the Isa and Iba57 proteins, which do not affect [2Fe-2S] protein maturation (Gelling et al., 2008; Muhlenhoff et al., 2011; Sheftel et al., 2012). In a similar manner, maturation and enzyme activity of the cytosolic Fe/S protein Leu1 were both strongly diminished in grx5Δ and Grx5-depleted Gal-GRX5 cells, similar to cells depleted of Ssq1 (Figure 5, C and D). Thus Grx5 is also required for cytosolic Fe/S protein biogenesis, unlike the Isa and Iba57 proteins (Gelling et al., 2008; Muhlenhoff et al., 2011). Consistent with this observation, depletion of yeast Grx5 showed a strong activation of the Aft1-dependent iron regulon (Gelling et al., 2008). This phenotype is observed in cells with defects in core members of the mitochondrial ISC assembly machinery but not in cells lacking late-acting ISC proteins, such as the Isa or Iba57 proteins. Taken together, these data strongly suggest that Grx5 belongs to the core part of the mitochondrial ISC assembly system that is involved in the maturation of all cellular Fe/S proteins and participates in cellular iron regulation.
FIGURE 5:
Grx5 is required for both mitochondrial and cytosolic Fe/S protein maturation. Wild-type (WT), Gal-SSQ1, Gal-GRX5, and grx5Δ cells (grown on SD medium) overproducing mitochondria-targeted (A) [4Fe-4S] (HiPIP) or (B) [2Fe-2S] (Bfd) cluster–containing bacterial proteins were radiolabeled with 55Fe. Protein-bound radioactivity was determined by immunoprecipitation as described in Figure 4B. (C) The 55Fe binding to endogenous Leu1 was determined by radiolabeling and immunoprecipitation as described in Figure 4B. The inset shows Leu1 protein levels in analyzed samples (1, WT; 2, Gal-SSQ1; 3, Gal-GRX5; 4, grx5Δ). (D) Leu1 enzyme activities were determined in whole-cell extracts from the indicated strains cultivated in YPD. Gal strains were depleted to critical ISC protein levels by cultivation in SD medium for 64 h before the experiments. Error bars, SEM (n > 4).
DISCUSSION
In this work we report several key observations that allow us to better define the function of the monothiol glutaredoxin Grx5 in the mitochondrial ISC assembly pathway. First, we show in vivo that Grx5 is an Fe/S protein and receives its Fe/S cluster from the Isu1 scaffold. This makes it unlikely that Grx5 per se functions as a scaffold protein as previously suggested (Bandyopadhyay et al., 2008; Rouhier et al., 2010). Second, we identified an as-yet-unknown protein interaction between Grx5 and the dedicated Hsp70 chaperone Ssq1. This specific complex formation was verified by several independent in vivo and in vitro approaches and appears to be crucial for the transfer of the Fe/S cluster synthesized on the scaffold protein Isu1 to target Fe/S apoproteins. Third, formation of the Ssq1-Grx5 complex was increased when Fe/S cluster synthesis was impaired. This indicates that already the apo form of Grx5 may bind to Ssq1. Because Fe/S cluster binding to Grx5 was dependent on the Ssq1-Jac1 chaperone system, it seems likely that apo-Grx5 binds to Ssq1 and is matured to its holo form in this bound state. Fourth, the Isu1peptide LPPVK and Grx5 were shown to bind simultaneously to the Ssq1 chaperone using independent binding sites. Most efficient Ssq1-Grx5 interaction was observed in the ADP state of Ssq1, that is, the conformation that tightly binds Isu1 (Dutkiewicz et al., 2003; Silberg et al., 2004; Vickery and Cupp-Vickery, 2007; Craig and Marszalek, 2011). In the bacterial ISC system, the conformational change of the U-type scaffold protein induced upon binding to the Ssq1 orthologue HscA induces the weakening of Fe/S cluster association to IscU (Bonomi et al., 2011). Thus the simultaneous binding of the holo U-type scaffold protein and the monothiol Grx5 on the dedicated Hsp70 chaperones Ssq1 or HscA likely facilitates the efficient Fe/S cluster transfer from the U-type scaffold to Grx5 in both bacteria and eukaryotes. In the bacterial ISC system, the presence of the Hsp70 HscA/HscB largely stimulates Fe/S cluster transfer from IscU to Grx5 (Shakamuri et al., 2012). Hence the formation of this trimeric complex likely provides a kinetic advantage for holo Grx5 maturation over the accidental collision of the two proteins. Finally, we show that the Grx5-bound Fe/S cofactor is crucial for Grx5 function, as mutation of the active-site cysteine of Grx5 impaired both Fe/S cluster binding and its physiological function (Belli et al., 2002; and this work). Our in vivo data are consistent with the idea that Grx5 binds the Fe/S cluster only transiently and further transfers it toward apoproteins as previously suggested from in vitro data (Bandyopadhyay et al., 2008; Rouhier et al., 2010). However, based on the available data, it cannot be excluded that, after maturation to its holo form, Grx5 fulfills an enzymatic function or serves as a redox partner within the ISC assembly pathway. The sum of our observations strongly supports the idea that the monothiol Grx5 1) receives its own Fe/S cluster from Isu1 during complex formation of both proteins with the Ssq1 chaperone, 2) functions in Fe/S cluster transfer from the site of cluster synthesis on the Isu1 scaffold to target apoproteins, and 3) operates after the Ssq1-Jac1 chaperone-mediated Fe/S cluster release from Isu1. The recent data from the bacterial system strongly suggest that this scheme is likely a general feature of all ISC systems (Bandyopadhyay et al., 2008; Bonomi et al., 2011; Shakamuri et al., 2012).
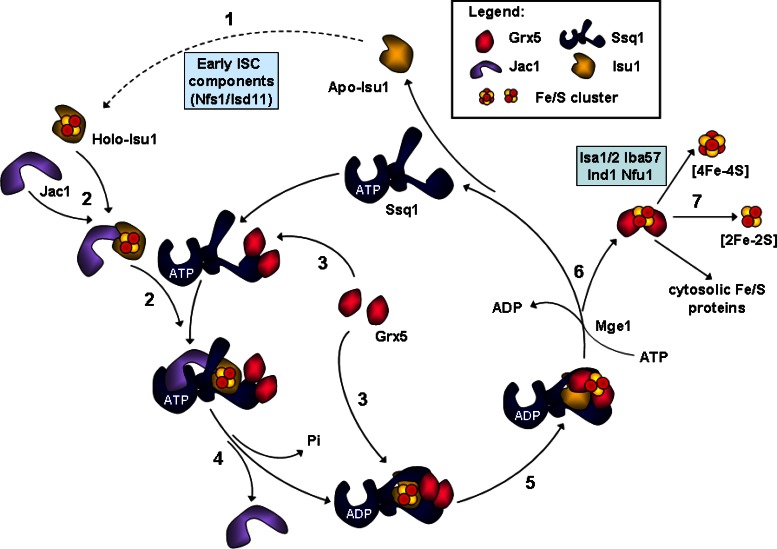
These findings suggest a detailed model of how Grx5 is integrated into the ISC assembly pathway, in particular into the well-established catalytic cycle of the dedicated Ssq1-Jac1 chaperones (Figure 6; Vickery and Cupp-Vickery, 2007; Kampinga and Craig, 2010). Unraveling of this Hsp70 cycle has largely benefited from numerous studies on the role of these chaperones in protein folding (Bukau and Horwich, 1998; Mayer and Bukau, 2005; Craig and Marszalek, 2011; Hartl et al., 2011; Schlecht et al., 2011). According to the current model for Hsp70 chaperone function (Vickery and Cupp-Vickery, 2007; Kampinga and Craig, 2010), Ssq1 in its ATP state binds the holo form of Isu1, which has been assembled by the cysteine desulfurase complex Nfs1-Isd11 and other early-acting components of the ISC assembly machinery (Lill et al., 2012; Figure 6, steps 1 and 2). Complex formation between Ssq1 and holo Isu1 is facilitated by the J-type cochaperone Jac1, which recruits Isu1 and targets it to Ssq1 (Figure 6, step 2). ATP hydrolysis, stimulated by both Isu1 and Jac1, induces a conformational change of Ssq1 to its closed ADP state, thus stabilizing the interaction between the Ssq1 peptide-binding domain and the LPPVK motif of Isu1, while Jac1 leaves the complex (Figure 6, step 4). This induces a conformational change on Isu1 that is, in analogy to the bacterial ISC system (Bonomi et al., 2011; Shakamuri et al., 2012), likely to destabilize Fe/S cluster binding on the Isu1 scaffold protein (Figure 6, step 5; see also later discussion). The nucleotide exchange factor Mge1 then supports the exchange of ADP to ATP, which in turn leads to dissociation of Isu1 and the closing of the cycle (Figure 6, step 6).
FIGURE 6:
Working model for the roles of the mitochondrial chaperones Ssq1-Jac1 and the glutaredoxin Grx5 in Fe/S protein maturation in eukaryotes. The model is based on the well-established working cycle of the Hsp70 chaperone system in the ISC assembly pathway (Vickery and Cupp-Vickery, 2007; Kampinga and Craig, 2010) and integrates the findings on Grx5 function from this work. 1) Initially, an ISC is synthesized de novo on the scaffold protein Isu1 involving early-acting ISC assembly components such as Nfs1-Isd11 (Lill et al., 2012). 2) The cochaperone Jac1 recruits the ISC-loaded Isu1 and targets it to the ATP-bound form of the Hsp70 chaperone Ssq1. 3) Grx5 binds to Ssq1 in either its ATP- and ADP-bound state but has a higher affinity for the ADP-bound form of Ssq1. Most likely Grx5 binding to Ssq1 precedes that of Isu1, but the presence of Grx5 on Ssq1 is no prerequisite for binding of Isu1 to the chaperone. 4) Jac1- and Isu1-induced ATP hydrolysis triggers a conformational change of the peptide-binding domain of Ssq1, leading to tight binding of the LPPVK peptide of Isu1 and of Grx5. 5) The simultaneous presence of these two proteins on Ssq1 facilitates efficient ISC transfer from Isu1 to Grx5. 6) The nucleotide exchange factor Mge1 mediates the exchange of ADP to ATP on Ssq1. The associated conformational switch leads to less stable binding of Isu1 and Grx5, resulting in dissociation of the trimeric Ssq1-Isu1-Grx5 complex. 7) Finally, Grx5 facilitates ISC integration into recipient apoproteins, a function performed in cooperation with late-acting ISC targeting factors such as Isa1-Isa2, Iba57, Ind1, and Nfu1. For further details see the text and Lill et al. (2012).
The monothiol Grx5 enters this cycle by associating with Ssq1 at a specific binding site that is independent of that of Isu1, since both proteins can interact simultaneously with Ssq1 (Figure 6, step 3). In keeping with this view, the LPPVK peptide of Isu1 increased rather than interfered with Ssq1-Grx5 association, making it unlikely that Grx5 binds to the canonical peptide-binding site of Hsp70. This fully explains why Grx5 was unable to stimulate the ATPase activity of Ssq1, unlike Jac1 and Isu1. Even though the ADP state of Ssq1 binds Grx5 most efficiently, the entry of Grx5 may also precede the association of Ssq1 and the Isu1 scaffold. This is evident from the fact that the in vivo interaction between Grx5 and Ssq1 was even stronger in the absence of Isu1. Although this shows that Grx5-Ssq1 can interact independently of Isu1, the observed increase in binding in vivo might be mostly due to low ATP levels prevailing in the absence of functional Isu1 and other ISC members such as Jac1 and Nfs1. In vitro, a twofold increase in Grx5-Ssq1 affinity was observed upon addition of the LPPVK peptide. At first glance, the in vivo and in vitro observations are contradictory. However, they can easily be understood in terms of the fact that in vivo depletion of Isu1 results in low ratios of ATP/ADP. Thus Grx5 is expected to be associated with the ADP-bound state of Ssq1, which represents the optimal binding partner for Grx5. In vitro, the nucleotide exchange factor Mge1 was not present. Hence Ssq1 will be mostly in the ADP conformation, where Isu1 and Grx5 are bound most efficiently. The tight binding and vicinity of both Isu1 and Grx5 on the ADP-state of Ssq1 may then facilitate Fe/S cluster transfer from Isu1 to Grx5 (Figure 6, step 5). As mentioned, Fe/S cluster binding to IscU is loosened up by ATP hydrolysis, favoring its transfer to Grx5 in the bacterial system (Bonomi et al., 2011; Shakamuri et al., 2012). After dissociation of the trimeric complex Ssq1-Isu1-Grx5 (Figure 6, step 6), holo Grx5 cooperates with the late-acting targeting factors of the ISC assembly machinery to deliver and assemble Fe/S clusters on target apoproteins (Figure 6, step 7). Thus Grx5 functions at the interface of the early phase of Fe/S protein biogenesis, leading to the synthesis of the Fe/S cluster and the late phase of cluster integration into apoproteins. This raised the interesting question of whether Grx5 belongs to the core ISC machinery involved in the maturation of all Fe/S proteins or fulfills a more specific function.
An important clue toward answering this question came from our finding that Grx5 was required for the maturation of mitochondrial [2Fe-2S] and [4Fe-4S] proteins, as well as of cytosolic Fe/S proteins. This behavior is similar to that of Nfs1, Isu1, Jac1, or Ssq1 and clearly defines Grx5 as a member of the core mitochondrial ISC assembly machinery, which is needed for maturation of all cellular Fe/S proteins (Lill et al., 2012). We therefore conclude that Grx5 is a late-acting component of the core ISC assembly machinery, yet precedes the function of the more specific ISC-targeting factors. These include the Isa and Iba57 proteins, which are involved in the biogenesis of [4Fe-4S] proteins by facilitating the conversion of [2Fe-2S] to [4Fe-4S] clusters (Gelling et al., 2008; Kim et al., 2010; Muhlenhoff et al., 2011; Sheftel et al., 2012; Figure 6, step 7). The [4Fe-4S] clusters generated by the Isa and Iba57 proteins are then believed to transiently bind to the ISC-targeting proteins Ind1 and Nfu1, which specifically deliver them to target apoproteins, such as respiratory complexes I and II or lipoate synthase (Bych et al., 2008; Sheftel et al., 2009; Cameron et al., 2011; Navarro-Sastre et al., 2011). In support of this sequence of events, maturation of the Fe/S cluster of Nfu1 requires Grx5 function (Navarro-Sastre et al., 2011).
Binding of Grx5 was specific for the dedicated chaperone Ssq1. Grx5 hardly bound to the general mitochondrial protein-folding and import Hsp70 chaperone Ssc1. Moreover, we did not observe any significant interaction of Ssq1 with canonical Fe/S apoproteins in mitochondrial extracts (Supplemental Figure S1). As mentioned, the Grx5 interaction does not involve the peptide-binding site of Ssq1. This unusual association is reminiscent of the interaction between the chaperone Ssc1 and Tim44 during mitochondrial protein import, in which the Ssc1-bound Tim44 is released from Ssc1 upon peptide binding before ATP hydrolysis (D'Silva et al., 2004; Chacinska et al., 2009). As a further example, the cochaperone CHIP has no effect on the basal ATPase activity of Hsp70s and Hsp90s (Stankiewicz et al., 2011). The observation that Grx5 can bind to Ssq1 in the absence of its client protein Isu1 is again reminiscent of the interaction of Ssc1 with Tim44 during mitochondrial protein import. Ssc1 binds to Tim44 independently of the ability of Ssc1 to associate with unfolded proteins in transit (D'Silva et al., 2004; Chacinska et al., 2009).
Taken together, our work establishes an unusual interaction between a monothiol glutaredoxin and a specialized mitochondrial Hsp70 chaperone, which both play a crucial role in the maturation of all cellular Fe/S proteins. The simultaneous association of both the scaffold protein Isu1 and Grx5 on this dedicated chaperone leads to Fe/S cluster transfer from Isu1 to Grx5. This scenario mechanistically explains the accumulation of Fe/S clusters on Isu1 in cells depleted of Ssq1, its cochaperone Jac1, or Grx5 (Muhlenhoff et al., 2003). Single-domain monothiol glutaredoxins are widespread in bacteria and eukaryotes, indicating that their function in ISC assembly is highly conserved (Lillig et al., 2008). Despite severe phenotypic consequences, the deletion of GRX5 is not lethal in yeast (Rodriguez-Manzaneque et al., 1999, 2002). This indicates that in this organism its function can be bypassed, likely through a direct yet inefficient transfer of Fe/S clusters from the Isu1 scaffold protein to late ISC assembly factors and/or target apoproteins. In multicellular organisms, however, mutations in Grx5 are lethal and associated with fatal human disease (Rodriguez-Manzaneque et al., 2002; Wingert et al., 2005; Camaschella et al., 2007; Ye et al., 2010). Of note, humans and most other eukaryotic species do not possess a dedicated Hsp70 chaperone for the mitochondrial ISC assembly pathway and instead use the multifunctional Ssc1 (Pukszta et al., 2010). It will be interesting to see whether Grx5 binds to the general mitochondrial Hsp70 chaperone in these organisms and how Grx5 and Hsp70 may have evolved to efficiently cooperate in Fe/S protein assembly.
MATERIALS AND METHODS
Strains and growth conditions
Yeast strains used in this study are listed in Supplemental Table S1. Cells were cultivated in rich medium (YP) or synthetic minimal medium (SC) supplemented with amino acids as required and 2% (wt/vol) glucose or galactose (Sherman, 2002). Iron-depleted minimal media were prepared using yeast nitrogen base without FeCl3 (ForMedium, Hunstanton, United Kingdom) and 20 μg/ml methionine. Regulatable GAL-promoter yeast strains were depleted for the respective ISC proteins by cultivation in SD medium for 16 h (Gal-ISU1/isu2Δ), 40 h (Gal-NFS1), or 64 h (Gal-SSQ1 and Gal-JAC1) before analysis (see Supplemental Table S1). Plasmids used in this study are listed in Supplemental Table S2. Plasmid constructs were verified by DNA sequencing and/or functional complementation of a corresponding yeast mutant.
Recombinant proteins
The N-terminally histidine (His)-tagged versions of S. cerevisiae Grx5, Cia1, and Cia2 were purified from E. coli strain BL21, which expressed indicated proteins from vector pET15b using nickel–nitriloacetic acid affinity chromatography (Qiagen, Valencia, CA). Recombinant, His-tagged versions of S. cerevisiae proteins Jac1 (Voisine et al., 2001), Mge1 (Horst et al., 1997), Ssq1, and Isu1 (Dutkiewicz et al., 2003) were purified as described. ATPase activities of Ssq1 were measured as described (Dutkiewicz et al., 2003), using 0.5 μM Ssq1, 0.5 μM Mge1, and 10 μM Isu1 or 0.5 μM Jac1 and variable amounts of Isu1, Jac1, or Grx5. For in vitro interaction studies, recombinant proteins were incubated at 4 μM concentrations each in buffer H (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–KOH, pH 7.5, 200 mM KCl, 1 mM MgCl2, 1 mM ADP) in 25ºC for 45 min. After incubation samples were subjected to immunoprecipitation with antibodies against Grx5 bound to protein A–Sepharose.
Miscellaneous methods
The following published methods were used: manipulation of DNA and PCR (Sambrook and Russel, 2001); transformation of yeast cells (Gietz and Woods, 2002); isolation of yeast mitochondria and postmitochondrial supernatants (Diekert et al., 2001); immunostaining (Harlow and Lane, 1988); determination of enzyme activities (Molik et al., 2007); and in vivo labeling of yeast cells with 55FeCl (Perkin Elmer) and measurement of 55Fe incorporation into Fe/S proteins by immunoprecipitation and scintillation counting (Molik et al., 2007). Coimmunoprecipitation experiments and affinity purification of GST-tagged mitochondrial proteins were performed as described using buffer A (20 mM Tris-HCl, pH 7.4, 2.5 mM EDTA, 150 mM NaCl, 10% glycerol) for detergent (0.5% Triton X-100) lysis of mitochondria (Gerber et al., 2003). Antibodies were raised in rabbits against recombinant proteins. Antibodies against c-Myc or HA were obtained from Santa Cruz Biotech (Heidelberg, Germany) and protein A–Sepharose from GE Healthcare (Piscataway, NJ).
Microscale thermophoresis
For MST, Grx5 or Cia1 was labeled using the Monolith NT Protein Labeling Kit RED (NanoTemper Technologies, Munich, Germany) with NT-647 dye as recommended by the manufacturer. Labeled bait proteins were mixed with serial dilutions of Cia2, Ssq1, or the Isu1-derived peptide AKELSLPPVKLHC (LPPVK), either alone or in combinations. Binding assays were performed in buffer T (50 mM KPi, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1 mM GSH) using Monolith NT.015 (NanoTemper Technologies) at 21°C (light-emitting diode power, 100%; infrared laser power, 75%). Three independent experiments were recorded at 680 nm, and data were processed by NanoTemper Analysis package 1.2.009 and Origin 8G software (OriginLab, Northampton, MA) to estimate the apparent Kd values.
Supplementary Material
Acknowledgments
We thank Tran Xuan Phong Nguyen and Gerhard Klebe (Marburg) for assistance with the MST experiments. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 593, Gottfried-Wilhelm Leibniz Program, and GRK 1216), the von Behring-Röntgen Stiftung, the LOEWE Program of the state Hessen, and the Max-Planck Gesellschaft. R.D. was supported by TEAM/2009-3/5.
Abbreviations used:
- Grx
glutaredoxin
- GST
glutathione S-transferase
- ISC
iron–sulfur cluster
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-09-0644) on April 24, 2013.
REFERENCES
- Andrew AJ, Dutkiewicz R, Knieszner H, Craig EA, Marszalek J. Characterization of the interaction between the J-protein Jac1p and the scaffold for Fe-S cluster biogenesis, Isu1p. J Biol Chem. 2006;281:14580–14587. doi: 10.1074/jbc.M600842200. [DOI] [PubMed] [Google Scholar]
- Ayala-Castro C, Saini A, Outten FW. Fe-S cluster assembly pathways in bacteria. Microbiol Mol Biol Rev. 2008;72:110–125. doi: 10.1128/MMBR.00034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Pilon M. Ancient and essential: the assembly of iron-sulfur clusters in plants. Trends Plant Sci. 2010;16:218–226. doi: 10.1016/j.tplants.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, et al. Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters. EMBO J. 2008;27:1122–1133. doi: 10.1038/emboj.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H. Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem. 2000;5:2–15. doi: 10.1007/s007750050002. [DOI] [PubMed] [Google Scholar]
- Belli G, Polaina J, Tamarit J, De La Torre MA, Rodriguez-Manzaneque MT, Ros J, Herrero E. Structure-function analysis of yeast Grx5 monothiol glutaredoxin defines essential amino acids for the function of the protein. J Biol Chem. 2002;277:37590–37596. doi: 10.1074/jbc.M201688200. [DOI] [PubMed] [Google Scholar]
- Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Studies on the mechanism of catalysis of iron-sulfur cluster transfer from IscU[2Fe2S] by HscA/HscB chaperones. Biochemistry. 2008;47:12795–12801. doi: 10.1021/bi801565j. [DOI] [PubMed] [Google Scholar]
- Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Facilitated transfer of IscU-[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry. 2011;50:9641–9650. doi: 10.1021/bi201123z. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Bych K, Kerscher S, Netz DJ, Pierik AJ, Zwicker K, Huynen MA, Lill R, Brandt U, Balk J. The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 2008;27:1736–1746. doi: 10.1038/emboj.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- Cameron JM, Janer A, Levandovskiy V, Mackay N, Rouault TA, Tong WH, Ogilvie I, Shoubridge EA, Robinson BH. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet. 2011;89:486–495. doi: 10.1016/j.ajhg.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli K, Johnson MK. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45:11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski SJ, Schilke BA, Osipiuk J, Bigelow L, Mulligan R, Majewska J, Joachimiak A, Marszalek J, Craig EA, Dutkiewicz R. Interaction of J-protein co-chaperone Jac1 with Fe-S scaffold Isu is indispensable in vivo and conserved in evolution. J Mol Biol. 2012;417:1–12. doi: 10.1016/j.jmb.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Marszalek J. eLS, Chichester. United Kingdom: John Wiley; 2011. Hsp70 chaperones. www.els.net, doi: 10.1002/9780470015902.a0023188 (accessed March 2011). [Google Scholar]
- D'Silva P, Liu Q, Walter W, Craig EA. Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat Struct Mol Biol. 2004;11:1084–1091. doi: 10.1038/nsmb846. [DOI] [PubMed] [Google Scholar]
- Diekert K, de Kroon AI, Kispal G, Lill R. Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 2001;65:37–51. doi: 10.1016/s0091-679x(01)65003-9. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz R, Marszalek J, Schilke B, Craig EA, Lill R, Muhlenhoff U. The hsp70 chaperone ssq1p is dispensable for iron-sulfur cluster formation on the scaffold protein isu1p. J Biol Chem. 2006;281:7801–7808. doi: 10.1074/jbc.M513301200. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz R, Schilke B, Cheng S, Knieszner H, Craig EA, Marszalek J. Sequence-specific interaction between mitochondrial Fe-S scaffold protein Isu and Hsp70 Ssq1 is essential for their in vivo function. J Biol Chem. 2004;279:29167–29174. doi: 10.1074/jbc.M402947200. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz R, Schilke B, Knieszner H, Walter W, Craig EA, Marszalek J. Ssq1, a mitochondrial Hsp70 involved in iron-sulfur (Fe/S) center biogenesis. Similarities to and differences from its bacterial counterpart. J Biol Chem. 2003;278:29719–29727. doi: 10.1074/jbc.M303527200. [DOI] [PubMed] [Google Scholar]
- Fontecave M. Iron-sulfur clusters: ever-expanding roles. Nat Chem Biol. 2006;2:171–174. doi: 10.1038/nchembio0406-171. [DOI] [PubMed] [Google Scholar]
- Fontecave M, Ollagnier-de-Choudens S. Iron-sulfur cluster biosynthesis in bacteria: mechanisms of cluster assembly and transfer. Arch Biochem Biophys. 2008;474:226–237. doi: 10.1016/j.abb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Fuzery AK, Oh JJ, Ta DT, Vickery LE, Markley JL. Three hydrophobic amino acids in Escherichia coli HscB make the greatest contribution to the stability of the HscB-IscU complex. BMC Biochem. 2011;12:3. doi: 10.1186/1471-2091-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RP, Vargo CJ, Cui X, Kurtz DM Jr. A [2Fe-2S] protein encoded by an open reading frame upstream of the Escherichia coli bacterioferritin gene. Biochemistry. 1996;35:6297–6301. doi: 10.1021/bi9600862. [DOI] [PubMed] [Google Scholar]
- Gelling C, Dawes IW, Richhardt N, Lill R, Muhlenhoff U. Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes. Mol Cell Biol. 2008;28:1851–1861. doi: 10.1128/MCB.01963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J, Muhlenhoff U, Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003;4:906–911. doi: 10.1038/sj.embor.embor918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies—A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Herrero E, de la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci. 2007;64:1518–1530. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff KG, Cupp-Vickery JR, Vickery LE. Contributions of the LPPVK motif of the iron-sulfur template protein IscU to interactions with the Hsc66-Hsc20 chaperone system. J Biol Chem. 2003;278:37582–37589. doi: 10.1074/jbc.M305292200. [DOI] [PubMed] [Google Scholar]
- Hoff KG, Ta DT, Tapley TL, Silberg JJ, Vickery LE. Hsc66 substrate specificity is directed toward a discrete region of the iron-sulfur cluster template protein IscU. J Biol Chem. 2002;277:27353–27359. doi: 10.1074/jbc.M202814200. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Uzarska MA, Berndt C, Godoy JR, Haunhorst P, Lillig CH, Lill R, Muhlenhoff U. The multidomain thioredoxin-monothiol glutaredoxins represent a distinct functional group. Antioxid Redox Signal. 2011;15:19–30. doi: 10.1089/ars.2010.3811. [DOI] [PubMed] [Google Scholar]
- Horst M, Oppliger W, Rospert S, Schonfeld HJ, Schatz G, Azem A. Sequential action of two hsp70 complexes during protein import into mitochondria. EMBO J. 1997;16:1842–1849. doi: 10.1093/emboj/16.8.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwema T, Picciocchi A, Traore DA, Ferrer JL, Chauvat F, Jacquamet L. Structural basis for delivery of the intact [Fe2S2] cluster by monothiol glutaredoxin. Biochemistry. 2009;48:6041–6043. doi: 10.1021/bi900440m. [DOI] [PubMed] [Google Scholar]
- Johansson C, Roos AK, Montano SJ, Sengupta R, Filippakopoulos P, Guo K, von Delft F, Holmgren A, Oppermann U, Kavanagh KL. The crystal structure of human GLRX5: iron-sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem J. 2011;433:303–311. doi: 10.1042/BJ20101286. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, Chung WH, Kim HJ, Lee KC, Roe JH. Monothiol glutaredoxin Grx5 interacts with Fe-S scaffold proteins Isa1 and Isa2 and supports Fe-S assembly and DNA integrity in mitochondria of fission yeast. Biochem Biophys Res Commun. 2010;392:467–472. doi: 10.1016/j.bbrc.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Knieszner H, Schilke B, Dutkiewicz R, D'Silva P, Cheng S, Ohlson M, Craig EA, Marszalek J. Compensation for a defective interaction of the hsp70 ssq1 with the mitochondrial Fe-S cluster scaffold ISU. J Biol Chem. 2005;280:28966–28972. doi: 10.1074/jbc.M503031200. [DOI] [PubMed] [Google Scholar]
- Lange H, Kaut A, Kispal G, Lill R. A mitochondrial ferredoxin is essential for biogenesis of cellular iron-sulfur proteins. Proc Natl Acad Sci USA. 2000;97:1050–1055. doi: 10.1073/pnas.97.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Saxena S, Pain D, Dancis A. Adrenodoxin reductase homolog (Arh1p) of yeast mitochondria required for iron homeostasis. J Biol Chem. 2001;276:1503–1509. doi: 10.1074/jbc.M007198200. [DOI] [PubMed] [Google Scholar]
- Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Muhlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta. 2012;1823:1491–1508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molik S, Lill R, Muhlenhoff U. Methods for studying iron metabolism in yeast mitochondria. Methods Cell Biol. 2007;80:261–280. doi: 10.1016/S0091-679X(06)80013-0. [DOI] [PubMed] [Google Scholar]
- Muhlenhoff U, Gerber J, Richhardt N, Lill R. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 2003;22:4815–4825. doi: 10.1093/emboj/cdg446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff U, Richter N, Pines O, Pierik AJ, Lill R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe-4S] proteins. J Biol Chem. 2011;286:41205–41216. doi: 10.1074/jbc.M111.296152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sastre A, et al. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet. 2011;89:656–667. doi: 10.1016/j.ajhg.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciocchi A, Saguez C, Boussac A, Cassier-Chauvat C, Chauvat F. CGFS-type monothiol glutaredoxins from the cyanobacterium Synechocystis PCC6803 and other evolutionary distant model organisms possess a glutathione-ligated [2Fe-2S] cluster. Biochemistry. 2007;46:15018–15026. doi: 10.1021/bi7013272. [DOI] [PubMed] [Google Scholar]
- Pukszta S, et al. Co-evolution-driven switch of J-protein specificity towards an Hsp70 partner. EMBO Rep. 2010;11:360–365. doi: 10.1038/embor.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque MT, Ros J, Cabiscol E, Sorribas A, Herrero E. Grx5 glutaredoxin plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:8180–8190. doi: 10.1128/mcb.19.12.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque MT, Tamarit J, Belli G, Ros J, Herrero E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol Biol Cell. 2002;13:1109–1121. doi: 10.1091/mbc.01-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N, Couturier J, Johnson MK, Jacquot JP. Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci. 2010;35:43–52. doi: 10.1016/j.tibs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning—A Laboratory Manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schilke B, Forster J, Davis J, James P, Walter W, Laloraya S, Johnson J, Miao B, Craig E. The cold sensitivity of a mutant of Saccharomyces cerevisiae lacking a mitochondrial heat shock protein 70 is suppressed by loss of mitochondrial DNA. J Cell Biol. 1996;134:603–613. doi: 10.1083/jcb.134.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilke B, Williams B, Knieszner H, Pukszta S, D'Silva P, Craig EA, Marszalek J. Evolution of mitochondrial chaperones utilized in Fe-S cluster biogenesis. Curr Biol. 2006;16:1660–1665. doi: 10.1016/j.cub.2006.06.069. [DOI] [PubMed] [Google Scholar]
- Schlecht R, Erbse AH, Bukau B, Mayer MP. Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat Struct Mol Biol. 2011;18:345–351. doi: 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donze M, Reutenauer L, Puccio H. Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLOS One. 2011;6:e16199. doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakamuri P, Zhang B, Johnson MK. Monothiol glutaredoxins function in storing and transporting [Fe2S2] clusters assembled on IscU scaffold proteins. J Am Chem Soc. 2012;134:15213–15216. doi: 10.1021/ja306061x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Pallesen LJ, Spang RJ, Walden WE. Cytosolic iron-sulfur cluster assembly (CIA) system: factors, mechanism, and relevance to cellular iron regulation. J Biol Chem. 2010;285:26745–26751. doi: 10.1074/jbc.R110.122218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Lill R. The power plant of the cell is also a smithy: the emerging role of mitochondria in cellular iron homeostasis. Ann Med. 2009;41:82–99. doi: 10.1080/07853890802322229. [DOI] [PubMed] [Google Scholar]
- Sheftel AD, Stehling O, Pierik AJ, Elsasser HP, Muhlenhoff U, Webert H, Hobler A, Hannemann F, Bernhardt R, Lill R. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Stehling O, Pierik AJ, Netz DJ, Kerscher S, Elsasser HP, Wittig I, Balk J, Brandt U, Lill R. Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol Cell Biol. 2009;29:6059–6073. doi: 10.1128/MCB.00817-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Wilbrecht C, Stehling O, Niggemeyer B, Elsasser HP, Muhlenhoff U, Lill R. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol Biol Cell. 2012;23:1157–1166. doi: 10.1091/mbc.E11-09-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ghosh M, Kovtunovych G, Crooks DR, Rouault TA. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim Biophys Acta. 2012;1823:484–492. doi: 10.1016/j.bbamcr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JJ, Hoff KG, Vickery LE. The Hsc66-Hsc20 chaperone system in Escherichia coli: chaperone activity and interactions with the DnaK-DnaJ-grpE system. J Bacteriol. 1998;180:6617–6624. doi: 10.1128/jb.180.24.6617-6624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JJ, Tapley TL, Hoff KG, Vickery LE. Regulation of the HscA ATPase reaction cycle by the co-chaperone HscB and the iron-sulfur cluster assembly protein IscU. J Biol Chem. 2004;279:53924–53931. doi: 10.1074/jbc.M410117200. [DOI] [PubMed] [Google Scholar]
- Stankiewicz M, Nikolay R, Rybin V, Mayer MP. CHIP participates in protein triage decisions by preferentially ubiquitinating Hsp70-bound substrates. FEBS J. 2011;277:3353–3367. doi: 10.1111/j.1742-4658.2010.07737.x. [DOI] [PubMed] [Google Scholar]
- Stemmler TL, Lesuisse E, Pain D, Dancis A. Frataxin and mitochondrial FeS cluster biogenesis. J Biol Chem. 2010;285:26737–26743. doi: 10.1074/jbc.R110.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley TL, Vickery LE. Preferential substrate binding orientation by the molecular chaperone HscA. J Biol Chem. 2004;279:28435–28442. doi: 10.1074/jbc.M400803200. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49:9132–9139. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- Vickery LE, Cupp-Vickery JR. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42:95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- Voisine C, Cheng YC, Ohlson M, Schilke B, Hoff K, Beinert H, Marszalek J, Craig EA. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2001;98:1483–1488. doi: 10.1073/pnas.98.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisine C, Schilke B, Ohlson M, Beinert H, Marszalek J, Craig EA. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol Cell Biol. 2000;20:3677–3684. doi: 10.1128/mcb.20.10.3677-3684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienken CJ, Baaske P, Rothbauer U, Braun D, Duhr S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat Commun. 2010;1:100. doi: 10.1038/ncomms1093. [DOI] [PubMed] [Google Scholar]
- Wingert RA, et al. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest. 2010;120:1749–1761. doi: 10.1172/JCI40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Rouault TA. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry. 2010;49:4945–4956. doi: 10.1021/bi1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillner K, Jerabek-Willemsen M, Duhr S, Braun D, Langst G, Baaske P. Microscale thermophoresis as a sensitive method to quantify protein: nucleic acid interactions in solution. Methods Mol Biol. 2012;815:241–252. doi: 10.1007/978-1-61779-424-7_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.