JNK1/2 has an obligatory role in mediating IL-1β–induced endoplasmic reticulum calcium release, its uptake into the mitochondria, and consequent apoptotic cell death in pancreatic β-cells. This is accompanied by attenuation of glucose-stimulated insulin release and abrogation of this event in the presence of JNK1/2 siRNA.
Abstract
Elevated interleukin-1β (IL-1β) induces apoptosis in pancreatic β-cells through endoplasmic reticulum (ER) stress induction and subsequent c-jun-N-terminal kinase 1/2 (JNK1/2) activation. In earlier work we showed that JNK1/2 activation is initiated before ER stress and apoptotic induction in response to IL-1β. However, the detailed regulatory mechanisms are not completely understood. Because the ER is the organelle responsible for Ca2+ handling and storage, here we examine the effects of IL-1β on cellular Ca2+ movement and mitochondrial dysfunction and evaluate the role of JNK1/2. Our results show that in RINm5F cells and human primary β-cells, IL-1β alters mitochondrial membrane potential, mitochondrial permeability transition pore opening, ATP content, and reactive oxygen species production and these alterations are preceded by ER Ca2+ release via IP3R channels and mitochondrial Ca2+ uptake. All these events are prevented by JNK1/2 small interfering RNA (siRNA), indicating the mediating role of JNK1/2 in IL-1β–induced cellular alteration. This is accompanied by IL-1β–induced apoptosis, which is prevented by JNK1/2 siRNA and the IP3R inhibitor xestospongin C. This suggests a regulatory role of JNK1/2 in modulating the ER-mitochondrial-Ca2+ axis by IL-1β in apoptotic cell death.
INTRODUCTION
Elevated levels of the proinflammatory cytokine interleukin 1β (IL-1β) are associated with pancreatic β-cell apoptosis (Corbett and McDaniel, 1994; Thomas et al., 2002; Larsen et al., 2007; Grunnet et al., 2009), and several signaling intermediates are believed to mediate this deleterious effect. IL-1β–mediated activation of NF-κB is believed to be a significant contributor to β-cell death (Welsh et al., 2005), and its suppression attenuates pancreatic β-cell cytotoxicity (Kim et al., 2007). Nitric oxide (NO) is also believed to contribute to IL-1β–induced β-cell damage that results from an initial event of internucleosomal DNA cleavage and nuclear condensation (Thomas et al., 2002, 2004). In addition, nitric oxide destroys iron–sulfur centers of ion-containing proteins, leading to mitochondrial dysfunction (Corbett et al., 1992). IL-1β–mediated calcium (Ca2+) influx into pancreatic β-cells also contributes to impaired β-cell function (Maedler et al., 2004; Dula et al., 2010). Although controlled flow of Ca2+ is required for normal functioning of the β-cell and for insulin release, aberrant Ca2+ influx is frequently encountered in the presence of IL-1β. This uninhibited increase in cellular Ca2+ is linked to classical mitochondrial dysfunction parameters, namely, loss of mitochondrial membrane potential (Δψm), ATP reduction, and increase in reactive oxygen species (ROS) production (Mbaya et al., 2010). Because it is responsible for Ca2+ storage and signaling, the endoplasmic reticulum (ER) contributes to maintaining cellular Ca2+ status, and therefore under conditions of ER stress, normal calcium homeostasis is severely altered (Deniaud et al., 2008). Such disruption is believed to be one of the factors underlying impaired β-cell function and decreased survival (Cardozo et al., 2005). Because pancreatic β-cells posses an intricate Ca2+ machinery that is especially important during insulin secretion (Chen et al., 2003), these alterations significantly contribute to decreased insulin release. IL-1β promotes such alterations within the pancreas (Gurzov et al., 2009), and an evident mediator is believed to be the induction of ER stress, which involves the participation of several key molecules, namely IRE-1α, PERK, ATF6, and their associated downstream intermediates (Nakatani et al., 2005; Marchetti et al., 2007). These act as proapoptotic signals, leading to caspase activation, which culminates in apoptosis of the pancreatic β-cells.
A very significant cellular phenomenon evident during these events is the cross-talk between the ER and mitochondria (Kornmann and Walter, 2010). These organelles exhibit direct physical contact regions that demonstrate dynamic interorganellar structural and functional correlation, and this is believed to be responsible for diverse arrays of cellular alterations that are critical during cell death (Pizzo and Pozzan, 2007; Csordas et al., 2010). In addition, both organelles are key players in buffering Ca2+ levels in general and in pancreatic β-cells in particular. Under pathological conditions, cellular Ca2+ imbalance leads to increased mitochondrial Ca2+ uptake, which initiates the apoptotic program within the cell. Reports suggest a role of Ca2+ in IL-1β–mediated pancreatic β-cell death, and the stress kinase JNK is believed to be critical in this event (Storling et al., 2005), with JNK inhibition preventing the IL-1β effect (Bonny et al., 2000; Major and Wolf, 2001). In addition, IL-1β induces ER stress, which activates JNK and initiates the apoptotic program in pancreatic β-cells (Collier et al., 2011).
We demonstrated that IL-1β induces ER stress and apoptosis in pancreatic cells in a JNK-dependent manner (Verma and Datta, 2010). Although JNK activation by ER stress induction is widely accepted, we showed that JNK1/2 mediates IL-1β–induced ER stress induction, as inhibition of JNK prevented IL-1β–induced ER stress (Verma and Datta, 2010). Our study demonstrated that JNK activation is upstream of IL-1β–mediated ER stress induction. Having described the central initiating role of JNK during IL-1β action, we report in this study that JNK1/2 mediates IL-1β–induced ER Ca2+ release and the consequent mitochondrial dysfunction in pancreatic RINm5F and human primary β cells.
RESULTS
IL-1β induces mitochondrial dysfunction in pancreatic RINm5F cells via JNK1/2
We previously showed that IL-1β mediates ER stress in a JNK1/2-dependent manner in Mia PaCa-2 cells (Verma and Datta, 2010). Here we demonstrate that also in insulin-producing rat pancreatic RINm5F cells, IL-1β–induced JNK1/2 activation precedes the induction of ER stress markers (Figure 1, A and B). Whereas JNK1/2 activation in the presence of IL-1β was visible at 2 h of incubation, the up-regulation of ER stress markers was observed starting only at 4 h of incubation. To further confirm this event, we used JNK1/2 small interfering RNA (siRNA; also written as JNK1/2 siRNA I) and evaluated the levels of ER stress markers. Figure 1C shows the dose-dependent effect of JNK1/2 siRNA I on total JNK1/2 levels. Maximum inhibition of total JNK1/2 levels was observed at 100 nM (Figure 1C). We therefore used this concentration of JNK1/2 siRNA I for all subsequent experiments. In addition, at this concentration of JNK1/2 siRNA, phosphorylation of the primary target of JNK, that is, c-jun, was markedly inhibited (Figure 1D). JNK1/2 also mediated IL-1β–induced c-jun phosphorylation, as inhibition of JNK1/2 with JNK1/2 siRNA I prevented this effect (Figure 1E). This pattern of c-jun phosphorylation by IL-1β was identical to IL-1β–induced JNK 1/2 phosphorylation (Figure 1A), suggesting that such JNK1/2 phosphorylation mediates c-jun phosphorylation. Further, in RINm5F cells pretreated with the JNK1/2 siRNA I before IL-1β, up-regulation of IL-1β–induced ER stress markers was completely prevented (Figure 1F). All of this suggests that in RINm5F cells, JNK1/2 mediates IL-1β–regulated ER stress induction.
FIGURE 1:
IL-1β–mediated JNK1/2 activation precedes ER stress induction. RINm5F cells were incubated with IL-1β (2 ng/ml) for 2, 4, 8, 12, and 24 h. On termination of incubation, cells were collected, and the levels of total and phospho-JNK1/2 (A) and Bip and CHOP (B) were evaluated by Western blot and quantitative RT-PCR, respectively. (C) RINm5F cells were transfected with scramble or JNK1/2 siRNA I (25–100 nM), and after 36 h, cells were lysed and the levels of JNK1/2 determined by Western blot analysis. (D) Cells transfected with either scramble or JNK1/2 siRNA I (100 nM) were evaluated for the levels of p-c-jun and c-jun. (E) RINm5F cells were transfected with either scramble or JNK1/2 siRNA I (100 nM) and then incubated with IL-1β (2 ng/ml) for the indicated times. On termination of incubation, the levels of p-c-jun were evaluated by Western blot. (F) Cells transfected with scramble or JNK1/2 siRNA I (100 nM) were incubated in the absence or presence of IL-1β as in A, and on termination of incubation, the levels of Bip and CHOP were evaluated by quantitative RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase and 18S rRNA were taken as the loading controls. All experiments were done thrice, and values are means ± SEM. ***p < 0.001, **p < 0.01, *p < 0.05 as compared with scramble or incubation at 0 h.
We further evaluated the effect of IL-1β on mitochondrial dysfunction and the contribution of JNK1/2–mediated ER stress to this. RINm5F cells were exposed to IL-1β for various times (0, 2, 8, 12, 24, and 36 h), and mitochondrial membrane potential, Δψm was measured using flow cytometry analysis of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole carbocyanide iodide (JC-1) fluorescence. Normal JC1 aggregates are measured by red fluorescence, and nonaggregate forms under stress are measured by increasing green fluorescence. As compared with control cells, in IL-1β–treated RINm5F cells, an increase in the nonaggregate form of JC-1 (as measured by increased green fluorescence) was observed, suggesting altered mitochondrial membrane potential (Figure 2, A and B). This increase was visible only at 36 h of incubation, and, surprisingly, in cells incubated with IL-1β in the presence of JNK1/2 siRNA, this disturbance in membrane potential was completely prevented and cells showed positive membrane potential similar to that of control cells (as evident by the presence of red J aggregates), suggesting that JNK1/2 is involved in IL-1β–induced alteration of Δψm (Figure 2, A and B). To substantiate these observed mitochondrial alterations, we evaluated the effect on mitochondrial permeability transition pore (mPTP) opening, a significant mitochondrial dysfunction event that leads to loss in Δψm and release of cytochrome c (Green and Kroemer, 2004; Tait and Green, 2010). mPTP opening was assessed by flow cytometry analysis, and in the presence of IL-1β, mitochondrial fluorescence (as detected by calcein-AM fluorescence in the presence of CoCl2) was significantly decreased at 36 h of incubation (Figure 2C). This suggests that IL-1β causes a significant increase in mPTP opening, which results in loss of mitochondrial fluorescence. This was prevented by the presence of JNK1/2 siRNA I, indicating a role of JNK1/2 in the increased opening of mPTP by IL-1β.
FIGURE 2:
IL-1β–induced mitochondrial dysfunction in RINm5F cells. (A) RINm5F cells were grown to confluence and incubated with IL-1β (2 ng/ml) for 2, 8, 12, 24, and 36 h. Incubation in the absence of IL-1β was taken as the control (0 h). On termination of incubation, mitochondrial membrane potential was assessed by flow cytometry using JC-1. The accumulation of green JC-1 monomers, which increased in the presence of IL-1β at 36 h, suggested a disruption of the mitochondrial membrane potential. In addition, confluent RINm5F cells were transfected with JNK1/2 siRNA I (100 nM) before IL-1β treatment and then evaluated for mitochondrial membrane potential. CCCP was used as a positive control for mitochondrial membrane depolarization. JNK1/2 siRNA I significantly prevented the increase in green JC-1 monomers by IL-1β. (B) These are depicted quantitatively. Values are presented with respect to the control (0h). (C) Confluent RINm5F cells were transfected with the scramble (Control) or JNK1/2 siRNA I and then incubated with IL-1β (2 ng/ml) for 0, 24, and 36 h. On termination of incubation, mitochondrial pore formation was evaluated as discussed in Materials and Methods. Incubated cells were loaded either calcein-AM alone or calcein-AM plus CoCl2. After washing of the cells, they were analyzed for calcein-AM fluorescence using flow cytometry. The arrow indicates the position of the peak fluorescence within the mitochondria in the presence of calcein-AM and CoCl2. (D) RINm5F cells incubated as in A were assessed for the ATP content using the Bioluminescent ATP Determination Kit. Experiments were done three times, and the values were normalized to the total protein content. (E) RINm5F cells (50,000) were incubated with IL-1β with or without JNK1/2 siRNA I for 0, 24, and 36 h. Cells were then incubated for 10 min at 37°C with 5 μM MitoSox fluorescent marker and also with the MitoTracker green dye. Cells were then visualized in a live-cell imaging system. H2O2 was used as a positive control. Experiments were done thrice, and representative figures are provided. The quantitation of the MitoTracker and MitoSox dyes is given below. Values in B, D, and E are means ± SEM of three independent experiments. **p < 0.001 and *p < 0.01 as compared with control (0 h incubation); #p < 0.01 and $p < 0.05 as compared with IL-1β alone at the same time point; ap < 0.001 as compared with similar time points in the presence of scramble.
IL-1β causes ATP depletion and ROS (superoxide) generation in a JNK1/2-dependent manner
To evaluate the effects of IL-1β and JNK1/2 on other mitochondrial parameters, we assessed the effect of these on its ATP content and ROS production. As shown in Figure 2D, IL-1β led to a significant decrease in mitochondrial ATP content in RINm5F cells as measured by ATP determination bioluminescence assay. A time-dependent decrease in ATP content was observed starting from 12 h of IL-1β treatment, which further significantly decreased at 24 h and then plateaued until 36 h. However, in the presence of JNK1/2 siRNA, this decrease was significantly prevented (Figure 2D), suggesting a critical role of JNK1/2 in this mitochondrial activity.
Because mitochondria contribute to a major part of cellular free radical generation, we studied the effect of IL-1β and JNK1/2 inhibition on this mitochondrial event. We used the mitochondrial ROS-detecting agent MitoSox Red in combination with MitoTracker Green FM (which localizes to the mitochondria) to identify mitochondrial ROS generation. In cells treated with IL-1β, there was a marked increase in MitoSox fluorescence that colocalized with mitochondria (as tracked by the MitoTracker Green dye), suggesting that mitochondrial ROS generation was significantly increased in the presence of IL-1β (Figure 2E). At 24 h, mitochondrial ROS generation increased, which was further enhanced at 36 h. JNK1/2 siRNA prevented this IL-1β–mediated increase in mitochondrial ROS generation (Figure 2E). Taken together, these data suggest that IL-1β caused mitochondrial dysfunction in RINm5F cells and that JNK1/2 is a significant mediator in the effect.
IL-1β depletes ER Ca2+ in RINm5F cells
Because cellular Ca2+ levels are believed to be critical during mitochondrial dysfunction and cellular bioenergetics (Mbaya et al., 2010), we further sought to determine the cellular calcium dynamics in the presence of IL-1β and JNK1/2 siRNA I that might influence mitochondrial dysfunction as observed in Figure 2. In addition to the JNK1/2 siRNA I described earlier, we used a second JNK1/2 siRNA (JNK1/2 siRNA II) to confirm the specificity of JNK1/2 inhibition. In the presence of JNK1/2 siRNA II, there was a dose-dependent decrease in total JNK1/2 levels (Figure 3A) in RINm5F cells. Incubation of RINm5F cells with IL-1β led to marginal increase in cytosolic Ca2+ ([Ca2+]c) levels at 4 h, and this greatly increased at 8 h of incubation and later decreased at 12 and 24 h (Figure 3B). This increase could be prevented by both JNK1/2 siRNA I and JNK1/2 siRNA II, and the gradual increase in cytosolic Ca2+ starting at 4 h is in accordance with our previous study (Verma and Datta, 2010), where we showed that induction of ER stress markers by IL-1β is evident starting from 4 h of incubation. Hence, we believe that IL-1β–induced ER stress is responsible for the release of Ca2+ from the ER to the cytosol. The decrease in cytosolic Ca2+ at later times, that is, 12 and 24 h, indicates that at these times, Ca2+ is either sequestered back into the ER or is taken up by the mitochondrion, which is evident during various pathophysiological conditions (Csordás et al., 2006).
FIGURE 3:
IL-1β promotes ER Ca2+ release and mitochondrial Ca2+ uptake in RINm5F cells. (A) RINm5F cells were transfected with the scramble or a second JNK1/2 siRNA-II (25–100 nM), and after 36 h, cells were lysed and the levels of JNK1/2 determined by Western blot analysis. (B) RINm5F cells were incubated with IL-1β (2 ng/ml) in the presence of the scramble (Control) or JNK1/2 siRNA I and II for 0, 2, 4, 8, 12, and 24 h. On termination of incubation, cells were loaded with Fluo-4; 5000 cells were counted, and the [Ca2+]c levels were read in on a multiplate reader. Values are presented with respect to the control (0 h of IL-1β). (C) To analyze whether the ER was depleted of Ca2+ by IL-1β, cells were plated in six-well plates and incubated with IL-1β as in B. On termination of incubation, cells were stained with Fluo-4 and triggered with thapsigargin (TG; 1 μM) to deplete the ER calcium. The Ca2+ released into the cytosol was evaluated (by Fluo-4 fluorescence) in a fluorimeter by monitoring the cytosolic Ca2+ increase for 10 min. (D) RINm5F cells incubated as in B were stained with the mitochondrial Ca2+ marker Rhod-2 (10 μM) and incubated for 10 min at 37°C. Rhod-2–stained cells (5000 cells) were read on a Tecan M200 multiplate reader. (E) Mitochondrial Ca2+ staining was also evaluated by live-cell imaging in which RINm5F cells (50,000) incubated with IL-1β in the absence and presence of JNK1/2 siRNA I for 0, 12, and 24 h were stained with the MitoTracker dye (50 nM) and Rhod-2 (10 μM) and incubated for 15 min at 37°C. Cells were then washed and visualized in a Nikon fluorescence microscope. CCCP was used as a reference for mitochondrial Ca2+ depletion. The quantitation of the MitoTracker and Rhod-2 fluorescence are given below. All experiments were done three times, and representative figures are given. Data in A, B, D and E are means of three independent experiments. ***p < 0.001, **p < 0.01, and *p < 0.05 as compared with scramble or control (0-h incubation); ##p < 0.001, #p < 0.01, and $p < 0.05 as compared with the incubation with IL-1β with scramble; ap < 0.001 for Rhod-2 as compared with similar time points in the presence of scramble + IL-1β.
To validate these results, we performed an independent experiment in RINm5F cells in which cells incubated with IL-1β for 2, 4, 8, 12, and 24 h were stained with Fluo-4 for 30 min at 37°C. Cells were then stimulated with thapsigargin (1 mM) to deplete ER Ca2+ stores. Cells incubated in the absence (0 h) or presence of IL-1β for 2 h showed a sharp increase in [Ca2+]c after exposure to thapsigargin, indicating that IL-1β at 2 h of incubation did not deplete the ER of endogenous Ca2+ levels (Figure 3C). Subsequently, at 4 h of incubation, thapsigargin induced a relatively blunt [Ca2+]c peak, which at later times pleateaued, suggesting that some leakage of ER Ca2+ had initiated in the presence of IL-1β at 4 h of incubation. Further, at 8, 12, and 24 h of IL-1β incubation, thapsigargin failed to increase [Ca2+]c levels, although a very small peak was observed at 8 h of incubation (Figure 3C). These results indicate that IL-1β starts depleting ER Ca2+ at 4 h, and by 8 h, almost complete depletion is observed. At 12 and 24 h, a completely flat pattern of [Ca2+]c in the presence of thapsigargin is observed, indicating that at these times, the ER is completely depleted of Ca2+. In addition, this proves that the decrease in [Ca2+]c observed at 12 and 24 h of IL-1β incubation (Figure 3B) is not because of the Ca2+ going back to the ER.
To validate this, we evaluated mitochondrial Ca2+ levels ([Ca2+]m) in the presence and absence of IL-1β using the specific mitochondrial Ca2+ dye Rhod-2. As depicted in Figure 3D, at 2–8 h of IL-1β incubation, [Ca2+]m is comparable to those of control cells; however, at 12 and 24 h of incubation, there is a significant increase. That this is also mediated by JNK1/2 is evident because JNK1/2 siRNA I and JNK1/2 siRNA II prevent these increases in mitochondrial Ca2+ uptake (Figure 3D). Taken together, these results suggest that the decrease in cytoplasmic Ca2+ that we observed at 12 and 24 h as shown in Figure 3B was presumably because they were taken up by the mitochondria. To further confirm the presence of calcium within the mitochondria, we incubated RINm5F cells in the presence and absence of IL-1β with or without JNK1/2 siRNA and double loaded them with MitoTracker Green dye (a mitochondrion-specific marker) and Rhod-2. An arrangement of mitochondrial threads was stained with MitoTracker Green dye (Figure 3E). Further, at 12 and 24 h of IL-1β treatment, [Ca2+]m staining also colocalized with these MitoTracker Green–stained mitochondrial threads (Figure 3E), suggesting the presence of Ca2+ within the mitochondria. This colocalized image validated our data of Figure 3D that at 12 and 24 h, the increased fluorescence of Rhod-2 reflects an increased presence of Ca2+ within the mitochondria. This was also prevented in cells preincubated with JNK1/2 siRNA, indicating that IL-1β–induced [Ca2+]m uptake is mediated by JNK1/2 (Figure 3E, right).
IL-1β–mediated decrease in [Ca2+]c is due to mitochondrial Ca2+ uptake
As shown in Figure 3, the decrease in [Ca2+]c at 12 and 24 h of IL-1β incubation was accompanied by a parallel increase in [Ca2+]m. Although this suggests a possible correlation between the decrease in [Ca2+]c and the increase in [Ca2+]m, it does not establish that the fall in [Ca2+]c is due to mitochondrial Ca2+ uptake. To determine this, we preincubated RINm5F cells with Ru 360, a mitochondrial Ca2+ uptake inhibitor, before IL-1β incubation. We then assessed [Ca2+]c and [Ca2+]m levels with Fluo-4 and Rhod-2, respectively. In the absence of Ru360, IL-1β–mediated Ca2+ movement demonstrated an identical pattern as in Figure 3. At 8 h of IL-1β incubation, there was a significant increase in [Ca2+]c, and this was followed by a decrease at 24 h of incubation that was accompanied by a concomitant increase in [Ca2+]m (Figure 4A). However, in cells pretreated with Ru360, [Ca2+]c levels demonstrated a similar pattern of increase at 8 h of incubation; at 24 h, the [Ca2+]m levels did not increase, and the elevated levels of [Ca2+]c were persistent until 24 h of incubation (Figure 4B). These results suggest that the decrease in [Ca2+]c observed at 12 and 24 h of IL-1β incubation is due to the Ca2+ being taken up by the mitochondria.
FIGURE 4:
IL-1β–mediated decrease in [Ca2+]c is due to [Ca2+]m uptake. RINm5F cells were preincubated with vehicle (A) or Ru360 (10 μM) (B) and then incubated in the absence or presence of IL-1β (2 ng/ml) for 8 and 24 h. On termination of incubation, cells stained with Fluo-4 and Rhod-2, and [Ca2+]c and [Ca2+]m, respectively, were imaged as described in Materials and Methods. The intensity of the dyes at each time point is shown in the inset, where the x-axis represents the fluorescence intensity. All experiments were done three times, and representative figures are given.
IL-1β increases [Ca2+]c and [Ca2+]m by ER Ca2+ release via IP3R
To further decipher the details of the contribution of the ER in these calcium movements, we studied ER Ca2+ release using specific ER Ca2+-channel blockers. Two types of Ca2+ channels are responsible for the release of Ca2+ from the ER: the ryanodine receptor (RyR) and the inositol triphosphate receptor (IP3R). We used channel-specific inhibitors, namely dantrolene and ryanodine (for RyR) and xestospongin C (for IP3R), to study their effects on [Ca2+]c and [Ca2+]m. Incubation of cells with either dantrolene or ryanodine (10 μM) could not prevent IL-1β–mediated increase in either cytoplasmic or mitochondrial Ca2+ levels (Figure 5, A and B). However, in the presence of xestospongin C (1 μM), IL-1β–mediated increase in Ca2+ levels in the cytosol and mitochondria was completely abrogated (Figure 5, C and D). These results indicate that the increase in [Ca2+]c and [Ca2+]m that we observed (Figure 3) was due to their increased release from the ER via the IP3R.
FIGURE 5:
IL-1β increases ER Ca2+ release through the IP3R. RINm5F cells were preincubated with the IP3R inhibitor xestospongin C (Xesto C, 1 μM) or the ryanodine receptor inhibitors dantrolene and ryanodine (10 μM) and then incubated without (0 h) or with IL-1β (2 ng/ml) for 2, 4, 8, 12, and 24 h. On termination of incubation, cells (5000 cells) were assessed for cytosolic (A, C) and mitochondrial (B, D) Ca2+ content using Fluo-4 and Rhod-2, respectively, in a multiplate reader. (E) RINm5F cells were grown to confluence and incubated in the absence or presence of IL-1β (2 ng/ml) for 8 and 12 h. On termination of incubation, total RNA was isolated and reverse transcribed, and the levels of IP3RI, II, and III were determined with quantitative RT-PCR using isoform-specific primers. Values were normalized to those of 18S rRNA. All experiments were done three times, and results presented with respect to control are means ± SEM of three independent experiments. ***p < 0.001, **p < 0.01, and *p < 0.05 as compared with control (0-h incubation); ##p < 0.001, #p < 0.01, and $p < 0.05 as compared with incubation with IL-1β alone at similar time points.
IL-1β increases the level of IP3R-I isoform, which increases [Ca2+]c
Because we observed that IL-1β–mediated increases in [Ca2+]c and [Ca2+]m were due to ER Ca2+ release through the IP3R receptor channels, we further sought to determine the mechanism of this increased Ca2+ release. As shown in Figure 5E, in the presence of IL-1β, there was a significant increase in the level of IP3R-I at 8 h of incubation, which increased further at 12 h. However, the levels of the other isoforms, that is, IP3R-II and IP3R-III, were unchanged at all the time points studied. In fact, use of IP3R-I siRNA prevented IL-1β–induced increases in [Ca2+]c and [Ca2+]m (unpublished data). Taken together, these results suggest that IL-1β induces elevations in [Ca2+]c and [Ca2+]m by up-regulating the levels of IP3R-I on the ER membrane.
IP3R inhibition abrogates IL-1β–mediated mitochondrial dysfunction
We observed that Ca2+ release from the ER through IP3R leads to increased cytoplasmic and mitochondrial Ca2+ levels during IL-1β action. To evaluate the role of IP3R inhibition in IL-1β–mediated mitochondrial dysfunction, we assessed the two critical events, mPTP opening and ∆ψm, in the presence of the IP3R inhibitor xestospongin C. RINm5F cells were preincubated with xestospongin C (1 μM) and then incubated with IL-1β (2 ng/ml) at various times. On termination of the incubation, ∆ψm and mPTP were studied. As shown in Figure 6, xestospongin C treatment before IL-1β prevented IL-1β–mediated disturbance in ∆ψm and opening of the mitochondrial pore, suggesting that inhibition of Ca2+ release from the ER could prevent IL-1β–mediated mitochondrial alterations. These results suggest that ER Ca2+ release via IP3R triggered by IL-1β leads to increased cytoplasmic and mitochondrial Ca2+ levels associated with mitochondrial dysfunction.
FIGURE 6:
Inhibition of IL-1β–induced ER Ca2+ release by xestospongin C prevents IL-1β–mediated mitochondrial dysfunctions. (A) Confluent RINm5F cells were preincubated with the IP3R inhibitor xestospongin C (1 μM) for 30 min and then incubated without (0 h) or with IL-1β (2 ng/ml) for 2, 8, 12, 24 and 36 h. On termination of incubation, mitochondrial membrane potential was evaluated by JC-1 as in Figure 2, A and B. (B) Quantitative data on the green JC-1 monomers, which are markers of disturbed mitochondrial membrane potential. (C). After incubation of RINm5F cells with IL-1β (0, 24, and 36 h) and xestospongin C, cells were loaded with calcein-AM alone or calcein-AM and CoCl2 and incubated for 15 min at 37°C. The calcein-AM fluorescence was then read by flow cytometry, and the IL-1β–induced decrease in calcein-AM fluorescence in the presence of CoCl2 suggested increased mitochondrial pore formation that was prevented in the presence of xestospongin C. The arrow indicates the position of the peak fluorescence within the mitochondria in the presence of calcein-AM and CoCl2. All experiments were done three times, and representative figures are given in A and C. Data in B are means of three independent experiments. *p < 0.05 as compared with control (0 h) and #p < 0.05 as compared with the incubation with IL-1β alone at 36 h.
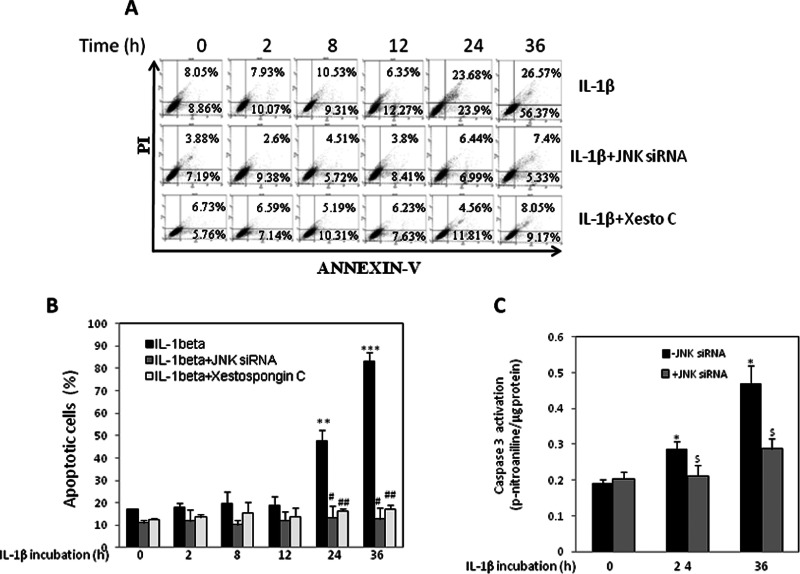
JNK1/2 siRNA and xestospongin C prevent IL-1β–mediated apoptosis
Further, to finally correlate these events with IL-1β–mediated apoptosis, we studied the effect of JNK1/2 and IP3R inhibition on these phenomena. RINm5F cells were treated with IL-1β in the absence or presence of either JNK1/2 siRNA I or xestospongin C. On termination of incubation, cells were labeled with annexin V–fluorescein isothiocyanate (FITC), and IL-1β treatment alone showed a significant increase in annexin V–positive cells in a time-dependent manner (at 24 and 36 h). As compared with the control cells, IL-1β incubation showed 80% of annexin V–positive cells, including early and late apoptotic cells (Figure 7, A and B) at 36 h. However, in cells preincubated with JNK1/2 siRNA I or xestospongin C, this increase in IL-1β–mediated annexin V–positive apoptotic cells was completely prevented, suggesting a critical role of JNK1/2 and IP3R in the apoptotic effect of IL-1β. In the presence of JNK1/2 siRNA I or the IP3R inhibitor, only 12 and 17%, respectively, of the cells were annexin V positive even in the presence of IL-1β, as opposed to 80% apoptotic cells in the presence of IL-1β alone.
FIGURE 7:
IL-1β induces apoptosis in RINm5F cells that is prevented in the presence of JNK1/2 siRNA and xestospongin C. (A) RINm5F cells were transfected with JNK1/2 siRNA I (100 nM) or preincubated with xestospongin C (1 μM) and then incubated in the absence (0 h) or presence of IL-1β (2 ng/ml) for 2, 8, 12, 24, and 36 h. On termination of incubation, cells were washed and labeled with annexin V and PI. After 15 min of incubation, cells were analyzed for apoptosis by flow cytometry. Experiments were done three times, and a representative figure is shown. (B) Quantitative data of apoptotic cells (percentage of control) of the incubation as shown in A. (C) Cells incubated with IL-1β with or without JNK1/2 siRNA I for 24 and 36 h were evaluated for apoptosis using the caspase 3 activation assay kit. Each value is the mean ± SEM of three experiments and is presented with respect to control (0 h). ***p < 0.001, **p < 0.01, and *p < 0.05 as compared with control (0-h incubation); ##p < 0.001, #p < 0.01, and $p < 0.05 as compared with the incubation with IL-1β plus scramble at the same time points.
The antiapoptotic effect of JNK1/2 siRNA I was also confirmed by caspase 3 activation assay. Whereas IL-1β led to significant caspase 3 activation at 24 and 36 h of incubation, this effect was completely prevented in cells incubated with JNK1/2 siRNA (Figure 7C). These observations suggest a protective effect of these inhibitors on IL-1β–induced apoptotic cell death.
JNK1/2 mediate IL-1β–induced alterations in human primary β-cells, and their inhibition restores glucose-mediated insulin release
All of the results described so far are for insulin-secreting RIN5mF cells. To validate them and substantiate their physiological relevance, we used human primary β-cells and incubated them with IL-1β (2 ng/ml) in the presence and absence of JNK1/2 siRNA I (100 nM). In the presence of JNK1/2 siRNA I, there was a significant decrease in the levels of JNK1/2 in primary human β-cells (Figure 8, A and B). Further, IL-1β significantly increased [Ca2+]c in primary β-cells at 4 h, which further increased at 8 h and then declined at 12 and 24 h (Figure 8C). This pattern of [Ca2+]c increase, in an identical manner as described for RINm5F cells, suggests that IL-1β–mediated ER Ca2+ release is initiated at 4 h of incubation. To further prove that the decrease in [Ca2+]c at 12 and 24 h is due to its import into the mitochondria, we evaluated human primary β-cells incubated with IL-1β in the absence and presence of JNK1/2 siRNA I for [Ca2+]m using Rhod-2. Increased [Ca2+]m signals were observed at 12 and 24 h of incubation (Figure 8D), suggesting delayed entry of Ca2+ into the mitochondria. Of interest, both the [Ca2+]c and [Ca2+]m increases were prevented in the presence of JNK1/2 siRNA I, indicating a mediating role of JNK1/2 during IL-1β action on Ca2+ movement within the β-cell. In the mPTP opening assay as determined by flow cytometry analysis, IL-1β led to a modest decrease in mitochondrial fluorescence (as seen in the presence of calcein-AM) at 24 h, which further decreased significantly at 36 h (Figure 8E); however, in the presence of JNK1/2 siRNA I, this decrease was completely prevented. Further, as seen in Figure 8, F and G, IL-1β significantly increased the amount of monomeric green JC1 (R2) at 36 h, which suggested altered mitochondrial membrane potential, and this was prevented in the presence of JNK1/2 siRNA I. Slight increases were observed at earlier times that significantly increased at 24 and 36 h, and they were prevented in the presence of JNK1/2 siRNA I. These results suggest that in primary β-cells, IL-1β alters cellular Ca2+ movement and induces mitochondrial dysfunction, specifically, altered mitochondrial membrane potential and mPTP opening, in a JNK1/2-dependent manner.
FIGURE 8:
IL-1β induces JNK1/2-mediated mitochondrial alterations in human primary pancreatic β-cells. Human primary β-cells were transfected with scramble or JNK1/2 siRNA I (100 nM), and after 36 h, cells were lysed and the levels of JNK1/2 determined by Western blot analysis. (A) A representative blot. (B) Its densitometric analyses. (C) Human primary β-cells were grown to confluence and incubated with IL-1β for 2, 4, 8, 12, and 24 h in the absence or presence of JNK1/2 siRNA I (100 nM). On termination of incubation, cytosolic Ca2+ content was evaluated fluorimetrically by incubating the cells in the presence of Fluo-4. (D) Cells incubated as in C were stained with Rhod-2, and the mitochondrial Ca2+ content was estimated fluorimetrically. (E) Confluent β-cells were incubated with IL-1β in the absence or presence of JNK1/2 siRNA I for 24 and 36 h and assessed for mitochondrial membrane pore formation using calcein-AM and CoCl2. (F) Mitochondrial membrane potential was determined in human primary β-cells incubated with IL-1β for 2, 8, 12, 24, and 36 h with or without JNK1/2 siRNA I as described in Materials and Methods. Green JC-1 monomers are in the R2 quadrant, whereas the red aggregates of JC-1 are in the R1 quadrant. (G) Quantitative values of green JC-1 monomers with respect to the red JC-1 aggregates. The solid lines are in the presence of IL-1β alone, and the dashed lines are in the presence of IL-1β and JNK1/2 siRNA I. (H). Confluent human primary β-cells were transfected with either scramble or JNK1/2 siRNA I (100 nM) and, after 36 h, incubated in the absence or presence of IL-1β (2 ng/ml) for 36 h. On termination of this incubation, cells were stimulated with basal or 25 mM glucose (Gluc), and after 2 h the media and cells were collected for determination of insulin and protein content, respectively. All experiments were done three times, and values are means of three independent experiments. **p < 0.01 and *p < 0.05 as compared with scramble (B); ***p < 0.001, **p < 0.01, and *p < 0.05 as compared with incubation in the absence of IL-1β (0h); $p < 0.05 and #p < 0.01 as compared with IL-1β alone at the same time point.
To evaluate physiological relevance, we incubated human primary β-cells with IL-1β for 36 h with and without JNK1/2 siRNA I and then stimulated them with basal or increased concentrations of glucose (25 mM). On termination of incubation, the media were collected, and the insulin released into the medium was assessed as described in Materials and Methods. Compared with control, there was a significant decrease in glucose-induced insulin release in the presence of IL-1β (Figure 8H). Although cells incubated with basal levels of glucose also showed this trend, the effect was more pronounced in cells incubated with 25 mM glucose. This decrease was completely abrogated by JNK1/2 siRNA I, and the values of insulin released by these cells were comparable to those of control cells (incubated in the presence of scramble; Figure 8H).
DISCUSSION
A previous study from our laboratory showed that IL-1β mediates pancreatic cell death via induction of ER stress in a JNK1/2-dependent manner (Verma and Datta, 2010). In the present study, we determined the consequent cellular effects of this IL-1β action and the contribution of JNK1/2 to it. To this end, we report the effect of IL-1β and JNK1/2 on ER Ca2+ release, mitochondrial dysfunction, and pancreatic β-cell apoptotic death. Elevated levels of IL-1β are believed to significantly contribute to β-cell death (Maedler et al., 2002), and this involves the participation of an intricate network of interacting factors (Mokhtari et al., 2008) that includes primarily NF-κB, mitogen-activated protein kinase (MAPK), NO, and so on that coordinate to lead to apoptotic cell death. Although it is well studied and reported that induction of ER stress activates JNK through activated IRE-1 (Urano et al., 2000), we reported that it is JNK1/2 activation that mediates IL-1β–mediated ER stress induction (Verma and Datta, 2010).
The present study demonstrates that JNK1/2 catalyzes IL-1β–mediated mitochondrial dysfunction. IL-1β–mediated decrease in mitochondrial ATP generation and increase in ROS production were completely prevented by JNK1/2 inhibition. The increase in mitochondrial pore opening and membrane potential disturbance also displayed similar patterns, suggesting that JNK1/2 mediates these mitochondrial alterations. IL-1β has been reported to disrupt mitochondrial membrane potential and cause ATP deprivation, leading to cell death in chondrocytes (Yasuhara et al., 2005; Kim et al., 2010). In insulin-secreting INS-1 cells, IL-1β markedly reduces mitochondrial membrane potential and metabolic activity (Veluthakal et al., 2005). These studies suggest that IL-1β induces mitochondrial dysfunction and initiates a cascade of cellular alterations that culminate in cell death.
In addition to the mitochondrial alterations, the ER also experiences stress that contributes to cell death. Many cellular disturbances that interfere with the normal functioning of the ER cause accumulation of unfolded proteins that trigger the unfolded protein response, which, when compromised, can eventually trigger apoptotic cell death. Diverse mechanisms are believed to mediate this ER stress–induced cell death, including but not limited to activation of proteases, transcription factors, kinases, and Bcl-2 family protein (Pirot et al., 2007).
In spite of being an independent organelle and exhibiting an independent contributory role during apoptosis, the ER also exerts typical frequent cross-talk with mitochondria. Close contacts between these two organelles are evident in cells, and they participate in important cellular functions, such as Ca2+ homeostasis and mitochondrial energy and lipid metabolism (Rizzuto et al., 1998; Wang et al., 2000; de Brito and Scorano, 2010). Therefore functional alterations in one organelle affect those of the other. However, the precise mechanisms of functional interplay between these two organelles are not completely understood.
Our data show that IL-1β–mediated ER stress leads to increased Ca2+ release via the IP3R. IP3Rs are the primary ER Ca2+ release channels in several tissues (Lee and Laychock, 2000). In mammals, there are primarily three subtypes of IP3R (IP3RI, II, and III), and their genes share 70–80% similarity in primary sequence (Marchi et al., 2012). However, they differ in localization, regulation, and modulation. Our data show that IL-1β leads to significant up-regulation of the levels of IP3RI without altering the levels of IR3RII and III in RINm5F cells. Such transcriptional regulation of IP3RI by IL-1β has also been shown in osteoblasts and astrocytic cell cultures (Pita et al., 1999; Bradford et al., 2000). These increases in IP3RI levels by IL-1β are responsible for increased ER Ca2+ release by IL-1β, which is taken up by the mitochondria, leading to mitochondrial dysfunction. The IP3R receptor blocker xestospongin C can prevent ER Ca2+ release, its uptake into the mitochondria, and subsequent mitochondrial dysfunction, indicating ER–mitochondrial interplay in the IL-1β–induced Ca2+ movements within the cell. The Ca2+ that is normally maintained at a low concentration in the cytosol increases as a result of its release from the ER (Filippin et al., 2003), which is then targeted to the mitochondria under cellular stresses, specifically through voltage-dependent anion channels and mitochondrial calcium uniporters (Rapizzi et al., 2002; Kirichok et al., 2004).
Increased Ca2+ release from the ER leads to the formation of microdomains on the mitochondrial membrane to additionally facilitate mitochondrial Ca2+ uptake (Giorgi et al., 2009; Rizzuto et al., 2009). Of interest, our results show that in the presence of JNK1/2 siRNA, both ER Ca2+ release and mitochondrial Ca2+ uptake are completely prevented. Taken together, our results indicate that IL-1β–mediated Ca2+ release and its consequent uptake by the mitochondria occurs in a JNK1/2-dependent manner. JNK is a stress-dependent MAPK and is a critical mediator of the cellular effects of IL-1β (Bonny et al., 2000). Three different isoforms of JNK have been identified, JNK1, JNK2, and JNK3 (Abdelli et al., 2009), and JNK1 and JNK2 are believed to be ubiquitous. The presence of JNK3 was previously believed to be restricted to the neurons (Davis, 2000), but recent reports suggest that its levels also are high in pancreatic β-cells (Abdelli et al., 2009; Abdelli and Bonny, 2012). However, whereas JNK1/2 have been involved in sensitizing β-cells to apoptotic cell death, JNK3 acts to protect β-cells against cytokine-induced cellular dysfunction and cell death mainly by maintaining a normal IRS2/Akt signaling cascade (Abdelli and Bonny, 2012). We earlier showed that JNK1/2 catalyzes IL-1β–induced ER stress. This observation was very significant in the midst of diverse reports that described the activation of JNK by ER stress–mediated activation of IRE-1 (Urano et al., 2000). The present study describes the mediating role of JNK1/2 during IL-1β induced ER stress, which is translated further into ER Ca2+ release, its uptake into the mitochondria, exertion of mitochondrial dysfunctions, and finally apoptotic cell death (Figure 9).
FIGURE 9:
Scheme of the role of JNK1/2 in IL-1β–mediated Ca2+movement between the ER and the mitochondria during pancreatic apoptosis. Binding of IL-1β to its receptor activates JNK1/2, which promotes ER Ca2+ release via the IP3R. This Ca2+ is then taken up by the mitochondria, which undergo mPTP opening and disturbance in membrane potential, which then initiates a cascade of events that leads to apoptotic cell death.
Persistent uptake of Ca2+ into the mitochondria leads to opening of the mPTP, disturbed mitochondrial membrane potential, and induction of apoptosis (Hajnóczky et al., 2006). On encountering any death stimuli, mitochondria release the apoptosis-inducing factor and generate ROS, which triggers cell death (Rizvi et al., 2011). Such regulatory roles of mitochondria and loss of its integrity and function are evident in diverse pathological states (Wang et al., 2008), and during pancreatic β-cell death, as is seen in the present study, IL-1β exerts its effect by inducing severe mitochondrial alterations. This event, which is accompanied by ER Ca2+ release and its uptake by the mitochondria, is regulated by JNK1/2; therefore JNK1/2 might be an attractive target for therapeutic intervention to prevent pancreatic β-cell death.
To conclude, our study reveals JNK1/2-dependent ER–mitochondrial Ca2+ cross-talk during IL-1β action in pancreatic β-cells. This identifies JNK1/2 as a central key molecule in the detrimental effects of IL-1β and therefore suggests that IL-1β–mediated JNK1/2 activation is a significant event that might be targeted to prevent the deleterious apoptotic effects of ER–mitochondrial Ca2+ cross-talk.
MATERIALS AND METHODS
Cell culture and incubations
All experiments were done in primary human β-cells (described later) and the rat islet–derived, insulin-producing cell line RINm5F. RINm5F cells were procured from the National Center for Cell Science (Pune, India) and were cultured in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (GIBCO Laboratory, Grand Island, NY), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B in the presence of 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and 2 mM l-glutamine at 37°C and 5% CO2. On attaining confluence, cells were transferred to serum-free medium containing IL-1β (2 ng/ml) and incubated for various time periods. For experiments with JNK1/2, cells were transfected with either the control (scramble) or JNK1/2 siRNA (25–100 nM; Cell Signaling Technology, Beverly, MA, or Sigma-Aldrich) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. To confirm the specificity of JNK1/2 inhibition, two different siRNAs, JNK1/2 siRNA I and siRNA II, were used in the experiments. After growing in fresh RPMI for 36 h, cells were incubated in the absence or presence of IL-1β as described. This dose of IL-1β and JNK1/2 siRNA incubation was chosen according to an earlier report from our laboratory (Verma and Datta, 2010).
Determination of JNK1/2 activation and ER stress induction
Confluent RINm5F cells were incubated without or with IL-1β (2 ng/ml) for 2, 4, 8, 12, and 24 h. On termination of incubation, cells were lysed, and lysates (60 μg of protein) were evaluated for the levels of p-JNK1/2 and total JNK1/2 levels by Western blot using specific antibodies (Cell Signaling Technology). In another set of experiments, total RNA was isolated from RINm5F cells identically incubated with IL-1β, and the levels of ER stress markers namely Bip and CHOP were determined by quantitative real-time (RT)-PCR using gene-specific primers (Bip: sense, 5′CTGGACTGAATGTCATGAGG3′, and antisense, 5′TATCCAGGCCATATGCAATAG3′; CHOP, sense, 5′CCAGCAGAGGTCACAAGCAC3′, and antisense, 5′CGCACTGA CCACTCTGTTTC3′). To determine the optimum concentration of JNK1/2 siRNA to be used, cells were transfected with 25–100 nM of the siRNA, and after 36 h, the levels of total JNK1/2 were determined by Western blot. The effectiveness of the JNK1/2 siRNA was corroborated by its effect on c-jun and p-c-jun, the immediate target of JNK, by Western blot using specific antibodies (Cell Signaling Technology). Immunoreactive bands were detected using 5-bromo-4-chloro-3′-indolyphosphate/nitro blue tetrazolium BCIP/NBT; the phosphorylated protein forms were detected using the ECL Western Blotting Kit (Pierce, Thermo Scientific, Rockford, IL), and the membranes were then stripped and reprobed identically for the respective total proteins. Glyceraldehyde-3-phosphate dehydrogenase was taken as the loading control.
Mitochondrial membrane potential determination
Mitochondrial membrane potential was measured using the mitochondrial voltage-sensitive dye JC-1 (Molecular Probes, Eugene, OR; red, aggregates, excitation 585 nm/emission 590 nm; green, nonaggregates, excitation 498 nm/emission 525 nm). RINm5F cells were cultured in 12-well plates and stimulated with IL-1β in the presence and absence of JNK1/2 siRNA I for 0, 2, 8, 12, 24, and 36 h. On termination of the incubation, mitochondrial membrane potential was evaluated using JC-1 according to the manufacturer's instructions. Briefly, cells were incubated with 2 μM JC-1 in RPMI 1640 media for 30 min at 37°C. Cells were then collected by scraping, washed in phosphate-buffered saline (PBS), and subjected to flow cytometry analysis (FACSCalibur; BD Biosciences, Franklin Lakes, NJ). An increase in the fluorescence of the green channel (FL1) was interpreted as decrease of Δψm. As a positive control to check the loss of Δψm, cells were treated with 10 µM carbonylcyanide-trifluoro-methoxyphenylene hydrazone (CCCP; Molecular Probes) at 37°C for 5 min and assessed identically for Δψm where a complete loss of the Δψm was observed.
Mitochondrial pore formation
Mitochondrial permeability transition pore formation was measured using the mitochondrial Transition Pore Assay Kit (Molecular Probes) according to the manufacturer's instructions. RINm5F cells were cultured in 12-well plates and stimulated with IL-1β (2 ng/ml) for 0, 24, and 36 h in the presence and absence of JNK1/2 siRNA I. The mPTP opening was measured by monitoring calcein-AM fluorescence in the absence and presence of CoCl2. After completion of incubation, cells were washed twice with PBS and resuspended in prewarmed RPMI 1640 medium. The cells were then loaded with calcein-AM for 15 min at 37°C as per the manufacturer's instructions. In another set, cells incubated identically were loaded with calcein-AM in the presence of CoCl2. Cells were then washed thoroughly and centrifuged, and cell pellets were resuspended in PBS. Cells were analyzed for calcein-AM fluorescence by flow cytometry (FACSCalibur). The change in calcein AM fluorescence between calcein-AM alone and in combination with CoCl2 indicates the continuous activation of mitochondrial permeability transition pores.
Mitochondrial ATP generation
The ATP concentration in the mitochondria was determined using the Bioluminescent ATP Determination Kit (Molecular Probes). Cells were incubated in the presence and absence of IL-1β with or without JNK1/2 siRNA I. On termination of incubation, mitochondrial proteins were isolated using a mitochondria isolation kit (Pierce), and equal amounts of mitochondrial proteins were assayed for ATP content according to the manufacturer's instructions. Luminescence was read on an Infinite M200 multiplate reader (Tecan, Mannedorf, Switzerland), and values were calculated from an ATP standard curve and normalized to the total mitochondrial protein content.
Determination of mitochondrial ROS (superoxide) generation
Mitochondrial ROS generation was detected using the MitoSox fluorescent marker (excitation 510 nm/emission 580 nm; Molecular Probes). RINm5F cells were seeded on Ibidi culture dishes (35 mm; Ibidi, Martinsried, Germany) and stimulated with IL-1β (2 ng/ml) in the presence and absence of JNK1/2 siRNA I. On termination of incubation, cells were loaded with MitoTracker Dye (50 nM; Molecular Probes; excitation 488 nm/emission 516 nm) and incubated for 20 min at 37°C, followed by 5 μM MitoSox (Invitrogen) in prewarmed RPMI 1640 medium for 10 min at 37°C. Cells were then washed twice with PBS and visualized under a live-cell imaging system (Eclipse Ti; Nikon, Melville, NY).
Intracellular Ca2+ concentration measurement
Intracellular cytosolic, [Ca2+]c, and mitochondrial, [Ca2+]m, calcium were measured using the fluorescent indicator Fluo-4 direct calcium assay kit and Rhod-2, respectively (Molecular Probes). RINm5F cells were cultured in 96-well (5000 cells) plates and were stimulated in the presence of 2 ng/ml IL-1β for 0, 2, 4, 8, 12, and 24 h. To evaluate the contribution of ER Ca2+ channels on [Ca2+]c and [Ca2+]m, cells were also treated with ER Ca2+-channel blockers dantrolene and ryanodine (ryanodine receptor blockers; 10 μM) for 5 min at 37°C and xestospongin C (IP3R blocker; 1 μM) for 15 min at 37°C. On termination of incubation, cells were loaded with 10 μM Rhod-2 and Flou-4 (2X) according to manufacturer's instructions. The fluorescence of [Ca2+]c by Flou-4 (excitation 494 nm/emission 516 nm) and [Ca2+]m by Rhod-2 (excitation 552 nm/emission 581 nm) was measured in a Tecan infinite M200 multiplate reader. To identify that the increases in [Ca2+]c were from the ER, intracellular [Ca2+]c levels were also measured using Fluo-4 in the presence of thapsigargin. Cells were plated in six-well plates and incubated in the presence and absence of IL-1β for 0, 2, 4, 8, 12, and 24 h and then stained with Fluo-4 according to the manufacturer's instruction. Cells were washed with Ca2+-free PBS and stimulated with thapsigargin (1 mM) after 60 s, and the influx of calcium into the cytosol was monitored using Fluorolog Tau-3 lifetime system fluorimeter (HORIBA Jobin Yvon, Edison, NJ) for 10 min. For the experiments with xestospongin C on mitochondrial physiology, cells were incubated with IL-1β with or without xestospongin C and evaluated for mPTP opening and for mitochondrial membrane potential as described.
Quantitative RT-PCR for IR3R isoforms
RINm5F cells were grown to confluence in six-well plates and incubated without or with IL-1β (2 ng/ml) for 8 and 12 h. On termination of incubation, total RNA was isolated using TRIzol (Invitrogen), and 2 μg of total RNA was reverse transcribed using random hexamers. The cDNA was subjected to quantitative RT-PCR using SYBR Green RT-PCR Master Mix (Applied Biosystems, Foster City, CA) and isoform-specific primers (Zhao et al., 2008; with slight modifications: IP3R-I, sense, 5′GAGATGAGCCTGGCTGAGGTTCAA3′, and antisense, 5′TGTTGCCTCCTTCCAGAAGTGCGA3′; IP3R-II, sense, 5′AGTACCTGGCCATCAACCAG3′, and antisense, 5′TCTTGGTGGGGATCTCAGTC3′; IP3R-III, sense, 5′AGACCCGCTGGCCTACTATGAGAA3′, and antisense, 5′GTCAGGAACTGGCAGATGGCAGGT3′) according to the manufacturer's instructions (StepOne Plus RT-PCR system; Applied Biosystems). Data were analyzed using the Pfaffl method (Pfaffl, 2001) and normalized to 18S rRNA.
Live-cell imaging of [Ca2+]m using Rhod-2
To further validate the increase in mitochondrial calcium levels, we performed live-cell imaging for [Ca2+]m by incubating RINm5F cells grown in Ibidi culture dishes (35 mm) in the presence of IL-1β with or without JNK1/2 siRNA. We plated 50,000 cells in each well for the experiments. Cells were then loaded with the MitoTracker Dye (50 nM) and Rhod-2 (10 μM) for 15 min at 37°C and washed twice with Ca2+-free PBS to completely remove the excess dye solution. Hank's balanced salt solution without phenol red was used as the imaging medium, and cells were then visualized in a fluorescence microscope (ApoChromatic, Nikon Eclipse Ti). CCCP was used as a reference control to deplete mitochondrial Ca2+ content. Images were taken at 60× magnification with a numerical aperture of 1.4 using a Nikon camera (DSQi1MC). Images were processed and quantified using Nikon Advanced element AR3.2 software.
In another experiment, cells were preincubated with Ru360 (a mitochondrial Ca2+ uptake inhibitor) at a dose of 10 μM and then incubated with IL-1β (2 ng/ml) for 8 and 24 h. On termination of incubation, cells were stained with Fluo-4 and Rhod-2, and [Ca2+]c and [Ca2+]m were imaged and their intensities quantified as described.
Detection of apoptosis by annexin V and caspase 3 activation
RINm5F cells were stimulated with IL-1β (2 ng/ml) for 0, 2, 8, 12, 24, and 36 h in the presence and absence of JNK1/2 siRNA I. They were then washed with PBS, scrapped, and centrifuged at 200 × g for 1 min. Apoptosis was detected using the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer's instructions. Briefly, the cell pellet from each incubation was dissolved in the supplied binding buffer and centrifuged at 200 × g for 1 min, and the pellet was resuspended in 200 ml of binding buffer containing 5 μl of annexin V FITC and 5 μl of PI conjugate. These were incubated for 15 min at 25°C and analyzed for apoptosis by flow cytometry (FACSCalibur). The effect of xestospongin C (1 μM) on IL-β–mediated cellular apoptosis was assessed in an identical manner.
RINm5F cells were transfected with either the scramble or the JNK1/2 siRNA I and then incubated without or with IL-1β (2 ng/ml) for 24 and 36 h. On termination of incubation, cells were lysed, and equal amounts of protein from each incubation were evaluated for caspase 3 activation using the Caspase 3 Assay Kit (Sigma-Aldrich).
Primary human β-cell culture
Primary human β-cells were purchased from Applied Biological Materials (Richmond, Canada) and grown in Prigrow II medium (Applied Biological Materials) containing fetal bovine serum (10%) and antibiotics according to the manufacturer's instructions. On attaining confluence, they were incubated in the absence or presence of IL-1β (2 ng/ml) for 2, 4, 8, 12, 24, and 36 h with or without JNK1/2 siRNA I (100 nM), and on termination of incubation, cells were evaluated for the presence of Ca2+ in the cytosol and mitochondria using Fluo-4 and Rhod-2 as described. Mitochondrial pore opening and determination of mitochondrial membrane potential in these cells was also studied under these conditions using the methods as described.
Glucose-induced insulin release assay
Human primary pancreatic β-cells were grown to confluence and transfected with JNK1/2 siRNA I (100 nM) using Lipofectamine 2000. After 36 h, cells were treated with IL-1β (2 ng/ml) for 36 h and then stimulated with basal or 25 mM glucose. After 2 h of incubation, the cells and media were collected for total protein content and insulin release, respectively. Insulin content was determined using the Insulin ELISA Kit (Glory Science, Del Rio, TX), and the values were normalized to the total protein content (measured by a BCA Kit; Pierce).
Statistical analysis
All incubations were done in triplicate, and statistical significance was calculated by the Student's t test. A value of at least p < 0.05 was considered as statistically significant.
Acknowledgments
We thank Saurabh Vig for help in preparation of the illustrations and Gunjan Purohit for help in capturing the microscopic images. This work was supported by Grant BSC0122 from the Council of Scientific and Industrial Research (New Delhi, India). G.V. and H.B. thank the Council of Scientific and Industrial Research for fellowships.
Abbreviations used:
- Akt
protein kinase B
- ATF6
activating transcription factor 6
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CHOP
CCAAT/enhancer-binding protein homologous protein
- IP3R
inositol 1,4,5-triphosphate receptor
- IRE-1α
inositol-requiring enzyme 1 alpha
- IRS2
insulin receptor substrate-2
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole carbocyanide iodide
- MCU
mitochondrial calcium uniporter
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum Ca+2 ATPase
- VDAC
voltage-dependent anion channel
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-12-0885) on April 24, 2013.
REFERENCES
- Abdelli S, Bonny C. JNK3 maintains expression of the insulin receptor substrate 2 (IRS2) in insulin-secreting cells: functional consequences for insulin signaling. PLoS One. 2012;7:e35997. doi: 10.1371/journal.pone.0035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelli S, Puyal J, Bielmann C, Buchillier V, Abderrahmani A, Clarke PG, Beckmann JS, Bonny C. JNK3 is abundant in insulin-secreting cells and protects against cytokine-induced apoptosis. Diabetologia. 2009;52:1871–1880. doi: 10.1007/s00125-009-1431-7. [DOI] [PubMed] [Google Scholar]
- Bonny C, Oberson A, Steinmann M, Schorderet DF, Nicod P, Waeber G. IB1 reduces cytokine-induced apoptosis of insulin-secreting cells. J Biol Chem. 2000;275:16466–16472. doi: 10.1074/jbc.M908297199. [DOI] [PubMed] [Google Scholar]
- Bradford PG, Maglich JM, Kirkwood KL. IL-1 beta increases type 1 inositol trisphosphate receptor expression and IL-6 secretory capacity in osteoblastic cell cultures. Mol Cell Biol Res Commun. 2000;3:73–75. doi: 10.1006/mcbr.2000.0194. [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van EF, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- Chen L, Koh DS, Hille B. Dynamics of calcium clearance in mouse pancreatic beta-cells. Diabetes. 2003;52:1723–1731. doi: 10.2337/diabetes.52.7.1723. [DOI] [PubMed] [Google Scholar]
- Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR. Pancreatic beta-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One. 2011;6:e22485. doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, McDaniel ML. Reversibility of interleukin-1 beta-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem J. 1994;299:719–724. doi: 10.1042/bj2990719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J. 1992;287:229–235. doi: 10.1042/bj2870229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Dula SB, Jecmenica M, Wu R, Jahanshahi P, Verrilli GM, Carter JD, Brayman KL, Nunemaker CS. Evidence that low-grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium. 2010;48:133–142. doi: 10.1016/j.ceca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippin L, Magalhaes PJ, Di BG, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- Giorgi C, De SD, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58:1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M, Cardozo AK, Eizirik DL. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ. 2009;16:1539–1550. doi: 10.1038/cdd.2009.99. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha RS, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Kwon KB, Song MY, Han MJ, Lee JH, Lee YR, Lee JH, Ryu DG, Park BH, Park JW. Flavonoids protect against cytokine-induced pancreatic beta-cell damage through suppression of nuclear factor kappaB activation. Pancreas. 2007;35:e1–e9. doi: 10.1097/mpa.0b013e31811ed0d2. [DOI] [PubMed] [Google Scholar]
- Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AW, Grishko V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Walter P. ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J Cell Sci. 2010;123:1389–1393. doi: 10.1242/jcs.058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lee B, Laychock SG. Regulation of inositol trisphosphate receptor isoform expression in glucose-desensitized rat pancreatic islets: role of cyclic adenosine 3’,5’-monophosphate and calcium. Endocrinology. 2000;141:1394–402. doi: 10.1210/endo.141.4.7421. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Storling J, Sturis J, Zuellig RA, Spinas GA, Arkhammar PO, Mandrup-Poulsen T, Donath MY. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2 +influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- Major CD, Wolf BA. Interleukin-1beta stimulation of c-Jun NH(2)-terminal kinase activity in insulin-secreting cells: evidence for cytoplasmic restriction. Diabetes. 2001;50:2721–2728. doi: 10.2337/diabetes.50.12.2721. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P. Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis. 2012;3:e304. doi: 10.1038/cddis.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaya E, et al. Calcium signalling-dependent mitochondrial dysfunction and bioenergetics regulation in respiratory chain complex II deficiency. Cell Death Differ. 2010;17:1855–1866. doi: 10.1038/cdd.2010.51. [DOI] [PubMed] [Google Scholar]
- Mokhtari D, Myers JW, Welsh N. MAPK kinase kinase-1 is essential for cytokine-induced c-Jun NH2-terminal kinase and nuclear factor-kappaB activation in human pancreatic islet cells. Diabetes. 2008;57:1896–1904. doi: 10.2337/db07-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirot P, Ortis F, Cnop M, Ma Y, Hendershot LM, Eizirik DL, Cardozo AK. Transcriptional regulation of the endoplasmic reticulum stress gene chop in pancreatic insulin-producing cells. Diabetes. 2007;56:1069–1077. doi: 10.2337/db06-1253. [DOI] [PubMed] [Google Scholar]
- Pita I, Jelaso AM, Ide CF. IL-1beta increases intracellular calcium through an IL-1 type 1 receptor mediated mechanism in C6 astrocytic cells. Int J Dev Neurosci. 1999;17:813–820. doi: 10.1016/s0736-5748(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi F, Heimann T, Herrnreiter A, O'Brien WJ. Mitochondrial dysfunction links ceramide activated HRK expression and cell death. PLoS One. 2011;6:e18137. doi: 10.1371/journal.pone.0018137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Storling J, Zaitsev SV, Kapelioukh IL, Karlsen AE, Billestrup N, Berggren PO, Mandrup-Poulsen T. Calcium has a permissive role in interleukin-1beta-induced c-jun N-terminal kinase activation in insulin-secreting cells. Endocrinology. 2005;146:3026–3036. doi: 10.1210/en.2005-0036. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51:311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes. 2004;53:113–121. doi: 10.2337/diabetes.53.1.113. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10:841–850. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–876. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Guay G, Pogan L, Sauve R, Nabi IR. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol. 2000;150:1489–1498. doi: 10.1083/jcb.150.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets. Diabetes. 2005;54:3238–3244. doi: 10.2337/diabetes.54.11.3238. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1beta induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389:315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]