Abstract
The essential amino acid tryptophan is not only a precursor of serotonin but is also degraded to several other neuroactive compounds, including kynurenic acid, 3-hydroxykynurenine and quinolinic acid. The synthesis of these metabolites is regulated by an enzymatic cascade, known as the kynurenine pathway, that is tightly controlled by the immune system. Dysregulations of the pathway, causing hyper- or hypofunction of active metabolites, are associated with neurodegenerative and other neurological disorders, as well as psychiatric diseases such as depression and schizophrenia. With recently developed pharmacological agents, it is now possible to restore metabolic equilibrium and envisage novel therapeutic interventions.
The discovery of kynurenic acid (KYNA) more than 150 years ago 1 and the subsequent elucidation of its biosynthesis and chemical structure initiated a series of discoveries that turned out to have significant implications for the neurosciences. KYNA was the first of the "kynurenines", a group of metabolically related compounds derived from the essential amino acid tryptophan. Biochemical studies during the first part of the 20th century elaborated the enzymatic steps linking the individual members of the kynurenine pathway (Fig. 1), which was found to account for >90% of peripheral tryptophan metabolism in mammals 2. The importance of the pathway was long thought to stem mainly from the fact that it is a source of the coenzyme NAD+, which plays a critical role in many fundamental biological processes, including redox reactions required for mitochondrial function.
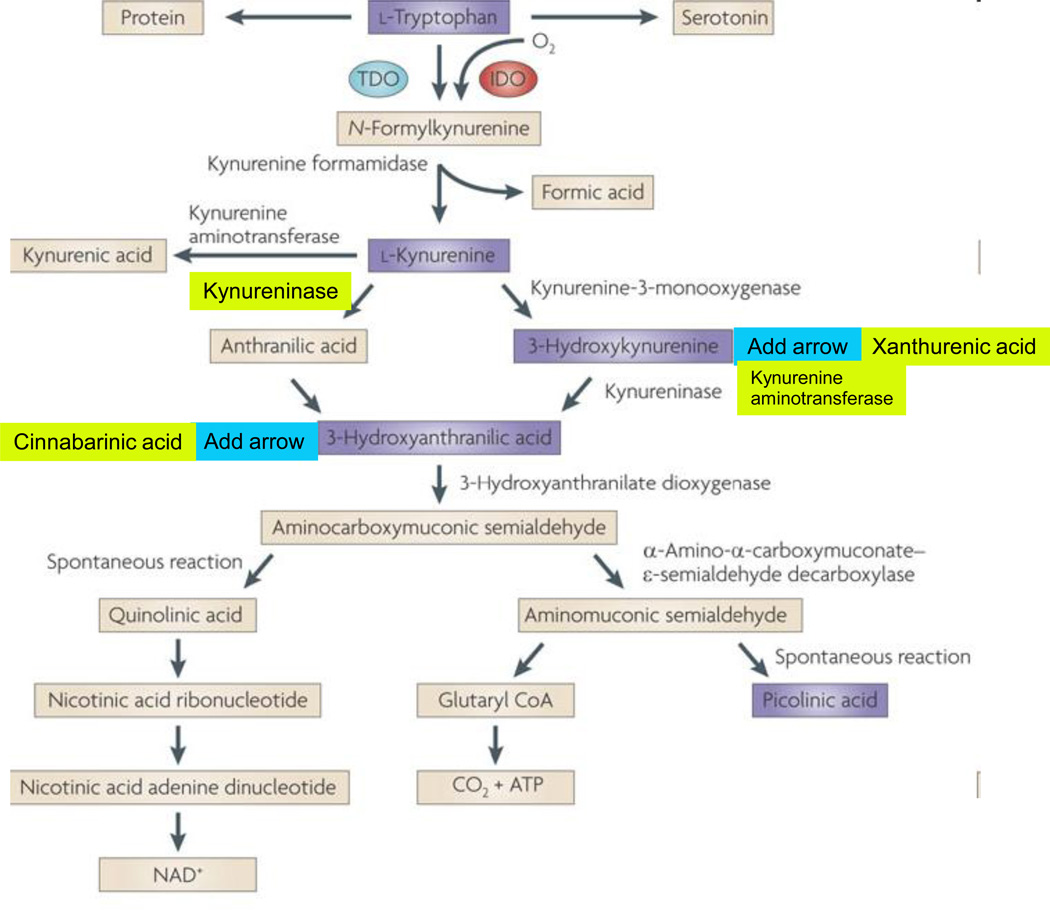
Figure 1. The kynurenine pathway of tryptophan degradation in mammals.
The kynurenine pathway is initiated by the transformation of tryptophan to N-formylkynurenine by indoleamine 2,3-dioxygenases (IDO) 1 186 and 2 187, and tryptophan 2,3-dioxygenase (TDO) 188. N-formylkynurenine is degraded by formamidase to yield the pivotal metabolite kynurenine. Compared to peripheral organs, the activities of these enzymes in the brain are normally low. Kynurenine undergoes irreversible transamination to form kynurenic acid (KYNA). Four kynurenine aminotransferases (KATs) have so far been shown to catalyze this reaction 189. In the mammalian brain, KAT II is thought to be the main biosynthetic enzyme of KYNA as it does not recognize competing abundant amino acids as substrates 190. No degradative enzyme of KYNA has yet been described in the mammalian brain.
A second branch of the kynurenine pathway, competing with KYNA synthesis by KATs, is initiated by kynurenine 3-monooxygenase (KMO) and kynureninase, which catalyze the degradation of kynurenine to 3-hydroxykynurenine (3-HK) and anthranilic acid, respectively 177. The in vivo disposition of kynurenine in the brain depends on the cellular localization, intracellular compartmentalization and kinetic characteristics of these two degradative enzymes, as well as KATs 41, 57. Like kynurenine, 3-HK serves as a substrate of competing enzymes: kynureninase and KAT II. Mammalian kynureninase preferentially recognizes 3-HK over kynurenine, catalyzing the formation of 3-HANA 191. Compared to peripheral organs and for unknown reasons, anthranilic acid is a far better precursor of 3-HANA than 3-HK within the brain 30. KAT II, and possibly other KATs, convert 3-HK to xanthurenic acid, which, like KYNA, appears to constitute a dead-end product of the kynurenine pathway.
In addition to being oxidized non-enzymatically to cinnabarinic acid, 3-HANA is the substrate of 3-hydroxyanthranilic acid 3,4-dioxygenase (3HAO), which is present in relative abundance in the brain 192, forming α-amino-α-carboxymuconic-ω-semialdehyde. The kynurenine pathway branches then branches into two arms. Under physiological conditions, α-amino-α-carboxymuconic-ω-semialdehyde spontaneously rearranges to form QUIN with a half-life of approximately 20 minutes. QUIN synthesis may therefore be regulated both at the level of 3HAO (for example by Fe2+ 193) and by changes in the intracellular milieu (pH, temperature) to influence the second, non-enzymatic step. Notably, the brain seems to contain very little α-amino-α-carboxymuconic-ω-semialdehyde carboxylase, an enzyme which deflects the metabolic cascade towards the production of picolinic and glutaric acids 194. The cerebral activity of QUIN's degradative enzyme, quinolinate phosphoribosyltransferase, is very low 195, making this enzyme one of the gatekeepers for the synthesis of NAD+ 196–197.
Numerous studies since the 1970s have demonstrated that kynurenines can influence brain function. The past years have witnessed a surge in new information regarding the roles of kynurenine pathway metabolites not only in brain physiology but also as potential causative factors in several devastating brain diseases. Here we first describe the properties of neuroactive kynurenines, their metabolism in the brain, and the communication between the peripheral and the central kynurenine pathways. Using selected examples and focusing largely on in vivo evidence, we then explain how fluctuations in kynurenine pathway metabolites can lead to the deterioration of physiological processes and the emergence of pathological states. Finally, we briefly review recent advances in drug discovery, which suggest exciting clinical applications of agents that are designed specifically to restore equilibrium in the cerebral kynurenine pathway.
Neuroactive kynurenines
Kynurenic acid
KYNA is a competitive, broad spectrum antagonist of glutamate receptors, inhibiting all three ionotropic excitatory amino acid receptors — NMDA, kainate and AMPA receptors — to approximately the same degree at high micromolar concentrations 3. KYNA has a greater affinity for the obligatory glycine co-agonist site of the NMDA receptor, so that this “glycineB“ receptor constitutes a preferred molecular target 4. Because of the competitive nature of KYNA’s action at this site, selective glycineB receptor antagonists can substitute for KYNA 5; and agonists can counter the actions of KYNA 6–7.
During the past few years, additional targets of KYNA have been identified. Only one of them, the α7 nicotinic acetylcholine receptor (α7nAChR), has so far been verified as a bona fide KYNA receptor in the brain 8–10, whereas the role of others, such as the former orphan G-protein coupled receptor GPR35 11 and the aryl hydrocarbon receptor 12, remains to be elaborated. Notably, the inhibitory action of KYNA at the α7nAChR is non-competitive in nature, and KYNA’s effector site on the α7nAChR overlaps with the allosteric potentiating site that can be occupied by the cognition-enhancing drug galantamine 13. Independent of its actions at receptors, KYNA also has antioxidant properties, which are related to the compound’s ability to scavenge hydroxyl, superoxide anion and other free radicals 14–15.
Kynurenine, 3-hydroxykynurenine and downstream kynurenine pathway metabolites
Electrophysiological investigations of other kynurenine pathway metabolites, including kynurenine, 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HANA) and anthranilic acid (Fig. 1), have failed to reveal direct effects on neuronal activity 16. In fact, there are only a few examples, such as the recently described action of kynurenine as an in vivo ligand of the human aryl hydrocarbon receptor 17, and the activation of metabotropic glutamate receptors by xanthurenic acid 18 and 3-HANA’s oxidation product cinnabarinic acid 19, of direct effects of these compounds on specific receptors within or outside the brain. However, several kynurenine pathway metabolites probably participate in complex pro- and anti-oxidative processes in the brain 20. 3-HK and 3-HANA, in particular, auto-oxidize readily under physiological conditions, producing H2O2 and highly reactive hydroxyl radicals in the process 21. But these properties are balanced by the related antioxidant capacity of these compounds, as well as xanthurenic acid, to scavenge peroxyl radicals 22.
Quinolinic acid
The demonstration that an intracerebroventricular injection of quinolinic acid (QUIN) causes seizure activity in mice 23 provided the first evidence that kynurenine pathway metabolites can have central effects. QUIN’s excitatory properties are due to its direct, selective stimulation of NMDA receptors, as originally demonstrated using selective NMDA receptor antagonists 24. Although its potency as an excitant is relatively low (ED50: >100 µM), QUIN is remarkable for its preference for NMDA receptors in the forebrain. This regional selectivity is due to the fact that QUIN has a >10-fold lower affinity to the hindbrain-specific NR2C subunit of the NMDA receptor compared to the NR2B subunit, which predominates in the forebrain 25. Differential subunit specificity notwithstanding, NMDA receptor activation seems to be obligatory for QUIN function as all neurobiological actions of QUIN described to date can be blocked by NMDA receptor antagonists.
QUIN also potently stimulates lipid peroxidation 26, and this effect is strictly dependent on the presence of Fe2+ ions 27. The generation of reactive oxygen species by QUIN is secondary to the formation and autooxidation of Fe2+-QUIN complexes and can be readily prevented by iron chelation 28. Moreover, endogenous iron also directly controls the binding of QUIN to the NMDA receptor29.
Metabolism and regulation of brain kynurenines
Formed by a sequence of mostly glial enzymes (Fig. 1), kynurenine pathway metabolites are normally present in the mammalian brain in concentrations in the nanomolar to low micromolar range 30–35. With only one noteworthy exception — the high levels of KYNA in the human brain, which possibly signify exceptional neuroactive properties 36 — species differences are relatively minor, although brain concentrations differ with age and between brain regions. The brain activities of the two tryptophan-degrading enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) are normally quite low 37, and approximately 60% of metabolism along the kynurenine pathway in the brain is initiated by the pivotal metabolite kynurenine 38, which readily enters the brain from the circulation (see below) and is taken up by glial cells 39. Intracerebral application of kynurenine in rats has revealed that approximately equal amounts of the primary degradation products 3-HK and KYNA are acutely produced from kynurenine under physiological conditions 40. Notably, the synthesis of 3-HK and further downstream kynurenine pathway metabolites occurs in microglia, whereas KYNA is formed in astrocytes 41. In other words, the two main arms of the kynurenine pathway are physically segregated in the brain (Fig. 2).
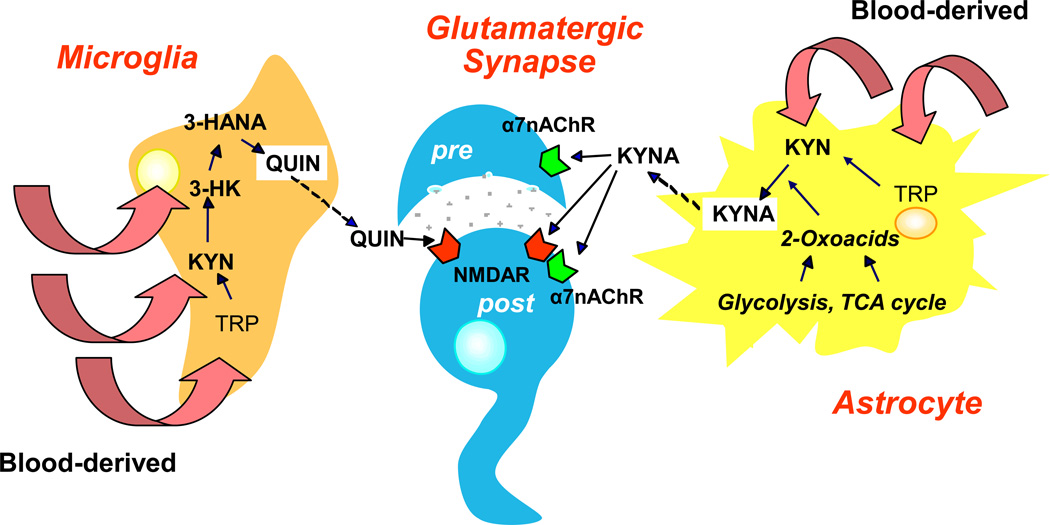
Figure 2. Segregation of the two Kynurenine Pathway Branches in the Brain.
Under physiological conditions, kynurenine pathway enzymes in the mammalian brain are preferentially, although not exclusively, localized in non-neuronal cells 57–58. Metabolism of the pathway is driven by blood-derived tryptophan (TRP), kynurenine (KYN) or 3-hydroxykynurenine (3-HK), or by locally formed metabolites. Of functional significance, the two branches of the pathway are physically segregated in the brain. Astrocytes, which harbor KATs but do not contain KMO and therefore cannot produce 3-HK from KYN 41, 198–199, account for kynurenic acid (KYNA) biosynthesis, which is regulated by intracellular metabolic events 46–48. 3-HK and its major downstream metabolites are synthesized in microglial and other cells of monocytic origin 118, 200. Once synthesized within glial cells, quinolinic acid (QUIN) and KYNA are promptly released into the extracellular milieu to affect their pre- and postsynaptic (“pre” and “post”) neuronal targets. 3-HANA: 3-Hydroxyanthranilic acid, α7nAChR: α7 nicotinic acetylcholine receptor, TCA: Tricarboxylic acid.
In contrast to several other kynurenine pathway metabolites, and because of their polar nature and the lack of transport processes, KYNA and QUIN do not cross the blood-brain barrier 42 and must be formed locally within the brain. Experimentally, both metabolites can be recovered from the extracellular milieu soon after their synthesis is stimulated by intracerebral infusions of their precursors kynurenine and 3-HANA, respectively 43–44. QUIN release appears to be controlled only by bioprecursor availability 45, whereas the release of KYNA can be modulated by several factors. For example, KYNA formation and release decrease substantially in the presence of depolarizing stimuli such as high K+ or veratridine, or under hypoglycemic conditions 46. The latter phenomenon is linked to cellular energy metabolism and can be effectively reversed by adding lactate and pyruvate 47. Like other 2-oxo acids, pyruvate also enhances the extracellular concentration of KYNA on its own by acting as a co-substrate in the transamination of kynurenine 48 (Fig. 2). Interestingly, dopamine may play a specific regulatory role as well, as D-amphetamine reduces KYNA levels in the brain but not in the periphery 49. Several of these effects are lost in the absence of neurons, indicating that KYNA production in, and release from, astrocytes is dependent on neuronal signals 46. Finally, although specific organic anion transporters for KYNA might exist in the brain 50, neither KYNA nor QUIN are efficiently removed from the brain’s extracellular compartment. Brain efflux therefore constitutes the major disposal mechanism for both metabolites 33, 51.
Peripheral influences on the brain kynurenine pathway
Brain kynurenines are not autonomous but are linked to, and influenced by, the peripheral kynurenine pathway (Fig. 3). In peripheral organs, kynurenine pathway enzymes are regulated by steroids, growth factors and, most notoriously, several signaling molecules of the immune system 52–55. It is uncertain to what extent these control mechanisms — including the best-known effect, the stimulation of IDO by interferon γ and other cytokines — operate in the brain under physiological conditions 34, 56. As tryptophan, kynurenine and 3-HK readily cross the blood-brain barrier 42, it is clear, however, that fluctuations in the blood levels of these metabolites directly affect metabolism in the kynurenine pathway, including the synthesis of KYNA and QUIN, in the brain. Of note, because of the rather low activity of brain IDO and TDO under physiological conditions (see above), the effects of systemic tryptophan on the brain kynurenine pathway are in part driven by its peripheral conversion to kynurenine and 3-HK, and the subsequent entry of these metabolites into the brain (Fig. 3).
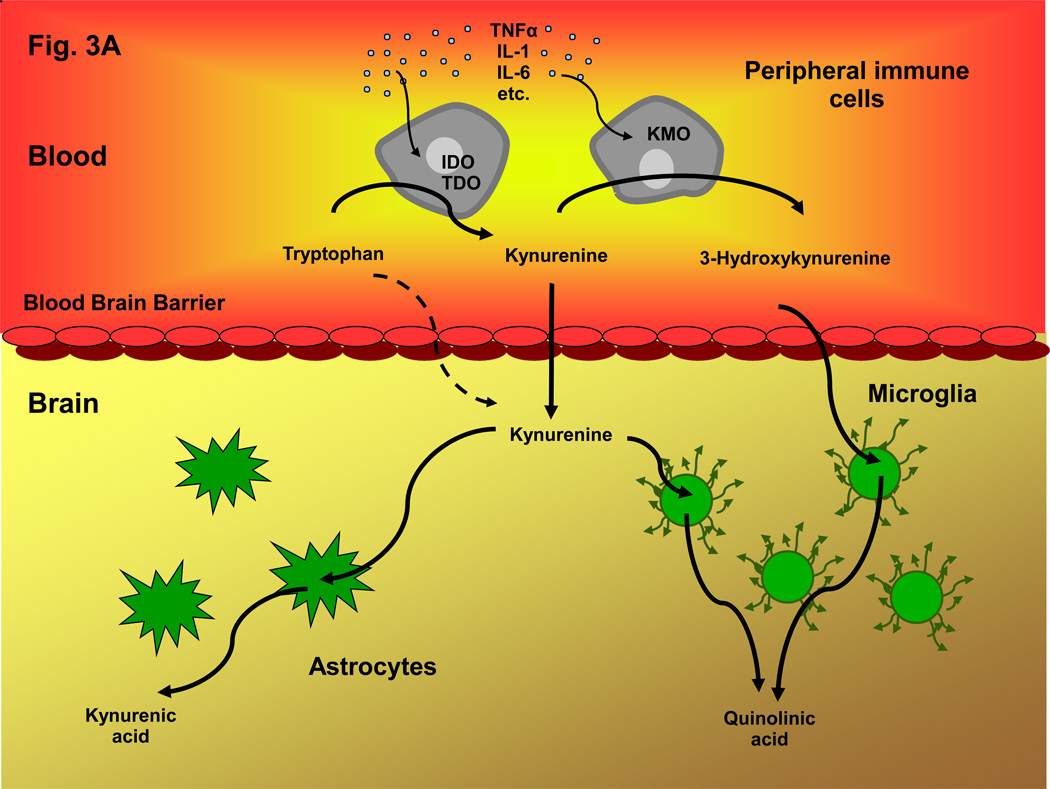
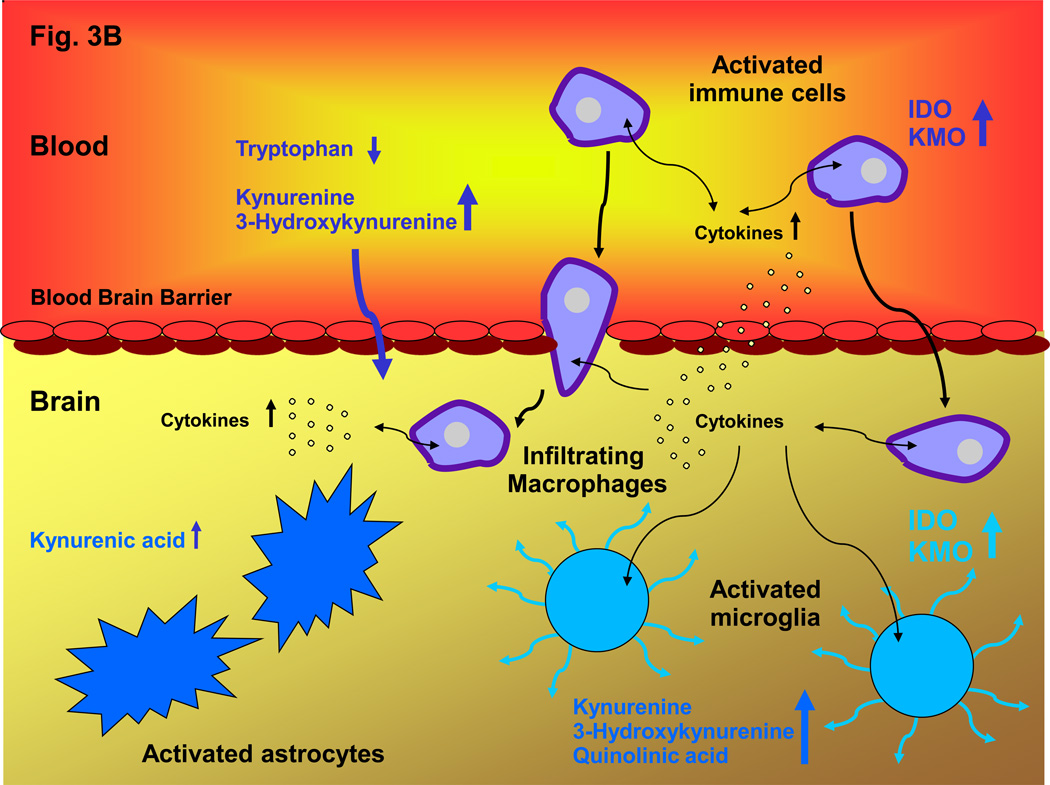
Figure 3. Communication between Peripheral and Central Kynurenine Pathways under Normal and Inflammatory Conditions.
A: In the periphery, the degradation of tryptophan and the subsequent formation of circulating kynurenines is normally regulated by steroid hormones, cytokines and growth factors. These factors stimulate IDO 123, 175, and also activate, or lead to the up-regulation of, other kynurenine pathway enzymes including TDO 201 and KMO 118, 132. Jointly, they control the levels of circulating tryptophan, kynurenine and 3-HK, which all readily cross the blood-brain barrier using the large neutral amino acid transporter 42. Together with kynurenine pathway metabolism within glial cells, brain uptake of these kynurenines therefore determines kynurenine pathway flux in the brain; B: Inflammatory conditions stimulate the kynurenine pathway both in the periphery and in the brain. Activation of kynurenine pathway enzymes, especially IDO and KMO, in peripheral immune cells – such as dendritic cells and macrophages – promotes the production of kynurenine and its downstream metabolites in the blood. Increased influx of the brain-permeable metabolites, in some cases aided by a leaky blood-brain barrier 173, as well as infiltration of macrophages or microbial pathogens 113, then leads to an excess of kynurenines in the brain parenchyma. Furthermore, infiltrating cytokines stimulate the kynurenine pathway in activated microglial cells and blood-borne cells within the brain 202. Together, these peripheral and central events connect neuroinflammatory mechanisms with abnormal metabolism along the kynurenine pathway, and brain pathology.
Kynurenine pathway metabolites in normal brain function
In early studies, the effects of KYNA and QUIN, the only neuroactive kynurenine pathway metabolites known at the time, were almost always tested at very high, non-physiological concentrations, i.e. the two compounds essentially functioned as pharmacological probes to examine the downstream effects of glutamate receptor manipulations. These experiments revealed, not unexpectedly, a multitude of chemical and functional consequences, but they were not overly informative with regard to a role of endogenous kynurenines in the mammalian brain. Owing to a lack of appropriate experimental approaches, these studies also failed to identify possible effects of a selective reduction in brain KYNA or QUIN levels.
The development of increasingly specific and potent pharmacological agents targeting individual enzymes in the kynurenine pathway (see below and Table 1) has gradually begun to transform our understanding of the functional roles of kynurenines in the brain. Most of these “kynurenergic” tools are still not ideal, and many of these compounds must be applied intracerebrally because they do not penetrate the blood-brain barrier after systemic administration. Nevertheless, the emerging new tools allow investigations of endogenous kynurenine pathway metabolites in situ, i.e. in an environment that maintains the natural anatomical arrangements between glial cells, which synthesize and release kynurenines, and adjacent neurons, which seem to play only a minor role in the production of kynurenine pathway metabolites 57–58.
Table 1.
Synthetic inhibitors of kynurenine pathway enzymes
| Enzyme | Inhibitor | Reference |
|---|---|---|
| IDO | 1-methyl-tryptophan | 203 |
| 4-amino-1,2,5-oxodiazole-3-carboximidamide | 176 | |
| TDO | (E)-6-fluoro-3-[2-(4’-pyridyl)-vinyl]-1H-indole | 178 |
| (E)-3-[2-(4’-pyridyl)-vinyl]-1H-indole | 178 | |
| KAT II | (S)-4-(ethylsulfonyl)benzoylalanine (ESBA) | 160 |
| [(S)-(-)-9-(4-aminopiperazine-1-yl)-8-fluoro-3-methyl-6-oxo-2,3,5,6-tetrahydro-4H-1-oxa-3a-aza-phenalene-5-carboxylic acid] (BFF122) | 204 | |
| KMO | 3,4-dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfonamide (Ro 61-8048) | 168 |
| [(1S,2S)-2-(3,4-dichlorobenzoyl)-cyclopropane-1-carboxylic acid] (UPF648) | 204 | |
| Kynureninase | o-methoxybenzoylalanine | 177 |
| 2-amino-4-[3'-hydroxyphenyl]-4-hydroxybutanoic acid | 205 | |
| 3HAO | 4-chloro-3-hydroxyanthranilic acid | 179 |
| 4,6-di-bromo-3-hydroxyanthranilic acid | 206 | |
Studies in which KYNA is administered in concentrations in the endogenous range, studies using selective inhibitors of KYNA’s main biosynthetic enzyme kynurenine aminotransferase II (KAT II), as well as studies using KAT II knockout mice 59, have revealed a broad neuromodulatory role of KYNA on brain chemistry. Thus, within various regions of the rodent forebrain, even modest increases in KYNA cause prompt reductions in extracellular glutamate and dopamine levels 60–61, whereas KYNA synthesis inhibition raises the levels of these neurotransmitters 10, 62, as well as acetylcholine levels63. Pharmacological experiments using selective receptor agonists and antagonists (such as galantamine and methyllicaconitine, respectively) indicate that α7nAChRs, rather than NMDA receptors, are the primary target of endogenous KYNA in the normal brain in vivo and mediate these bi-directional effects of KYNA on neurotransmitter levels 10, 60–61. This suggests, conversely, that α7nAChRs and their many physiological functions 64 are controlled by endogenous KYNA.
Fluctuations in brain KYNA levels have distinct behavioral consequences, especially related to cognition. Administered to normal rats and following conversion to nanomolar or low micromolar concentrations of KYNA in the brain, systemic administration of kynurenine causes impairments in visuospatial working memory, contextual learning, sensory gating, and prepulse inhibition of the acoustic startle reflex — all behaviors linked to cholinergic, glutamatergic and dopaminergic neurotransmission 65–68. Notably, the effect of KYNA on dopaminergic neurotransmission has also been convincingly demonstrated electrophysiologically. Thus, systemic administration of kynurenine, or relatively moderate elevation of brain KYNA levels by pharmacological means, reliably affects the firing of dopaminergic neurons in the midbrain, influencing downstream projection areas such as the striatum, the nucleus accumbens and the prefrontal cortex 69–70. Conversely, specific reductions in brain KYNA, caused by genetic elimination of KAT II or acute administration of a KAT II inhibitor, improve contextual memory and spatial discrimination 71 and enhance spatial memory 72 (see below).
Additional mutant mice and enzyme inhibitors (Table 1) have recently become available for studying the complex interplay of peripheral and central kynurenines. These experimental tools are beginning to be used to systematically examine the physiological roles of kynurenine pathway metabolites in the brain 57, 73–75.
Kynurenines in neurological diseases
Kynurenines did not attract significant attention from neurobiologists until the pathological effects of QUIN in the brain were recognized three decades ago. In addition to its usefulness as a readily available, inexpensive excitotoxin, QUIN became recognized for its ability to model neuropathological, neurochemical and clinical features of human diseases such as Huntington's disease and temporal lobe epilepsy 76, raising the specter that an abundance of endogenous QUIN might play a causative role in the pathophysiology of these and other neurological diseases. The discovery of KYNA’s neuroprotective and anticonvulsant effects 77 then led to the idea that a reduction in endogenous KYNA or, more generally, any process resulting in an abnormal increase in the QUIN:KYNA ratio in the brain might trigger neuronal lesions in various conditions linked to excitotoxic phenomena 78–80. However, skepticism concerning a possible pathogenic role of endogenous kynurenine pathway metabolites abounded, especially because endogenous concentrations of QUIN and KYNA in the brain are orders of magnitude lower than those needed to model neurological diseases acutely in rodents or primates in vivo.
Huntington’s disease
Evidence for a pathophysiologically significant role of abnormal metabolism in the kynurenine pathway is most compelling in the case of Huntington’s disease, the first and prototypical disease linked to an excitotoxic mechanism. Studies in post-mortem brains revealed that QUIN levels are substantially elevated in the initial stages of the disease, and especially in those brain regions (neostriatum, cortex) that suffer the most damage 81. This increase, which coincides with early activation of microglia 82, generates QUIN concentrations that are clearly capable of producing excitotoxic neuronal damage 83–84. Notably, the elevation in brain QUIN levels in Huntington’s disease is accompanied by a quantitatively similar increase in cerebral 3-HK levels 81, 85. The concentrations of 3-HK reached in the disease are neurotoxic to striatal neurons but, interestingly, not to cerebellar neurons 86. Of possible pathogenic significance, the neurotoxic properties of these two microglia-derived kynurenine pathway metabolites are synergistic 87–88. Reductions in brain KYNA levels in Huntington’s disease 89 could be physiologically relevant as well, as a deficiency in KYNA enhances vulnerability to QUIN neurotoxicity 90. Similar changes in the cerebral kynurenine pathway — most reliably the increase in 3-HK levels —, are also seen in mouse models of Huntington’s disease 91–92.
The most persuasive arguments favoring a causative link between abnormal kynurenine pathway function and Huntington’s disease pathology comes from work in lower model organisms. Thus, a genome-wide loss-of-function screen in yeast showed that the deletion of kynurenine 3-monooxygenase (KMO) potently suppresses the toxicity of a mutant huntingtin fragment, and that elimination of KAT exacerbates toxicity 93. Interestingly, deletions in the genes encoding 3-hydroxyanthranilic acid dioxygenase (3HAO) and nicotinate phosphoribosyltransferase suppress and enhance, respectively, mutant huntingtin toxicity. Surprisingly, the majority of functionally unrelated genetic suppressors of mutant huntingtin toxicity in yeast decrease the levels of 3-HK and QUIN, indicating that the kynurenine pathway plays a critical downstream role in the toxic effects of mutant huntingtin 94. As yeast do not have glutamate receptors, abnormal mitochondrial function and reactive oxygen species, rather than excitotoxicity, link kynurenine pathway manipulations to cell death in this model of Huntington’s disease. These factors may therefore also contribute to some aspects of neurodegeneration in the human disease 93. In a transgenic Drosophila melanogaster model of Huntington’s disease, genetic or pharmacological KMO inhibition increased levels of KYNA relative to 3-HK (flies do not produce QUIN) and ameliorated neurodegeneration. Underscoring the roles of 3-HK and KYNA in neuropathology, the neuroprotective effect of KMO inhibition is reduced by feeding the flies 3-HK, whereas feeding KYNA attenuates neurodegeneration. These interventions have no obvious effects in wild-type control flies, indicating that vulnerable organisms are excessively sensitive to manipulations of the kynurenine pathway 95.
Other neurological diseases
Cerebral kynurenine pathway changes also occur in other neurodegenerative diseases but have not been studied comprehensively. Thus, in the basal ganglia of patients with Parkinson’s disease, the concentration of 3-HK is increased, whereas kynurenine and KYNA levels are slightly reduced 96. In Alzheimer’s disease, QUIN immunoreactivity is strongest in glial cells in close proximity to amyloid plaques and also labels neurofibrillary tangles, suggesting that QUIN-induced excitotoxicity or oxidative stress might participate in the pathogenic process 97. Immunocytochemical studies of IDO and 3-HK provide further evidence that the kynurenine pathway is upregulated in the disease 98. Also indicative of a role of the kynurenine pathway in Alzheimer’s disease, QUIN induces tau phosphorylation in cultured primary human neurons 99.
As predicted from studies in rodent models 23, 100–102, human seizure disorders are also associated with abnormal cerebral kynurenine pathway activity. Of special interest, this impairment can be documented non-invasively by positron emission tomography using 11C-α-methyl-L-tryptophan as a tracer. This in vivo imaging methodology, which has so far been used mainly in glioma and epilepsy research, allows the simultaneous assessment of serotonin and kynurenine synthesis in the human brain with high specificity and good sensitivity. The technology has revealed enhanced tryptophan retention and subsequent activation of kynurenine pathway metabolism in gliotic tissue, and can be used to identify epileptogenic lesions interictally and to guide tissue resection during surgery in patients with epilepsy 103. Once kynurenine pathway metabolites downstream from kynurenine can be reliably identified through further methodological improvements, such imaging techniques — applied longitudinally and in combination with assessments of kynurenine pathway metabolites in blood and cerebrospinal fluid (CSF) — could revolutionize our understanding of kynurenine pathway neurobiology in health and disease.
Studies in animal models have shown that connections between abnormal kynurenine pathway metabolism and neuropathology also exist in animals experiencing acute metabolic or physical insults, such as hypoglycemia 104, cerebral ischemia 105, perinatal hypoxia 106 or traumatic spinal cord injury 107, even though these non-specific events clearly induce and probably involve a multitude of additional pathogenic mechanisms.
Because of the complex interactions between peripheral and central kynurenine pathways, studies describing kynurenine pathway abnormalities in blood, cultured cells and CSF in various human neurological disorders (see 108–110 for reviews) are not unequivocally instructive about the contribution of these abnormalities to the disease. Nevertheless, such studies, including the numerous demonstrations of peripheral IDO activation in neurological disorders, are often useful as predictors and indicators of abnormal kynurenine pathway function within the brain (see below). They can also help to assess the involvement of kynurenine pathway abnormalities in models of neurological diseases, especially those induced by known pathogens, for example malaria or trypanosome parasites 111–113.
The role of inflammatory mechanisms
Together with other, disease-specific etiological factors, chronic neuroinflammation plays an increasingly appreciated role in the pathogenesis of a large number of neurological diseases 114–115. Underlying events include brain infiltration of circulating immune cells, activation of resident microglia and other non-neuronal cells, and brain influx of blood-derived, pro-inflammatory cytokines and other immune activators. Alone or jointly, these mechanisms are responsible for the enhanced synthesis of kynurenine from tryptophan within the brain 34, 56 and the subsequent activation of the cerebral kynurenine pathway. In fact, both systemic and central immune stimulation, including cytokine-induced IDO activation, may contribute to cerebral kynurenine pathway activation in neurological disorders (Fig. 3). Not unexpectedly, therefore, the brain kynurenine pathway is often turned on in diseases of viral origin. Common to all these diseases, increases in 3-HK and QUIN levels in the brain outweigh the elevation in KYNA by substantial margins — most dramatically in the case of HIV-1-infected individuals 116–117. This robust increase in neurotoxic metabolites suggests that blood-derived or locally produced kynurenine is preferentially degraded within activated microglial cells and macrophages 118, but is less available for sequestration to KYNA in astrocytes (Fig. 3).
Kynurenines in psychiatric disorders
Major depressive disorders
Speculation about a role of kynurenines in major depressive disorders dates back several decades to the time when the connection between depression, tryptophan and serotonin was first recognized 119. However, the exceptional success of serotonin re-uptake inhibitors as antidepressants, paired with the lack of consistent indications of kynurenine pathway changes in depression, essentially stifled efforts to explore a possible involvement of kynurenine pathway metabolites in pathophysiology. However, studies performed during the past 10 years have led to a significant paradigm shift in this regard, and kynurenine pathway dysfunction is now increasingly recognized as a major player in the development and symptomatology of depressive disorders. Similar in principle to the above-mentioned neuro-immune hypothesis of neurological disorders, the pathophysiological concept is based on the realization that the kynurenine:tryptophan ratio in blood is significantly enhanced in patients with depression and correlates with anxiety and cognitive deficits, but not with neurovegetative or somatic symptoms 120–121. Several lines of evidence indicate that immune system activation of IDO plays a central role here. First, a number of pro-inflammatory cytokines that are known to stimulate IDO, such as interferon α, induce depressive symptoms in patients suffering from cancer or chronic hepatitis C 121–122. Second, following bacterial infection in mice, chronic up-regulation of IDO by interferon γ or tumor necrosis factor α (TNFα) induces long-lasting depressive-like behaviors, and these effects are largely attenuated when the increase in IDO activity is diminished 123. Finally, vulnerability to cytokine-induced depression is associated with a polymorphism in IDO 124, providing a possible explanation for clinical heterogeneity in the occurrence of depressive symptoms following stimulation of the immune system. Interestingly, bacterial infection also stimulates IDO activity in the placenta 125 and may thus contribute to post partum-depression 126.
How systemic IDO activation — i.e. an excess of circulating kynurenine levels — might lead to depressive behaviors is unclear. Immunohistochemical studies show some microglial activation (and QUIN immunoreactivity) as well as a reduction in astrocyte numbers in animal models of depression and in post-mortem brains of patients with major depression 127–130. Like in neurological diseases, but to a lesser extent, enhanced kynurenine influx from the periphery may therefore enhance ratio between the neurotoxic metabolites, i.e. 3-HK and QUIN, and the neuroprotective KYNA. This could increase neuronal vulnerability and adversely affect, for example, the viability of newly generated hippocampal neurons, which are arguably necessary to avert or attenuate depression 131–132. However, in humans, QUIN and KYNA levels are equally enhanced in the CSF after immune stimulation with interferon α 122, suggesting a more complicated scenario. Moreover, any changes in the cerebral kynurenine pathway in depression may be brain region-specific, as 3-HK levels are reduced in the cortex but enhanced in the striatum and amygdala in an established mouse model of the disease 133. Definitive resolution of the respective roles of the two kynurenine pathway branches in depression must therefore await improved imaging of brain kynurenine pathway metabolism (see above) and targeted pharmacological interventions in humans and appropriate animal models.
Schizophrenia
Assessment of kynurenine pathway involvement in schizophrenia initially followed a similar trajectory as that for major depression, with early studies failing to establish pathophysiologically significant links 134. The realization that the psychotomimetic drug ketamine, which produces schizophrenia-like symptoms in normal individuals and exacerbates psychotic features in patients with schizophrenia 135–136, functions as a NMDA receptor antagonist 137, led to the idea that excessive KYNA (by reducing NMDA receptor activity) or an increased ratio of KYNA on one hand, and 3-HK and its downstream metabolites on the other, might play a role in schizophrenia pathology 138. Brain levels of KYNA are indeed elevated in postmortem brains of patients with schizophrenia, and this increase, which is also seen in the CSF, is unrelated to chronic medication with antipsychotic drugs 139–142. Brain levels of 3-HK levels, on the other hand, are unchanged 140. Measurements of the expression and activity of kynurenine pathway enzymes in brain areas known to be involved in schizophrenia pathology (i.e. frontal and anterior cingulate cortices) suggest a scenario in which a reduction in KMO 142–143, combined with an increase in TDO 144–145, increases kynurenine levels 140 and thereby shifts kynurenine pathway metabolism towards enhanced KYNA formation (see Fig. 1). Kynurenine levels are indeed increased in the prefrontal cortex and CSF in patients with schizophrenia 140, 146, possibly as a consequence of peripheral neuroinflammation, which exists in schizophrenia as well (Fig. 3) 147–148. As explained earlier, a preferential elevation in KYNA levels, leading to tissue concentrations in the high nanomolar range, would then result in increased inhibition of α7nAChRs, subsequently causing an imbalance in glutamate, dopamine and acetylcholine systems, all of which have been linked to schizophrenia pathology 91–95, 149.
Studies in animals and humans indicate that elevated KYNA levels may be especially relevant for the cognitive deficits seen in schizophrenia. Thus, in rats, acute KYNA elevation in the prefrontal cortex causes the characteristic impairment in cognitive flexibility seen in schizophrenia 150–151. Moreover, experimentally induced increases in KYNA synthesis in the developing brain, perhaps mimicking prodromal events in schizophrenia, produce long-lasting deficits in spatial and contextual learning and memory, as well as impaired cognitive flexibility, in the adult animal 152–155. Finally, in schizophrenia patients, a single nucleotide polymorphism (SNP) in the KMO gene is associated with smooth pursuit eye movement and visuospatial working memory 143, 156. The study of kynurenine pathway metabolism in schizophrenia, and possibly also in other psychiatric diseases (such as bipolar disorder, drug abuse and autism spectrum disorders; 157–159), may therefore be particularly rewarding using an approach focused on distinct sub-domains of psychopathology (endophenotypes).
Targeting the kynurenine pathway
An increasingly diverse armamentarium of pharmacological agents has been developed for the study of the kynurenine pathway in health and disease (Table 1). Alone or in combination with exogenously supplied kynurenines, specific enzyme inhibitors can now be used to examine physiological events in the brain and to normalize cerebral kynurenine pathway function when needed. Two classes of inhibitors, targeting KAT II and KMO, respectively, have so far contributed most to our understanding of kynurenine pathway neurobiology.
KAT II inhibitors
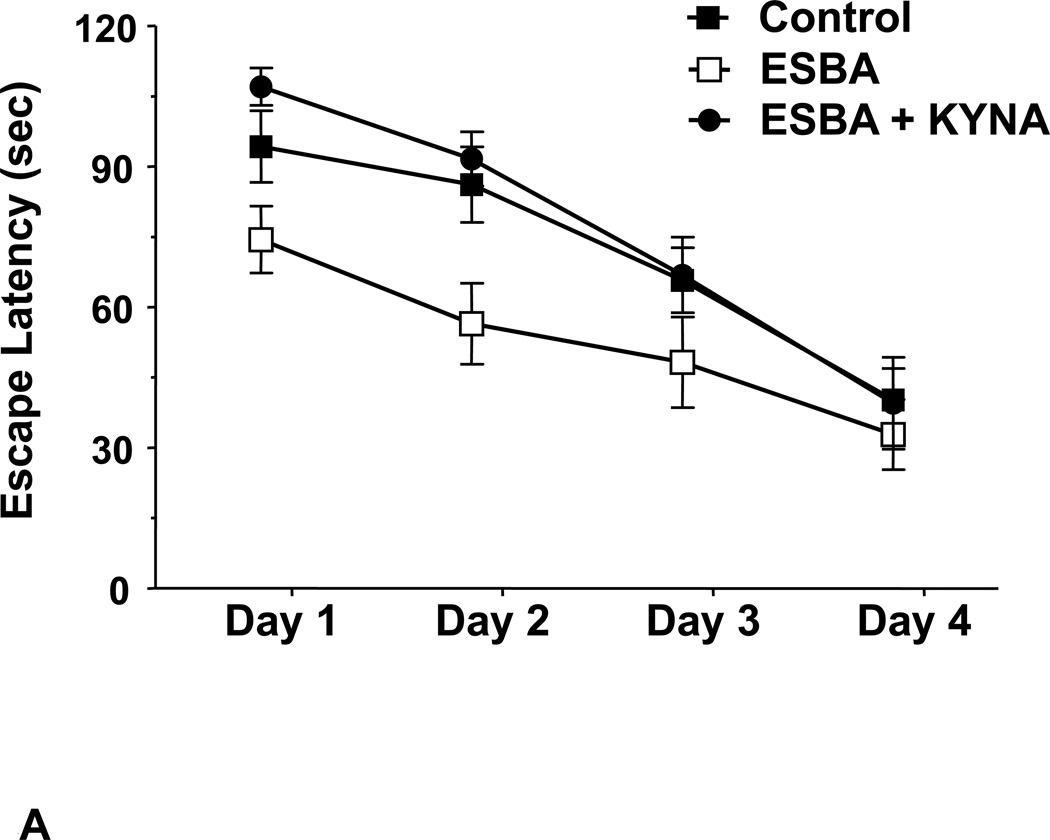
The first-generation specific KAT II inhibitor, (S)-4-(ethylsulfonyl)benzoylalanine (ESBA), was introduced in 2006 160 and turned out to be an excellent tool for examining the disposition and function of endogenous KYNA in the rat brain. Irrespective of brain region and dosage, intracerebral application of ESBA or other KAT II inhibitors causes no more than a 30–40% reduction in extracellular KYNA, indicating that KAT II controls only a fraction of KYNA in the extracellular milieu in vivo. This relatively small decrease is sufficient, however, to significantly increase the extracellular concentrations of glutamate, dopamine and acetylcholine, i.e. the same neurotransmitters that are reduced when KYNA levels are even moderately elevated (see above). This suggests that the KAT II-dependent pool of KYNA, which can be readily mobilized, provides tonic inhibition of its central receptor target(s). Notably, preliminary data demonstrate that the pro-cognitive effects of genetic elimination of KAT II 71 or acute application of ESBA 72 (Fig. 4) mentioned above can be duplicated by administration of second-generation, brain-penetrable KAT II inhibitors 161–162, and that this intervention also attenuates ketamine-induced working memory deficits in monkeys 163–164. These results bode well for the use of KAT II inhibitors in humans, especially in diseases like schizophrenia, where cognitive deficits might be related to high brain KYNA levels (Fig. 5).
Figure 4. Effects of Kynurenine Pathway Manipulation on Cognition and Neurodegeneration.
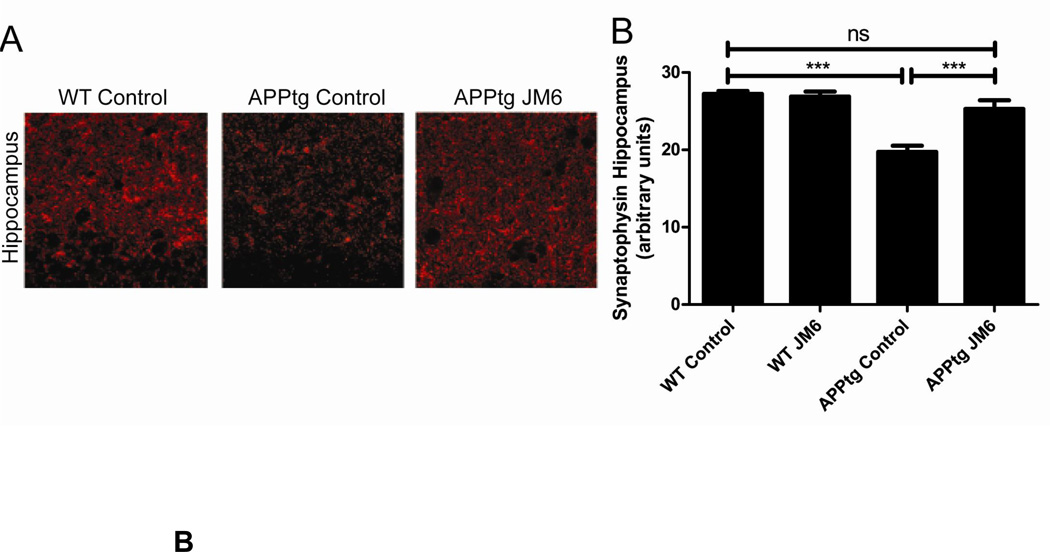
A: Inhibition of endogenous KYNA synthesis with the KAT II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (ESBA), administered intracerebroventricularly 90 min prior to daily training (Days 1–4), facilitates learning in a spatial working memory task (the Morris water maze test) in normal rats. Co-administration of KYNA eliminates this effect (see 72 for details); B: JM6, a peripherally acting pro-drug of the KMO inhibitor Ro 61-8048, prevents synaptic loss in a transgenic mouse model of Alzheimer’s disease (APPtg mice). JM6 was administered orally for 120 days, and the brain was evaluated in 8-month-old APPtg mice. Left: Representative image (630X) of serial sections of the hippocampus of wild-type or APPtg mice immunostained with an antibody for synaptophysin. Right: Quantification of synaptophysin levels in the hippocampus of APPtg mice treated with JM6. Synaptophysin levels in APPtg mice treated with JM6 are not significantly different from those found in wild-type mice (see 172 for details).
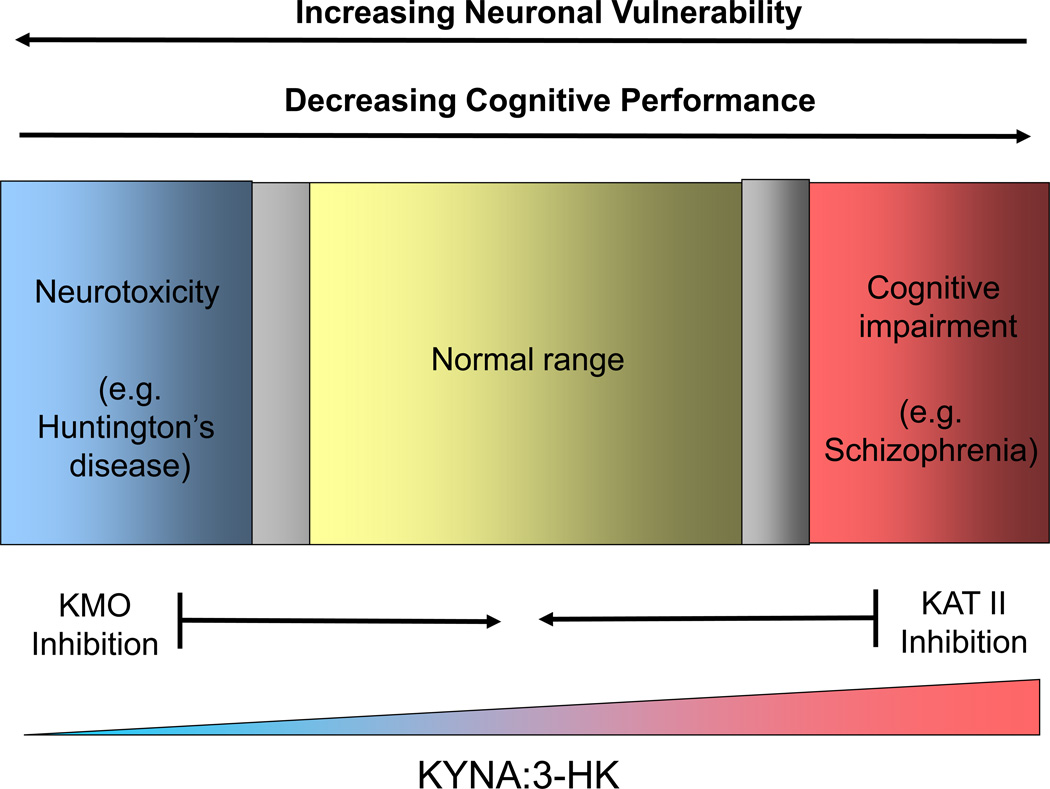
Figure 5. Targeting Brain Kynurenines Pharmacologically: Focus on KAT II and KMO.
As KAT II and KMO are directly responsible for the formation of KYNA and 3-HK, respectively, specific inhibitors of these enzymes can be used to manipulate the ratio between these two neuroactive metabolites pharmacologically. Increases in the KYNA:3-HK ratio are desirable in neurodegenerative diseases such as Huntington’s disease, which present with an excess of neurotoxic 3-HK (and QUIN) in the brain 81, 85, 92. Conversely, lowering of the KYNA:3-HK ratio in the brain will improve cognitive capabilities, providing special benefits in diseases such as schizophrenia, which show enhanced brain levels of KYNA 140. In the normal brain, fluctuations in the KYNA:3-HK ratio will have moderate effects on neuronal viability and cognition, limited to the physiological range. Pharmacological interventions with specific “kynurenergic” agents, such as KAT II or KMO inhibitors, should therefore be tailored to achieve the intended benefits and minimize potentially harmful consequences. The gray areas indicate transition zones between the physiological range and pathologies.
KMO inhibitors
First-generation KMO inhibitors demonstrated anticonvulsant and neuroprotective efficacy in various model systems in vivo 90, 95, 165–167. Ro 61-8048, the most widely used KMO inhibitor, was introduced in 1997 168. This compound has pronounced neuroprotective and anti-dyskinetic effects when used systemically in animal models of focal or global ischemia 169 and L-dopa-induced dyskinesia 170, respectively. Ro 61-8048 also has substantial anti-inflammatory effects in an animal model of sleeping sickness 113 and delays death in a mouse model of cerebral malaria 171. Like all KMO inhibitors tested so far, Ro 61-8048 raises brain KYNA levels following systemic application, and this increase is probably responsible for the remarkable central effects of the compound. In normal animals, the increase in brain KYNA is not accompanied by a reduction in the levels of brain 3-HK and QUIN, suggesting that the enzyme inhibitor does not cross the blood-brain barrier. This was verified in the course of a recent study demonstrating neuroprotection with JM6, a pro-drug of Ro 61-8048, in mouse models of Huntington’s disease and Alzheimer’s disease (Fig. 4). Thus, the beneficial effects of JM6 treatment were achieved exclusively through peripheral KMO inhibition, leading to moderate, persistent elevations of brain KYNA levels 172. Interestingly, application of a KMO inhibitor in conditions of strong immune stimulation, perhaps involving KMO blockade in activated endothelial cells and pericytes of the blood-brain barrier, causes a reduction in brain 3-HK and QUIN levels within the brain parenchyma 173–174. This may provide additional benefit to patients with neurodegenerative diseases (Fig. 5).
Other kynurenine pathway inhibitors
Inhibitors of IDO and TDO, prominent as potential cancer therapies 175–176, have so far not been used to study the cerebral kynurenine pathway directly, although these enzyme inhibitors might offer innovative, novel treatment options for brain tumors and for neurological and psychiatric diseases that are caused or exacerbated by immune system dysfunction. Of other agents targeting individual kynurenine pathway enzymes specifically, kynureninase inhibitors 177–178 and the 3HAO inhibitor 4-Cl-3-hydroxyanthranilic acid 179 have been sparingly but successfully used in neurobiological research. Most interestingly, 4-Cl-3-hydroxyanthranilic acid, an effective blocker of microglial QUIN neosynthesis in vivo 180, preserves white matter and effects functional recovery in a model of spinal cord injury, without, however, enhancing KYNA levels 181.
Challenges for future research
It is clear that the kynurenine pathway, a highly conserved metabolic machinery, has evolved to serve important regulatory functions in the mammalian brain. As we explore the largely unknown role(s) of kynurenines in cell biology, we are likely to uncover the functional significance of distinct intracellular processes they might influence. Specific questions abound. For example, does the strict association of KMO with the outer mitochondrial membrane 182 signify a specific mitochondrial function of 3-HK or downstream metabolites? More generally, is there a physiological role for the pro- and anti-oxidative effects of KYNA, 3-HK and 3-HANA 14–15, 20–22 or for the ability of QUIN 28 or picolinic acid 183 to chelate bioactive metal ions? Are any of these intracellular functions brain-specific, and do they differ between various glial cells or between resting and activated glia? And if so, what are the functional consequences of this diversity?
Another promising line of investigation, which has been only sparingly pursued so far 125, 184–185, will explore the possibility that the role of brain kynurenines changes over the course of the life span. Do kynurenines derived from the mother shape metabolism along the kynurenine pathway, and the function of pathway metabolites, in the fetal brain? Do kynurenines, maybe through their involvement in mitochondrial processes and oxidative stress, contribute to apoptotic cell death in the aged brain? Or, more generally, are the neuromodulatory effects of kynurenines age-specific, perhaps involving qualitatively distinct intra- and intercellular mechanisms during brain development and during ageing?
For several reasons, further elucidation of the mechanisms that control and maintain equilibrium between peripheral and central kynurenine pathways in mammals will probably remain a major research objective. First, normal homeostasis between the two compartments is delicate and complex, and influenced by a considerable number of environmental and physiological factors. These mechanisms are still poorly understood. Second, as briefly reviewed here, changes in the levels of circulating kynurenines under inflammatory conditions can either increase (in neurological disorders) or reduce (in schizophrenia) the ratio between neurotoxic and neuroprotective kynurenine pathway metabolites in the brain. Resolution of this puzzling quandary, which has pathophysiologically relevant implications, will require a thorough assessment of kynurenine pathway function in multiple brain cell types and various disease models. These studies will clarify, in essence, how physiology can be usurped to produce distinct brain pathologies. Finally, new information is needed to confidently predict the desired physiological effects or clinical improvements caused by selective kynurenine pathway enzyme inhibitors (see above). To optimize efficacy and minimize the risk of adverse side effects, especially when used in conjunction with other drugs, it will be especially important to discriminate between the consequences of peripheral and central enzyme inhibition and to compare acute and chronic effects of novel pharmacological agents. As shown in Fig. 5, illustrating the effects of manipulating the KYNA:3-HK ratio in the brain under physiological and pathological conditions, interventions with specific “kynurenergic” drugs must then be carefully tailored to achieve the intended benefit and avoid harmful outcomes. Successful improvement of brain function or effective treatment of brain disorders by selective targeting of individual kynurenines in the human brain would constitute the ultimate reward of these efforts.
Acknowledgements
Studies in our laboratories have been supported by grants from the US National Institutes of Health (NIMH, NINDS, NICHD), the National Alliance for Research in Schizophrenia and Affective Disorders, the Hereditary disease Foundation/High Q and CHDI, Inc. We are grateful to the pre- and postdoctoral associates, who were instrumental in the design and completion of the work conducted in our laboratories. We feel special gratitude to our friend Paolo Guidetti, who passed away prematurely on December 28, 2007.
References
- 1.Liebig J. Über Kynurensäure. Justus Liebig's Ann. Chem. 1853;86:125–126. [Google Scholar]
- 2.Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am. J. Clin. Nutr. 1971;24:659–672. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 4.Parsons CG, et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997;283:1264–1275. [PubMed] [Google Scholar]
- 5.Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. Some central effects of kynurenic acid, 7-chlorokynurenic acid and 5,7-dichloro-kynurenic acid, glycine site antagonists. Pol. J. Pharmacol. 1994;46:115–124. [PubMed] [Google Scholar]
- 6.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988;154:85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 7.Russi P, et al. Nicotinylalanine increases the formation of kynurenic acid in the brain and antagonizes convulsions. J. Neurochem. 1992;59:2076–2080. doi: 10.1111/j.1471-4159.1992.tb10097.x. [DOI] [PubMed] [Google Scholar]
- 8.Hilmas C, et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grilli M, et al. Modulation of the function of presynaptic alpha7 and non-alpha7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain. Br. J. Pharmacol. 2006;149:724–732. doi: 10.1038/sj.bjp.0706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu HQ, et al. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 12.DiNatale BC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes C, et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J. Pharmacol. Exp. Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- 14.Hardeland R, et al. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Adv. Exp. Med. Biol. 1999;467:389–395. doi: 10.1007/978-1-4615-4709-9_49. [DOI] [PubMed] [Google Scholar]
- 15.Lugo-Huitron R, et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011;33:538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993;45:310–379. [PubMed] [Google Scholar]
- 17.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 18.Copeland CS, Neale SA, Salt TE. Soc. Neurosci. Abstr. 2011;Vol. 36 [Google Scholar]
- 19.Fazio F, et al. Cinnabarinic Acid, an Endogenous Metabolite of the Kynurenine Pathway, Activates Type-4 Metabotropic Glutamate Receptors. Mol. Pharmacol. 2012 doi: 10.1124/mol.111.074765. doi:mol.111.074765 [pii] 10.1124/mol.111.074765. [DOI] [PubMed] [Google Scholar]
- 20.Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem. Biophys. Res. Commun. 2003;300:719–724. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein LE, et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry (Mosc) 2000;39:7266–7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- 22.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J. Neural Transm. 1978;42:37–43. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- 24.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981;72:411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 25.De Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunit. Neurochem. Int. 1996;28:445–452. doi: 10.1016/0197-0186(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 26.Ríos C, Santamaría A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 27.Stípek S, Stastný F, Pláteník J, Crkovská J, Zima T. The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochem. Int. 1997;30:233–237. [PubMed] [Google Scholar]
- 28.Pláteník J, Stopka P, Vejrazka M, Stípek S. Quiniolinic acid - iron (II) complexes: slow autoxidation, but enhanced hydroxyl radical production in the fenton reaction. Free Radic. Res. 2001;34:445–459. doi: 10.1080/10715760100300391. [DOI] [PubMed] [Google Scholar]
- 29.St'astny F, Hinoi E, Ogita K, Yoneda Y. Ferrous iron modulates quinolinate-mediated [3H]MK-801 binding to rat brain synaptic membranes in the presence of glycine and spermidine. Neurosci. Lett. 1999;262:105–108. doi: 10.1016/s0304-3940(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 30.Baran H, Schwarcz R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. J. Neurochem. 1990;55:738–744. doi: 10.1111/j.1471-4159.1990.tb04553.x. [DOI] [PubMed] [Google Scholar]
- 31.Guidetti P, Walsh JL, Schwarcz R. A fluorimetric assay for the determination of anthranilic acid in biological materials. Anal. Biochem. 1994;220:181–184. doi: 10.1006/abio.1994.1316. [DOI] [PubMed] [Google Scholar]
- 32.Gobaille S, et al. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J. Neurochem. 2008;105:982–993. doi: 10.1111/j.1471-4159.2008.05219.x. [DOI] [PubMed] [Google Scholar]
- 33.Moroni F, Russi P, Lombardi G, Beni M, Carla V. Presence of kynurenic acid in the mammalian brain. J. Neurochem. 1988;51:177–180. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 34.Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 35.Smythe GA, et al. ECNI GC-MS analysis of picolinic and quinolinic acids and their amides in human plasma, CSF, brain tissue. Adv. Exp. Med. Biol. 2003;527:705–712. doi: 10.1007/978-1-4615-0135-0_83. [DOI] [PubMed] [Google Scholar]
- 36.Turski WA, et al. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988;454:164–169. doi: 10.1016/0006-8993(88)90815-3. [DOI] [PubMed] [Google Scholar]
- 37.Dang Y, Dale WE, Brown OR. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic. Biol. Med. 2000;28:615–624. doi: 10.1016/s0891-5849(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 38.Gál EM, Sherman AD. L-Kynurenine: its synthesis and possible regulatory function in brain. Neurochem. Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 39.Speciale C, Schwarcz R. Uptake of kynurenine into rat brain slices. J. Neurochem. 1990;54:156–163. doi: 10.1111/j.1471-4159.1990.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 40.Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J. Neurochem. 1995;65:2621–2632. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- 41.Guillemin GJ, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 42.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 43.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speciale C, Ungerstedt U, Schwarcz R. Production of extracellular quinolinic acid in the striatum studied by microdialysis in unanesthetized rats. Neurosci. Lett. 1989;104:345–350. doi: 10.1016/0304-3940(89)90601-0. [DOI] [PubMed] [Google Scholar]
- 45.Speciale C, Schwarcz R. On the production and disposition of quinolinic acid in rat brain and liver slices. J. Neurochem. 1993;60:212–218. doi: 10.1111/j.1471-4159.1993.tb05840.x. [DOI] [PubMed] [Google Scholar]
- 46.Gramsbergen JB, et al. Brain-specific modulation of kynurenic acid synthesis in the rat. J. Neurochem. 1997;69:290–298. doi: 10.1046/j.1471-4159.1997.69010290.x. [DOI] [PubMed] [Google Scholar]
- 47.Hodgkins PS, Schwarcz R. Interference with cellular energy metabolism reduces kynurenic acid formation in rat brain slices: reversal by lactate and pyruvate. Eur. J. Neurosci. 1998;10:1986–1994. doi: 10.1046/j.1460-9568.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 48.Hodgkins PS, Wu HQ, Zielke HR, Schwarcz R. 2-Oxoacids regulate kynurenic acid production in the rat brain: studies in vitro and in vivo. J. Neurochem. 1999;72:643–651. doi: 10.1046/j.1471-4159.1999.0720643.x. [DOI] [PubMed] [Google Scholar]
- 49.Rassoulpour A, Wu H-Q, Poeggeler B, Schwarcz R. Systemic d-amphetamine administration causes a reduction of kynurenic acid levels in rat brain. Brain Res. 1998;802:111–118. doi: 10.1016/s0006-8993(98)00577-0. [DOI] [PubMed] [Google Scholar]
- 50.Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 2011 doi: 10.1016/j.phrs.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Foster AC, Miller LP, Oldendorf WH, Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp. Neurol. 1984;84:428–440. doi: 10.1016/0014-4886(84)90239-5. [DOI] [PubMed] [Google Scholar]
- 52.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Biochem. J. 1985;229:499–504. doi: 10.1042/bj2290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espey MG, Namboodiri MAA. Selective metabolism of kynurenine in the spleen in the absence of indoleamine 2,3-dioxygenase induction. Immunol. Lett. 2000;71:67–72. doi: 10.1016/s0165-2478(99)00179-0. [DOI] [PubMed] [Google Scholar]
- 54.Belladonna ML, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 55.Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int. Rev. Immunol. 2010;29:133–155. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito K, et al. Kynurenine pathway enzymes in brain: respones to ischemic brain injury versus systemic immune activation. J. Neurochem. 1993;61:2061–2070. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- 57.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 58.Guillemin GJ, et al. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu P, et al. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol. Cell. Biol. 2004;24:6919–6930. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpenedo R, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 61.Rassoulpour A, Wu HQ, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 62.Amori L, et al. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zmarowski A, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur. J. Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 64.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- 66.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav. Brain Res. 2009;201:325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Linderholm KR, et al. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: a pharmacological analysis. Neuropharmacology. 2007;53:918–924. doi: 10.1016/j.neuropharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Erhardt S, Oberg H, Mathe JM, Engberg G. Pharmacological elevation of endogenous kynurenic acid levels activates nigral dopamine neurons. Amino Acids. 2001;20:353–362. doi: 10.1007/s007260170032. [DOI] [PubMed] [Google Scholar]
- 71.Potter MC, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pocivavsek A, et al. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giorgini F, et al. Soc. Neurosci. Abstr. 2011;Vol. 36 [Google Scholar]
- 74.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Kanai M, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- 77.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984;48:273–278. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 78.Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 79.Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science. 1985;230:681–683. doi: 10.1126/science.2996146. [DOI] [PubMed] [Google Scholar]
- 80.Schwarcz R, Foster AC, French ED, Whetsell WO, Jr, Köhler C. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984;35:19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- 81.Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol. Dis. 2004;17:455–461. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Tai YF, et al. Microglial activation in presymptomatic Huntington's disease gene carriers. Brain. 2007;130:1759–1766. doi: 10.1093/brain/awm044. [DOI] [PubMed] [Google Scholar]
- 83.Whetsell WO, Jr, Schwarcz R. Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci. Lett. 1989;97:271–275. doi: 10.1016/0304-3940(89)90609-5. [DOI] [PubMed] [Google Scholar]
- 84.Kerr SJ, Armati PJ, Guillemin GJ, Brew BJ. Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. AIDS. 1998;12:355–363. doi: 10.1097/00002030-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Pearson SJ, Reynolds GP. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington's disease. Neurosci. Lett. 1992;144:199–201. doi: 10.1016/0304-3940(92)90749-w. [DOI] [PubMed] [Google Scholar]
- 86.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 87.Guidetti P, Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999;11:3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 88.Chiarugi A, Meli E, Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J. Neurochem. 2001;77:1310–1318. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 89.Beal MF, et al. Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J. Neurol. Sci. 1992;108:80–87. doi: 10.1016/0022-510x(92)90191-m. [DOI] [PubMed] [Google Scholar]
- 90.Sapko MT, et al. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington's disease. Exp. Neurol. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Guidetti P, et al. Elevated brain 3-hydroxykynurenine and quinolinate levels in Huntington disease mice. Neurobiol. Dis. 2006;23:190–197. doi: 10.1016/j.nbd.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Guidetti P, Reddy PH, Tagle DA, Schwarcz R. Early kynurenergic impairment in Huntington's disease and in a transgenic animal model. Neurosci. Lett. 2000;283:233–235. doi: 10.1016/s0304-3940(00)00956-3. [DOI] [PubMed] [Google Scholar]
- 93.Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat. Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giorgini F, et al. Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. J. Biol. Chem. 2008;283:7390–7400. doi: 10.1074/jbc.M708192200. [DOI] [PubMed] [Google Scholar]
- 95.Campesan S, et al. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr. Biol. 2011;21:961–966. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogawa T, et al. Kynurenine pathway abnormalities in Parkinson's disease. Neurology. 1992;42:1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 97.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol. Appl. Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 98.Bonda DJ, et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer's disease. Redox Rep. 2010;15:161–168. doi: 10.1179/174329210X12650506623645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rahman A, et al. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baran H, Gramer M, Honack D, Loscher W. Systemic administration of kainate induces marked increases of endogenous kynurenic acid in various brain regions and plasma of rats. Eur. J. Pharmacol. 1995;286:167–175. doi: 10.1016/0014-2999(95)00443-o. [DOI] [PubMed] [Google Scholar]
- 101.Lehrmann E, et al. Glial activation precedes seizures and hippocampal neurodegeneration in measles virus-infected mice. Epilepsia 49 Suppl. 2008;2:13–23. doi: 10.1111/j.1528-1167.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- 102.Wu HQ, Schwarcz R. Seizure activity causes elevation of endogenous extracellular kynurenic acid in the rat brain. Brain Res. Bull. 1996;39:155–162. doi: 10.1016/0361-9230(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 103.Chugani DC. alpha-methyl-L-tryptophan: mechanisms for tracer localization of epileptogenic brain regions. Biomark. Med. 2011;5:567–575. doi: 10.2217/bmm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heyes MP, Papagapiou M, Leonard C, Markey SP, Auer RN. Effects of profound insulin-induced hypoglycemia on quinolinic acid in hippocampus and plasma. Adv. Exp. Med. Biol. 1991;294:679–682. doi: 10.1007/978-1-4684-5952-4_92. [DOI] [PubMed] [Google Scholar]
- 105.Saito K, Nowak TSJ, Markey SP, Heyes MP. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J. Neurochem. 1993;60:180–192. doi: 10.1111/j.1471-4159.1993.tb05836.x. [DOI] [PubMed] [Google Scholar]
- 106.Ceresoli-Borroni G, Schwarcz R. Neonatal asphyxia in rats: acute effects on cerebral kynurenine metabolism. Pediatr. Res. 2001;50:231–235. doi: 10.1203/00006450-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 107.Blight AR, Saito K, Heyes MP. Increased levels of the excitotoxin quinolinic acid in spinal cord following contusion injury. Brain Res. 1993;632:314–316. doi: 10.1016/0006-8993(93)91167-q. [DOI] [PubMed] [Google Scholar]
- 108.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 109.Németh H, Toldi J, Vecsei L. Kynurenines, Parkinson's disease and other neurodegenerative disorders: preclinical and clinical studies. J. Neural Transm. 2006;70:285–304. doi: 10.1007/978-3-211-45295-0_45. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int. J. Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miu J, Ball HJ, Mellor AL, Hunt NH. Effect of indoleamine dioxygenase-1 deficiency and kynurenine pathway inhibition on murine cerebral malaria. Int. J. Parasitol. 2009;39:363–370. doi: 10.1016/j.ijpara.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodgers J, Stone TW, Barrett MP, Bradley B, Kennedy PG. Kynurenine pathway inhibition reduces central nervous system inflammation in a model of human African trypanosomiasis. Brain. 2009;132:1259–1267. doi: 10.1093/brain/awp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. doi:nrneurol.2010.17 [pii] 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 115.Steinman L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004;5:575–581. doi: 10.1038/ni1078. doi:10.1038/ni1078 ni1078 [pii] [DOI] [PubMed] [Google Scholar]
- 116.Heyes MP, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 117.Heyes MP, Mefford IN, Quearry BJ, Dedhia M, Lackner A. Increased ratio of quinolinic acid to kynurenic acid in cerebrospinal fluid of D retrovirus-infected rhesus macaques: relationship to clinical and viral status. Ann. Neurol. 1990;27:666–675. doi: 10.1002/ana.410270614. [DOI] [PubMed] [Google Scholar]
- 118.Alberati-Giani D, Ricciardi-Castagnoli P, Köhler C, Cesura AM. Regulation of the kynurenine metabolic pathway by interfon-ç in murine clone macrophages and microglial cells. J. Neurochem. 1996;66:996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- 119.Lapin IP. Kynurenines as probable participants of depression. Pharmakopsychiatr. Neuropsychopharmakol. 1973;6:273–279. doi: 10.1055/s-0028-1094391. [DOI] [PubMed] [Google Scholar]
- 120.Maes M, et al. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- 121.Capuron L, et al. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol. Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 122.Raison CL, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O'Connor JC, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith AK, et al. Association of a polymorphism in the indoleamine-2,3-dioxygenase gene and interferon-alpha-induced depression in patients with chronic hepatitis C. Mol. Psychiatry. 2011 doi: 10.1038/mp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manuelpillai U, et al. Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: up-regulation by inflammatory stimuli and with clinical infection. Am. J. Obstet. Gynecol. 2005;192:280–288. doi: 10.1016/j.ajog.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 126.Kohl C, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J. Affect. Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 127.Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 129.Tynan RJ, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain. Behav. Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. doi:S0889-1591(10)00043-7 [pii] 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Steiner J, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. doi:1742-2094-8-94 [pii] 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zunszain PA, et al. Interleukin-1beta: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2011;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laugeray A, et al. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav. Brain Res. 2010;210:84–91. doi: 10.1016/j.bbr.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 134.Frazer A, Pandey GN, Mendels J. Metabolism of tryptophan in depressive disease. Arch. Gen. Psychiatry. 1973;29:528–535. doi: 10.1001/archpsyc.1973.04200040070012. [DOI] [PubMed] [Google Scholar]
- 135.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 136.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 137.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schwarcz R, et al. Kynurenic acid: a potential pathogen in brain disorders. Ann. N.Y. Acad. Sci. 1992;648:140–153. doi: 10.1111/j.1749-6632.1992.tb24532.x. [DOI] [PubMed] [Google Scholar]
- 139.Erhardt S, et al. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 140.Schwarcz R, et al. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 141.Ceresoli-Borroni G, Rassoulpour A, Wu HQ, Guidetti P, Schwarcz R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J. Neural Transm. 2006;113:1355–1365. doi: 10.1007/s00702-005-0432-z. [DOI] [PubMed] [Google Scholar]
- 142.Sathyasaikumar KV, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011;37:1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wonodi I, et al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry. 2011;68:665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 145.Miller CL, et al. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 146.Linderholm KR, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Potvin S, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol. Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 148.Müller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox. Res. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- 149.Erhardt S, Engberg G. Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta Physiol. Scand. 2002;175:45–53. doi: 10.1046/j.1365-201X.2002.00962.x. [DOI] [PubMed] [Google Scholar]
- 150.Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol. Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 151.Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) doi: 10.1007/s00213-011-2539-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexander K, et al. Soc. Neurosci. Abstr. 2011;Vol. 36 [Google Scholar]
- 153.Trecartin KV, Bucci DJ. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr. Res. 2011;133:156–158. doi: 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur. J. Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08064.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aoyama N, et al. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes Brain Behav. 2006;5:364–368. doi: 10.1111/j.1601-183X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 157.McFarlane HG, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 158.Justinova Z, et al. Soc. Neurosci. Abstr. 2011;Vol. 36 [Google Scholar]
- 159.Olsson SK, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatry Neurosci. 2010;35:195–199. doi: 10.1503/jpn.090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pellicciari R, et al. Modulators of the kynurenine pathway of tryptophan metabolism. Synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- 161.Chapin DS, Campbell B, Strick CA, Kozak R. Soc. Neurosci. Abstr. 2010;Vol. 35 [Google Scholar]
- 162.Kozak R, et al. Soc. Neurosci. Abstr. 2010;Vol. 35 [Google Scholar]
- 163.Abbott A, et al. Soc. Neurosci. Abstr. 2010;Vol. 35 [Google Scholar]
- 164.Koshy Cherian A, et al. Soc. Neurosci. Abstr. 2011;Vol. 36 [Google Scholar]
- 165.Connick JH, et al. Nicotinylalanine increases cerebral kynurenic acid content and has anticonvulsant activity. Gen. Pharmacol. 1992;23:235–239. doi: 10.1016/0306-3623(92)90017-e. [DOI] [PubMed] [Google Scholar]
- 166.Carpenedo R, et al. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience. 1994;61:237–244. doi: 10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 167.Speciale C, et al. (R,S)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur. J. Pharmacol. 1996;315:263–267. doi: 10.1016/s0014-2999(96)00613-9. [DOI] [PubMed] [Google Scholar]
- 168.Röver S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-Phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 1997;40:4375–4385. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- 169.Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-Alanine (mNBA) and 3,4-dimethoxyl-[-N-4-(nitrophenyl)thiazol-2YL-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J. Cereb. Blood Flow Metab. 1999;19:771–777. doi: 10.1097/00004647-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 170.Gregoire L, et al. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 2008;186:161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 171.Clark CJ, et al. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect. Immun. 2005;73:5249–5251. doi: 10.1128/IAI.73.8.5249-5251.2005. doi:73/8/5249 [pii] 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zwilling D, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Owe-Young R, et al. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J. Neurochem. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 174.Chiarugi A, Moroni F. Quinolinic acid formation in immune-activated mice: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(-3-nitrophenyl) thiazol-2yl]-benzenesulfonamide (Ro 61-8048), two potent and selective inhibitors of kynurenine hydroxylase. Neuropharmacology. 1999;38:1225–1233. doi: 10.1016/s0028-3908(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 175.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr. Med. Chem. 2011;18:2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yue EW, et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J. Med. Chem. 2009;52:7364–7367. doi: 10.1021/jm900518f. [DOI] [PubMed] [Google Scholar]
- 177.Chiarugi A, et al. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J. Neurochem. 1995;65:1176–1183. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- 178.Madge DJ, Hazelwood R, Iyer R, Jones HT, Salter M. Novel tryptophan dioxygenase inhibitors and combined tryptophan dioxygenase/5-HT reuptake inhibitors. Bioorg. Med. Chem. Lett. 1996;6:857–860. [Google Scholar]
- 179.Parli CJ, Krieter P, Schmidt B. Metabolism of 6-chlorotryptophan to 4-chloro-3-hydroxyanthranilic acid: a potent inhibitor of 3-hydroxyanthranilic acid oxidase. Arch. Biochem. Biophys. 1980;203:161–166. doi: 10.1016/0003-9861(80)90164-2. [DOI] [PubMed] [Google Scholar]
- 180.Lehrmann E, Molinari A, Speciale C, Schwarcz R. Immunohistochemical visualization of newly formed quinolinate in the normal and excitotoxically lesioned rat striatum. Exp. Brain Res. 2001;141:389–397. doi: 10.1007/s002210100887. [DOI] [PubMed] [Google Scholar]
- 181.Yates JR, Heyes MP, Blight AR. 4-chloro-3-hydroxyanthranilate reduces local quinolinic acid synthesis, improves functional recovery, and preserves white matter after spinal cord injury. J. Neurotrauma. 2006;23:866–881. doi: 10.1089/neu.2006.23.866. [DOI] [PubMed] [Google Scholar]
- 182.Uemura T, Hirai K. Kynurenine 3-monooxygenase activity of rat brain mitochondria determined by high performance liquid chromatography with electrochemical detection. Adv. Exp. Med. Biol. 1991;294:531–534. doi: 10.1007/978-1-4684-5952-4_61. [DOI] [PubMed] [Google Scholar]
- 183.Minakata K, Fukushima K, Nakamura M, Iwahashi H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe and NADPH. J Clin Biochem Nutr. 2011;49:207–215. doi: 10.3164/jcbn.11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ceresoli-Borroni G, Guidetti P, Amori L, Pellicciari R, Schwarcz R. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. J. Neurosci. Res. 2007;85:845–854. doi: 10.1002/jnr.21183. [DOI] [PubMed] [Google Scholar]
- 185.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J. Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4:199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- 187.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int. J. Biochem. Cell Biol. 2009;41:467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 188.Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch. Biochem. Biophys. 2000;377:195–203. doi: 10.1006/abbi.2000.1755. [DOI] [PubMed] [Google Scholar]
- 189.Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- 191.Kawai J, Okuno E, Kido R. Organ distribution of rat kynureninase and changes of its activity during development. Enzyme. 1988;39:181–189. doi: 10.1159/000469117. [DOI] [PubMed] [Google Scholar]
- 192.Foster AC, White RJ, Schwarcz R. Synthesis of quinolinic acid by 3-hydroxyanthranilic acid oxygenase in rat brain tissue in vitro. J. Neurochem. 1986;47:23–30. doi: 10.1111/j.1471-4159.1986.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 193.Stachowski E, Schwarcz R. Regulation of quinolinic acid neosynthesis in mouse, rat, and human brain by iron and iron chelation in vitro. J. Neural Transm. 2012;119:123–131. doi: 10.1007/s00702-011-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pucci L, Perozzi S, Cimadamore F, Orsomando G, Raffaelli N. Tissue expression and biochemical characterization of human 2-amino 3-carboxymuconate 6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. FEBS J. 2007;274:827–840. doi: 10.1111/j.1742-4658.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 195.Foster AC, Zinkand WC, Schwarcz R. Quinolinic acid phosphoribosyltransferase in rat brain. J. Neurochem. 1985;44:446–454. doi: 10.1111/j.1471-4159.1985.tb05435.x. [DOI] [PubMed] [Google Scholar]
- 196.Bellac CL, Coimbra RS, Christen S, Leib SL. Inhibition of the kynurenine-NAD+ pathway leads to energy failure and exacerbates apoptosis in pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 2010;69:1096–1104. doi: 10.1097/NEN.0b013e3181f7e7e9. [DOI] [PubMed] [Google Scholar]
- 197.Braidy N, Guillemin GJ, Grant R. Effects of Kynurenine Pathway Inhibition on NAD Metabolism and Cell Viability in Human Primary Astrocytes and Neurons. Int. J. Tryptophan Res. 2011;4:29–37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guillemin GJ, et al. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000;5:108–111. doi: 10.1179/135100000101535375. [DOI] [PubMed] [Google Scholar]
- 199.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 200.Heyes MP, et al. Human microglia convert L-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996;320:595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liao M, et al. Impaired dexamethasone-mediated induction of tryptophan 2,3-dioxygenase in heme-deficient rat hepatocytes: translational control by a hepatic eIF2alpha kinase, the heme-regulated inhibitor. J. Pharmacol. Exp. Ther. 2007;323:979–989. doi: 10.1124/jpet.107.124602. [DOI] [PubMed] [Google Scholar]
- 202.Connor TJ, Starr N, O'Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci. Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]