Abstract
Recreational use of the cathinone derivative 3,4-methylenedioxypyrovalerone (MDPV; “bath salts”) has increased worldwide in past years, accompanied by accounts of health and legal problems in the popular media and efforts to criminalize possession in numerous jurisdictions. Minimal information exists on the effects of MDPV in laboratory models. This study determined the effects of MDPV, alongside those of the better studied stimulant d-methamphetamine (METH), using rodent models of intravenous self-administration (IVSA), thermoregulation and locomotor activity. Male Wistar rats were trained to self-administer MDPV or METH (0.05 mg/kg/infusion, i.v.) or were prepared with radiotelemetry implants for the assessment of body temperature and activity responses to MDPV or METH (0–5.6 mg/kg s.c.). METH and MDPV were consistently self-administered within 10 training sessions (mg/kg/hour; METH Mean=0.4 and Max = 1.15; MDPV Mean=0.9 and Max = 5.8). Dose-substitution studies demonstrated that behavior was sensitive to dose for both drugs, but MDPV (0.01–0.50 mg/kg/inf) showed greater potency and efficacy than METH (0.1–0.25 mg/kg/inf). In addition, both MDPV and METH increased locomotor activity at lower doses (0.5–1.0 mg/kg, s.c.) and transiently decreased activity at the highest dose (5.6 mg/kg, s.c.). Body temperature increased monotonically with increasing doses of METH but MDPV had a negligible effect on temperature. Stereotypy was associated with relatively high self-administered cumulative doses of MDPV (~1.5 mg/kg/hr) as well as with non-contingent MDPV administration wherein the intensity and duration of stereotypy increased as MDPV dose increased. Thus, MDPV poses a substantial threat for compulsive use that is potentially greater than that for METH.
Keywords: Bath Salts, Locomotor, Thermoregulation, Self-Administration
1. Introduction
The novel cathinone derivative drug 3,4-methylenedioxypyrovalerone (MDPV, a common constituent of “bath salts”) is increasingly associated with emergency department visits (Benzie et al., 2011) and clinical reports of adverse effects (Penders and Gestring, 2011; Spiller et al., 2011). MDPV is a derivative of cathinone which is a natural product found in khat (Catha edulis); cathinone, methcathinone and mephedrone (4-methylmethcathinone) function as reinforcers in intravenous self-administration (IVSA) paradigms in rats (Aarde et al., 2013; Gosnell et al., 1996; Hadlock et al., 2011) and nonhuman primates (Johanson and Schuster, 1981; Kaminski and Griffiths, 1994; Schuster and Johanson, 1979; Woolverton and Johanson, 1984).
In comparison with other stimulant drugs such as amphetamine, methamphetamine and cocaine, the literature on MDPV is limited. Although there was no information published on the combined locomotor and thermoregulatory effects or the reinforcing efficacy of MDPV at the initiation of this study (Aarde et al., 2011), a number of relevant reports have recently been published. It has been reported that MDPV can act as a reinforcer in self-administration in rats and furthermore, that when under a progressive-ratio schedule of reinforcement, self-administration breakpoints were higher for higher per-infusion doses (Watterson et al., 2012). MDPV also dose-dependently decreased reward thresholds in intra-cranial self-stimulation (Watterson et al., 2012). However, it is remains to be determined how the self-administration dose-response curves of MDPV and METH compare (only one dose of METH was used in the aforementioned report) and how well these reinforcing properties generalize to different experimental procedures and subjects.
In addition, MDPV acts a locomotor stimulant and has been shown to dose-dependently increase activity, stereotypy, heart rate and blood pressure in rats (Baumann et al., 2013), and to increase locomotor activity (Fantegrossi et al., 2013; Fuwa et al., 2009; Marusich et al., 2012) and body temperature (Fantegrossi et al., 2013) in mice. These effects were potentiated under a relatively high ambient temperature (28° C) as compared to a relatively low ambient temperature (20 °C) (Fantegrossi et al., 2013). Effects of MDPV on thermoregulation, and the timecourse of locomotor stimulation, have not been previously reported in the rat. The substituted amphetamines 3,4-methylenedioxymethamphetamine (MDMA) and METH have been shown to substitute for MDPV in drug discrimination experiments in mice (Fantegrossi et al., 2013). Lastly, MDPV has a biphasic effect on voluntary wheel running in rats; increased running at low doses but decreased running at high doses (Huang et al., 2012). This effect was similar to that of methamphetamine but dissimilar to that of MDMA or mephedrone – both of which monotonically decreased running (Huang et al., 2012). The high dose effects on wheel activity are consistent with the observation that higher dose MDPV induces stereotypies in mice (Marusich et al., 2012) but one prior report indicated only monotonic dose-dependent increases in locomotor distance traveled up to 3 mg/kg in rats (Baumann et al, 2013).
These behavioral and physiological observations are consistent with the known actions of MDPV on monoamine systems. Like methamphetamine and cocaine, MDPV is a monoamine transporter inhibitor with relative potencies (IC50; nM; from rat synaptosomes and human transporters expressed by HEK 293 cells, respectively) at the dopamine transporter (DAT), noradrenaline transporter (NET) and serotonin transporter (SERT) as follows: DAT (4.1, 31) > NET (26, 44) >> SERT (3349, 9300) (Baumann et al., 2013; Simmler et al., 2013). Furthermore, MDPV has a larger DAT/SERT inhibition ratio, estimated as >100; as compared to >10 and 3.1 for METH and cocaine, respectively (Simmler et al., 2013); this ratio may indicate high abuse potential (Bauer et al., 2013). However, like cocaine and unlike the amphetamines, MDPV is ineffective as a monoamine releaser (Baumann et al., 2013; Simmler et al., 2013).
An investigation was designed to further determine the in vivo effects of MDPV, and to contrast these with the effects of METH, within a single animal model. Assessment of intravenous self-administration and unrestrained temperature/activity responses were conducted in groups of male Wistar rats. Groups of animals were trained and tested on drug self-administration under varied reinforcement schedules and additional animals were monitored via radiotelemetry after non-contingent drug administration. Dose-response curves for MDPV were compared to those for METH given the structural similarity between cathinones and amphetamines and our prior finding of similar effects on wheel activity (Huang et al, 2012). Observations of stereotypy after MDPV self-administration and non-contingent administration at various doses were quantified.
2. METHODS
2.1 Animals
Male Wistar rats (Charles River, New York; self-administration, N = 24; non-contingent administration, N = 16) were housed in humidity and temperature-controlled (23±1 °C) vivaria on 12:12 hour light:dark cycles. Animals entered the laboratory at 10–13 weeks of age and weighed 350–400 grams at the start of the study. Animals had ad libitum access to food (save for pellet training, see below) and water in their home cages. All procedures were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and in a manner consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Clark et al., 1996).
2.2 Drugs
The racemic 3,4-methylenedioxypyrovelarone HCl used for this study was synthesized as described below. d-Methamphetamine HCl was provided by RTI International (Research Triangle Park, NC) under contract from the National Institute on Drug Abuse (Bethesda, MD).
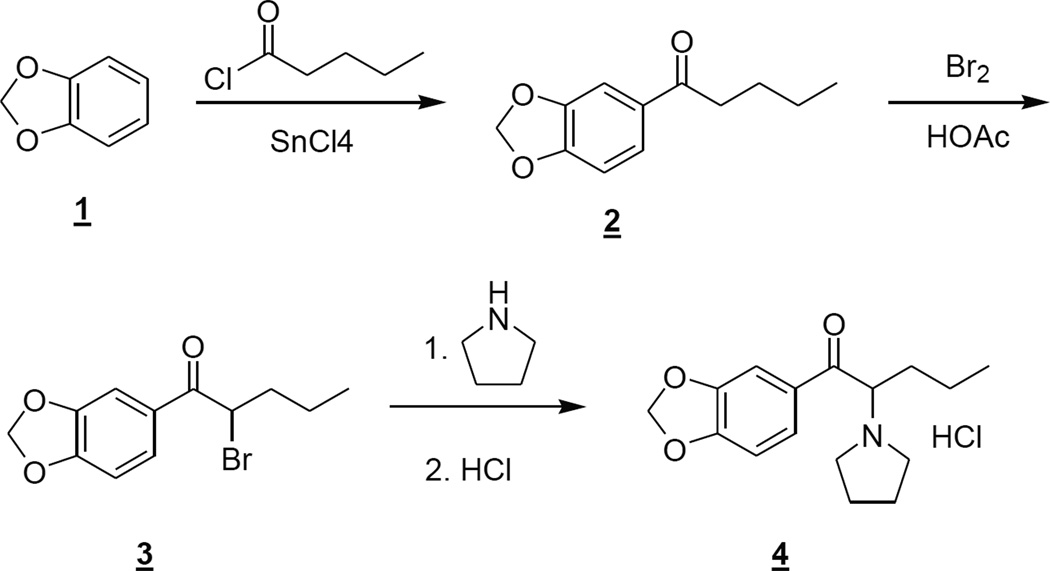
2.2.1 Synthesis of 3,4-methylenedioxypyrovalerone (MDPV; 1-(1,3-benzodioxol-5-yl)-2-(1-pyrrolidinyl)- 1-pentanone)
2.2.1.1 1-Benzo[1,3]dioxol-5-yl-pentan-1-one (2)
To a solution of benzo[1,3]dioxole 1 (30 g, 246 mmol) and pentanoyl chloride (31.5 mL, 266 mmol) in dichloromethane (220 mL) at 3 °C was added tin (IV) chloride (36 mL, 307 mmol) dropwise over 30 minutes, keeping the temperature < 10 °C. After stirring for an additional 15 minutes, the reaction mixture was poured into a mixture of concentrated hydrochloric acid (150 mL) and ice (200 g) with vigorous stirring. The layers were separated and the organic layer was washed with water (100 mL). The organic layer was dried over sodium sulfate, filtered and concentrated at reduced pressure. The crude product was purified via flash column chromatography using a silica gel cartridge (330 g), eluting with hexanes/ethyl acetate (99:1 to 95:5) to give the product as a colorless oil (26 g, 51%). 1H NMR (CDCl3) δ 7.55 (dd, J = 8.2, 1.7 Hz, 1H), 7.43 (d, J = 1.8 Hz, 1H), 6.83 (d, J = 8.2 Hz, 1H), 6.02 (s, 2H), 2.87 (t, J = 7.4 Hz, 2H), 1.69 (m, 2H), 1.39 (m, 1H), 0.94 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3) δ 198.8, 151.7, 148.3, 132.2, 124.4, 108.1, 108.0, 102.0, 38.3, 27.0, 22.7, 14.1.
2.2.1.2 1-Benzo[1,3]dioxol-5-yl-2-bromo-pentan-1-one (3)
To a stirring solution of 1-benzo[1,3]dioxol-5-yl-pentan-1-one 2 (5 g, 24.5 mmol) in acetic acid (15 mL) was added bromine (1.26 mL, 24.5 mmol) dropwise which resulted in a mild exotherm which was kept below 35 °C via water bath. After 1 hour at am bient temperature water (50 mL) was added and the mixture was extracted with dichloromethane (2 × 100 mL) and the combined organic layers were concentrated at reduced pressure. The residue was dissolved in dichloromethane (100 mL) and washed with saturated aqueous sodium hydrogen carbonate (75 mL) and the organic layer was concentrated at reduced pressure. The crude product was recovered in quantitative yield and was used without purification in the next step. 1H NMR (CDCl3) δ 7.61 (dd, J = 8.1, 1.7 Hz, 1H), 7.48 (d, J = 1.8 Hz, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.06 (s, 2H), 5.06 (app t, J = 6.8 Hz, 1H), 2.11 (m, 2H), 1.49 (m, 2H), 0.97 (t, J = 7.2 Hz, 3H).
2.2.1.3 1-(1,3-Benzodioxol-5-yl)-2-(1-pyrrolidinyl)- 1-pentanone hydrochloride (4)
To a stirring solution of pyrrolidine (3.17 mL, 38.6 mmol) in dichloromethane (25 mL) was added 1-benzo[1,3]dioxol-5-yl-2-bromo-pentan-1-one 3 (5 g, 17.5 mmol) in dichloromethane (15mL) and the resulting solution was stirred at ambient temperature for 16 hours. The reaction mixture was diluted with dichloromethane (50 mL), washed with water (50 mL), dried over sodium sulfate, filtered and concentrated at reduced pressure. The crude product was dissolved in 2-propanol (50 mL) and to this stirring solution was added hydrogen chloride (1 N solution in ethyl ether (20 mL) dropwise, producing a white precipitate. After 1 hour, the mixture was cooled to 5 °C and filtered. The solid was rinsed wit h cold 2-propanol (10 mL) and dried to give the hydrochloride salt as a white solid (4.1 g, 75%). 1H NMR (DMSO-d6) δ 10.60 (br s, 1H), 7.76 (dd, J = 8.2, 1.8 Hz, 1H), 7.56 (d, J = 1.8 Hz, 1H), 7.13 (d J = 8.2 Hz), 1H), 6.18 (s, 2H), 5.48 (br q, 6.5 Hz, 1H), 3.59 (m, 1H), 3.39 (m, 1H), 3.20 (m, 1H), 2.98 (m, 1H), 2.10-1.81 (m, 4H), 1.20 (m, 1H), 1.05 (m, 1H), 0.78 (t, J = 7.3 Hz, 3H). 13C NMR (DMSO-d6) δ 195.2, 153.7, 1489.0, 129.9, 126.8, 109.2, 108.5, 67.5, 54.1, 52.6, 32.7, 23.6, 18.2, 14.4.
2.3 Ki Determinations
The Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA (http://pdsp.med.unc.edu/). Details can be found in the program’s Assay Protocol Book (http://pdsp.med.unc.edu/UNC-CH%20Protocol%20Book.pdf).
2.4 Surgery
2.4.1 Intravenous Catheterization
Rats (MDPV, N = 16; METH, N = 8) were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described elsewhere (Caine et al., 1993) with minor modifications described previously (Emmett-Oglesby and Lane, 1992). Briefly, the catheters consisted of an 18-cm length of silastic tubing fitted to a guide cannula (Plastics One, Roanoke, VA) bent at a right angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal's back to the right jugular vein. Silastic tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB). A minimum of 7 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin; 0.4 g/ml; 2.0 ml/kg, s.c.) and an analgesic (flunixin; 2.5 mg/ml; 2.0 ml/kg, s.c.) were administered daily. During testing and training, intravenous catheters were flushed with sterile physiological saline containing timentin (before sessions; 0.1 g/ml; 0.2–0.3 ml/rat) and heparinized saline (after sessions; 10 USP units/ml; 0.2–0.3 ml/rat).
Catheter patency was assessed nearly once a week after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic Brevital sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec after infusion. Animals that failed to display these signs were considered to have faulty catheters and were discontinued from the study. Data that was taken prior to failing this test and after the previous passing of this test were excluded from analysis.
2.4.1 Radiotelemetry Probe Implantation
Sterile radiotelemetry transmitters (Data Sciences International; CTA-F40) were implanted in the abdominal cavity thru an incision along the abdominal midline posterior to the xyphoid space. Absorbable sutures were used to close the abdominal muscle incision and the skin incision was closed with the aforementioned tissue adhesive. Post-operative care and recovery time was the same as that for i.v. catheterization. One animal in the group assigned to the methamphetamine study did not survive surgery (MDPV, N=8; METH, N=7).
2.5 Drug Administration Procedures
2.5.1 Self-administration
Animals underwent the following four sequential phases (detailed below); Lever-Press Training, Drug self-administration acquisition, fixed-ratio dose-response testing and progressive-ratio dose-response testing. For lever-press training and drug self-administration acquisition/testing, each subject was transported to an experimental room (ambient temperature 23 ± 1 °C; illuminated by red light) and placed into operant boxes (Med Associates) located inside sound-attenuating chambers. For drug self-administration, catheter fittings on the animals' backs were connected to polyethylene tubing contained inside a protective spring suspended into the operant chamber from a liquid swivel attached to a balance arm. Drugs were delivered via syringe pump. Each operant session started with the extension of two retractable levers into the chamber. Following each completion of the response requirement (response ratio), a white stimulus light (located above the reinforced lever) signaled delivery of the reinforcer and remained on during a 20-sec post-infusion timeout, during which responses were recorded but had no scheduled consequences. Sessions began between 0.5–4 hours after the beginning of the vivarium dark cycle.
2.5.1.1 Lever-Press Training
Prior to catheterization surgery, rats were food-restricted (~15 g chow/rat/day) and trained to press the reinforced lever (always left lever) for delivery of 45-mg food pellets (TestDiet; 1811156) under a fixed-ratio, one-press-per-reinforcer schedule of reinforcement (FR1). Once advancement criterion was achieved (50 reinforcers earned), the ratio requirement was increased to FR2 then to FR5 (same advancement criteria). On average, this training required 4±1 days. Rats were returned to ad libitum feeding immediately after completion of training and 1–3 days before surgery.
2.5.1.2 Drug Self-administration Acquisition
After recovery from surgery, animals were again food restricted (~17 g chow/rat/day). On the second day of food restriction began a sequence of ten, one-hour self-administration sessions (run 5 days per week) in which drug infusions (0.05 mg/kg/inf; 0.1 ml/inf; 4 sec/inf) were made available on an FR5 schedule of reinforcement. Rats were returned to ad libitum feeding immediately after the 3rd session.
2.5.1.3 Fixed-Ratio (FR), Dose-Response Testing
After completion of drug self-administration acquisition, subjects were given testing sessions wherein the per-infusion dose was varied from session to session. The doses of METH were chosen to be similar to precedent literature (Wee et al., 2007) while the doses of MDPV were chosen from pilot studies in our lab in rats previously trained in i.v. self-administration of other drugs (METH or MDMA). Sessions were given in a block-randomized design wherein each block consisted of four sessions; each at one of the four doses tested. Subjects were given 2–3 blocks of testing.
2.5.1.3 Progressive-Ratio (PR), Dose-Response Testing
After completing the fixed-ratio, dose-response testing, subjects were given testing sessions of varied per-infusion dose under a progressive-ratio schedule of reinforcement. Sessions were given in a block-randomized design wherein each block consisted of four sessions; each at one of the four doses tested (same doses as those for fixed-ratio, dose-response testing). Subjects were given 2–3 blocks of testing.
In this self-administration paradigm, the required response ratio increases after each reinforcer delivery (Hodos, 1961; Segal and Mandell, 1974). The sequence of response ratios started with one response then progressed thru ratios determined by the following equation (rounded to the nearest integer): Response Ratio = 5e^(injection number * j) – 5 (Richardson and Roberts, 1996). The value of “j” for METH was 0.3 and was chosen to be slightly higher than that from precedent literature (j = 0.2) wherein a significant effect of per-infusion dose was not observed under a similar range of doses and conditions (Wee et al., 2007). The value of “j” for MDPV was 0.4 and was based upon extrapolation from studies in our lab in rats previously trained in i.v. self-administration of other drugs (METH or MDMA) so as to observe a “breakpoint” in ~3 hrs at 0.10 mg/kg/inf.
“Breakpoint” was defined as the observation of an inter-infusion interval > 60 min. If a “breakpoint” was observed within a session, the session terminated after that observation. However, sessions wherein METH was the available drug were also time limited to prevent the escalation of drug taking observed after extended-access sessions (i.e., 6 hour sessions) (Kitamura et al., 2006); thus, a “breakpoint” was not always observed in METH sessions at the highest per-infusion dose (0.25 mg/kg). As predicted from the aforementioned pilot studies, all rats with access to MDPV reached a breakpoint in less than 3 hrs for per-infusion doses at or below 0.1 mg/kg. For the highest per-infusion dose of MDPV (0.5 mg/kg), a “breakpoint” was observed within 5 hours for all except 2 sessions – each for a different subject (Figure 3c). To make the sampling interval for analyses of measures the same across drug type, analyses were limited to the 1st 3 hours of PR testing.
Figure 3.
Fixed-ratio (FR5), dose-response testing. Means of infusion rate (infusions/hr; Graph A) and post-reinforcement pause length (average time in seconds between an infusion and the next correct response; Graph B) as a function of the type of drug available (MDPV, N=10; METH, N=9) and per-infusion dose (mg/kg). For comparisons between drug types as a function of per-infusion dose, significant differences are indicted by the “*” symbol. For the effect of per-infusion dose as a function of drug type, significant post hoc comparisons are indicted by the “#” symbol for MDPV and the “@” symbol for METH. Error bars represent SEM. Also, infusion number as a function of the time within the session that that infusion occurred (Graph C) are presented to show records of individual rats’ behavior within each session. Within a particular drug type, each unique symbol represents a specific subject. For each per-infusion dose, each session of a particular subject at that dose is represented by a single curve.
2.5.2 Non-contingent Administration
Animals were evaluated in clean standard plastic homecages (thin layer of bedding) in a separate testing room approximately 1 hour after the start of the vivarium dark cycle. Radiotelemetry transmissions were collected via telemetry receiver plates (Data Sciences International; RPC-1) placed under the cages as in prior investigations (Miller et al., 2013; Miller et al., 2012; Wright et al., 2012). Food and water were available throughout a session. Test sessions started with a 30-minute baseline period followed by subcutaneous injection of drug. Sessions were given twice a week with no less than two days between sessions. Prior to these sessions, animals were habituated to the procedure (i.e., 2–3 saline sessions). The ambient temperature (TA) for administration of MDPV was 23±1 °C while the TA for METH was experimentally varied to a low (20±2 °C) a nd a moderate (25±2 °C) temperature. Drug dose and TA (for METH only) were varied between sessions and within-subjects in pseudorandom order. Each dose was administered to each subject once for MDPV and 1–2 times for METH (if the same dose and TA was administered to a subject twice, the two sessions were pooled for that subject).
2.5.3 Behavioral observations after MDPV administration: Stereotypy
Stereotypy was operationally defined by the observation of the following types of behaviors: Repetitive licking, biting or sniffing the walls/bars of the homecage [IVSA] or test cage [non-contingent]. Stereotypy was scored in the fixed-ratio, dose-response testing phase of IVSA (i.e., the phase wherein the cumulative dose per session varied greatly but session duration and reinforcement schedule was a constant) as either “present” or “absent” during the first 20 sec following return to the home cage. Stereotypy was assessed for all MDPV conditions in the telemetry study during a 20 second observation interval every 15 min of the session. If behaviors operationally defined above as stereotypy were observed, but those behaviors could be disrupted by tapping on the side of the cage opposite the location of the rat, a score of “1” was recorded. If this tapping could not disrupt those behaviors a score of “2” was recorded. If the rat did not display stereotypy, a score of “0” was recorded. The experimenter remained blind to the cumulative dose under self-administration and the dose administered under non-contingent administration.
2.6 Designs and Data Analyses
2.6.1 Self-administration
Measures collected during self-administration sessions included: 1) Infusion rate (per hour). 2) Total correct lever presses (for PR; 1st 3 hours only). 3) Lever discrimination (100% * correct lever presses ÷ total lever presses; correct lever presses ≠ 0). 4) Post-reinforcement pause length (average time interval between an infusion and the next correct lever press counting toward completion of the next response ratio; dose-response testing only; 40% trimmed mean; (Killeen et al., 2009)). For dose-response testing, measures were averaged (pooled) across testing block.
Acquisition analyses included separate analysis of food-restricted sessions (1–3) and of non-food-restricted sessions (4–10); both analyses included the within-subjects factor of session number and the between-subjects factor of the type of drug available for self-administration. Dose-response testing included the within-subjects factor of per-infusion dose and the between subjects factor of the type of drug available when permitted by comparable dose conditions (see below).
Statistical analyses were conducted using a mixed effects linear model (MELM) under the assumption of compound symmetry in covariance structure (Kincaid, 2005; Wang and L. A. Goonewardene, 2004); under MELM, all observations are retained and the error degrees of freedom are decreased in accordance with the missing observations. Missing observations were encountered in some data sets due to 1) loss of catheter patency before the end of an experimental phase (1 METH rat during the dose-response curve for PR), 2) when a post-reinforcement pause was not observed (i.e., when less than 2 infusions were earned in a session; for FR dose-response testing, 6 cases each for METH and MDPV, for PR dose-response testing, 6 cases for METH and 10 cases for MDPV) and 3) for acquisition sessions wherein the tether became unattached (one rat on day 8 and a different subject on day 9 for the METH study).
Significant main effects and interactions were explored with post hoc comparisons using Tukey’s honest significant difference method (Tukey HSD). For dose response testing, separate MELM analyses were performed for each drug type that included the within-subject factor of per-infusion dose. Comparisons of measures between drug types at the 3 common per-infusion doses were performed using independent, two-tailed t-tests with a Bonferroni correction for multiple comparisons.
SPSS (IBM Corporation) was used for MELM and rmANOVA analyses. StatView (SAS Institute, Inc.) was used for post hoc and pre-planned comparisons as well as graph generation. For all analyses, Type I error rate (α) was set at p(u1≠u2|H0=0) < 0.05.
2.6.2 Non-contingent administration
Measures included the following: 1) Average locomotor activity (represents counts per minute of changes in signal strength from the radiotelemetry transmitter; represented as percent of baseline; baseline was the average counts per minute for the 30 min pre-injection period). 2) Body temperature (represents the average body cavity temperature in °C over a 30-second sample period taken at the end of each 5-min interval). Both measures were calculated using Dataquest A.R.T. system™ software (Data Sciences International). Additionally, to compare measures between drug types at common doses, the above telemetry measures were collapsed across temperature (METH average ~23 °C) and an area under the curve (AUC) was calculated and normalized to the vehicle condition (i.e., sum of deviations from mean of vehicle).
Analyses of radiotelemetry data were performed using repeated-measures analysis of variance (rmANOVA) including within-subjects factors of dose and time post injection (averaged in 30-minute bins). The additional within-subjects factor of average TA (20±2 °C vs. 25±2 °C) was included in the METH analyses. When the assumption of sphericity was violated (i.e., when the p-value for Mauchly’s Test of sphericity was < 0.05 or, when a p-value could not be calculated, the Huynh-Feldt ε value was less than 0.75), a Huynh-Feldt correction was applied (for presentational purposes, degrees of freedom were rounded to the nearest whole number). Effects confirmed by rmANOVA were delineated with post hoc comparisons using Tukey’s HSD (effect of dose and TA) or Dunnett’s test (effect of time from injection; comparison to pre-injection baseline). Pre-planned comparisons of AUCs between drug types at the common doses were made using independent, two-tailed t-tests with Bonferroni corrections.
SPSS (IBM Corporation) was used for rmANOVA analyses and sphericity tests/calculations. StatView (SAS Institute, Inc.) was used for post hoc and pre-planned comparisons as well as graph generation. For all analyses, Type I error rate (α) was set at p(u1≠u2|H0=0) < 0.05.
2.6.3 Stereotypy
For analysis of post-session stereotypy in the self-administration FR dose-response series, the average cumulative MDPV dose (mg/kg) was calculated for each rat for the sessions after which stereotypy was scored as “present” or “absent” (see subsection 2.5.3). The average cumulative dose per score was analyzed with a rmANOVA for which “score” was the within-subjects factor.
For non-contingent sessions, stereotypy scores (0, 1 or 2; see subsection 2.5.3) were analyzed with a rmANOVA (same procedural considerations and post hoc analysis described in subsection 2.6.2 for non-contingent administration; baseline was 1st post-injection time bin) that included the within-subjects factors of injection dose and time from injection (in 15 min bins).
3. Results
3.1 Self-Administration
3.1.1 Acquisition
Rats readily self-administered MDPV or METH at a per-infusion dose of 0.05 mg/kg on an FR5 schedule of reinforcement with lever presses consistently favoring the drug-paired lever (Figure 2). Across acquisition, the infusion rate of both drugs declined through the first three food-restricted sessions after which it was stable for the non-food-restricted sessions. Over these 7 non-food-restricted sessions, the infusion rate (inf/hr) of MDPV (M = 19; SD = 22; Max-Min = 115-0) was consistently higher than that of METH (M = 8; SD = 7; Max-Min = 23-0) while lever discrimination for MDPV (M = 86%; SD = 22%; Max-Min = 100%-8%) and METH (M = 83%; SD = 14%; Max-Min = 100%-33%) was similar.
Figure 2.
Infusion Rate (filled symbols; inf/hr; 0.05 mg/kg/inf)and lever discrimination (unfilled symbols; value = 100% * drug-paired lever presses/total lever presses; lever presses during post-reinforcement time out not included) of MDPV (N=13) or METH (N=9) under a fixed-ratio schedule of reinforcement (FR5) as a function of self-administration session (1st 3 sessions under food restriction).For the effect of session, significant differences between pairs of means are indicated by a # symbol. Error bars represent SEM.
MELM analysis of infusion rate for the food-restricted sessions confirmed a main effect of session (F(2,40)= 12.1, p<0.0001) but not of drug group (F(1,20)= 4.1, p = 0.057) or a drug*session interaction (F(2,40)= 0.5, p = 0.6). Post hoc comparisons confirmed that infusion rate for the first session was higher than that of the next 2 sessions while the rate was equivocal for sessions 2 and 3. MELM analysis of lever discrimination did not confirm a main effect of drug group (F(1,20)= 1.0, p = 0.3), session (F(2,40)= 0.3, p = 0.8) or a drug*session interaction (F(2,40)= 0.1, p =0.9).
For the non-food-restricted sessions (4–10), MELM analysis of infusion rate did not confirm main effects of drug group (F(1,20)= 2.5, p = 0.13), session (F(6,118)= 0.6, p = 0.7) or a drug*session interaction (F(6,118)= 0.3, p = 0.96). Similarly, MELM analysis of lever discrimination did not confirm a main effect of drug group (F(1,20)= 0.3, p = 0.6), session (F(6,116)= 0.3, p = 0.9) or a drug*session interaction (F(6,116)= 0.5, p = 0.8).
3.1.2 Dose-response analysis under a fixed-ratio schedule of reinforcement
Altering the per-infusion dose changed both the infusion rate and the average post-reinforcement pause when either MDPV or METH was available for self-administration (Figure 3). These changes were dose-dependent: As the per-infusion dose increased, the infusion rate decreased and the post-reinforcement pause increased. Analysis of infusion rate (infusions/hour) confirmed an effect of dose for MDPV (F(4,17)= 15.4, p < 0.001) but only a trend toward an effect of dose for METH (F(4,15)= 2.96, p = 0.055) (Figure 3a). The post hoc comparisons for MDPV confirmed that the infusion rate was higher when the per-infusion dose (mg/kg) was 0.01 (M = 35; SD = 28) than when it was 0.05 (M = 13; SD = 6), 0.10 (M = 8; SD = 6) or 0.50 (M = 4; SD = 2); the rate was equivalent for other comparisons. For METH, infusion rates were as follows: 0.01 (M = 8; SD = 13), 0.05 (M = 6; SD = 7), 0.10 (M = 5; SD = 5) and 0.25 (M = 4; SD = 2). Planned comparisons of infusion rate as a function of drug type available confirmed that for the per-infusion dose of 0.01 mg/kg the rate was higher when MDPV was available than when METH was available; the rate was equivalent for other comparisons.
Analysis of post-reinforcement pause confirmed main effects of dose for MDPV (F(4,17)= 51.8, p < 0.0001) and for METH (F(4,8)= 10.3, p < 0.01) (Figure 3b). The post hoc comparisons of pause length for MDPV confirmed that the pause was longer when the per-infusion dose (mg/kg) was 0.50 (M = 572; SD = 212) than when it was 0.10 (M = 223; SD = 96), 0.05 (M = 107; SD = 48) or 0.01 (M = 48; SD = 37). Also, the post-reinforcement pause was longer for the per-infusion dose of 0.10 than 0.01, but equivalent for other comparisons. For METH, post hoc comparisons of pause duration confirmed that the pause was longer when the per-infusion dose was 0.25 (M = 162; SD = 71) than when it was 0.01 (M = 94; SD = 35) but not when it was 0.10 (M = 170; SD = 225) or 0.05 (M = 106; SD = 65); the length was equivalent for other comparisons. Preplanned comparisons did not confirm any effects of the type of drug available at common per-infusion doses.
Qualitatively, individual session infusion rate curves (i.e., traces of the relationship between the time from the start of the session and the time at which an infusion was delivered; Figure 3c) for both MDPV and METH showed an overall linear (rather than curvilinear or accelerating) pattern of consumption and the slopes of these curves appeared to decrease as per-infusion dose increased.
3.1.3 Dose-response analysis under a progressive-ratio schedule of reinforcement
Changing the per-infusion dose altered the total number of correct lever presses per session and the average post-reinforcement pause length when either MDPV or METH was available for IVSA (Figure 4). In general, as the per-infusion dose increased both the number of correct lever presses and length of the average post-reinforcement pause increased.
Figure 4.
Progressive-ratio, dose-response testing (ratio = 5e[injection#*j], except for 1st ratio which was always 1; MDPV, j = 0.4; METH, j = 0.3). Means of total correct lever presses (1st 3 hours of the session only; includes lever presses during post-reinforcement time out; Graph A) and post-reinforcement pause length (average time in seconds between an infusion and the next correct response; 1st 3 hours of the session only; Graph B) as a function of the type of drug available (MDPV, N=8; METH, N=7) and per-infusion dose (mg/kg). For comparisons between drug types as a function of per-infusion dose, significant differences are indicted by the “*” symbol. For the effect of per-infusion dose as a function of drug type, significant post hoc comparisons are indicted by the “#” symbol for MDPV. Error bars represent SEM.
Analysis of the number of correct lever presses (1st 3 hours of the session only) confirmed main effects of dose for MDPV (F(4,13) = 13.7, p < 0.001) and for METH (F(4,11) = 5.7, p = 0.01) (Figure 4a). For MDPV, post hoc comparisons of lever presses confirmed that there were more lever presses when the per-infusion dose (mg/kg) was 0.05 (M = 610; SD = 565) or 0.10 (M = 933; SD = 496) than when it was 0.01 (M = 53; SD = 57). The mean number of lever presses for the 0.50 mg/kg (M = 286; SD = 291) dose was not reliably different from the other doses. For METH, post hoc comparisons of the number of lever presses did not confirm any differences between per-infusion doses; 0.01 (M = 60; SD = 97), 0.05 (M = 74; SD = 74), 0.10 (M = 250; SD = 222) and 0.25 (M = 135; SD = 97). Planned comparisons of lever presses as a function of drug type confirmed that for the per-infusion dose of 0.10 mg/kg, there were more lever presses when MDPV was available than when METH was available; means were equivalent for other comparisons at common per-infusion doses.
Analysis of post-reinforcement pause duration (1st 3 hours of session only) confirmed main effects of dose for MDPV (F(4,13) = 14.0, p < 0.0001) and for METH (F(4,12) = 6.3, p < 0.01) (Figure 3b). Post hoc comparisons confirmed that the pause length after MDPV infusions was longer when the per-infusion dose was 0.50 (M = 820; SD = 462) than when it was 0.10 (M = 179; SD = 103), 0.05 (M = 110; SD = 114) or 0.01 (M = 225; SD = 339). Although the pattern was similar for METH, with longer lengths observed when the per-infusion dose was 0.25 (M = 490; SD = 303) than when it was 0.10 (M = 113; SD = 62), 0.05 (M = 103; SD = 64) or 0.01 (M = 276; SD = 449), post hoc analyses did not confirm these differences. Planned comparisons did not confirm any effects of the type of drug available at any common per-infusion dose.
3.2 Non-contingent administration
3.2.1.1 MDPV: Locomotor Activity
Non-contingent administration of MDPV under normal (23±1°C) ambient temperature (TA) increased locomotor activity in a dose-dependent and time-dependent manner (Figure 5a; top left). The analysis confirmed an interaction between MDPV dose and time after injection (F(10,71) = 3.3, p < 0.01) as well as main effects of dose (F(4,28)= 3.9, p< 0.05) and time (F(4,26) = 6.6, p < 0.01).The post hoc test confirmed that activity was higher early in the session after MDPV injections of 1.0 mg/kg (30- and 60-min post-injection time bins) compared with the vehicle condition as well as higher after injections of 1.0 mg/kg compared to 5.6 mg/kg for most of the session (30–120 min bins) and 3.2mg/kg early in the session (60 min bin). Activity counts were also higher early in the session (30–60-min post-injection time bins) after 1.0 mg/kg compared to the pre-injection baseline.
Figure 5.
A) Average locomotor activity (% change in activity counts from 30-min pre-injection interval)and body temperature (°C) in 30-min time intervals (“bins”) as a function of injection dose and time from injection (s.c.) of either MDPV (N=8) or METH (N=7). Top Graphs: Locomotor activity after injection of MDPV under a room temperature of 23±1 °C (Left) or METH under room temperatures of 20±2 °C or 25±2 °C (Three Rightmost; the first of which shows the data pooled across room temperatures; the effect of room temperature was not significant nor did it interact with other factors). Bottom Graphs: Body temperature after injection of MDPV under a room temperature of 23±1 °C (Left) or METH under room temperatures of 20±2 °C or 25±2 °C (Three Rightmost – the first of which shows the data pooled across room temperatures of 20±2 °C and 25±2 °C; 3-way interaction was not significant; other 2-way interactions graphed in “C”). B) Means for area under the curve (AUC) calculations (summed deviations from the mean of vehicle) for locomotor activity (Left) and body temperature (Right) as functions of drug type. C) Body temperature after METH injections. Left Graph: Effect of injection dose as a function of room temperature. Middle Graph: Effect of room temperature as a function of injection dose. Right Graph: Effect of room temperature as a function of time from injection. Symbols (all graphs): For the effect of time, mean values that were significantly different from the baseline mean within dose or room temperature are indicated by open symbols. For the effect of dose (mg/kg),mean values that were significantly different are indicated by the following symbols: α= vehicle vs. 0.5; β = vehicle vs. 1.0; δ = vehicle vs. 3.2; ψ = vehicle vs. 5.6; ¢ = 0.5 vs. 1.0; $ = 0.5 vs. 3.2; € = 0.5 vs. 5.6; * = 1.0 vs. 3.2; # = 1.0 vs. 5.6; § = 3.2 vs. 5.6. For the effect of drug type and room temperature, significant differences are indicated by the “*” symbol. Error bars represent SEM.
3.2.1.2 MDPV: Body Temperature
Non-contingent administration of MDPV (23±1°C TA) changed body temperature modestly in a dose- and time-dependent manner (Figure 5a; bottom left) as was confirmed by a significant interaction between MDPV dose and time following injection (F(9,64) = 2.5, p < 0.05); main effects of dose (F(4,28) = 2.4, p = 0.078) or of time (F(2,14) = 2.8, p = 0.098) were not confirmed. Body temperature was higher after MDPV injections of 1.0 mg/kg than after 5.6 mg/kg by the end of the session (180-min post-injection time bin) but no other comparisons between doses met statistical criterion. Body temperature was also higher late in the session after 1.0 only (180-min post-injection time bin) compared to the pre-injection baseline.
3.2.2.1 METH: Locomotor Activity
Non-contingent administration of d-methamphetamine (METH) produced similar dose-dependent and time-dependent effects on locomotor activity across the two ambient temperatures (TA) under which it was tested (20±2 or 25±2 °C) (Figure 5a; top rig ht). Analysis of activity counts confirmed main effects of METH dose (F(2,12) = 13.7, p < 0.01), of time after injection (F(4,24) = 11.7, p < 0.001) and the interaction between injection dose and time after injection (F(5,30) = 6.4, p < 0.001). The analysis did not confirm a main effect of TA (F(1,6) = 2.7, p = 0.15), an interaction between TA and dose (F(2,12) = 0.9, p = 0.4), an interaction between TA and time (F(6,36) = 1.9, p = 0.10) or the three-way interaction (F(321) = 1.1, p = 0.4).
Post-hoc comparisons confirmed that, compared to vehicle injections, activity counts after METH injections were higher for the 1.0 dose (30-, 90- and 120-min post-injection time bins) and lower for the 5.6 dose toward the middle of the session (60–150-min post-injection time bins). Activity was greater for the 1st 30-min time bin compared to the pre-injection baseline for all dosing conditions and also higher than baseline values after the 1.0 dose in the 90 min time bin.
3.2.2.2 METH: Body Temperature
Subcutaneous administration of METH changed body temperature in a dose-, time- and ambient-temperature- (TA) dependent manner (Figure 5a; bottom right). Analysis of temperature data confirmed main effects of METH dose (F(2,12) = 4.7, p < 0.05) and time after injection (F(6,36) = 21.6, p < 0.001), but not of their interaction (F(2,14) = 3.3, p = 0.06). Although there was no main effect of TA (F(1,6) = 0.5, p = 0.5), the analysis confirmed two-way interactions between TA and dose (F(2,12) = 8.6, p < 0.01) as well as TA and time post injection (F(4,22) = 7.1, p < 0.01); the three-way interaction was not significant (F(5,32) = 1.6, p = 0.2).
Post hoc comparisons confirmed that body temperature was higher after METH injections of 5.6 mg/kg, compared to vehicle (60- to150-min time bins) as well as compared to 1.0 mg/kg at the 90-min time bin (Figure 5a; middle). Post hoc delineation of the effect of dose as a function of TA (Figure 4c; left) confirmed that average body temperature significantly differed (5.6 > 1.0 > vehicle) under TA of 25±2 °C but did not vary as a function of drug condition at 20±2 °C TA. Post hoc delineation of the effect of TA as a function of dose (Figure 5c; middle) confirmed that body temperature was lower after vehicle injections in 25±2 °C TA compared to 20±2 °CTA, higher following 5.6 mg/kg METH under a TA of 25±2 °C compared to 20±2 °C and equivalent between TA conditions after 1.0 mg/kg.
Post hoc delineation of the effect of time post-injection as a function of METH dose (Figure 5a; bottom right) confirmed that body temperature was higher than pre-treatment baseline values after injections of 1.0 mg/kg (30- to120-min post-injection time bins) or of 5.6 mg/kg (60- to 90-min post-injection time bins) but unchanged after vehicle injection. Post hoc delineation of the effect of time as a function of TA (Figure 5c; right) confirmed significant increases in body temperature compared to the pre-injection baseline occurred earlier in the session under 20±2 °C TA (30- to 60-min post-injection) than under 25±2 °C TA (60- to 90-min post-injection).
3.2.3 MDPV vs. METH: Area under the curve (AUC) analyses for Locomotor Activity and Body Temperature
AUC measures (summed deviations from the mean of the vehicle condition) were calculated to compare the effects of MDPV and METH on locomotor activity and body temperature (Figure 5b). The AUC for activity was significantly higher for MDPV than METH for the 5.6 mg/kg dose but not for the 1.0 mg/kg dose. The AUC for body temperature was higher for METH than for MDPV following the 5.6 mg/kg dose but not for the 1.0 mg/kg dose.
3.3 Behavioral Observations after Administration of MDPV
3.3.1 Stereotypy after self-administration
The average cumulative dose of MDPV (mg/kg per one hour session) during the fixed-ratio dose-response testing phase of self-administration was larger when post-session stereotypy was observed (M = 1.4, SD = 0.5) than when it was not observed (M = 0.6, SD = 0.4) (Figure 6; left). Analysis of average cumulative dose confirmed a main effect of post-session stereotypy (F(1,9) = 18.4, p < 0.01).
Figure 6.
Stereotypy. Left Graph: Cumulative dose (mg/kg; N = 10) as a function of the observation of post-session stereotypy (repetitive behaviors: biting, licking and sniffing) after self-administration sessions (1 hour per session; FR5) (*p < 0.01). Right Graph: Stereotypy scores (range of 0–2; N=8) as a function of injection dose of MDPV and time from injection in 15 min time bins. For the effect of time, mean values that significantly different from the first observation period (immediately after injection) within dose are indicated by open symbols. For the effect of dose (mg/kg), mean values that significantly differed as a function of time are indicated by the following symbols: α= vehicle vs. 0.5; β = vehicle vs. 1.0; δ = vehicle vs. 3.2; ψ = vehicle vs. 5.6; ¢ = 0.5 vs. 1.0; $ = 0.5 vs. 3.2; € = 0.5 vs. 5.6; * = 1.0 vs. 3.2; # = 1.0 vs. 5.6; § = 3.2 vs. 5.6.Error bars represent SEM.
3.3.2 Stereotypy after non-contingent administration
Stereotypy scores increased in maximum values and remained above “0” (i.e., above vehicle scores) for longer periods of time, as the per-injection dose increased, following non-contingent administration of MDPV (Figure 6; right). Analysis of stereotypy scores confirmed a main effect of injection dose (F(4,28) =64.0, p < 0.001) and time from injection (F(4,26) = 38.1, p < 0.001) as well as an interaction between dose and time (F(15,106) = 6.8, p < 0.001).
For the effect of dose as a function of time from injection, in comparison to vehicle (wherein stereotypy was never observed) stereotypy scores were higher after injections of either 5.6 mg/kg or 3.2 mg/kg for the entire session, were higher after injections of 1.0 mg/kg for the first 60 min and were higher after injection of 0.5 mg/kg for only 15 min. Additionally, the scores were higher after injections of 5.6 mg/kg or 3.2 mg/kg than after injections of 1.0 mg/kg or 0.5 mg/kg for the entire session.
For the effect of time from injection as a function of dose, scores were higher than baseline (immediately post-injection wherein stereotypy was never observed) for the rest of the session after injections of 5.6 mg/kg and for most of the session (15–135-min bins) after injections of 3.2 mg/kg. Additionally, the scores were higher than baseline after injections of 1.0 mg/kg for the 1st 60 min of the session and after injections of 0.5 mg/kg for the 1st 15 min of the session.
3.4 Ki Determinations
Across the range of targets tested by the PDSP, significant affinities were only confirmed for the dopamine transporter (Ki = 8.8 nM; against positive control GBR12909 Ki = 3.1 nM), noradrenergic transporter (Ki = 166.5nM; positive control desipramine Ki = 2.1 nM), serotonin transporter (Ki = 1541.5 nM; positive control amitriptyline Ki = 8.7 nM) and sigma-1 receptor (Ki = 860 nM; positive control haloperidol Ki = 0.88 nM).
4. Discussion
These experiments show that MDPV supports stable intravenous self-administration (IVSA) in Wistar rats, resulting in consistent levels of drug intake from session to session (~1 mg/kg/hr) and high reward-lever selectivity (well in excess of 80% for most rats). The amount of MDPV self-administered was greater than that for METH (~0.5 mg/kg/hr) during acquisition however these differences were not confirmed in the statistical analysis. In the only other available MDPV IVSA report (Watterson et al., 2012), the intake rate was observed to be much higher (~5 mg/kg/hr) at the same per-infusion dose (0.05 mg/kg) after 10 acquisition sessions. Thus, as yet unexplored differences in these two methods may determine the capacity of MDPV to reinforce responding in IVSA. The two studies (Watterson vs. Aarde) differed in rat strain (Sprague-Dawley vs. Wistar), rat age (~8 vs. ~11 weeks old), rat weight (~250 vs. ~370 grams), session length (2 vs. 1 hour), response ratio (1 vs. 5), infusion volume (0.06 vs. 0.1 ml), infusion cue (light+tone vs. light) and the manner in which the animals were trained to lever press (single 16-hr session at FR1 for sucrose pellets vs. 4±1 progressive sessions from FR1–5 for grain pellets).
The dose-substitution studies showed that as the per-infusion dose of MDPV and METH was increased, the infusion rate decreased under a fixed-ratio (FR) schedule, the number of correct responses increased under a progressive-ratio (PR) schedule and the post-reinforcement pause increased under both schedules. However, compared with METH, the effect of MDPV on drug-reinforced behavior was of greater potency (more responding under lowest dose under fixed-ratio schedule) and greater efficacy (more responding under optimal dose under a progressive-ratio schedule) despite similar dose-response functions for post-reinforcement pause length. The similarity of the post-reinforcement pause lengths indicates, however, that the duration of the hedonic actions of these two drugs are similar. Nevertheless, since the overall amount of behavior put forth when MDPV was available was much greater than when METH was available, MDPV appears to have greater reward value. These findings contrast with the report of Watterson et. al. (2012) which found equal responding obtained for MDPV and METH at the one common dose (0.05 mg/kg) under a PR procedure; however, that dose was the only one reported for METH and the per-infusion dose was manipulated between subjects as opposed to within subjects as reported here. Other noteworthy differences in method between these two studies were (Watterson vs. Aarde) in the value of “j” (0.2 vs. 0.4), breakpoint definition (2 hours with no response vs. 1 hour inter-reward interval) and session time limit (16 vs. 7 hrs).
The locomotor stimulant effects of MDPV in this study were comparable to those of METH. Activity was maximally increased by lower doses to about 4-fold over the baseline and/or vehicle activity levels and was suppressed following higher doses; the high-dose suppressive effects on locomotion were replaced three hours after injection with additional increases in activity. The duration of action of MDPV was about 30 min shorter than that of METH since significant low-dose locomotor stimulation lasted 90 vs. 120 minutes post-injection and resolution of high-dose suppression appeared 120–150 versus 150–180 minutes post-injection. These findings are consistent with a prior report that MDPV and METH have similar effects on wheel activity (Huang et al., 2012).
The MDPV effect on temperature was modestly hyper-thermic when evaluated at normal laboratory TA of about 23°C; additional data from our lab (not shown) indicate that MDPV fails to significantly elevate rat body temperature even at TA of 30°C. Thus, MDPV is interpreted to be a less potent disruptor of thermoregulation compared with either METH or MDMA. However, this result does not appear to generalize across species as mice show hyperthermia when MDPV given in a warm environment (30 °C) at doses > 3.0 mg/kg i.p. (Fantegrossi et al., 2013).
A modest ambient temperature manipulation was included for determining body temperature data for METH because a prior report found that 5 mg/kg METH i.p. initially lowered telemetered body temperature (nadir ~60 min post-injection) then elevated body temperature (peak ~180 min post-injection) when administered at 20±2 °C in Sprague- Dawley rats (Myles et al., 2008) but in normal ambient temperature it elevates rodent body temperature across a range of doses in a manner independent of locomotor activity (Phelps et al., 2010). These prior observations are consistent with the current findings wherein low TA attenuated the magnitude of METH-induced hyperthermia in male Wistar rats but did not convert animals to a sustained hypothermic response.
The results for stereotyped behavior (dose-dependent observations of repetitive licking, biting and sniffing) indicate that MDPV is potentially psychotomimetic since it can produce stimulant-typical stereotypy (Fog and Randrup, 2002) and increase striatal dopamine levels (Fuwa et al., 2009). This finding is consistent with a prior report in Sprague-Dawley rats wherein stereotypies were reported for doses above 0.3 mg/kg, s.c. (Baumann et al., 2013), however no effects on locomotion were apparent, the dose-response function was linear. The sterotypy-inducing effect of MDPV appears to generalize across rodent species since mice have also been reported to exhibit stereotypies after doses above 1–3 mg/kg i.p. (Marusich et al., 2012).
The pharmacological data show that MDPV is a relatively selective monoamine transporter ligand with affinity for these transporters as follows: dopamine >> noradrenaline >> serotonin, consistent with reports from Simmler et al (2013) and Baumann et al (2013). A search of the UNC PDSP Ki database (Roth et al., 2000) confirms that for d-methamphetamine these relationships are as follows: noradrenaline > dopamine >> serotonin (Rothman et al., 2001; Rothman et al., 2003); METH also exhibits low affinity binding for alpha adrenergic receptors. These results are consistent with the interpretation that behavioral and thermoregulatory effects of both MDPV and METH are influenced primarily by indirect agonist action at the dopamine transporter.
5. Conclusions
MDPV is a classical psychomotor stimulant with in vivo activity that is similar to that of METH in rats. Both drugs support robust self-administration behavior, induce stereotypy following higher doses and increase locomotor activity. These data support a clear inference that the relatively novel stimulant drug of abuse, MDPV, poses a high risk of abuse liability. Moreover, the abuse liability of MDPV may be greater than that of METH since this novel substituted cathinone exhibited greater potency and efficacy on self-administration behavior.
Figure 1.
Route for MDPV synthesis
Highlights.
The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is underexplored in scientific studies.
MDPV is more potent and efficacious than d-methamphetamine (METH) in rat self-administration.
MDPV produces biphasic dose-dependent effects on locomotor activity, similar to those of METH.
Stereotyped behavior was observed after self-administration of over 1.5 mg/kg/hr of MDPV.
Acknowledgements
This work was supported by USPHS grants DA018418, DA024105 and DA024705; the NIH/NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors are grateful to Glen Dickinson for technical assistance. The MDPV was synthesized by Garry R. Smith, PhD at Fox Chase Chemical Diversity Center (Doylestown, PA) from routes designed by T.J.D. under contract from T.J.D. and M.A.T. Portions of this study were presented in preliminary form at scientific meetings (Aarde et al., 2012; Aarde et al., 2011). This is manuscript #21601 from The Scripps Research Institute.
Abbreviations
- MDMA
3,4-methylenedioxymethamphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- METH
d-methamphetamine
- DAT
Dopamine transporter
- FR
Fixed Ratio
- IVSA
Intravenous self-administration
- MELM
Mixed-effects linear model
- NET
Norepinephrine transporter
- PR
Progressive Ratio
- SERT
Serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors do not have any financial or other conflicts of interest to declare for this work.
References Cited
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addiction biology. 2013 doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang P-K, Creehan KM, Vaillancourt BD, Vandewater SA, Wright MJ, Miller ML, Taffe MA. Methylenedioxypyrovalerone (MDPV): Self-administration and acute drug challenges in rats. The FASEB Journal. 2012;26:1040–1045. [Google Scholar]
- Aarde SM, Wright JMJ, Buczynski MW, Angrish D, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Behavioral and Thermoregulatory Effects of Novel Cathinone Derivative Drugs 4-MMC and MDPV. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:S441. [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British journal of pharmacology. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive 'Bath Salts' Products. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie F, Hekman K, Cameron L, Wade DR, Smolinske S. Emergency department visits after use of a drug sold as "bath salts" --- michigan, november 13, 2010--march 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:624–627. [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience: A Practical Approach. New York, NY: Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Washington, DC: Institute of Laboratory Animal Resources, National Research Council; 1996. p. 125. [Google Scholar]
- Emmett-Oglesby MW, Lane JD. Tolerance to the reinforcing effects of cocaine. Behavioural pharmacology. 1992;3:193–200. [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo Effects of Abused 'Bath Salt' Constituent 3,4-methylenedioxypyrovalerone (MDPV) in Mice: Drug Discrimination, Thermoregulation, and Locomotor Activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog R, Randrup A. Commentary on: "Stereotyped activities produced by amphetamine in several animal species and man" Psychopharmacologia (1967) 11:300–310. A landmark publication from the early days of the dopamine hypothesis. Psychopharmacology. 2002;162:349–350. doi: 10.1007/s00213-002-1089-z. [DOI] [PubMed] [Google Scholar]
- Fuwa T, Kodama T, Honda Y, Tanaka T, Kubo Y, Ohashi N, Nakae D, Ogata A. Influence of Methylenedioxypyrovalerone on Central Nervous System Using Microdialysis Method. ChemoBio Integrated Management. 2009;5:62–72. [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behavioural pharmacology. 1996;7:526–531. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science (New York, NY) 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and alcohol dependence. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A comparison of the behavioral effects of l- and dl-cathinone and d-amphetamine. The Journal of pharmacology and experimental therapeutics. 1981;219:355–362. [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Posadas-Sanchez D, Johansen EB, Thrailkill EA. Progressive ratio schedules of reinforcement. Journal of Experimental Psychology Animal Behavior Processes. 2009;35:35–50. doi: 10.1037/a0012497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid C. Guidelines for Selecting the Covariance Structure in Mixed Model Analysis. Philadelphia, Pennsylvania: SAS Institute, Inc; 2005. [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in"bath salts" on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, Taffe MA. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug and alcohol dependence. 2013;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. A Methamphetamine Vaccine Attenuates Methamphetamine-Induced Disruptions in Thermoregulation and Activity in Rats. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles BJ, Jarrett LA, Broom SL, Speaker HA, Sabol KE. The effects of methamphetamine on core body temperature in the rat--part 1: chronic treatment and ambient temperature. Psychopharmacology. 2008;198:301–311. doi: 10.1007/s00213-007-1061-z. [DOI] [PubMed] [Google Scholar]
- Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: "Bath Salts". Gen Hosp Psychiatry. 2011;33:525–526. doi: 10.1016/j.genhosppsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Phelps G, Speaker HA, Sabol KE. Relationship between methamphetamine-induced behavioral activation and hyperthermia. Brain Res. 2010;1357:41–52. doi: 10.1016/j.brainres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roth BL, Lopez E, Patel S, Kroeze WK. The Multiplicity of Serotonin Receptors: Uselessly diverse molecules or an embarrasment of riches? The Neuroscientist. 2000;6:252–262. [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. The Journal of pharmacology and experimental therapeutics. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Behavioral studies on cathinone in monkeys and rats. NIDA Res Monogr. 1979;27:324–325. [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of"bath salts" and"legal highs" (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Wang LA, Goonewardene Z. The use of MIXED models in the analysis of animal experiments with repeated measures data. Canadian Journal of Animal Science. 2004;84:1–11. [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2012 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. Journal of the experimental analysis of behavior. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PloS one. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]