Abstract
Child neglect is the most prevalent form of child maltreatment in the United States, and poses a serious public health concern. Children who survive such episodes go on to experience long-lasting psychological and behavioral problems, including higher rates of post-traumatic stress disorder symptoms, depression, alcohol and drug abuse, attention-deficit/hyperactivity disorder, and cognitive deficits. To date, most research into the causes of these life-long problems has focused on well-established targets such as stress responsive systems, including the hypothalamus–pituitary–adrenal axis. Using the maternal separation and early weaning model, we have attempted to provide comprehensive molecular profiling of a model of early-life neglect in an organism amenable to genomic manipulation: the mouse. In this article, we report new findings generated with this model using chromatin immunoprecipitation sequencing, diffuse tensor magnetic resonance imaging, and behavioral analyses. We also review the validity of the maternal separation and early weaning model, which reflects behavioral deficits observed in neglected humans including hyperactivity, anxiety, and attentional deficits. Finally, we summarize the molecular characterization of these animals, including RNA profiling and label-free proteomics, which highlight protein translation and myelination as novel pathways of interest.
Child maltreatment is an important public health concern with long-term ramifications for both the psychological welfare of the individual involved (Anda et al., 2006; DeBellis & Thomas, 2003; Watts-English, Fortson, Gibler, Hooper, & DeBellis, 2006), and the problems it creates for the education, health-care, and welfare systems (Bari & Robbins, 2011; DeBellis, 2005; Hildyard & Wolfe, 2002; Lehman, Taylor, Kiefe, & Seeman, 2009; US Department of Health and Human Services, 2011). Child neglect is currently the most common form of maltreatment in the United States, accounting for over 75% of reported cases of maltreatment, and is solely responsible for over 30% of maltreatment-related deaths (US Department of Health and Human Services, 2011). Positive interactions with caregivers and peers are critical for normal neurobiological development (DeBellis, 2005; Sánchez, Ladd, & Plotsky, 2001), and, as a result of early life maltreatment, many survivors experience long-lasting behavioral and cognitive difficulties (DeBellis, 2005; DeBellis & Thomas, 2003). These difficulties include inappropriate aggression (Hildyard & Wolfe, 2002) and anxiety (Mathews, Kaur, & Stein, 2008), postraumatic stress disorder (PTSD; DeBellis & Thomas, 2003), depression (Famularo, Kinscherff, & Fenton, 1992; Heim & Nemeroff, 2001; Kaufman, 1991), bipolar disorder and schizophrenia (Agid et al., 1999), alcohol and substance abuse (DeBellis, 2001), and attention-deficit/hyperactivity disorder (ADHD; Famularo et al., 1992; Ford et al., 2000; Hinshaw, 2002; Ouyang, Fang, Mercy, Perou, & Grosse, 2008). Children diagnosed with PTSD as a result of maltreatment have deficits in attention and abstract reasoning, and there are many reports of neglected children underperforming academically (Beers & DeBellis, 2002; Moradi, Doost, Taghavi, Yule, & Dalgleish, 1999).
Human studies thus far have focused on anatomical findings, using magnetic resonance imaging (MRI) and the stress-related hypothalamus–pituitary–adrenal axis (HPA), because levels of most involved hormones or their metabolites can be easily detected in urine and saliva (DeBellis & Thomas, 2003; Heim & Nemeroff, 2001). Neuroimaging findings in this domain are many and varied, but one of the major underlying themes concerns decreases in volume across large brain regions and myelin tracts, particularly the corpus callosum (Teicher et al., 1997), concomitant with enlarged ventricles (DeBellis et al., 1999; DeBellis & Thomas, 2003). Regions affected by volumetric changes include the prefrontal cortex (PFC; Bremner, 2006a; DeBellis et al., 2002; Frodl, Reinhold, Koutsouleris, Reiser, & Meisenzahl, 2010; Tomoda et al., 2009; van Harmelen et al., 2010), an area strongly associated with higher processes including attention and executive function, the right temporal lobe, and potentially the hippocampus, although these findings remain relatively controversial (Teicher, Anderson, & Polcari, 2012; Woon & Hedges, 2008). Assessment of stress-related hormones in affected individuals demonstrates increased catecholamines (dopamine and norephinephrine) and cortisol, similar to those described in adults diagnosed with PTSD (reviewed in DeBellis & Thomas, 2003).
From these human studies, it is difficult to assign causality, define critical periods of vulnerability, or to comprehensively investigate the molecular changes occurring in relevant brain regions as a result of early-life stress. For this reason, we developed the maternal separation with early weaning (MSEW) paradigm, which has allowed for the assessment of molecular changes underpinning the response to early-life stress in genetically characterized animals, which are amenable to behavioral assessment and targeted genetic and pharmacological manipulation. Given the comprehensively annotated genome and relative ease of creating transgenic lines, we attempted to design a manipulation that consistently altered behavior in the relatively stress resistant C57BL/6 inbred mouse line (Anisman, Zaharia, Meaney, & Merali, 1998; Millstein, Ralph, Yang, & Holmes, 2006; Parfitt, Walton, Corriveau, & Helmreich, 2007).
To date, most separation-based rodent models involve the use of rats, because it seems easier to elicit consistent stressrelated behavior (Holmes et al., 2005; de Kloet, Sibug, Helmerhorst, Schmidt, & Schmidt, 2005). Millstein, Ralph, Yang, and Holmes (2006) used an accepted rat protocol of 3 hr of separation between postnatal days (PDs) 0–13, and failed to elicit changes in prepulse inhibition (PPI) due to separation in any mouse strain tested, despite large baseline differences between strains. PPI is a measure of sensorimotor gating commonly disrupted in certain psychiatric patients and their first-degree relatives (Braff, Geyer, & Swerdlow, 2001; Gottesman & Gould, 2003). Although not consistent, it has been possible to observe PPI differences in certain rat models of early-life stress (Ellenbroek & Cools, 2002; Ellenbroek, de Bruin, van den Kroonenburg, van Luijtelaar, & Cools, 2004; Lovic & Fleming, 2004). Mouse separation studies to date are briefly reviewed in a table in Millstein and Holmes (2007), but the resulting effects appear to be inconsistent, and are both strain and sex dependent. In their study, they were unable to elicit main effects of separation on the elevated plus maze, light dark exploration, novel open field, and forced swim tests, again despite large differences on each test between strains (Millstein & Holmes, 2007). Macrì and Laviola (2004), exposed CD-1 mice to a single 24-hr period of deprivation early in development, which resulted in decreased latency to assume an immobility posture in the forced swim test, indicative of behavioral despair. Parfitt et al. (2004) observed short-term differences in anxiety-related behavior in C57Bl/6J mice exposed to 10 min of separation per day during PDs 0–10. Although these changes did not persist into adulthood, adult mice did show altered levels of corticosterone in response to acoustic stimuli (Parfitt et al., 2004). In addition, CD-1 mice subjected to 3 hr of separation between PDs 2–14 later exhibit increases in aggression, nonsocial behavior, and locomotor activity in a novel environment (Venerosi, Cirulli, Capone, & Alleva, 2003). Likewise, Romeo et al. (2003) were able to demonstrate anxiety-like behavior in the open field and elevated plus maze in adult male C57Bl/6J mice after exposure to 3 hr of separation per day on PDs 1–14. None of these models, however, have been used to investigate prefrontal dysfunction, despite the high prevalence of cortical abnormalities observed in human survivors of early-life maltreatment, and these models have not been subjected to comprehensive expression profiling.
We previously published the MSEW model in order to describe both the behavioral and molecular consequences of this early-life neglect (see George, Bordner, Elwafi, & Simen, 2010). In brief, this model involves timed separations of the whole litter from the dam for 4 hr on PDs 2–5, increasing to 8 hr on PDs 6–16. The pups are then weaned early on PD17. Our previous findings demonstrate that this separation paradigm elicits a consistent behavioral phenotype in both C57Bl/6J and DBA/2J mice, including increases in anxietylike behavior in the elevated plus maze and hyperactivity in the novel environment of the open field (George et al., 2010). What is important is that this behavior is consistent with a number of models for ADHD (reviewed by Davids, Zhang, Tarzai, & Baldessarini, 2003) and that observed by Venerosi et al. (2003) in the elevated plus maze. In Duque et al. (2012), we extensively characterized the brain anatomy of these mice, using immunohistochemistry, unbiased stereology, and diffusion tensor imaging (DTI), a technique that allows estimation of the integrity of axons and white matter tracts (Hulvershorn, Cullen, & Anand, 2011). Again, the brain anatomy of these mice is highly reflective of that observed in human survivors of early-life maltreatment (DeBellis et al., 1999; DeBellis & Thomas, 2003). Finally, in Bordner, George, et al. (2011), we described a comprehensive label-free proteomic analysis and genome-wide characterization of messenger RNA (mRNA) expression in these animals, which points to a large-scale negative bias in protein expression and downregulation of myelin-associated genes. Together these results show intriguing relevance of MSEW for modeling the effects of early-life stress on the developing brain, and the behaviors these changes may later induce.
In this article, we present the results of new behavioral studies intended to further examine the validity of the MSEW model regarding PFC function, using the 5 choice Serial Reaction Time Test, but with 3 choices (3CSRTT). Using this well-established test of spatial divided attention (Robbins, 2002), we found subtle deficits related to attention in MSEW animals, increasing the validity for the use of MSEW as an ADHD type model. Furthermore, DTI was used to investigate the connectivity of the frontal pole regions of the brain. The results of this DTI analysis demonstrated changes in fractional anisotropy (FA) in rostral areas known to be involved in executive function and inhibitory control (Buschman & Miller, 2007; Lee, Rushworth, Walton, Watanabe, & Sakagami, 2007; Thompson-Schill et al., 2002). The findings in these brain regions may explain the mild deficits we observe in attention-related behavior. Finally, we examined two correlates of the most notable molecular findings from the proteomics analysis of Bordner, George, et al. (2011): the upregulation of mixed lineage leukemia 1 (Mll1, a histone methyl transferase) protein in the PFC of MSEW brains, and the negative bias to protein expression. Thus, we conducted chromatin immunoprecipitation sequencing (ChIP-seq) for Mll1, as well as assessment of genome-wide changes in histone 3 lysine 4 trimethylation, a key epigenetic mark associated with transcriptional activation, in these animals. Although we did not find evidence of changes in Mll1 occupancy of genomic targets at PD30, we did observe a significant genome-wide decrease in the enrichment of promoter sites by trimethylate histone 3 lysine 4 (H3K4Me3). This finding requires further investigation, but may go some way to explain the negative bias observed in protein expression in MSEW animals, and suggests a possible epigenetic mechanism for the behavioral and anatomical changes we observed in the MSEW model.
Methods
Animals
Experimental C57Bl/6J mice were bred in house. Entire litters were randomized at birth to MSEW or control on PD 0. During PDs 2–5, the dam was removed from the home cage for 4 hr, and placed in a clean cage with access to ad lib. food and water. A heating blanket set at 32°C was placed underneath the pups to assist with thermoregulation. From PDs 6 to 16, the period of separation was increased to 8 hr. On PD 17, MSEW animals were weaned, then left undisturbed aside from daily brief checks for dehydration on PDs 18 and 19. They were housed in a single cage until PD 30, when the animals were separated by sex. Control animals were left undisturbed aside from normal animal facility care taking, which was likely to involve two cage changes. Animals were weaned at PD 23, the facility standard, then the group was housed till PD 30 as in MSEW (George et al., 2010). Only male mice were used for the work described in this article. For behavioral testing, we used 10 control and 13 MSEW mice. (The DTI study used 8 control and 8 MSEW mice. ChIP studies used 4 control and 4 MSEW samples.) This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and approved by the Yale Institutional Animal Care and Use Committee.
Open field testing
To assess locomotor activity in a novel setting, animals were placed in the center of a circular arena 55 cm in diameter with 30 cm high walls. The open field was placed in a dimly lit room with no obvious visual cues, and was lit by a single overhead light source. The animal’s movements were recorded by a video camera suspended 1 meter above the field, and animals were tested for 15 min on 2 consecutive days. Behavior was scored offline using custom software written by the authors (see George et al., 2010).
3CSRTT
In order to assess prefrontal cortical function in the MSEW model, we employed a modified version of the 5-choice Serial Reaction Time Test, designed to test rodent attention and response inhibition (Dalley, Cardinal, & Robbins, 2004; Robbins, 2002; our method is explained in detail in Bordner, Kitchen, et al., 2011). In brief, all testing occurred in a standard operant chamber (Med Associates) equipped with a liquid dipper, house light, reward light, clicker, and three apertures positioned opposite the liquid dipper. The liquid dipper provided a reward of approximately 30 μl of commercially available chocolate milk. The 3CSRTT consisted of four stages: magazine training, pre-CSRTT, and CSRTT testing, and, finally, a random stimulus presentation time protocol.
In the magazine training phase, all apertures were closed, the house light would flash, the reward light illuminated, and the dipper was raised in order to provide 5-s access to the reward. Animals were subjected to multiple reward presentations for 15 min, allowing the association of auditory and visual cues with reward availability. Animals in this experiment beg anmagazine training at PD 30. In the pre-CSRTT training phase, a continuous light was presented randomly in one of the three apertures, and a nose poke in the correct hole elicited presentation of the reward dipper concomitant with the appropriate cues. Once the reward was retrieved, or after 5 s had expired, the light was presented from a new aperture, and the task recommenced. Animals were tested for 30 min each day, until 40% of responses were made into the correct aperture.
In the CSRTT-testing phase, aperture stimulus presentation times were systematically reduced, beginning at 6 s to 5, 4, 3, 2, and 1 s, followed by 750, 500, 250, 125, and 75 ms. During this phase, animals had to make more than 10 responses, and achieve over 80% correct responses for 2 consecutive days. Once criterion was met, animals would progress to a shorter stimulus presentation time. Errors were punished with a 2-s timeout. At PD 60, after 30 days of 3CSRTT testing, the animals were assessed in a single 30-min variable presentation time session. In this test session, the stimulus was presented for one of the following time periods: 10, 6, 3, or 1 s or 500, 250, or 100 ms at random, with multiple trials at each stimulus presentation time. Animals would respond as usual for the reward, after which the next trial was automatically initiated.
All reported statistics were obtained from repeated measures analysis of variance (ANOVA) implemented in the “aov” package in the R statistical software (R Team, 2011). Time and group were defined as fixed effects, including their interaction, and mouse a random effect. Linear mixed-effects models were also applied to these data, implemented in the R package “nlme” (v. 3.1–103; Pinheiro, 2000) but, due to the relatively balanced design of our experiment, the results were practically identical to those obtained via the repeated measures ANOVA. For the variable stimulus presentation time data, proportion correct scores were calculated individually for each mouse at each of the stimulus presentation times tested. These values were then analyzed using a linear mixed effects model with a structured covariance matrix using SAS PROC MIXED, with stimulus presentation time and group (MSEW vs. control) as fixed effects. A variance components covariance structure was used.
Progressive ratio
Once the animals had completed the 3CSRTT, they were subjected to the progressive ratio paradigm, a measurement of motivation to work for the caloric reward (Bordner, Kitchen, et al., 2011; Hodos & Kalman, 1963). In this phase of testing, one aperture was illuminated, and the animals were required to respond on an n + 2 schedule in order to achieve the reward. If animals failed to exhibit the necessary number of responses within a 15-min time period, the session was terminated. Animals were tested on 2 consecutive days, and the higher number of responses recorded as the breakpoint.
Tissue collection
For ChIP, PD 30 mice were anesthetized with chloral hydrate (1500 mg/kg in sterile saline, intraperitoneal injection) and rapidly decapitated. A frontal block was dissected by slicing the brain coronally at Bregma +1.3, and the ventral part of the frontal block removed (Chiu, Lau, Lau, So, & Chang, 2007). This frontal block was placed in ice-cold Hibernate-A (Invitrogen) before trituration on ice to a single-cell suspension.
For DTI studies, mice were anesthetized with chloral hydrate (1500 mg/kg in sterile saline, intraperitoneal injection) and transcardially perfused with ice-cold phosphate buffered saline (PBS), followed by 4% paraformaldehyde (PFA) in PBS. Whole brains were collected and postfixed in 4% PFA at 4°C for 2 weeks. One hour before the DTI scans, the brains were washed 3 times for 10 min in 10 mL of PBS to remove the PFA solution. The brains were placed into a custom-built MRI-compatible tube filled with Fluorinert, an MRI susceptibility-matching fluid (Sigma-Aldrich, Inc., St. Louis, MO), and scanned.
DTI and analysis
A full description of the DTI data collection and analysis protocols can be found in Duque et al. (2012). In brief, data sets were obtained on a 9.4 T horizontal bore magnet (Bruker, Billerica, MA) with a custom-made 1H radiofrequency coil (Chahboune et al., 2007). Twenty contiguous coronal slices of 0.5 mm thickness were acquired from each brain. Anatomical images were acquired using a spin-echo sequence with a repetition time (TR) of 3000 ms and an echo time (TE) of 10 ms. Twelve averages of 256×256 images were acquired for each slice, resulting in an in-plane resolution of 100×100 μm. The Stejskal–Tanner spin-echo diffusion-weighted sequence with a diffusion gradient of 5 ms and a delay between the two diffusion gradients of 15 ms was used. TR was 2000 ms and TE 25.1 ms. Two Shinnar–Le Roux pulses of 1 ms each were used for excitation and inversion, respectively. Eighteen averages were acquired for each slice and the 128 ×64 images were zero filled to 256×256. Sixteen different images were acquired for each slice, 15 corresponding to various noncollinear diffusion weighting directions with the same b = 1000 s/mm2 and one with no diffusion weighting. The six elements of the diffusion tensor were calculated for each voxel from the intensities of the 16 diffusion-weighted images. The tensor eigenvalues and the corresponding eigenvectors were obtained by matrix diagonalization (Hasan, Basser, Parker, & Alexander, 2001; Jones, Horsfield, & Simmons, 1999). FA, composite FA images, Gaussian smoothing, and other aspects of FA results were as previously described (Chahboune, Ment, et al., 2009; Chahboune, Mishra, et al., 2009).
ChIP
The dissected tissue was mechanically dissociated by trituration to a single-cell suspension, and fixed for 8 min in 1% formaldehyde in PBS. The fixation reaction was stopped by the addition of glycine to a final concentration of 125 mM. Cells were washed twice with cold PBS and stored at −80°C. Cells were thawed for 1 hr at 4°C in PBS containing the following inhibitors: complete protease inhibitor (Roche), 500 μM phenylmethanesulfonylfluoride, 1 mMdithiothreitol, and 10 mM sodium butyrate. Cells were resuspended in hypotonic buffer (20 mM HEPES, 10 mM potassium chloride, 1 mM EDTA, 10% glycerol) containing all inhibitors, and swollen on ice for 10 min. Cells were Dounce homogenized with 30 strokes. Dounced cells were centrifuged at 2400×g for 5 min, and the nuclear pellet washed once with hypotonic buffer. Nuclear pellets were resuspended in 3 ml of RIPA buffer (Millipore) plus all inhibitors and incubated on ice for 30 min, with vortexing every 5min. Samples were then sonicated on ice for 25 cycles of 20 s at 15% (Fisher Scientific Model 500 Sonicator). Lysates were clarified by centrifugation at 13,000 rpm on a desktop centrifuge for 5 min. Sample volume was adjusted to 10 ml with RIPA plus all inhibitors, and 1 ml aliquots stored at 80°C. Aliquot concentration was determined by pico-green assay (Invitrogen, according to manufacturer’s instructions), and each IP loaded with 120 μg of sheared DNA. H3K4Me3 or Mll1–DNA complexes were immunoprecipitated overnight at 4°C with appropriate antibodies (H3K4me3, Active Motif, 1:200; Mll1, Active Motif, 1:200). The next day, 300 μL of washed protein A agarose (Invitrogen) for H3K4me3 or protein G agarose (Invitrogen) for Mll1, was added to each sample, and incubated with agitation for 1 hr. Postincubation samples were washed 3 times with RIPA plus all inhibitors, and once with ice-cold PBS. TF-DNA complexes were eluted at 65°C using 1×TE with 1% sodium dodecyl sulfate, followed by a second elution at 65°C using 1×TE with 0.67% sodium dodecyl sulfate. All samples, plus 1 aliquot of input DNA per animal were decross-linked overnight at 65°C. Samples were RNaseA and proteinase K treated, then purified using phenol chloroform extraction. The output was then processed for ChIP sequencing.
ChIP data analysis
Raw sequence reads (34 nt long) were obtained from two lanes of a flow cell analyzed using the Illumina HiSeq 2000. Samples were prepared and multiplexed by barcoding using the standard Illumina TruSeq protocol. All H3K4me3 samples were run on the first lane and all Mll1 samples were run on the second lane. A total of eight mice were used (four controls, four MSEW), and two samples were obtained from the H3K4 and Mll1 IPs from each mouse. In addition to the IP samples, two samples of “input” DNA (which were not subject to IP) were run on each lane as a measure of the technical background signal of the assay.
Basic quality assessment of the raw sequence reads was performed using the FastQC software package (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The data exhibited no obvious shortcomings based on per-base quality, per-read quality, per-base sequence content, GC content, or sequence duplication. In several thousand reads, a large portion of the Illumina TruSeq 3′ adapter (GATCGGAAGAGCACACGTCTGAACTCCAGTCACCG) was detected in the read sequence. We therefore removed this sequence from the 3′ end of all reads in which it could be reliably detected using the fastx_clipper application (Pearson, Wood, Zhang, & Miller, 1997). Clipped reads were again assessed for quality using FastQC and no further problems were reported.
Clipped sequence reads were mapped to the mouse reference genome (mm9) using Bowtie (v. 0.12.7; Langmead, Trapnell, Pop, & Salzberg, 2009) using the default seed length and the default maximum of two-base mismatch in valid alignments. The “-best” and “-strata” options were used to improve the quality of the alignments and up to five valid alignments for each read were considered; in the rare case that the quality score was tied, one of the valid alignments was chosen at random.
Bowtie-read alignments were processed by the pipeline required by the PeakSeq peak-calling software (Rozowsky et al., 2009). In brief, PeakSeq attempts to more closely reflect the composition of the presequenced mixture by extending the reads in the 3′ direction so that the size of the extended sequences is closer to that expected following DNA fragmentation post-IP. PeakSeq uses the input DNA control to estimate both the local and global mapping profile that can be attributed to nonspecific random background noise in the assay. Peaks are identified in the IP samples by compensating for this background and peak significance and FDR empirically computed based on the number of IP fragments, the characteristics of the peak, and the local background.
Reads from each of the eight samples from each IP were independently subjected to peak calling along with their corresponding sample of input DNA. The peak regions reported for each of the eight samples were collapsed to create a master set of regions representing the union of all regions observed in all eight samples. A bespoke Java application was written to provide counts of the number of reads for each of the eight biological replicates falling into any of these master peak regions. Read-count data for each sample was assessed for differences between the groups of control and MSEW animals using negative-binomial statistical models implemented in the DESeq package (v. 1.6.1; Anders & Huber, 2010) within the R statistical software (v. 2.14.1; R Team 2011).
In order to identify genomic features proximal to detected peaks, we downloaded the Ensembl mouse annotation (release 66) corresponding to the NCBI genome build (release 37). Peaks were associated with Ensembl features (including, but not exclusively protein-coding transcripts) based on proximity to the start of the 5′-most annotated exon. Peaks were allowed to reside within a region ±1.5 kb of these positions to account for inaccuracies in the annotation and in the peak identification procedure described above. Peaks associated with multiple transcripts of the same gene were collapsed so that associations reported correspond to individual Ensembl genes.
Results
Behavior
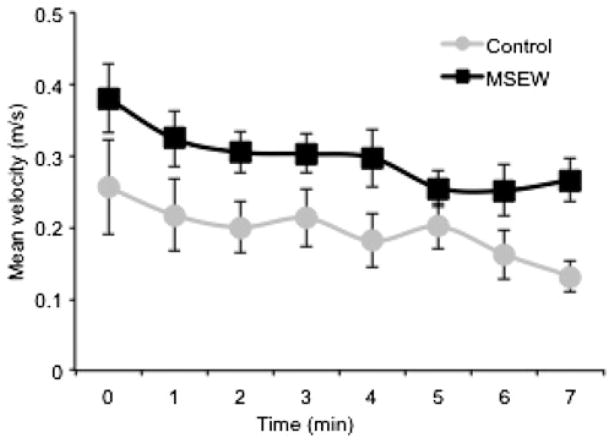
Mice subjected to the MSEW paradigm have previously been assessed for a number of behaviors pertaining to early-life neglect associated deficits, including locomotor activity, anxiety, and behavioral despair. MSEW mice are relatively unusual for models of maternal separation, in that they demonstrate increased activity in the open field, a finding that we replicate in the current cohort of animals (Figure 1), F (1, 21) = 5.89, p = .0244, and time, F (1, 159) = 15.28, p < .0001, but no Time × Condition, F (1, 159) = 0.0, p = .9890, effect. This difference is only apparent on the first day of training, suggesting that their hyperactivity is a response to the novel environment of the open field. Our previous data showed that MSEW animals display anxiety-like behavior in the elevated plus maze, where they made fewer entries into the open arms of the maze than their control counterparts. They also made more entries into the closed portions of the maze, and more protected exploratory head dips than controls. Finally, DBA/2J MSEW mice displayed behavioral despair, exhibiting increased immobile time in the forced swim test (George et al., 2010).
Figure 1.
Locomotor speed (velocity) in the open field. Mixed-effects repeated measures analysis revealed significant effects of condition, F (1, 21) = 5.89, p=.0244, and time, F (1, 159)=15.28, p < .0001, but no Time × Condition, F (1, 159) = 0.0, p = .9890, effect, suggesting higher locomotor activity in MSEW animals than controls.
Due to the differences seen in MRI scans of humans with histories of neglect (DeBellis et al., 2002; Tomoda et al., 2009), in the current study we wished to examine the PFC function of the MSEW mice. The 3CSRTT is an adapted version of the 5CSRTT, a well-established test of PFC mediated attention and response inhibition (Bari & Robbins, 2011; Robbins, 2002). Throughout the task there are multiple indicators of attentive capacity, including rate of progression through the task, percentage correct responses, and omission errors (the failure to nose poke in response to the cue). Indicators of failure in response inhibition include premature responses (response before the stimulus), responses during time out, and perseverative errors (animal continues to nose poke in the previous hole).
In the pre-3CSRTT, when all mice are exposed to a continuous stimulus, repeated measures ANOVA shows a trend toward MSEW animals making increased responses, demonstrated by a trend to a main effect of group on correct responses, F (1, 21) = 3.005, p = 0.098, a trend to a group by time interaction effect in incorrect responses, F (9, 189) = 1.85, p = .062, and total trials completed, F (1, 21) = 4.018, p = .058 (Figure 2a–c), over the first 10 days. Performance measured by percentage of correct responses shows no difference between the two groups (Figure 2d) over this time period. This suggests that the MSEW mice exhibit increased exploratory behavior early in the task, but are not more successful in the cognitive component required to receive the reward.
Figure 2.
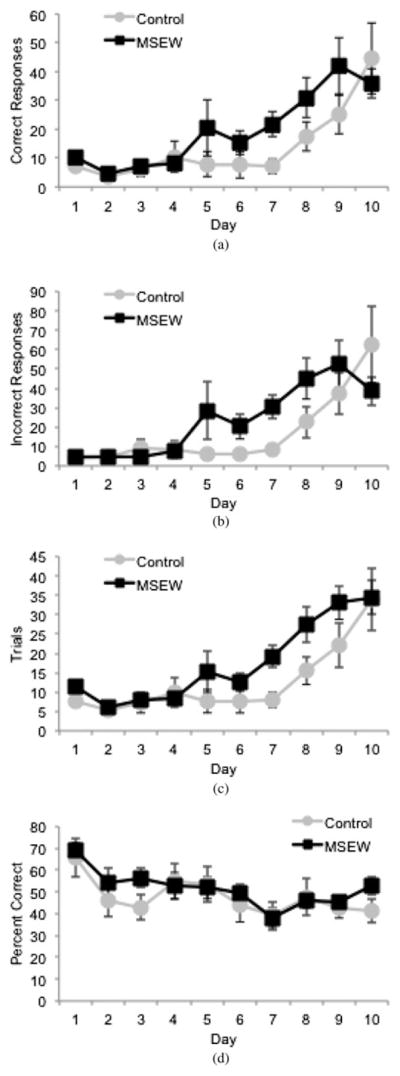
Responses of (a) animals during the first 10 days of the 3 choice serial reaction time test (3CSRTT), (b) MSEW animals trend toward a main effect of group on number of correct responses, (c) a group by time interaction on number of incorrect responses, and (d) a main effect of group on number of trials undertaken. There is no difference between groups concerning percentage of correct responses.
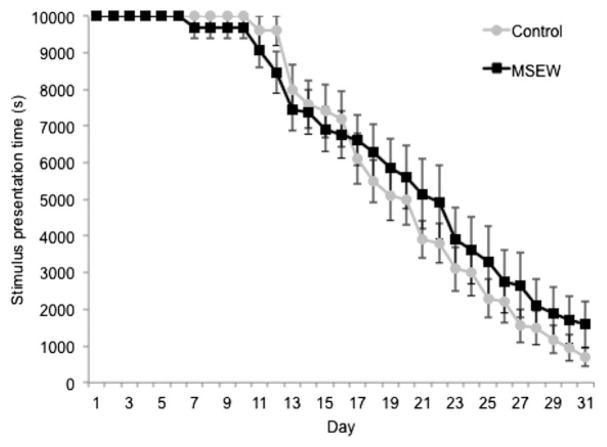
Over the full duration of the 3CSRTT testing, there is a highly significant group by time interaction effect, with MSEW mice exhibiting a reduced rate of progression through the task compared to controls, F (1, 688) = 14.94, p, .001 (Figure 3a), suggesting a slowing in the rate of task acquisition. There were no significant differences in the number of errors of omission, premature responses, or responses during timeout measured in the 3CSRTT test stage (Figure 4a–c). There was a significant group by time interaction concerning the number of perseverative errors, F (1, 19) = 10.25, p, < .005 (Figure 4d), with MSEW animals making more of these errors as stimulus presentation time decreased, suggesting a degree of failure in response inhibition. A high rate of perseverative errors is also associated with compulsive behavior (Robbins, 2002). Only two control and three MSEW animals, however, progressed to this range of short stimulus presentation times during the 30 days of testing.
Figure 3.
Progression through the 3 choice serial reaction time test (3CSRTT) demonstrated by stimulus presentation time by day of testing shows a significant Group × Time interaction effect. **p, < .001.
Figure 4.
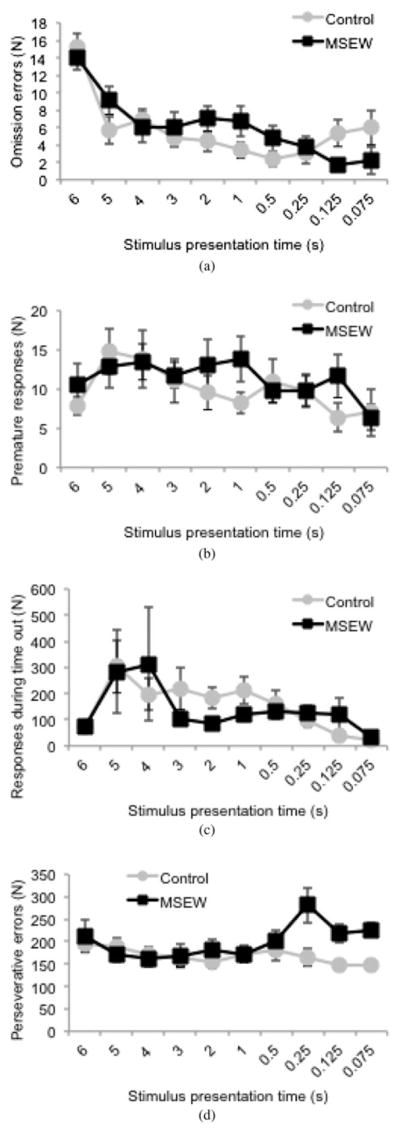
An analysis of error types during the testing phase of the 3 choice serial reaction time test (3CSRTT). There is no effect of (a) errors of omission, (b) premature responses, or (c) responses during time out. (d) Perseverative errors show a significant Time × Group interaction effect. **p, < .001.
In the variable stimulus presentation time trial, linear mixed effects modeling shows a significant main effect of group, F (1, 20) = 5.75, p = .026, in which MSEW animals made fewer correct responses than controls (Figure 5a), suggesting a decreased ability to cope with the cognitive demands of altering stimulus presentation times. There was a trend toward a main effect of group on percentage of premature responses, F (1, 21) = 3.039, p = .096 (Figure 5b), with MSEW mice making more premature responses, suggesting a degree of impulsivity to their responding. There was no main effect of group on omission, F (1, 21) = 1.32, p = .263 (Figure 5c). Throughout the test there were very few perseverance errors, resulting in no significant differences between groups (Figure 5d).
Figure 5.
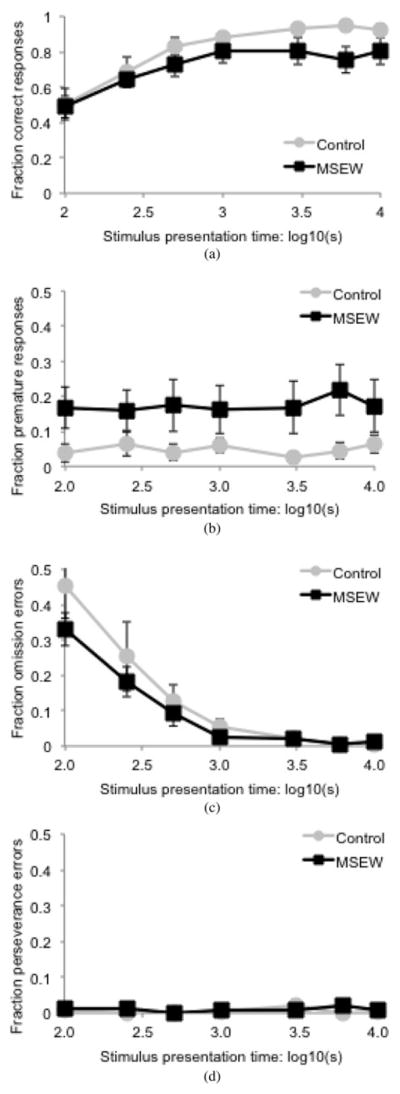
An analysis of responding and errors during the variable stimulus presentation time trial (a) percentage correct responses shows a main effect of group. *p, < .05. Error analysis accounting for stimulus presentation time shows a trend to a main effect of group in (b) premature responses and (c) omission errors. (d) There is no main effect of group or interaction effect on perseverance errors.
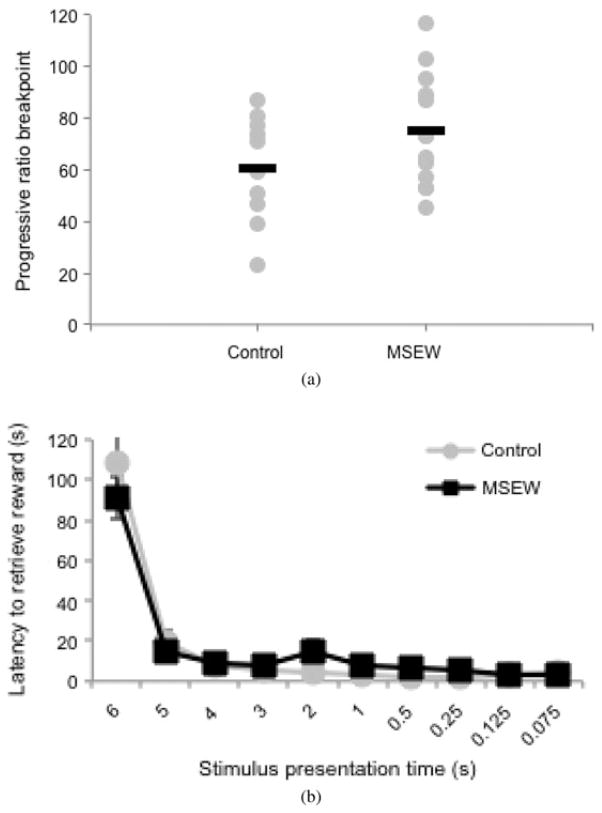
The final stage of behavioral testing was an n + 2 progressive ratio schedule, to test the motivation of the animals to work for the caloric reward (Hodos & Kalman, 1963). In the first trial, animals would nose poke once into a lit aperture to receive the chocolate milk reward. In the second trial, three responses would be required per reward, then five per reward, until they failed to make the necessary number of responses in a 15-min time period. Animals were tested on two consecutive days, and the highest number of responses made (the “breakpoint”) of the recorded 2 days. There was a trend toward an increased breakpoint of MSEW animals in the progressive ratio schedule, but this failed to reach significance using the Student t test (p = 0.14, two tailed, Figure 6a). Motivation to retrieve the reward can also be assessed by the latency to retrieve the reward in the 3CSRTT (Davids et al., 2003) and, in this respect, MSEW mice also showed no differences to controls (Figure 6b). Thus, despite no statistically significant differences in motivation to work for a caloric reward, and an early increase in exploratory behavior, the MSEW mice show subtle deficits in the cognitive components of the 3CSRTT, which suggests alterations in attention and impulsivity that may result from a deficit in PFC function.
Figure 6.
(a) An analysis of motivation to work for the caloric reinforcer. (b) The progressive ratio shows a trend toward an increased breakpoint in MSEWanimals. Latency to retrieve the rewards in the 3 choice serial reaction time test (3CSRTT) shows no main effect of group or interaction effect.
DTI Imaging
In an earlier study, we described anatomical alterations in MSEW animals, as compared to controls, that included (a) smaller brain size, (b) abnormal interhemispheric asymmetry, (c) decreased cortical thickness, (d) abnormalities in subcortical structures, (e) white matter disorganization and atrophy severely affecting the left hemisphere (LH), and (f) alterations in Olig2 (a marker of immature oligodendrocytes) densities in different cortical areas (Duque et al., 2012). In the present study, we confirm these previous results in a new and larger sample and expand our analysis to cover aspects of the mouse brain rostral pole in more detail.
FA values in motor (M2) and cingulate cortex together were higher for MSEW than for controls but the opposite relationship was observed in the forceps minor of the corpus callosum, that is, higher FA for controls and lower for the MSEW cases. These differences were not statistically significant and they were asymmetrical, that is, much more salient for the LH than for the right hemisphere (RH). Moving caudally, the Student t tests of FA values for the medial septum and the anterior commissure indicate significantly lower FA values in MSEW animals compared to controls (p = .041 and .001, respectively). However, note that the differences seem to be mostly attributable to changes in the LH of the MSEW animals, because FA values for the corresponding RH are predominantly similar to those of both hemispheres in controls, and no statistically significant difference was found between them. Statistically significant differences between the FA values of the RH and those of the LH for the MSEW cases were particularly salient in the anterior commissure and the forceps minor of the corpus callosum. Given that there are large differences within the forceps minor of the corpus callosum in fiber density in different subregions, we decided to further analyze the forceps minor by dividing it into three subregions: one most medial (genu), one central (body), and the tails (external capsule; see most caudal slice in the MSEW illustrated in Figure 7). Statistically significant differences were found in all subregions analyzed (Table 1). It must be cautioned, however, that particularly for the external capsule subregion of the forceps minor, because these areas are very thin, the possibility of partial volume effects arising from non corpus callosum tissue is possible and needs further investigation at even higher resolution than what we have been able to obtain thus far. Overall, MSEW seems to primarily affect the LH but more subtle effects in the RH cannot be ruled out, especially given the inherent connectivity between the two hemispheres. See Figure 7 for illustration of the FA changes and Table 1 for a summary of the corresponding statistical results.
Figure 7.
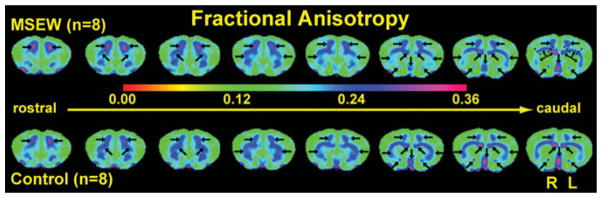
Diffusion tensor imaging (DTI) indicates differences in fractional anisotropy in several brain areas of MSEW mice as compared to age and gender matched controls. Salient differences are indicated by arrows. Each image is an averaged FA map for five contiguous slices, where each slice was 100-μm thick and spaced apart by 200 μm. Here we focus in changes in the frontal lobe particularly in regions of the motor (M1 and M2), area 1 of the cingulate cortex, prelimbic and infralimbic areas (prefrontal cortex), rostral striatum, the septal nuclei and nucleus of the vertical limb of the diagonal band (at more caudal levels in the series), the forceps minor of the corpus callosum, and the anterior commissure. The forceps minor of the corpus callosum was subdivided into three subregions for the last four caudal slices shown in this series and these subregions were analyzed separately. Subregion borders are indicated by the black dashed lines and thinner arrows in the most caudal section of the maternal separation with early weaning (MSEW) case. The FA scalewas contracted between 0 and 0.36 (instead of 0 and 1) to allow better visualization of differences. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Table 1.
Average FA values (±SD) in the rostral pole of the mouse brain
| ROI | Average FA Values
|
||
|---|---|---|---|
| MSEW (n = 8) | Control (n = 8) | p | |
| Motor (M2) and cingulate cortex | 0.27 ± 0.02 | 0.25 ± 0.02 | .075 |
| Forceps minor of corpus callosum | 0.25 ± 0.03 | 0.26 ± 0.02 | .192 |
| Medial septum | 0.23 ± 0.03 | 0.26 ± 0.03 | .041 |
| Anterior commissure | 0.21 ± 0.01 | 0.23 ± 0.01 | .001 |
|
| |||
| MSEW Left | MSEW Right | ||
|
| |||
| Corpus callosum forceps minor | |||
| Medial part (genu) | 0.24 ± 0.01 | 0.26 ± 0.02 | .053 |
| Center (body) | 0.24 ± 0.01 | 0.25 ± 0.01 | .022 |
| Tail (external capsule) | 0.23 ± 0.01 | 0.21 ± 0.01 | .005 |
| Anterior commissure | 0.21 ± 0.01 | 0.23 ± 0.01 | .022 |
Note: The values in bold are considered significant (p ≤.05; t test). FA, fractional anisotropy; MSEW, maternal separation and early weaning; ROI, region of interest.
Genomic and Proteomic Studies
In our previous work, we performed a significant amount of genomic, epigenomic, and proteomic characterization on the PFC of adult male MSEW mice (PD 75; Bordner, George, et al., 2011). Microarray analysis of MSEW mice from C57Bl/6J (B6) and DBA/2J (D2) strains showed a total of 4,375 probes in 4,031 genes to be dysregulated with respect to MSEW with a false-discovery correct p value (Q value) of Q, < 0.01. We found 235 genes to be differentially regulated by MSEW with respect to the strain of mouse tested (C57 and DBA2). The genes with a Q, < 0.01 for the effect of MSEW were subjected to gene ontology analysis, and the most significant category affected by MSEW was genes involved in translation. These genes included many translation initiation factors (Eif3i, Eif3k, Eif5a, Eif6; Das & Maitra, 2000; Kapp & Lorsch, 2004; Sun et al., 2011) and ribosomal components (Rpl19, Rpl29, Rpl5; Bordner, George, et al., 2011).
We also previously characterized mRNA expression in B6 animals by RNA-sequencing pooled samples, with each pool equally representing five individual animals. In this analysis, there were 1,239 significant genes with Q, < 0.01, and evidence was again seen for dysregulation of translation, with multiple initiation factors and ribosomal components represented (Bordner, George, et al., 2011). It is interesting, given the recent associations of white matter changes in human subjects exposed to early-life maltreatment, that many genes associated with myelin were also dysregulated by MSEW. Inparticular, genes characteristically expressed by mature, myelinating oligodendrocytes such as Mag, Mbp, Mog, Omg, and Plp1 were found to be downregulated by RNA sequencing (Bradl & Lassmann, 2010; Dugas, Tai, Speed, Ngai, & Barres, 2006).
Label Free Proteomics is one of the only mass spectrometry based protein analysis tools that allows for a hypothesis-free approach to protein quantification, performing a broad assessment of highly expressed proteins (Bantscheff, Schirle, Sweetman, Rick, & Kuster, 2007). Considering the evidence at the RNA level for widespread dysregulation of translation in MSEW animals, it is of great interest that the majority of proteins showing differential expression by Label Free Proteomics (37 out of 46 total) were observed to be downregulated. These proteins included Mbp, a key component of myelin (Readhead et al., 1987) found to be downregulated by microarray, RNA-seq, label-free, and multiple reaction monitoring (MRM) proteomic analyses. Downregulation of myelin- related proteins including 2′,3′-cyclic nucleotide 3′ phosphodiesterase, myelin basic protein, oligodendrocyte myelin glycoprotein, and reticulon 4 was confirmed using targeted MRMproteomics. Considering the results of our DTI studies, which likely reflect large changes in the integrity of myelin and fiber tracts, investigation of myelin related molecules and pathways would appear to be promising for future studies. Furthermore, there was evidence of a decrease of the GABAergic interneuron marker Calb1 by Label Free, concomitant with decreases in neuropeptide Y and somatostatin at the RNA level, suggesting effects of MSEW on inhibitory interneurons in the PFC of MSEW mice.
One of the few proteins found to be upregulated by label free proteomics was mixed lineage leukemia 1 (Mll1). Mll1 is a histone methyl transferase enzyme that trimethylates histone 3 lysine 4 (H3K4me3) in a subset of DNA targets, many of which are related to early development, including numerous Fox and Hox family genes (Wang et al., 2009). It is essential for normal GABAergic neuron function (Huang et al., 2007). For this reason, it was of great interest to us that it was found to be twofold upregulated in PD 75 MSEW animals (Bordner, George, et al., 2011). To assess whether this upregulation was causing differential binding of Mll1 to DNA targets in MSEW animals, as well as changes in H3K4me3, which is downstream of Mll1 and is an epigenetic marker of actively transcribed DNA (Li, Carey, & Workman, 2007), we performed ChIP-seq on PD 30 frontal tissue using antibodies recognizing Mll1 and H3K4Me3. Sequencing reads from ChIP were compared to input reads using Peak-Seq software (Rozowsky et al., 2009) to examine which regions were enriched by Mll1 IP. Observed peaks were mapped to ENSEMBL genes within 1.5 kb of the start of the first coding exon, and the resulting gene list was significantly enriched for the promoter regions of published targets of Mll1 (Wang et al., 2009; Fisher exact test p < 5−10; online-only, supplementary Table S.1). These results confirm that 211 of the 525 published targets ofMll1 in mouse embryonic fibroblast cells are shared in PD 30 mouse frontal cortex. When peaks were examined for differential enrichment by Mll1 IP between the control and MSEW groups, however, there were no peaks within promoter regions that reached genome- wide statistical significance (Q, < 0.05).
Likewise, none of the published targets ofMll1 showed differences in H3K4Me3 enrichment by H3K4Me3 IP. H3K4Me3 is generally accepted to be a marker of active genes across the genome (Li et al., 2007), and given our previous observation of a negative bias to protein expression in our label- free proteomics analysis, we compared genome-wide enrichment for H3K4Me3 in MSEW versus control. The total number of mapped reads per MSEWH3K4me3 IP was significantly lower than those from control samples (Student t test, two tailed, p, < 0.003). This finding will need further investigation to fully confirm the biological plausibility. Given these bulk differences in global read mapping statistics, no significant changes in any given peak were detected following normalization by total number of mapped reads per sample. If the data is not normalized by total number of mapped reads, however, making the assumption that the difference in reads obtained between MSEW and Control has biological cause (e.g., widespread transcriptional repression), 86 genes show a significant decrease in H3K4me3 enrichment with Q, < 0.01 (online-only supplementary Table S.2).
Discussion
Behavior
We developed the MSEW model of early-life neglect in an attempt to generate a reproducible and consistent murine model of early-life neglect that would be amenable to comprehensive genomic and proteomic characterization, plus genetic and pharmacological manipulations. There are many such models in rats, but mice appear to be relatively resilient to early-life perturbations, resulting in inconsistent and subtle effects exhibited during adulthood (Anisman et al., 1998; Millstein et al., 2006; Parfitt et al., 2007). Although mice subjected to the MSEW treatment are separated from the dam for considerable amounts of time, there are no apparent differences in pup mortality, weight change, or metabolic parameters, as measured by beta-hydroxybutyrate, glucose or nonesterified fatty acid serum levels as a result of this treatment (George et al., 2010). In a previous study, we reported behavioral changes that are significant across two different strains of mice (B6 and D2), although there was evidence for Strain × MSEW interactions (George et al., 2010).
To further assess the validity of the MSEW model, we performed a series of behavioral characterizations. Thus far, our characterization suggests that the model shows validity for modeling early-life neglect, particularly with regard to an ADHD-like phenotype. ADHD is characterized by attentional dysfunction, hyperactivity, and poor impulse control (Davids et al., 2003). Previous cohorts and the current cohort of MSEW animals exhibit hyperactivity in the novel environment of the open field in comparison to controls, and this difference is not apparent once they are habituated to the test area (George et al., 2010, Figure 1). Similar behavior can be observed in the most well-studied models of ADHD: the spontaneously hypertensive rat (Cierpial et al., 1989; Gentsch, Lichtsteiner, & Feer, 1987), the acallosal mouse (Magara, Ricceri, Wolfer, &Lipp, 2000), and stunted cerebellar mouse models (Ferguson, 1996) of the disorder. Although these models mostly fail to provide construct validity, in that their etiology is unlikely to be shared with that of ADHD, it is agreed that they model certain aspects of the disorder, and show some predictive validity in terms of the effect of stimulants (Bari & Robbins, 2011; Davids et al., 2003). The MSEW model, therefore, is of increasing interest because it provides some construct validity, with early-life maltreatment, including neglect, in humans, resulting in an increased incidence of ADHD (DeBellis & Thomas, 2003; Ouyang et al., 2008).
In our previous work, the MSEW mice showed a mild anxiety-related phenotype: they made fewer entries into the open arm of the elevated plus maze, and made more “protected” head dips compared to controls (George et al., 2010). Elevated anxiety is a clear feature of human survivors of early maltreatment (DeBellis & Thomas, 2003; Gibb, Chelminski, & Zimmerman, 2007; Mathews et al., 2008; Veenema, 2009). Also, in earlier work, we reported that the MSEW mice show an indication of behavioral despair demonstrated by increased immobility in the forced swim test (George et al., 2010).
In this latest cohort of animals, MSEW mice trend toward an increased response rate in the first 10 days of the 3CSRTT. These results suggest exploratory hyperactivity within the novel environment of the operant chamber, which is also consistent with hyperactivity in the open field. There was a statistically significant group by time interaction as animals progressed through the whole duration of the 3CSRTT, with MSEW animals progressing through the task at a decreased rate, reflecting slowed task acquisition. The MSEW mice, tested under a variable stimulus presentation time protocol, made fewer correct responses, and make more premature responses. This is despite a trend toward increased motivation to work for the caloric chocolatemilk reward, as evidenced by the progressive ratio test. This mild deficit MSEW mice exhibit in 3CSRTT performance suggests a subtle attentional and inhibitory control deficit, as expected for an animal model of ADHD (Bari & Robbins, 2011). Analysis of further cohorts of animals will be needed to fully establish the specific nature of the deficit in MSEW animals. It may also be of interest to assess other behaviors such as aggression, startle deficits, and deficits in working memory. Specifically, this may help to further establish similarities and differences from an ADHD-like phenotype in these animals.
Anatomy
We have previously described a strong downregulation of markers of mature, myelinating oligodendrocytes in the PFC of MSEW animals (Bordner, George, et al., 2011), which corresponds with our observations of statistically significant differences in the mean number of Olig2-positive cells in the PFC and rostral corpus callosum of the MSEW cases as compared to controls. We have also described FA differences in several structures at mid-rostrocaudal levels (Duque et al., 2012), suggesting changes in axon and fiber tract integrity in MSEW mice. In this article, we demonstrate anatomical differences in the frontal pole of MSEW animals versus controls and also interhemispheric asymmetries, in that the left and RHs of MSEW cases are not affected to the same degree. These results would appear to correlate with changes in behaviors observed in the 3CSRTT known to have rostral anatomical substrates such as the PFC, including attention and response inhibition (Buschman & Miller, 2007; Lee et al., 2007; Thompson-Schill et al., 2002). In addition, human early-life neglect and abuse has been associated with asymmetry in the frontal lobes (Carrion et al., 2001) and volume loss and other abnormalities in the PFC (Bremner, 2006b; Tomoda et al., 2009), anterior cingulate gyrus (DeBellis, Keshavan, Spencer, & Hall, 2000), corpus callosum (Teicher et al., 2004), and other structures. Asymmetries in cortical thinning, with left medial PFC being most affected, have been shown in children with ADHD (Shaw et al., 2006) and cortical changes, affecting layer II/III pyramidal cells that give rise to callosal fibers, have been shown in the PFC after early maternal deprivation (Pascual & Zamora-León, 2007). Taken together, we interpret the FA changes in the rostral pole of MSEWanimals compared to controls as indicative of anatomical changes likely to affect white matter integrity and axonal organization. Alterations in the involved circuits may underlie the behavioral changes observed in MSEW animals.
Genomics
One of the real attractions of the MSEW model is that consistent outcomes can be obtained from a model that is amenable to comprehensive genomic characterization across multiple time points: the mouse. Our goals are to use this model to identify pathways that may be causing the negative effects of early-life neglect. The MSEW model is particularly interesting in that it bears many of the behavioral and anatomical changes found in survivors of maltreatment. Although much of the field has focused on the role of dysregulation of the HPA axis, we found that MSEW animals do not show large changes in cortisol levels during the stressed period (George et al., 2010). Cortisol is a glucocorticoid hormone that forms the backbone of the HPA axis (reviewed by Vermetten & Bremner, 2002), which is maturing during the MSEW manipulation, and has been the focus of investigation in most models of neglect (DeBellis & Thomas, 2003; Veenema, 2009). Our findings have identified numerous molecular changes that are not classically associated with HPA axis dysregulation, and we therefore hope to highlight novel targets for manipulation in trying to improve the outcome of individuals who have experiences early-life stress.
The first time point investigated on a comprehensive scale in the MSEW model was of adult male mice at PD 75. These samples were subjected to RNA analysis by both microarray and RNA sequencing, and two forms of proteomics: hypothesis free label-free analysis and target driven MRM analysis. Analysis of RNA expression showed a large number of expression differences persisting into adulthood in MSEW mice. The most interesting functional gene categories affected at the RNA level were those involved in protein translation, and in oligodendrocyte myelination and development (Bordner, George, et al., 2011). Both arrays and the RNA sequencing results were highly enriched for genes involved in translation, encompassing many initiation factors and ribosomal components, both in the cytoplasm and mitochondria. It was particularly striking that most of the observed differences were in the direction of a decrease in MSEW animals. RNA sequencing also highlighted a general decrease in genes involved in myelination, particularly those expressed in mature myelinating oligodendrocytes (Bradl &Lassmann, 2010; Dugas et al., 2006). Given the observed changes in FA in the MSEW animals and the increasing interest in white matter changes in numerous psychiatric disorders, this finding is particularly interesting (Fields, 2008; Mamah et al., 2010; Sprooten et al., 2011; Sussmann et al., 2009; Togao et al., 2010).
The hypothesis-free approach to proteomic profiling, label- free analysis, highlighted the importance of myelin basic protein (Readhead et al., 1987), which was consistently downregulated in MSEW animals by this technique, and also at the RNA level by micro array and RNA-seq. Considering the decreases seen in the translation machinery at the RNA level, it was of interest that the majority of differences in protein expression as measured by label free were decreases in expression. We were unable to find reports of similar occurrences in the literature, but it is well accepted that the mammalian target of rapamycin (mTOR) complex functions upstream of protein translation, controlling the cellular response to stress, nutrient availability, growth factors, and availability of energy (reviewed by Sengupta, Peterson, & Sabatini, 2010). mTOR has a well-established function in neurons in LTP (Hoeffer & Klann, 2010), and may also play a role in the response to anxiety (Blundell, Kouser, & Powell, 2008). It would therefore be of interest to test the application of an mTOR inhibitor, such as rapamycin, in this period of brain development.
Another important functional gene category of interest affected by MSEW involves markers of GABAergic interneurons. Calbindin1 is a commonly employed marker of these interneurons, and was seen to be downregulated by MSEW at the protein level. Two other markers, somatostatin and neuropeptide Y, were not detected in the label-free assay, but were downregulated at the mRNA level in our RNA sequencing analysis. Given that reciprocal interactions between oligodendrocytes and neurons are critical for both the developing and the mature brain (Bartsch, Montag, Schachner, & Bartsch, 1997; Edgar et al., 2004; Fields, 2008; Griffiths et al., 1998; Inoue, 2005; Lappe-Siefke et al., 2003; Nave & Trapp, 2008; Schnaar & Lopez, 2009), this potential loss or damage to interneurons may well be secondary to the deficits we are observing with myelination. Inhibitory interneuron deficits and changes to the white matter present in the PFC may well be responsible for the subtle deficits we observe on the 3CSRTT, particularly concerning failures of response inhibition (Robbins, 2002).
To take the molecular characterization further, we performed ChIP with anti-Mll1 antibodies, because Mll1 is the second most highly upregulated protein on our proteomics screen. We also performed ChIP using anti-H3K4Me3, the histone modification target of Mll1 and a general marker of actively transcribed genes (Li et al., 2007). The peaks we detected were highly enriched for the promoter regions of genes in both pulldowns: 84% of 10,000 detected peaks in the H3K4Me3 experiment, 85% of 4,000 detected peaks in the Mll1 experiment. Furthermore, we observed a significant enrichment for the promoter regions of known published targets of Mll1 (Wang et al., 2009). In the Mll1 pulldown, a large amount of between sample variation limited our ability to detect differences in Mll1 binding between our sample groups. Although power may have been an issue, the timepoints at which we performed the proteomics assay (PD 75) and ChIP assays (PD 30) were also different. It may be that the upregulation of Mll1 seen in the adult MSEW mouse is actually an endogenous compensatory mechanism caused by deficits in development in the MSEW mouse. This is especially pertinent, given our observations of decreased reads in the H3K4Me3 experiment. Mll1 knockdown in cortical neurons results in decreased occupancy of the glutamate decarboxylase 1 (GAD1) promoter by Mll1, and a corresponding decrease in H3K4Me3 modification at this promoter (Huang et al., 2007). GAD1 is highly related to the function of GABAergic interneurons in the PFC. Moreover, the atypical antipsychotic clozapine increases the occupancy of Mll1 and H3K4Me3 modifications at the GAD1 promoter, suggesting that increases in Mll1 may form part of the antipsychotic function of this drug by affecting function of GABAergic interneurons (Huang et al., 2007). Upregulation of Mll1 in the adult brain therefore clearly has potential to compensate for the GABAergic interneuron deficits that we appear to be observing in the MSEW model.
The H3K4Me3 IP is more difficult to interpret. Total reads obtained by the IPs from the MSEW subjects were consistently and significantly lower in number than those obtained from the control subjects. We are encouraged that the IP was significantly enriched for promoter regions of protein coding genes. However, the bulk difference in the number of sequence reads obtained from MSEW and control samples compromised all attempts to normalize and statistically assess group differences. Further work must be performed to assess whether this large-scale loss of H3K4Me3 at promoters is a true biological effect of MSEW. There is a biological precedent for decreased trimethylation of H3K4 in GABAergic genes in schizophrenia (Huang et al., 2007), and 7 days of restraint stress led to subtly decreased H3K4me3 levels in CA1 of the hippocampus in adult rats (Hunter, McCarthy, Milne, Pfaff, & McEwen, 2009). However, we are unable to find further evidence in the literature of such large-scale downregulation of a histone modification after an experimental manipulation. Significant H3K4 remodeling occurs during early postnatal development in human PFC, and it is possible that stress may adversely affect this process (Cheung et al., 2010).
Furthermore, there is emerging evidence that histone H3 demethylase inhibitors bear structural similarity to the nonspecific monoamine oxidase class of antidepressants (Lee, Wynder, Schmidt, McCafferty, & Shiekhattar, 2006), and therefore H3K4me3 demethylation may actually represent a previously unanticipated target of these medications (Szyf, 2009). It is possible that H3K4me3 demethylation acts to suppress the transcription of “nonessential” developmental genes in response to early-life stress, and results in the deficits we observe in our model. It would therefore be interesting to test a histone H3 demethylase inhibitor in MSEW animals, to investigate this theory. Finally, mutations in the X linked gene JARID1C, a H3K4me3 specific demethylase, has been linked to mental retardation and autism in humans (Akbarian & Huang, 2009). It is clearly possible that widespread changes in H3K4me3 result from early-life stress and contribute to the resulting behavioral abnormalities.
If we presume therefore that the decreased number of reads obtained by H3K4me3 ChIP has biological plausibility, 86 genes show significant genome-wide decreases in enrichment, at Q, < 0.01 level of significance. Supplementary Table S.2 contains a list of these peaks based on unnormalized read count data. Among these genes are CDK5r1, a CDK5 regulator that plays a role in the specification of cortical layers (Gupta & Tsai, 2003), which is of interest given the disarray we note in our DTI and other anatomical studies. Also relevant to the anatomical phenotype is Hes5, a Notch target gene with a role in oligodendrocyte development (Wu et al., 2012), and Brd2, which is associated with a seizure disorder and GABAergic interneuron physiology (Velíšek et al., 2011).
Conclusion
Further work is required to characterize the MSEW model and its utility for studying the many behavioral changes associated with early-life stress. The behavioral phenotype currently shows some similarity to ADHD and anxiety-related disorders, but given the widespread occurrence of other disorders with human abuse and neglect, additional behavioral changes are also likely to be present. The model shows strong construct validity, because human neglect can result in a number of different, and apparently unrelated, disorders that share aspects of the behaviors we have noted. This may actually be where the real value of the model lies, in detailing an anatomical and molecular susceptibility for disorders that interact with genetic and other environmental insults to manifest as disorders in the human. The predictive validity of the model is yet to be tested. We have performed the first comprehensive molecular characterization of this model and highlighted protein translation, H3K4 trimethylation, and particularly myelination and white matter integrity as potential key pathways in the etiology of the negative changes associated with early-life stress. We believe, therefore, that the MSEW model has highlighted three pathways of real interest to translational neuroscience focused on treating, or better still, preventing the sequelae of early-life neglect in humans.
Supplementary Material
Acknowledgments
This work was supported by NIDA Grant DA022251 (to A.A.S.), a pilot grant from the Yale Center for Translational Study of Alcoholism (NIAAA Grant P50 AA A06377 to A.A.S.), and a grant from the Yale Center for Genomics (to A.A.S.). The genomics work was also supported by NINDS Grant 5U24NS051869, the proteomics work was supported by NIA Grant DA018343, and the DTI work and Daniel Coman were supported by Grant P30 NS052519 from the QNMR Program (to F.H.). The second and third authors contributed equally to this article. The authors thank Professor Angus Nairn for his helpful conversations in preparing the manuscript.
Footnotes
Supplementary Materials and Methods
The supplementary material referred to in this article can be found online at http://journals.cambridge.org/dpp
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Molecular Psychiatry. 1999;4:163–72. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Epigenetic regulation in human brain-focus on histone lysine methylation. Biological Psychiatry. 2009;65:198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? International Journal of Developmental Neuroscience. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Analytical and Bioanalytical Chemistry. 2007;389:1017–1031. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Animal models of ADHD. Current Topics in Behavioral Neurosciences. 2011;7:149–185. doi: 10.1007/7854_2010_102. [DOI] [PubMed] [Google Scholar]
- Bartsch S, Montag D, Schachner M, Bartsch U. Increased number of unmyelinated axons in optic nerves of adult mice deficient in the myelin-associated glycoprotein (MAG) Brain Research. 1997;762:231–234. doi: 10.1016/s0006-8993(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Beers SR, DeBellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiology of Learning and Memory. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordner KA, George ED, Carlyle BC, Duque A, Kitchen RR, Lam TT, et al. Functional genomic and proteomic analysis reveals disruption of myelin-related genes and translation in a mouse model of early life neglect. Frontiers in Psychiatry/Frontiers Research Foundation. 2011;2:18. doi: 10.3389/fpsyt.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordner KA, Kitchen RR, Carlyle B, George ED, Mahajan MC, Mane SM, et al. Parallel declines in cognition, motivation, and locomotion in aging mice: Association with immune gene upregulation in the medial prefrontal cortex. Experimental Gerontology. 2011;46:643–659. doi: 10.1016/j.exger.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: Biology and pathology. Acta Neuropathologica. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues in Clinical Neuroscience. 2006a;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006b;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, et al. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. NeuroImage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahboune H, Ment LR, Stewart WB, Ma X, Rothman DL, Hyder F. Neurodevelopment of C57B/L6 mouse brain assessed by in vivo diffusion tensor imaging. NMR in Biomedicine. 2007;20:375–382. doi: 10.1002/nbm.1130. [DOI] [PubMed] [Google Scholar]
- Chahboune H, Ment LR, Stewart WB, Rothman DL, Vaccarino FM, Hyder F, et al. Hypoxic injury during neonatal development in murine brain: Correlation between in vivo DTI findings and behavioral assessment. Cerebral Cortex. 2009;19:2891–2901. doi: 10.1093/cercor/bhp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, et al. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu K, Lau WM, Lau HT, So KF, Chang RCC. Micro- dissection of rat brain for RNA or protein extraction from specific brain region. Journal of Visualized Experiments. 2007;7:269. doi: 10.3791/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cierpial MA, Shasby DE, Murphy CA, Borom AH, Stewart RE, Swithers SE, et al. Open-field behavior of spontaneously hypertensive and wistar-kyoto normotensive rats: Effects of reciprocal crossfostering. Behavioral and Neural Biology. 1989;51:203–210. doi: 10.1016/s0163-1047(89)90827-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Das S, Maitra U. Mutational analysis of mammalian translation initiation factor 5 (eIF5): Role of interaction between the beta subunit of eIF2 and eIF5 in eIF5 function in vitro and in vivo. Molecular and Cellular Biology. 2000;20:3942–3950. doi: 10.1128/mcb.20.11.3942-3950.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Research Brain Research Reviews. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2001;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A. E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. American Journal of Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Thomas LA. Biologic findings of post-traumatic stress disorder and child maltreatment. Current Psychiatry Reports. 2003;5:108–117. doi: 10.1007/s11920-003-0027-z. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV, Schmidt M. Stress, genes and the mechanism of programming the brain for later life. Neuroscience and Biobehavioral Reviews. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. Journal of Neuroscience. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, Coman D, Carlyle BC, Bordner KA, George ED, Papademetris X, et al. Neuroanatomical changes in a mouse model of early life neglect. Brain Structure and Function. 2012;217:459–472. doi: 10.1007/s00429-011-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Barrie JA, McCulloch MC, Garbern J, Griffiths IR. Age-related axonal and myelin changes in the rumpshaker mutation of the Plp gene. Acta Neuropathologica. 2004;107:331–335. doi: 10.1007/s00401-003-0808-9. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Early maternal deprivation and prepulse inhibition: The role of the postdeprivation environment. Pharmacology, Biochemistry, & Behavior. 2002;73:177–184. doi: 10.1016/s0091-3057(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, de Bruin NMWJ, van den Kroonenburg PTJM, van Luijtelaar ELJM, Cools AR. The effects of early maternal deprivation on auditory information processing in adult Wistar rats. Biological Psychiatry. 2004;55:701–707. doi: 10.1016/j.biopsych.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: Preliminary findings. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Ferguson SA. Neuroanatomical and functional alterations resulting from early postnatal cerebellar insults in rodents. Pharmacology, Biochemistry, & Behavior. 1996;55:663–671. doi: 10.1016/s0091-3057(96)00253-5. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JD, Racusin R, Ellis CG, Daviss WB, Reiser J, Fleischer A, et al. Child maltreatment, other trauma exposure, and posttraumatic symptomatology among children with oppositional defiant and attention deficit hyperactivity disorders. Child Maltreatment. 2000;5:205–217. doi: 10.1177/1077559500005003001. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of Psychiatric Research. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Open field and elevated plus-maze: A behavioural comparison between spontaneously hypertensive (SHR) and Wistar–Kyoto (WKY) rats and the effects of chlordiazepoxide. Behavioural Brain Research. 1987;25:101–107. doi: 10.1016/0166-4328(87)90003-9. [DOI] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neuroscience. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depression and Anxiety. 2007;24:256– 263. doi: 10.1002/da.20238. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–163. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Gupta A, Tsai LH. Cyclin-dependent kinase 5 and neuronal migration in the neocortex. Neurosignals. 2003;12:173–179. doi: 10.1159/000074618. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Basser PJ, Parker DL, Alexander AL. Analytical computation of the eigenvalues and eigenvectors in DT-MRI. Journal of Magnetic Resonance. 2001;152:41–47. doi: 10.1006/jmre.2001.2400. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hildyard KL, Wolfe DA. Child neglect: Developmental issues and outcomes. Child Abuse and Neglect. 2002;26:679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Preadolescent girls with attention-deficit/hyperactivity disorder: I. Background characteristics, comorbidity, cognitive and social functioning, and parenting practices. Journal of Consulting and Clinical Psychology. 2002;70:1086–1098. doi: 10.1037//0022-006x.70.5.1086. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. Journal of the Experimental Analysis of Behavior. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends in Neurosciences. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neuroscience & Biobehavioral Reviews. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. Journal of Neuroscience. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proceedings of the National Academy of Sciences. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. PLP1-related inherited dysmyelinating disorders: Pelizaeus– Merzbacher disease and spastic paraplegia type 2. Neurogenetics. 2005;6:1–16. doi: 10.1007/s10048-004-0207-y. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42:515–525. [PubMed] [Google Scholar]
- Kapp LD, Lorsch JR. The molecular mechanics of eukaryotic translation. Annual Review of Biochemistry. 2004;73:657–704. doi: 10.1146/annurev.biochem.73.030403.080419. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:257– 265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lee D, Rushworth MFS, Walton ME, Watanabe M, Sakagami M. Functional specialization of the primate frontal cortex during decision making. Journal of Neuroscience. 2007;27:8170–8173. doi: 10.1523/JNEUROSCI.1561-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chemistry and Biology. 2006;13:563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychology. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task-reversal of effects with maternal-like licking stimulation. Behavioural Brain Research. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Macrì S, Laviola G. Single episode of maternal deprivation and adult depressive profile in mice: Interaction with cannabinoid exposure during adolescence. Behavioural Brain Research. 2004;154:231–238. doi: 10.1016/j.bbr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Magara F, Ricceri L, Wolfer DP, Lipp HP. The acallosal mouse strain I/LnJ: A putative model of ADHD? Neuroscience & Biobehavioral Reviews. 2000;24:45–50. doi: 10.1016/s0149-7634(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizo- MSEW model and novel insights into neglect 1415 phrenia: A diffusion-tensor imaging study. Psychiatry Research. 2010;183:144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews CA, Kaur N, Stein MB. Childhood trauma and obsessive– compulsive symptoms. Depression and Anxiety. 2008;25:742–751. doi: 10.1002/da.20316. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes, Brain, & Behavior. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience & Biobehavioral Reviews. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. Everyday memory deficits in children and adolescents with PTSD: Performance on the Rivermead Behavioural Memory Test. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:357–361. [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annual Review of Neuroscience. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Fang X, Mercy J, Perou R, Grosse SD. Attention- deficit/hyperactivity disorder symptoms and child maltreatment: A population-based study. Journal of Pediatrics. 2008;153:851–856. doi: 10.1016/j.jpeds.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL. Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Research. 2004;1016:111–118. doi: 10.1016/j.brainres.2004.04.077. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Walton JR, Corriveau EA, Helmreich DL. Early life stress effects on adult stress-induced corticosterone secretion and anxiety-like behavior in the C57BL/6 mouse are not as robust as initially thought. Hormones and Behavior. 2007;52:417–426. doi: 10.1016/j.yhbeh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiologiae Experimentalis. 2007;67:471–479. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Wood T, Zhang Z, Miller W. Comparison of DNA sequences with protein sequences. Genomics. 1997;46:24–36. doi: 10.1006/geno.1997.4995. [DOI] [PubMed] [Google Scholar]
- Pinheiro DBJ. NLME: Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Readhead C, Popko B, Takahashi N, Shine HD, Saavedra RA, Sidman RL, et al. Expression of a myelin basic protein gene in transgenic shiverer mice: Correction of the dysmyelinating phenotype. Cell. 1987;48:703–712. doi: 10.1016/0092-8674(87)90248-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Hormones and Behavior. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Rozowsky J, Euskirchen G, Auerbach RK, Zhang ZD, Gibson T, Bjornson R, et al. PeakSeq enables systematic scoring of ChIP-seq experiments relative to controls. Nature Biotechnology. 2009;27:66–75. doi: 10.1038/nbt.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Lopez PHH. Myelin-associated glycoprotein and its axonal receptors. Journal of Neuroscience Research. 2009;87:3267–3276. doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Molecular Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TWJ, et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biological Psychiatry. 2011;70:350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Sun C, Todorovic A, Querol-Audí J, Bai Y, Villa N, Snyder M, et al. Functional reconstitution of human eukaryotic translation initiation factor 3 (eIF3) Proceedings of the National Academy of Sciences. 2011;108:20473–20478. doi: 10.1073/pnas.1116821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Muñoz Maniega S, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annual Review of Pharmacology and Toxicology. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- Team, R. D. C. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Togao O, Yoshiura T, Nakao T, Nabeyama M, Sanematsu H, Nakagawa A, et al. Regional gray and white matter volume abnormalities in obsessive–compulsive disorder: A voxel-based morphometry study. Psychiatry Research. 2010;184:29–37. doi: 10.1016/j.pscychresns.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage. 2009;47(Suppl 2):T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Child maltreatment 2010. Washington, DC: US Government Printing Office; 2011. [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models? Frontiers in Neuroendocrinology. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Shang E, Velíšková J, Chachua T, Macchiarulo S, Maglakelidze G, et al. GABAergic neuron deficit as an idiopathic generalized epilepsy mechanism: The role of BRD2 haploinsufficiency in juvenile myoclonic epilepsy. PloS One. 2011;6:e23656. doi: 10.1371/journal.pone.0023656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Cirulli F, Capone F, Alleva E. Prolonged perinatal AZT administration and early maternal separation: Effects on social and emotional behaviour of periadolescent mice. Pharmacology, Biochemistry, & Behavior. 2003;74:671–681. doi: 10.1016/s0091-3057(02)01068-7. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depression and Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Wang P, Lin C, Smith ER, Guo H, Sanderson BW, Wu M, et al. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Molecular and Cellular Biology. 2009;29:6074–6085. doi: 10.1128/MCB.00924-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts-English T, Fortson BL, Gibler N, Hooper SR, De Bellis MD. The psychobiology of maltreatment in childhood. Journal of Social Issues. 2006;62:717–736. [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Wu M, Hernandez M, Shen S, Sabo JK, Kelkar D, Wang J, et al. Differential modulation of the oligodendrocyte transcriptome by Sonic Hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. Journal of Neuroscience. 2012;32:6651–6664. doi: 10.1523/JNEUROSCI.4876-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.