Abstract
Impaction allograft is an established method of securing initial stability of an implant in arthroplasty. Subsequent bone integration can be prolonged, and the volume of allograft may not be maintained. Intermittent administration of parathyroid hormone has an anabolic effect on bone and may therefore improve integration of an implant.
Using a canine implant model we tested the hypothesis that administration of parathyroid hormone may improve osseo-integration of implants surrounded by bone graft. In 20 dogs a cylindrical porous-coated titanium alloy implant was inserted into normal cancellous bone in the proximal humerus and surrounded by a circumferential gap of 2.5 mm. Morsellised allograft was impacted around the implant. Half of the animals were given daily injections of human parathyroid hormone (1-34) 5 μg/kg for four weeks and half received control injections. The two groups were compared by mechanical testing and histomorphometry. We observed a significant increase in new bone formation within the bone graft in the parathyroid hormone group. There were no significant differences in the volume of allograft, bone-implant contact or in the mechanical parameters.
These findings suggest that parathyroid hormone improves new bone formation in impacted morsellised allograft around an implant and retains the graft volume without significant resorption. Fixation of the implant was neither improved nor compromised at the final follow-up of four weeks.
Initial primary stability and subsequent bone integration is critical for long-term survival of uncemented total joint replacement.1 Although most implants are placed in healthy cancellous bone in press-fit, the presence of bone defects may represent a particular challenge to integration. Loss of bone stock in primary arthroplasties may be seen as subchondral peri-articular cysts while osteolysis and cavitary defects induced by loosening of the prosthesis may be present at revision surgery.2 The initial stability of the implant in these situations may be improved by impacting morsellised bone around it. Autograft is preferred as bone graft, but it is sometimes difficult to harvest sufficient bone at revision and donor site morbidity may be a problem. Allograft has been used successfully as an alternative.3,4 In an ideal situation the morsellised and impacted allograft gives structural support to the prosthesis while allowing cancellous osseo-integration and bone remodelling without compromising the initial mechanical stability. Although the graft is particulate in nature,5 early stability can be achieved.6 However, remodelling may only be partial and may take between six and 72 months.7-9 Ingrowth into the morsellised impacted graft may be delayed or limited to a few millimetres because of impaction.10,11
Parathyroid hormone (PTH) is anabolic to bone when administered intermittently as human (h) PTH(1-84) or PTH(1-34). It increases bone mass and the strength of intact remodelling cancellous and cortical bone.12,13 It is used for the prevention of fractures in patients with severe osteoporosis.14 It has also generated improvement in bone healing in fracture and distraction studies in animals,15-19 in spinal fusion20 and in the healing of structural bone grafts.21 Doses of 5 μg/kg to 200 μg/kg per day of PTH (1-34) increase the volume and formation of callus, bone mineral density (BMD) and the mechanical strength of long bone fractures in rodents. In higher primates, at a lower dosage, a similar effect is seen following femoral osteotomy, although less callus is seen.18
PTH has only recently been introduced as an adjuvant in experimental implant surgery. Treatment with PTH has shown improved peri-implant bone formation and fixation.22-29 Enhancement of allograft incorporation with PTH could improve the outcome of joint replacement surgery by enhancing new bone formation within the allograft and improving the mechanical properties.
We have examined whether PTH improves early integration and fixation of the implant by augmenting the healing of peri-implanted bone allograft. We tested the hypothesis that systemic, intermittent administration of hPTH(1-34) increases new bone formation in allograft, preserves allograft and increases the mechanical fixation of porous-coated implants when surrounded with impacted morsellised bone allograft.
Materials and Methods
We carried out a randomised, controlled study in 20 dogs, inserting a porous-coated titanium implant in the metaphyseal cancellous bone of the proximal humerus (Fig. 1). Each implant was surrounded by a 2.5 mm gap filled with impacted, morsellised allograft. Half of the animals were treated with intermittent hPTH(1-34) (Bachem Holding, Bubendorf, Switzerland) and the other half were given control injections of a PTH drug delivery vehicle. The study was approved by the Animal Care and Use Committee of the Minneapolis Medical Research Foundation. Surgery and observation was undertaken at the Animal Care facilities of the Research Foundation and the Hennepin County Medical Centre, Minnesota, according to the regulations of the National Institute of Health. Processing of the bone specimens, mechanical testing and histomorphomentry were undertaken at the Orthopaedic Research Laboratory of Aarhus University Hospital, Denmark. The animals were purpose-bred, skeletally mature, male American Hound Dogs (HRP Covance Research Products, Kalamazoo, Michigan), with a mean age of 13.8 months (11.6 to 20.0) and a mean weight of 24.8 kg (21.0 to 28.9). The animals were housed individually but socialisation took place daily with exercise groups in the large housing room.
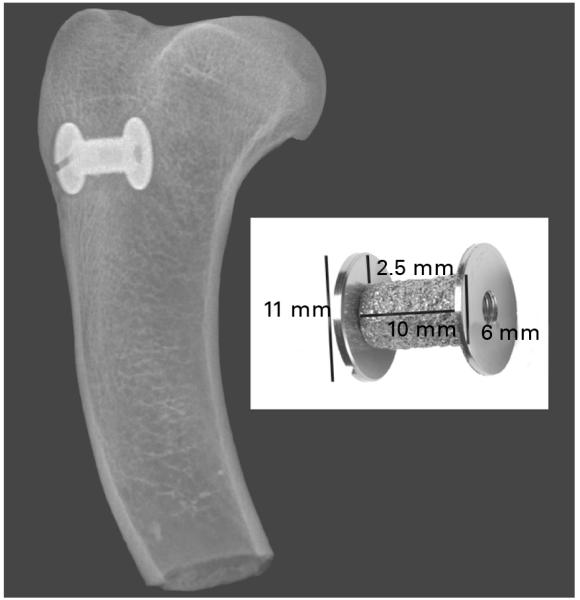
Fig. 1.
The implant lies in cancellous bone in the proximal humerus. The radiograph was taken at the time of bone harvest after four weeks treatment with parathyroid hormone or a control. The implant measures 10 mm × 6 mm and is surrounded by a 2.5 mm gap impacted with morsellised allograft bone secured by an 11 mm washer.
Implants
We used custom-made cylindrical porous-coated implants (Biomet Inc., Warsaw, Indiana) (Fig. 1). A plasma-sprayed titanium alloy porous-coated surface (Ti6Al4V ELI, ASTM F136) was superimposed on a titanium alloy core. The coating was applied in the same manner as for the clinical implants of the manufacturer. The mean diameter of the implants was 5.95 mm (5.85 to 6.13) and the length 10.94 mm (10.90 to 11.03). An end cap with a diameter of 11 mm centralised the implant in its bed, creating a 2.5 mm circumferential surrounding defect. Before sterilisation by autoclave, the implants were cleaned in an ultrasonic bath of trichlorethylene with final baths in alcohol. An additional four implants were randomly chosen and evaluated for quantitative topography (Somicronic Surfascan, 3CS; Hommel Somicronic, Saint-André-de-Corc, France) (The Danish Technological Institute, Copenhagen, Denmark). The implant roughness was assessed by measuring the Ra (the mean deviation from the mean line over a sampling length), the Rz (the mean height difference between the five highest peaks and the five lowest valleys), the Rq (the mean square root value of the profile departure) and the Rmax (maximum peak-to-valley height). The mean Ra = 66.3 μm (62.6 to 70.9), Rz = 322.5 μm (311.8 to 336.4), Rq = 80.0 μm (76.3 to 85.1), and Rmax = 322.5 μm (311.8 to 336.4).
Surgery
The operations were performed under general anaesthesia in sterile conditions with buprenorphine used to control post-operative pain. Ceftriaxon was given as a prophylactic antibiotic pre-operatively and for the next three days. A skin incision, 5 cm long, was made on the right lateral proximal humerus from the upper border of the greater tuberosity. Blunt dissection under the deltoid muscle exposed the periosteum. A 2.5 mm Kirschner guide-wire was inserted anterolateral and perpendicular to the surface at a distance of 1.7 mm from the tip of the greater tuberosity. The cylindrical implant bed was drilled with a cannulated 11 mm drill at a speed of less than 2 Hz and cooled with a sterile saline irrigation drip. The implant with the footplate was inserted and the bone graft impacted. A top washer was mounted onto the implant to contain the graft and maintain the concentricity of the gap. The soft tissue was closed in layers. All procedures were undertaken by the same surgeon (HD).
The dogs were allowed unrestricted weight-bearing post-operatively. The position of the implant was verified radiologically. Fluorochrome labelling of the mineralising bone was carried out on days 14 and 24 using 20 mg/kg per day tetracycline (Sigma, St. Louis, Missouri). Other unrelated non-graft implant studies on PTH hormone treatment were conducted at other bone sites in this set of test animals.
The bone graft
Cancellous allograft bone was harvested under sterile conditions from the two humeral heads of a single dog which was not included in the study. The preparation of graft for the whole study was undertaken in one sitting. Using a bone mill (L160, 3M, St. Paul, Minnesota) on a fine setting, bone from the donor was milled once and mixed before being divided into portions of 2 ml. The bone was kept frozen at −20°C. At operation the bone graft was thawed for one hour prior to use and inserted into the peri-implant gap with custom-made impaction tools. The amount of allograft in the peri-implant gap was evaluated by weight with a mean of 1.99 g (1.72 to 2.27). All pre- and per-operative handling of the allograft was carried out by the same surgeon (HD) in order to standardise impaction.
Administration of PTH
After operation the animals were randomly divided in two groups of ten. The intervention group was injected with hPTH(1-34) (Bachem, Bubendorf, Switzerland), 5 ?g/kg body-weight daily subcutaneously (with the exception of day 11, as explained below) between 8 am and 10 am, starting on the day after operation until completion of the four-week observation period. The animals were weighed once a week, and the doses were adjusted to body weight in increments of 0.5 kg. On day 11, injections were paused and hPTH(1-34) was changed from research grade to ‘GMP’ grade (Bachem, Torrance, California) with unaltered dosage due to concern as to the content of tri-fluoroacetic acid in the research grade. We were obliged to change the PTH because of ethical considerations over animal welfare. The control group was injected with a drug vehicle of the same volume. The PTH and drug vehicle were prepared in a sterile environment according to Andreassen et al17 using a vehicle of heat inactivated (56°C, 1h) 2% canine serum (S-1757, Sigma-Aldrich) in 0.9% NaCI adjusted to pH 5. The drugs for the whole study were prepared in one sitting and stored at −20°C.
Preparation of the specimens
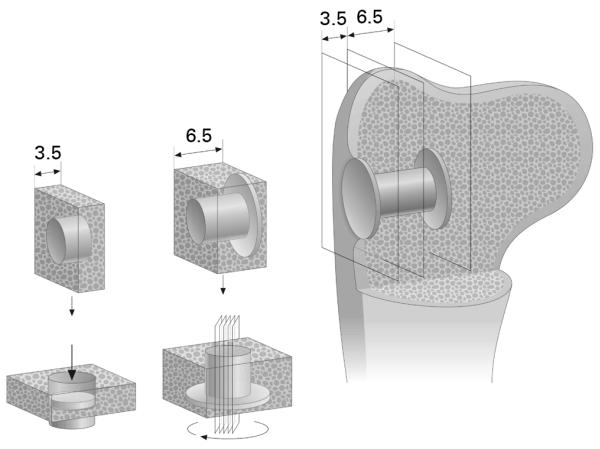
The animals were sedated then killed by a 10 ml injection of a solution of pentobarbital sodium and phenytoin sodium. Joint fluid from the shoulder was cultured and the bones with the implants in situ were removed. Preparation of the specimens was performed blinded as to the treatment group. A block of bone containing the implant and surrounding bone was cut from the proximal humerus (Fig. 2). After removing the top washer, two bone-implant sections were cut perpendicular to the long axis of each implant, using a water-cooled diamond band saw and an implant-based alignment post (Exact Apperatebau, Nordstedt, Germany). The inner 6.5 mm section of the implant was processed for undecalcified histomorphometric evaluation with the implant in situ and the outer 3.5 mm section stored at −20°C until assessed by mechanical testing.
Fig. 2.
Drawing showing the technique of sectioning. Implant in-situ in humerus metaphyseal bone. An outer section of 3.5 mm is taken for mechanical testing. The inner section of 6.5 mm is for histomorphometry with four serial sections around the centre of the implant after random rotation around its axis.
The specimens for histomorphometry were dehydrated in graded ethanol (70% to 100%) containing 0.4% basic fuchsin (Merck, Damstadt, Germany) and embedded in methyl-methacrylate (MMA, Merck, Hohenbruun, Germany). Four vertical random uniform sections were cut with a hard-tissue microtome (KDG-95, MeProTech, Heerhugoward, The Netherlands) around the centre part of each implant.30 Before making the sections parallel to the long axis of the implant, the specimen blocks were randomly rotated around the axis. The sections were cut parallel to this axis at the centre part of the implant, and serially with a separation distance of 400 ?m as the minimal space achieved due to the kerf of the saw.31 This technique provides highly reliable results with minimal bias.32 Previous studies have determined the specimen thickness to be 32 ?m to 41 ?m.22,29 After sectioning the specimens, the surface was counterstained with 2% light green (Light Green SF, BDH Laboratory Supplies, Poole, England). The staining technique made it possible to distinguish bone at the specimen surface (green) and fibrous tissue/marrow-like tissue (red). The depth of penetration of light green was determined to be 4 ?m to 10 ?m. This enabled definition of the superficial focus plane for tissue parameter sampling, reducing overestimation of tissue from deeper planes in the section.
Histological analysis
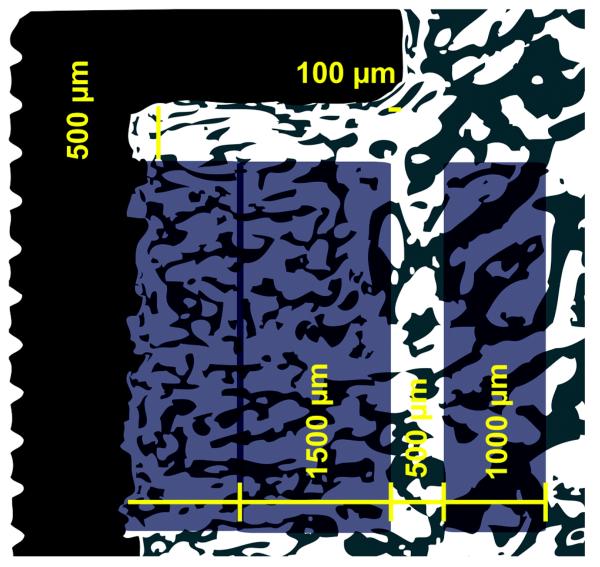
We quantified the area fractions of tissue in contact with the surface of the implant and the fractions of the volume of tissue around it. Analysis was computer-assisted and blinded in random specimen order using a light microscope (Olympus BX50; Olympus Optical, Tokyo, Japan) and stereologic image analysis system (newCast, Visiopharm Integrator System VIS Version 2.16.1.0, Visiopharm, Horsholm, Denmark). Regions were defined along the whole section by dividing the 2.5 mm gap into an outer region of 1500 ?m and an inner region reaching the implant (Fig. 3). A concentric peri-implant region of 1000 ?m defined host bone not subjected to implantation. Tissue ongrowth was defined as tissue directly at the implant surface and estimated by a line intercept technique with randomly disposed sine-weighted lines. Peri-implant tissue fractions were estimated using point counting with randomly disposed points. The test system was calibrated with a minimum of 100 line interceptions and points per region and meander sampling. The specimen preparation, stereological software and systematic uniform random sampling made it possible to obtain unbiased estimates, although anisotropy of cancellous bone exists.32
Fig. 3.
Histomorphometry – The region of interest (ROI) is defined on both sides of the implant. The ROIs are illustrated on one side of the implant above. Tissue ongrowth (surface fraction) at the surface of the implant, the tissue volume (volume fraction) in the grafted gap of 2.5 mm (divided into an outer region (1500 μm) and inner region reaching the surface of the implant) and tissue volume in region of intact non-implanted bone (1000 μm) are defined. The regions were defined from the end-washer margin as a fixed point with a 100 μm clearance at the gap-intact-bone-interface and 500 μm below the washer. ROI were defined at magnification × 1.25. Total assessment magnification × 30.3. For histomorphometric assessment, the corresponding magnifications were × 20 and × 402.
Each zone was evaluated independently by the same author (HD). Each specimen was divided into relevant zones, as shown in Figure 3. Each specimen was sampled in one session with one region after the other. Bone discrimination was based on light-microscopic morphology with polarised light and fluoroscopic confirmation. Discrimination was defined by a multiple of trabecular structure, the presence of osteocytes, and the shape, size and polarisation of the lacunae with scattering either randomly or on line. Bone ongrowth was expressed as the percentage of tissue cover of the implant surface (surface fraction) and the peri-implant tissue as the volume (volume fraction). Reproducibility was estimated by histomorphometric double measurements by the same author (HD) of 16 randomly selected specimens. The coefficient of variation for new bone/old bone at the interface for new bone/old bone was 8%/3% and for bone volume fraction was 4%/3%.
Mechanical testing
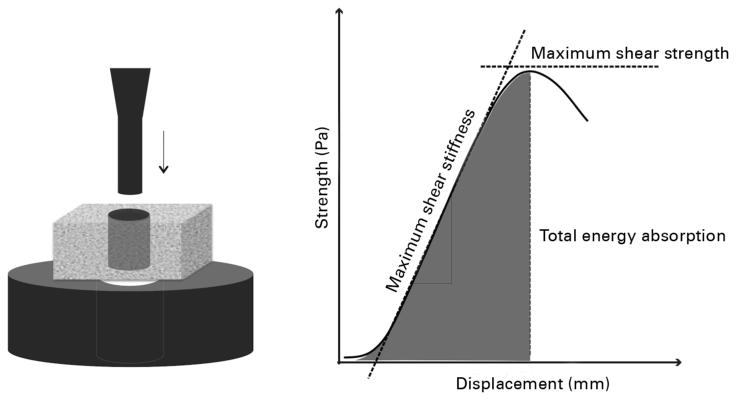
The implants were tested with an axial push out load to failure test (MTS 858 Mini Bionix, MTS System Corporation, Minneapolis, Minnesota) (Software MTS Test Star 790.00 Version 4.00) (Fig. 4). The testing was performed blinded in all specimens in one session. The specimens were placed on a metal support jig and the implant centred over a 7.4 mm circular opening assuring a 0.7 mm distance between the implant and support jig as recommended by Dhert et al.33 The piston diameter was 5.25 mm. A preload of 2 N was applied to standardise the contact position before initiating loading. A displacement velocity of 5 mm/min was used with a 10 kN load cell, and continuous load-displacement data were recorded. The direction of loading was from the external side of the bone inwards.
Fig. 4.
Drawing showing mechanical testing. An axial push-out test is performed with the specimen placed on a metal platform with a central opening. The specimen thickness = 3.5 mm, the implant diameter = 6 mm, the support hole diameter = 7.4 mm and the preload = 2 N. Displacement velocity is 5 mm/min. The load (Pa) displacement (mm) curve enables calculation of the ultimate shear strength (MPa), apparent shear stiffness (MPa/mm) and the total energy absorption (J/m2).
The maximum shear strength (MPa) was determined from the maximum force applied until failure of the bone-implant interface (Fig. 4). The maximum shear stiffness (MPa/mm) was obtained from the slope of the linear section of the load-displacement curve, and the total energy absorption (J/m2) calculated as the area under the load-displacement curve until failure. All push-out parameters were normalised by the cylindrical surface area of the transverse implant section. Determination of the reproducibility for a push-out test is not possible due to its destructive nature.
Statistical analysis
STATA statistical software (Stata 10.1, StataCorp, College Station, Texas) was used. Since data were not normally distributed, statistical analysis was non-parametric. A two-sample Wilcoxon rank-sum (Mann-Whitney) test was applied to assess the difference between treatment groups. Estimates are given as medians and inter-quartile ranges (IQRs), and p < 0.05 was considered statistically significant. A sample size calculation was performed prior to the study.
Results
After operation
All animals were fully weight-bearing the day after surgery, but two in the PTH group died on the sixth and eighth days after operation. Autopsy revealed ventricular hypertrophy and myositis in both animals. All other animals completed the observation period with no abnormal serum calcium levels or other complications. No clinical infections were observed. All bacterial cultures taken at death were negative.
Histology
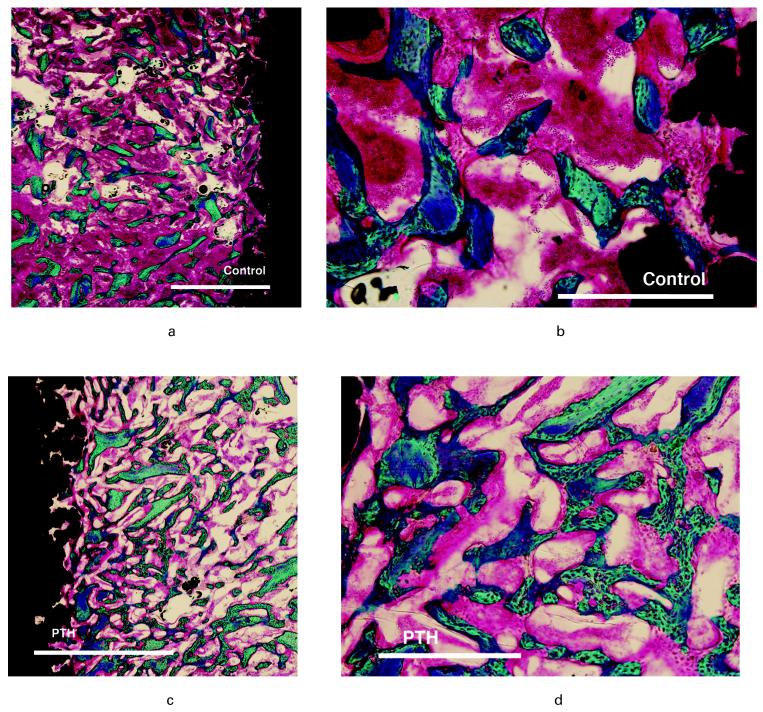
New bone and graft filled the gap in both the control and PTH groups (Fig. 5). A common observation in the PTH group was of allograft chips embedded in new bone with trabeculae bridging to neighbouring graft chips and thereby displaying high connectivity. Resorption lacunae in the allograft were sparse. At the interface, new bone dominated contact as an intervening bone layer between the graft and the surface of the implant. In the control group, new bone formation generally had a lesser degree of connectivity, and bridging trabeculae were sparse.
Fig. 5.
Photomicrographs of representative histological samples showing a-b) control and c-d) parathyroid hormone (PTH). Left images a-c) bar 2000 μm and right images b-d) bar 500 μm. Staining technique 0.4% basic fuchsin (red) and 2% light green (green = bone) and black = implant. PTH shows increase in bone in the morsellised impacted gap with numerous connective trabeculae of wo ven bone and elements of bone allograft. In the control group bone graft appears with sparse new bone.
Histomorphometry
There was a significant increase in the amount of bone in the gap in the PTH group (Table I). A 1.4-fold increase in new bone and 1.1- to 1.3-fold increase in total bone was seen. There was no significant difference in the amount of allograft in the parathyroid hormone group compared with the control (allograft in the inner group, p = 0.93; allograft in the outer group, p = 0.42). At the surface of the implant, PTH did not increase bone formation and the amount of allograft was unaffected.
Table I.
Histomorphometric results; fraction of new, old and total bone, marrow-like and fibrous tissues at implant surface (surface fraction), and in a concentric peri-implant gap (volume fraction) at 2.5 mm divided in an outer gap (1500 μm) and inner gap. n (control = 10, n (PTH) = 8. Mann-Whitney test (%, range.
| Total bone | New bone | Old bone | Marrow | Fibrous | |
|---|---|---|---|---|---|
| Implant interface | |||||
| Control | 12 (8 to 16) | 10 (7 to 15) | 1 (1 to 2) | 87 (84 to 92) | 0 (0 to 0) |
| PTH* | 12 (9 to 16) | 12 (8 to 14) | 1 (1 to 2) | 86 (83 to 88) | 0 (0 to 1) |
| Inner gap | |||||
| Control | 26 (23 to 30) | 14 (13 to 16) | 13 (9 to 17) | 73 (70 to 75) | 0 (0 to 0) |
| PTH | 33 (28 to 34) | 20 (16 to 22)† | 13 (11 to 15) | 67 (65 to 70) | 0 (0 to 0) |
| Outer gap | |||||
| Control | 26 (23 to 27) | 15 (14 to 16) | 11 (8 to 12) | 74 (73 to 77) | 0 (0 to 0) |
| PTH | 30 (27 to 32)‡ | 21 (17 to 23)‡ | 8 (7 to 12) | 70 (68 to 73) | 0 (0 to 0) |
PTH, parathyroid hormone
p = 0.07, PTH compared with control within region
p < 0.05
PTH did not improve the volume of the intact host bone as no differences were observed in the 1000 ?m circumferential region outside the original drill hole. The total median bone volume for PTH was 31% (IQR 27 to 36) and for the control 33% (IQR 22 to 38). The volume of marrow tissue for PTH was 69% (IQR 64 to 73) and for the control 67% (IQR 62 to 78). No fibrous tissue was observed in either treatment groups.
Mechanical testing
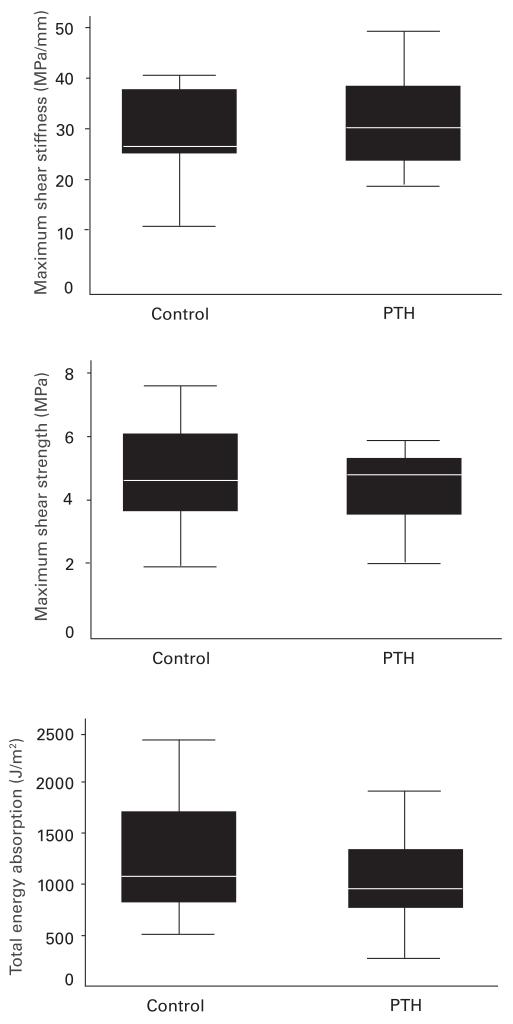
One specimen in the control group revealed incomplete exposure of the implant after sectioning and was excluded at the time of testing. We found no significant differences in the mechanical parameters of implants surrounded by allograft treated with parathyroid hormone or control (maximum shear strength, p = 0.63; total energy absorption, p = 0.50; maximum shear stiffness, p = 0.56) (Fig. 6).
Fig. 6.
Mechanical testing - Box plots showing results from mechanical testing push-out-to-failure. Maximum shear stiffness, maximum shear strength, and total energy absorption. n(control) = 9, n(PTH) = 8. Mann-Whitney test, p < 0.05 PTH compared to control (PTH, parathyroid hormone).
Discussion
We have investigated the effect of treatment with parathyroid hormone PTH(1-34) on the osseo-integration and mechanical fixation of implants impacted with morsellised allograft. We found that intermittent PTH (1-34) treatment increased formation of new bone within the allografted gap and increased total bone without significantly affecting the allograft amount. The mechanical implant fixation was unaltered. PTH did not influence bone at the bone-implant interface.
Although impaction allografting is a well established method for restoring acetabular34 and femoral bone loss during joint replacement, concerns exist regarding the remodelling of the allograft into viable bone tissue and whether the mechanical stability of the prosthetic implant is maintained during bone integration. Our experimental model was designed to reproduce the impaction allografting of the uncemented endoprosthesis in cancellous bone in humans.35 The implants were inserted in healthy metaphyseal cancellous bone in a well-defined closed osseous gap in which morsellised bone graft was impacted. The dog was chosen as the testing model because its bone structure most closely resembles human bone, although the effect of PTH in dogs is less pronounced than in smaller animals.36-38 Using only single sex (male) dogs reduced inter-individual biological variation in bone structure. The implants had a porous coating similar to that of implants in clinical use. In order to determine the degree of early fixation a period of four weeks was chosen as clinically relevant, based on previous implant studies in this model. The model was non-articular and was not directly loaded during walking thereby avoiding the effect of graft interlocking movement on implant loading.5,39 The anabolic effect of PTH is both dose and time dependent.17,18,40 We chose 5 ?g/kg body weight of hPTH(1-34), which is within the range of previous studies using similar animal models.12,13,41-43 Two test animals died and concern arose over the tri-fluoroacetic acid present in research grade PTH. As a precaution we changed from research grade PTH to GMP grade (same dosage) from day 11 and all animals completed the course uneventfully.
Our study demonstrated that PTH produced a significant increase in bone formation within the allografted defect around the implant, but with no improvement of the mechanical properties, as suggested in our hypothesis, which may be attributed to the insignificant formation of bone at the implant interface itself. Secure fixation of the implant is dependent on the formation of bone in the supportive bed and on the connection between bone and implant. Mechanical testing assesses the static bone adherent to the surface of the implant and the dynamic micro-interlocking of bone along the porous surface. Bone morphology at the implant surface revealed new bone, but allograft was less prevalent at the surface of the implant. When the allograft bordered on the interface, the level of resolution at sampling made it possible to discriminate the formation of new bone as a condensed intervening layer at the graft and implant interface. Our data imply that at this time point, PTH initially integrated bone in the impacted allograft around the implant and retained the graft volume without stimulating resorption. The mechanical properties at the implant were preserved without compromising fixation.
The effect of PTH on the fixation of implants has already been evaluated experimentally.22-29 In our previously published series, porous-coated implants inserted in normal cancellous bone improved fixation and the healing of peri-implant bone defects with PTH stimulation.22,29 When inserted as press-fit into canine bone the formative effect is more subtle because it is less dependent on bone regeneration around the implant.22,40 Substantial integration is seen in rats with transcortical insertion of screws in long bones and in bone of low density.23-28,44 No studies have evaluated the effect of PTH on implant integration with impacted morsellised allograft.
We found a 1.4-fold increase in new bone formation in the allograft around the implant of animals treated with PTH compared with controls which is less than we found in a companion study in a similar model of implants inserted into empty bone defects.29 This may be related to the efficacy of the dosage regime when allografting is used or not used, but only one dose was included in this study and further investigation is needed. Also, PTH may have been inhibited from exerting its biological activity owing to the impacted nature of the allograft. In an open gap the PTH in the bed of implant bone has immediate access to blood supply and osteoprogenitor cells, which are necessary for the differentiation of bone cells.45 Within impacted allograft, the migration of cells into the graft may be compromised and vascularisation delayed. In bone conduction chamber studies in rats, a reduction of bone ingrowth into the graft due to impaction alone was seen at six weeks after impaction.46 In a goat study on HA-coated implants inserted into the femoral canal, vascularisation in impacted grafts was first seen after six weeks. Similar findings have been demonstrated in human studies of retrieved or biopsied impacted grafts at arthroplasty.47,48 A study on open fractures by Tägil et al,49 although not directly comparable with impacted and morsellised allograft, showed that PTH did not increase the rate of union of bone defects induced by fractures. This was ascribed to inherent damage of blood supply and was in contrast to the previous reports in closed fractures.
The mechanisms by which PTH enhances healing and incorporation of graft are complex. Studies indicate that PTH stimulates the proliferation and differentiation of osteogenic cells, increases the number of osteoblasts, collagen I synthesis, and the rate of apposition of mineral.50,51 Observations on the genesis of osteoclasts during the remodelling phase of repair are diverse.15,45,52 The influence of PTH on resorption is in part influenced by changes in the ratio of Receptor Activator for Nuclear Factor κ B Ligand to osteoprotegerin (RANKL/OPG ratio).8 Up-regulating RANKL induces resorption of bone, whereas OPG causes a decrease.53 In intact bone, intermittent administration of PTH initially causes bone formation as demonstrated by the increase in bone formative markers. Subsequently, an increase in the RANKL/OPG ratio is seen which leads to an increase in bone resorption and in the markers of resorption, although the overall effect remains anabolic. Bone resorption with concurrent formation has been observed in models of bone healing. In a model of spinal arthrodesis in the rat using autograft without an implant, the bone volume improved and no grafted elements were observed in the fusion mass four weeks after administering PTH(1-34) 40 μg/kg/day.52 The study supports our aim of using PTH to encourage bone formation in a bone graft environment. However, in our study the PTH group compared with the control showed no significant differences in graft volume at this time point. This may be attributed to a different animal model and the use of a graft in a spinal fusion in an open space with inherent mobility, as compared to our closed osseous gap around an implant.54 A more important factor may be the differing PTH potency in time and magnitude within animal models,44 and within the bone site inter- and intraskeletally. In our implant model PTH initially integrates bone in the allograft around the implant and retains the graft volume without it being significantly resorbed and thereby impaired. A further study is required to investigate longer periods of observation of the integration of impacted morsellised allograft around an implant. Adjuvant therapy with parathyroid hormone shows promise in joint replacement surgery with morselised bone graft. Stabilising the implant and allowing implant-bone-healing at the same time are one of the more challenging situations employing bone grafting in arthroplasties. Our study determined that PTH treatment compared to control integrates bone in the allograft around an implant, and maintains the graft volume without compromising implant fixation. Bearing in mind the interspecies variation in the bone anabolic response to PTH, adjuvant therapy with PTH may be considered in human arthroplasties with impaction allografting.
Acknowledgments
The authors would like to acknowledge funding for this study received from the E. Horslev Foundation, Foundation 1870, Hede Nielsen Foundation, Velux Foundation, NIH (AR 42051).The authors would also like to thank A. Lamberg and Histomorphometry technicians J. Pauli and A. Milton.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributor Information
H. Daugaard, Department of Orthopaedic Surgery, Orthopaedic Research Laboratory, Aarhus University Hospital, Norrebrogade 44, Building 1A, DK-8000 Aarhus C, Denmark.
B. Elmengaard, Department of Orthopaedic Surgery, Orthopaedic Research Laboratory, Aarhus University Hospital, Norrebrogade 44, Building 1A, DK-8000 Aarhus C, Denmark.
T. T. Andreassen, Institute of Anatomy, The Faculty of Health Sciences, Aarhus University, Wilhelm Meyers Allé, Building 1231, DK-8000 Aarhus C, Denmark.
J. Baas, Department of Orthopaedic Surgery, Orthopaedic Research Laboratory, Aarhus University Hospital, Norrebrogade 44, Building 1A, DK-8000 Aarhus C, Denmark.
J. E. Bechtold, Orthopaedic Biomechanics Laboratory, Excelen Center for Bone and Joint Research and Minneapolis Medical Research Foundation, 914 South Eighth Street, Minneapolis, Minnesota 55404, USA.
K. Søballe, Department of Orthopaedic Surgery, Orthopaedic Research Laboratory, Aarhus University Hospital, Norrebrogade 44, Building 1A, DK-8000 Aarhus C, Denmark.
References
- 1.Kärrholm J, Borssén B, Löwenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter?: 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg [Br] 1994;76-B:912–17. [PubMed] [Google Scholar]
- 2.Rubash HE, Sinha RK, Shanbhag AS, Kim SY. Pathogenesis of bone loss after total hip arthroplasty. Orthop Clin North Am. 1998;29:173–86. doi: 10.1016/s0030-5898(05)70316-3. [DOI] [PubMed] [Google Scholar]
- 3.Gie GA, Linder L, Ling RS, et al. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg [Br] 1993;75-B:14–21. doi: 10.1302/0301-620X.75B1.8421012. [DOI] [PubMed] [Google Scholar]
- 4.Babis GC, Sakellariou VI, O’Connor MI, Hanssen AD, Sim FH. Proximal femoral allograft-prosthesis composites in revision hip replacement: a 12-year follow-up study. J Bone Joint Surg [Br] 2010;92-B:349–55. doi: 10.1302/0301-620X.92B3.23112. [DOI] [PubMed] [Google Scholar]
- 5.Brodt MD, Swan CC, Brown TD. Mechanical behavior of human morselized cancellous bone in triaxial compression testing. J Orthop Res. 1998;16:43–9. doi: 10.1002/jor.1100160108. [DOI] [PubMed] [Google Scholar]
- 6.Malkani AL, Voor MJ, Fee KA, Bates CS. Femoral component revision using impacted morsellised cancellous graft: a biomechanical study of implant stability. J Bone Joint Surg [Br] 1996;78-B:973–8. doi: 10.1302/0301-620x78b6.1288. [DOI] [PubMed] [Google Scholar]
- 7.Nelissen RG, Bauer TW, Weidenhielm LR, LeGolvan DP, Mikhail WE. Revision hip arthroplasty with the use of cement and impaction grafting: histological analysis of four cases. J Bone Joint Surg [Am] 1995;77-A:412–22. doi: 10.2106/00004623-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Ling RS, Timperley AJ, Linder L. Histology of cancellous impaction grafting in the femur: a case report. J Bone Joint Surg [Br] 1993;75-B:593–6. doi: 10.1302/0301-620X.75B5.8376422. [DOI] [PubMed] [Google Scholar]
- 9.Whiteside LA, Bicalho PS. Radiologic and histologic analysis of morselized allograft in revision total knee replacement. Clin Orthop. 1998;357:149–56. doi: 10.1097/00003086-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Heekin RD, Engh CA, Vinh T. Morselized allograft in acetabular reconstruction: a postmortem retrieval analysis. Clin Orthop. 1995;319:184–90. [PubMed] [Google Scholar]
- 11.Tägil M. The morselized and impacted bone graft: animal experiments on proteins, impaction and load. Acta Orthop Scand Suppl. 2000;290:1–40. [PubMed] [Google Scholar]
- 12.Jerome CP, Burr DB, Van Bibber T, Hock JM, Brommage R. Treatment with human parathyroid hormone (1-34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis) Bone. 2001;28:150–9. doi: 10.1016/s8756-3282(00)00430-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Takahashi HE, Inoue J, et al. Effects of intermittent administration of low dose human PTH(1-34) on cancellous and cortical bone of lumbar vertebral bodies in adult beagles. Bone. 1997;21:501–6. doi: 10.1016/s8756-3282(97)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 15.Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg [Am] 2005;87-A:731–41. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 16.Komatsubara S, Mori S, Mashiba T, et al. Human parathyroid hormone (1-34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone. 2005;36:678–87. doi: 10.1016/j.bone.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960–8. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 18.Manabe T, Mori S, Mashiba T, et al. Human parathyroid hormone (1-34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007;40:1475–82. doi: 10.1016/j.bone.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Seebach C, Skripitz R, Andreassen TT, Aspenberg P. Intermittent parathyroid hormone (1-34) enhances mechanical strength and density of new bone after distraction osteogenesis in rats. J Orthop Res. 2004;22:472–8. doi: 10.1016/j.orthres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 20.O’Loughlin PF, Cunningham ME, Bukata SV, et al. Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine. 2009;34:121–30. doi: 10.1097/BRS.0b013e318191e687. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Shigetomi M, Ohno T, et al. Sequential treatment with intermittent low-dose human parathyroid hormone (1-34) and bisphosphonate enhances large-size skeletal reconstruction by vascularized bone transplantation. Calcif Tissue Int. 2007;81:232–9. doi: 10.1007/s00223-007-9056-7. [DOI] [PubMed] [Google Scholar]
- 22.Daugaard H, Elmengaard B, Lamberg A, Bechtold JE, Soballe K. Osseointegration of implants inserted pressfit with parathyroid hormone as adjuvant therapy; 10th EFORT Conference; 2009. [Google Scholar]
- 23.Shirota T, Tashiro M, Ohno K, Yamaguchi A. Effect of intermittent parathyroid hormone (1-34) treatment on the bone response after placement of titanium implants into the tibia of ovariectomized rats. J Oral Maxillofac Surg. 2003;61:471–80. doi: 10.1053/joms.2003.50093. [DOI] [PubMed] [Google Scholar]
- 24.Gabet Y, Müller R, Levy J, et al. Parathyroid hormone 1-34 enhances titanium implant anchorage in low-density trabecular bone: a correlative micro-computed tomographic and biomechanical analysis. Bone. 2006;39:276–82. doi: 10.1016/j.bone.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg [Br] 2001;83-B:437–40. doi: 10.1302/0301-620x.83b3.10256. [DOI] [PubMed] [Google Scholar]
- 26.Skripitz R, Aspenberg P. Early effect of parathyroid hormone (1-34) on implant fixation. Clin Orthop. 2001;392:427–32. doi: 10.1097/00003086-200111000-00056. [DOI] [PubMed] [Google Scholar]
- 27.Mair B, Tangl S, Feierfeil J, et al. Age-related efficacy of parathyroid hormone on osseointegration in the rat. Clin Oral Implants Res. 2009;20:400–5. doi: 10.1111/j.1600-0501.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohkawa Y, Tokunaga K, Endo N. Intermittent administration of human parathyroid hormone (1-34) increases new bone formation on the interface of hydroxyapatite-coated titanium rods implanted into ovariectomized rat femora. J Orthop Sci. 2008;13:533–42. doi: 10.1007/s00776-008-1275-x. [DOI] [PubMed] [Google Scholar]
- 29.Daugaard H, Elmengaard B, Lamberg A, Bechtold JE, Søballe K. The effect of parathyroid hormone on bone ongrowth of implants with a peri-implant gap: a canine study. Nordic Orthopaedic Federation; 54th Conference; Amsterdam, The Netherlands. 2008. [Google Scholar]
- 30.Overgaard S, Søballe K, Jørgen H, Gundersen G. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res. 2000;18:313–21. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 31.Gotfredsen K, Budtz-Jørgensen E, Jensen LN. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol. 1989;64:121–7. doi: 10.3109/10520298909106984. [DOI] [PubMed] [Google Scholar]
- 32.Baas J. Adjuvant therapies of bone graft around non-cemented experimental orthopedic implants stereological methods and experiments in dogs. Acta Orthop Scand. 2008;79:1–43. [PubMed] [Google Scholar]
- 33.Dhert WJ, Verheyen CC, Braak LH, et al. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26:119–30. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 34.Emms NW, Buckley SC, Stockley I, Hamer AJ, Kerry RM. Mid- to long-term results of irradiated allograft in acetabular reconstruction: a follow-up report. J Bone Joint Surg [Br] 2009;91-B:1419–23. doi: 10.1302/0301-620X.91B11.22274. [DOI] [PubMed] [Google Scholar]
- 35.Søballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bünger C. Bone graft incorporation around titanium-alloy- and hydroxyapatite-coated implants in dogs. Clin Orthop. 1992;274:282–93. [PubMed] [Google Scholar]
- 36.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–70. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 37.Eitel F, Klapp F, Jacobson W, Schweiberer L. Bone regeneration in animals and in man: a contribution to understanding the relative value of animal experiments to human pathophysiology. Arch Orthop Trauma Surg. 1981;99:59–64. doi: 10.1007/BF00400911. [DOI] [PubMed] [Google Scholar]
- 38.Reeve J. PTH: a future role in the management of osteoporosis? J Bone Miner Res. 1996;11:440–5. doi: 10.1002/jbmr.5650110404. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge JD, Smith EJ, Hubble MJ, Whitehouse SL, Learmonth ID. Massive early subsidence following femoral impaction grafting. J Arthroplasty. 1997;12:535–40. doi: 10.1016/s0883-5403(97)90176-5. [DOI] [PubMed] [Google Scholar]
- 40.Skripitz R, Andreassen TT, Aspenberg P. Strong effect of PTH (1-34) on regenerating bone: a time sequence study in rats. Acta Orthop Scand. 2000;71:619–24. doi: 10.1080/000164700317362271. [DOI] [PubMed] [Google Scholar]
- 41.Inoue J, Takahashi H, Konno T. Histomorphometric evaluation on ilium of beagle dogs with long term administration of low dose 1-34 HPTH. Bone. 1985;6:401. [Google Scholar]
- 42.Boyce RW, Paddock CL, Franks AF, Jankowsky ML, Eriksen EF. Effects of intermittent hPTH(1-34) alone and in combination with 1,25(OH)(2)D(3) or risedronate on endosteal bone remodeling in canine cancellous and cortical bone. J Bone Miner Res. 1996;11:600–13. doi: 10.1002/jbmr.5650110508. [DOI] [PubMed] [Google Scholar]
- 43.Ma YF, Chen YY, Ijiri K, et al. PTH in combination with risedronate in aged beagle dogs resulted in bone balance similar to PTH alone. Bone. 1997;20(Suppl):99. [Google Scholar]
- 44.Tashjian AH, Jr, Chabner BA. Commentary on clinical safety of recombinant human parathyroid hormone 1-34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res. 2002;17:1151–61. doi: 10.1359/jbmr.2002.17.7.1151. [DOI] [PubMed] [Google Scholar]
- 45.Nishida S, Yamaguchi A, Tanizawa T, et al. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone. 1994;15:717–23. doi: 10.1016/8756-3282(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 46.Tägil M, Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop. 1998;352:231–8. [PubMed] [Google Scholar]
- 47.Buma P, Lamerigts N, Schreurs BW, et al. Impacted graft incorporation after cemented acetabular revision: histological evaluation in 8 patients. Acta Orthop Scand. 1996;67:536–40. doi: 10.3109/17453679608997751. [DOI] [PubMed] [Google Scholar]
- 48.Ullmark G, Linder L. Histology of the femur after cancellous impaction grafting using a Charnley prosthesis. Arch Orthop Trauma Surg. 1998;117:170–2. doi: 10.1007/s004020050221. [DOI] [PubMed] [Google Scholar]
- 49.Tägil M, Godfrey C, Little D, et al. NMP-7, bisphosphonates and PTH in a rat open fracture model: fundamental differences in their L effects on union and strength. Calcif Tissue Int. 2007;80(Suppl):87. [Google Scholar]
- 50.Pettway GJ, Meganck JA, Koh AJ, et al. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806–18. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abe Y, Takahata M, Ito M, et al. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1-34) in a rat spinal arthrodesis model. Bone. 2007;41:775–85. doi: 10.1016/j.bone.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama T, Saxon LK, Zaman G, et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43:238–48. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]