Abstract
α, β, and γ adducins mediate F-actin remodeling of plasma membrane structures as heterotetramers. Here, we present two new functions of γ-adducin. (1) Overexpression of γ-adducin promoted formation of neurite-like processes in non-neuronal fibroblast COS7 cells. Conversely, overexpression of the C-terminal 38 amino acids of γ-adducin (γAddC38) acting as a dominant negative inhibited formation of neurites/processes in Neuro2A cells and anterior pituitary AtT20 cells. (2) γ-Adducin appears to facilitate pro-opiomelanocortin (POMC) exit from the trans-Golgi network (TGN) by re-organizing the actin network around the Golgi complex. Filamentous actins (F-actins) which formed puncti around the Golgi complex in control cells were dispersed in AtT20 cells stably transfected with γAddC38. Furthermore, γAddC38-transfectants showed significant accumulation of POMC/adrenocorticotropin (ACTH) in the Golgi complex and diminished POMC/ACTH vesicles in the cell processes. The C-terminal 38 amino acids of γ-adducin interacted with F-actins around the Golgi complex, to facilitate F-actin-mediated budding of POMC/ACTH vesicles from the TGN. Thus, we propose that γ-adducin, via its interaction with F-actins, plays a critical role in actin remodeling to facilitate process/neurite outgrowth, as well as budding of POMC/ACTH vesicles from the TGN via its interaction with peri-Golgi F-actins.
Keywords: γ-Adducin, F-actins, Neurite outgrowth, Vesicle budding, TGN, POMC/ACTH
Introduction
γ-Adducin was first cloned from a rat kidney cDNA library (Dong et al. 1995) and belongs to the adducin family, together with α-adducin and β-adducin. The amino acid sequences are generally well conserved between adducins (Matsuoka et al. 2000). These isoforms form two types of tetramers consisting of either α- and β- or α-and γ- adducins (Matsuoka et al. 2000). Adducins are ubiquitously expressed and enriched in synapses of different brain areas including hippocampus and hypothalamus (Yang et al. 2002), and in dendrites and dendritic spines of the CA1 and CA3 regions of the hippocampus (Seidel et al. 1995; Matsuoka et al. 1998), implying their possible roles in the central nervous system. As such, knockout of β-adducin impairs both long-term potentiation and depression, resulting in deficits in learning and memory in mice (Rabenstein et al. 2005; Porro et al. 2009). A recent report indicated that β-adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment (Bednarek and Caroni 2011). However, the role of γ-adducin is less well understood.
Adducins control growth of filamentous actins (F-actins) via their interaction with the fast-growing barbed ends of F-actins, spectrin, protein kinase C, protein kinase A, and calmodulin kinase, thereby affecting actin remodeling at the cortex underneath the plasma membrane (Kuhlman et al. 1996; Matsuoka et al. 2000; Barkalow et al. 2003). The C terminus of adducins contains myristoylated alanine-rich C-kinase substrate (MARCKS) domain that interacts with those proteins involved in remodeling of F-actins (Matsuoka et al. 2000). γ-Adducin is highly expressed in progenitor cells that undergo morphogenetic migration during neural tube closure (Akai and Storey 2002), and its overexpression or mutation induces development of capillary-like tube structures from endothelial cells (Cappuzzello et al. 2007; Matou-Nasri et al. 2009). In addition to F-actin remodeling, adducins also affect vesicle exocytosis (Miyazaki et al. 1994; Burns et al. 1998). Rabphilin-3A implicated in activity-dependent neurotransmitter release interacts with β-adducin (Miyazaki et al. 1994; Burns et al. 1998). Furthermore, we recently demonstrated that the cytoplasmic tail of transmembrane carboxypeptidase E (CPE) present in hypothalamic synaptic vesicles interacted with F-actins via γ-adducin. This interaction was required for localization of the synaptic vesicles in the pre-active zone of hypothalamic presynaptic terminals for proper stimulated secretion of glutamate (Lou et al. 2010). Thus, γ-adducin, via interactions with F-actins, plays dual roles, one in plasma membrane remodeling and the other in synaptic vesicle localization at the synaptic bouton.
In this report, we demonstrate two new roles of γ-adducin. Our study shows that exogenous expression of γ-adducin in non-neuronal COS7 cells induced neurite-like process outgrowth, while overexpression of its C-terminal 38 amino acids (γAddC38) to compete for interaction of endogenous γ-adducin with F-actins inhibited neurite-like process outgrowth in Neuro2A (N2A) cells and anterior pituitary AtT20 cells. Additionally, γ-adducin appears to be involved in the re-organization of the actin network around the Golgi complex to facilitate vesicular exit of POMC/ACTH out of the Golgi complex in AtT20 cells. The F-actin reorganization around the Golgi complex may be mediated by an interaction of γ-adducin. γ-Adducin was also found to bind to the cytoplasmic tail of the transmembrane form of CPE (Lou et al. 2010) which is present in membranes of the trans-Golgi network and POMC containing secretory vesicles in AtT-20 cells (Dhanvantari and Loh 2000; Dhanvantari et al. 2002). This binding may also participate in promoting POMC vesicle budding from the trans-Golgi network (TGN).
Materials and Methods
DNA Constructs
The cDNA of full-length γ-adducin was generated by PCR and subcloned into EcoRI/XhoI sites of pcDNA 3.1+ (Invitrogen, Carlsbad, CA). The cDNA of C-terminal 38 amino acids of γ-adducin (γAddC38) was subcloned into Kpn1/Not1 sites of pcDNA 3.1+ (γAddC38/pcDNA3.1+). Green fluorescence protein (GFP)-tagged full-length γ- adducin (GFP-γ-adducin) and GFP-tagged C-terminal 38 amino acids of γ-adducin (GFP-γAddC38) were generated by subcloning at the XmaI/BglII sites in pEGFP-C1 (Clontech, Mountain View, CA). GST-tagged C-terminal 10-amino-acid of CPE (GST-CPEC10) and GST-tag constructs were described previously (Park et al. 2008).
Antibodies for Immunocytochemistry and Western Blot
Rabbit antibodies against α-adducin (H-100), β-adducin (N-19), and goat antibody to γ-adducin (H-16) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antibody to α-tubulin and p115 was from BD Bioscience (San Jose, CA). Rabbit anti-CPE and POMC/ACTH antibodies were made in our laboratory. Mouse antibodies to actin were from Abcam, Inc. (Cambridge, MA). The Alexa Fluor@ conjugated secondary antibodies used for immunocytochemistry were purchased from Invitrogen. The IRDye™700 and IRDye™800 conjugated secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA) were used for Western blotting.
Cell Culture, Transfection, and Immunocytochemistry
COS7 cells, Neuro2A (N2A) cells, and AtT20 cells (all from A.T.C.C., Manassas, VA) were grown in DMEM containing 10% fetal bovine serum and transfected with cDNA constructs (full-length γ-adducin/pcDNA3.1+, pcDNA3.1+, GFP-γ-adducin, GFP-γADDC38, or GFP vector control) using Lipofectamine2000 (Invitrogen). To select AtT20 cells that were stably expressing γAddC38/pcDNA3.1+, G418 (500 µg/ml) was added to the media of the transiently transfected cells after 48 h. Stable colonies were selected after 3 weeks of transfection.
To study the effect of exogenous full-length γ-adducin on the morphology of the cells, immunocytochemistry was preformed to detect exogenous γ-adducin as described previously after 48 h transfection (Lou et al. 2007). The percent of cells expressing the full-length γ-adducin or its dominant negative form, γAddC38, was calculated with respect to elongated or shortened processes. GFP-expressing cells were examined as control. To examine the effect of γAddC38 on the intracellular distribution of POMC/ACTH vesicles, AtT20 cells were transfected with GFP or GFP-γAddC38 and immunostained for endogenous POMC/ACTH and p115 (a Golgi marker). The number of cells with accumulated POMC/ACTH immunostaining in the cell processes, or with extensive POMC/ACTH staining in the Golgi complex was counted and expressed as a percent of total transfected cells. The mean±standard error of mean (SEM) was obtained from at least three separate experiments. Immunocytochemistry was also used to investigate the localization of endogenous γ-adducin and α-adducin or p115 in AtT20 cells.
To investigate an effect of expression of γAddC38 on microtubule organization and F-actin distribution, we used stably transfected AtT20 cells. A pool of antibiotic-resistant cells was batch-selected from either γAddC38 or vector transfected cells for the experiments. AtT20 cells were immunostained with anti-tubulin, and images were captured on a confocal microscope. The interphase in these cells usually shows a radial arrangement of microtubules in the peri-nuclear region. The number of cells with the radial microtubule arrangement was counted in three independent experiments (416 cells for control and 415 cells for γAddC38 groups). The percent of cells showing the interphase microtubules was calculated. Immunocytochemistry using double-staining of p115 and rhodamine-labeled phalloidin (Cytockeleton, Denver, CO) was performed to investigate F-actin distribution in stably transfected AtT20 cells. The images of F-actin distribution were captured by confocal microscopy. The average intensity and the area of F-actin puncti around the Golgi complex were analyzed using ‘Metamorph’ software. The peri-Golgi region showing F-actin puncti was chosen by a selecting tool for free shape and subject to region measurement tool of Metamorph for calculation of average intensity and area (pcDNA3.1+ alone, n=83; γAddC38/pcDNA3.1+, n=101). In addition, in order to examine the effect of expression of γAddC38 on F-actins inside of cells, we counted the number of stable transfectant cells showing F-actin fibrils or no fibrils in the cytoplasm (pcDNA3.1+ alone, n=313; γAddC38/pcDNA3.1+, n=222).
Western Blotting for Adducins
To examine the protein levels of adducins, cell lysates were prepared in M-PER lysis buffer (Thermo Scientific) and protein concentration were determined with protein assay reagent (Bio-Rad, Hercules, CA, USA). Multiple aliquots of 20 µg of proteins from each cell lysate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After blotting, the nitrocellulose membrane was cut into three pieces and incubated separately with α-, β-, or γ-adducin antibodies and β-actin antibody. The corresponding protein bands were compared for the expression of different isoforms of adducin for each cell line.
Interaction of γ-Adducin with Different Domains of the CPE Cytoplasmic Tail
CPE C-terminal ten amino acids tagged with GST (GST-CPEC10) or GST-tag control were used in co-precipitation with AtT20 cell cytosol as described previously (Park et al. 2008). To identify the region of γ-adducin that interacts with CPE, co-precipitation was performed in the presence and absence of three peptides derived from the C-terminal 38 residues of γ-adducin (Peptide 2.0, Chantilly, VA). AtT20 cell cytosol was pre-incubated with the peptides at two concentrations for 30 min at 4°C prior to co-precipitation. The presence of endogenous γ-adducin in GST-CPEC10 pulldown was detected by Western blotting.
Statistical Analysis
All quantifications in immunocytochemistry and Western blot experiments were carried out at least in three separate experiments (n=3) in each group, unless stated otherwise. Data are presented as mean±SEM. Analyses of statistical significance were performed using student’s t test with two tails to establish statistical significance between experimental groups at the p<0.05 level.
Results
γ-Adducin Promotes Growth of Neurite-like Processes in COS7 Cells
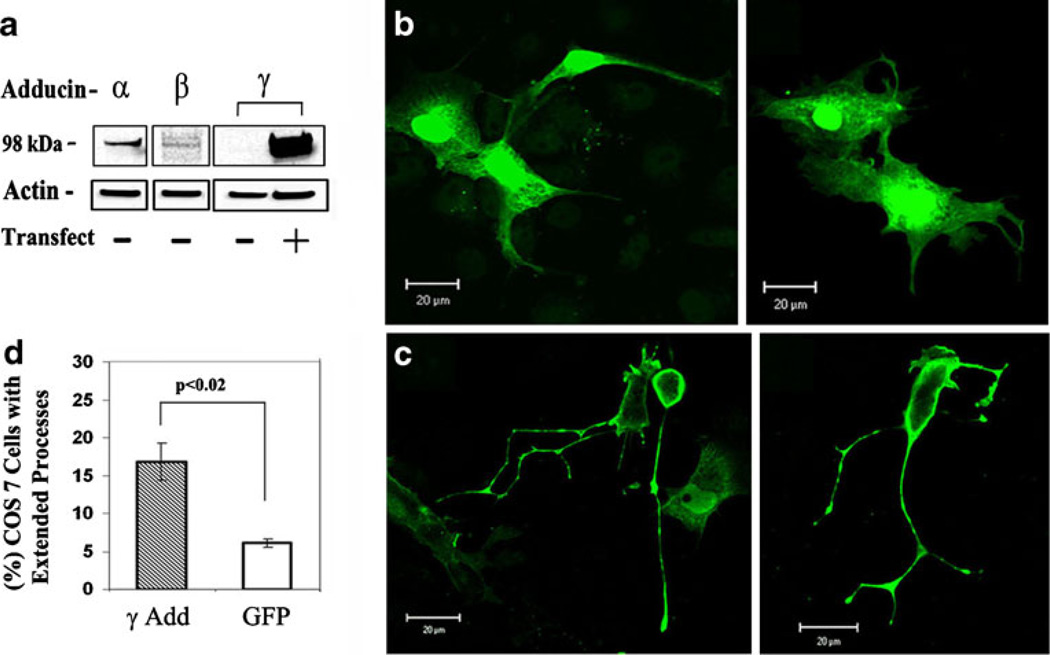
Based on the reports of enrichment of adducins in the dendrites of neurons (Seidel et al. 1995; Matsuoka et al. 1998) and their involvement in capillary tube formation from endothelial cells (Cappuzzello et al. 2007; Matou-Nasri et al. 2009), we speculated that adducins might promote neurite/process outgrowth. To test this hypothesis, we examined the effect of exogenous expression of γ-adducin, an adducin isoform that was detected at very low levels in the fibroblast COS7 (Fig. 1a), on the morphology of the cells which do not normally form long processes. Western blot analysis confirmed that COS7 cells expressed α- adducin and β-adducin but little γ-adducin (Fig. 1a). Full-length γ-adducin transiently transfected into COS7 cells was expressed at high levels (Fig. 1a). We examined the morphology of cells expressing GFP-tagged γ-adducin. In 539 control cells transfected with GFP tag alone, only a few cells (6.1%±0.1, n=3) formed long processes (Fig. 1b), which is similar to untransfected COS7 cells (4.0%±0.2 in 328 cells, p>0.05). In contrast, in 904 cells expressing exogenous GFP-γ-adducin, the percent of cells that formed neurite-like processes (thin processes longer than 1 cell body length and/or have branches) was increased to 16.8%±0.2 (n=5, Fig. 1c). Statistical analysis (Fig. 1d) confirmed that a significantly higher percentage of COS7 cells expressing GFP-γ-adducin formed long processes compared with cells expressing GFP alone (p<0.02). Thus, the exogenous expression of γ-adducin promotes formation of neurite-like processes in non-neuronal fibroblasts.
Fig. 1. COS7 cells with or without transfected γ-adducin.
a The expression of endogenous α, β, and γ-adducins in COS7 cells before (−) or after (+) transfection with cDNA encoding full-length γ-adducin. Twenty micrograms of proteins were analyzed by SDS-PAGE and immunoblotting for adducins and β-actin (loading control). b Representative images of COS7 cells expressing GFP (green). c COS7 cells expressing exogenous GFP-tagged full-length γ-adducin (green). d Bar graphs showing the mean percentage±SEM of the cells that developed elongated processes (processes longer than one cell body length and with branches) among the cells transfected with GFP (n=539 cells) and cells transfected with GFP-γ-adducin (γAdd, n=904 cells) from three experiments. Scale bar=20 µm
C-terminal Domain of γ-Adducin Inhibits Neurite Elongation in N2A Cells
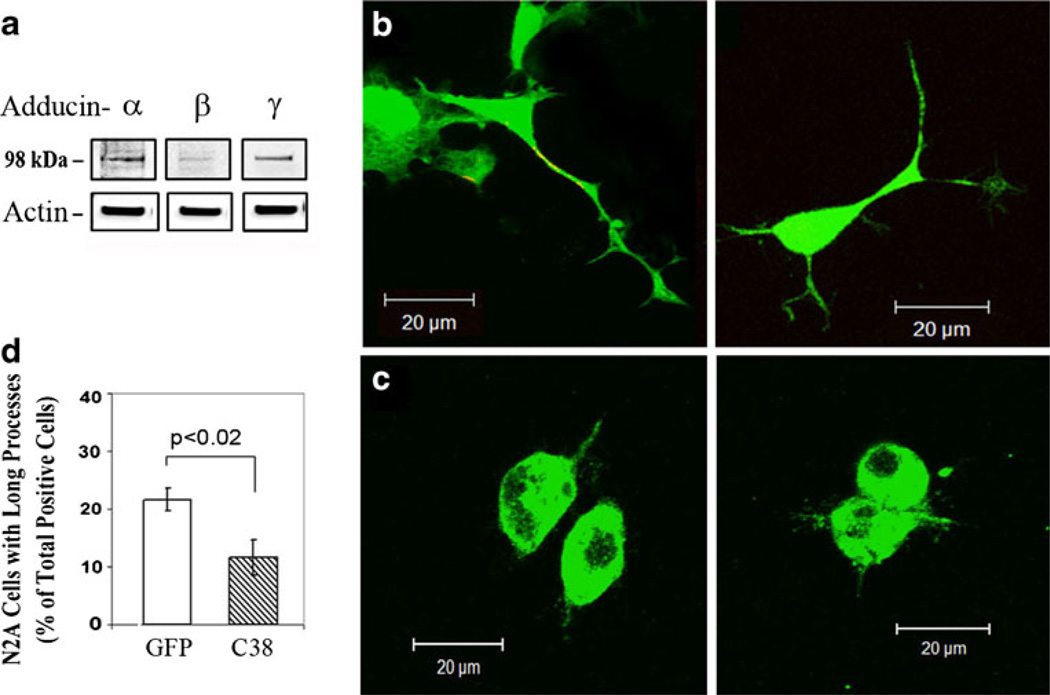
We examined whether we could block neurite/process outgrowth using the C-terminal domain of γ-adducin that mediates most of the protein–protein interactions for F-actin remodeling (Akai and Storey 2002; Cappuzzello et al. 2007; Chen et al. 2007; Lavaur et al. 2009; Matou-Nasri et al. 2009). The C-terminal 38 amino acids of γ-adducin construct was N-terminally tagged with GFP (GFP-γAddC38) and transfected as a dominant negative into neurite-forming neuro2A (N2A) cells that contained all three adducin isoforms endogenously (Fig. 2a). Compared with GFP expression in control N2A cells (n=363) which form normal long neurites (Fig. 2b), overexpression of GFP-γAddC38 (C38, n=375) (Fig. 2c, d) decreased the percent of cells with long neurites by ~50% in three experiments (GFP=21.6%±2.0, vs. C38=11.6%±3.1, p<0.05). These results suggest that the C terminus of γ-adducin is involved in neurite outgrowth in N2A cells.
Fig. 2. N2A cells expressing the C-terminal 38 amino acid residues of γ-adducin (GFP-γAddC38) or GFP controls.
a Expression of endogenous α-, β-, and γ-adducins in N2A cells. Twenty micrograms of proteins in cell lysate were separated by SDS-PAGE gels and analyzed by Western blotting for adducins and β-actin. Representative images of N2A cells expressing GFP (b) or GFP-tagged γ-adducin C-terminal 38 amino acids (GFP-γAddC38) (c). d Bar graphs showing the percentage of N2A cells with long processes in GFP-expressing cells (n=363) or GFP-γAddC38-expressing cells (C38, n=375) from three experiments
C-terminal Domain of γ-Adducin Inhibits Process Elongation in AtT20 Cells
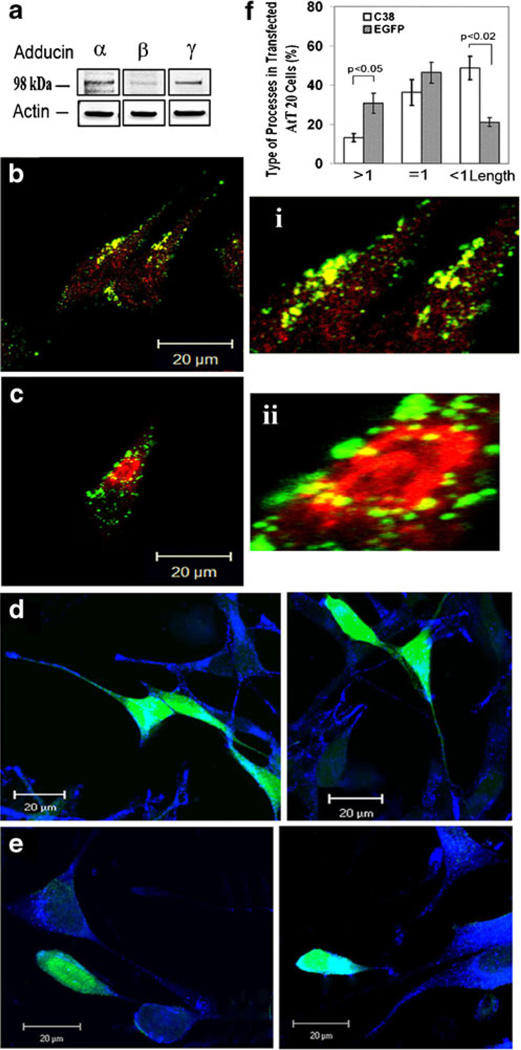
We examined where γ-adducin and its isoforms (α, β) reside in AtT20 cells, which express all three adducin isoforms endogenously (Fig. 3a). α-Adducin (red, Fig. 3b) was ubiquitously expressed throughout the cell, while γ-adducin (green) was found mostly along the plasma membrane, at the process tip, and around the peri-nuclear region of the cells (Fig. 3b). Labeling with antibody to p115, a Golgi marker, showed that γ-adducin (green) resided around the Golgi apparatus (red) (Fig. 3c). Then, we examined whether overexpression of GFP-γAddC38 interfered with formation of processes in AtT20 cells. Processes were visualized by staining with antibodies against secretory granule proteins, POMC/ACTH (blue), which are localized in vesicles and transported along processes to the tips. Among the GFP-expressing cells (n=341), 35.8%±4.1 of them showed processes of longer than one cell body length (Fig. 3d). In contrast, only 12.2% ±3.6 cells expressing GFP-γAddC38 (C38, n=616) showed such long processes (p<0.05) (Fig. 3e and f). Conversely, the percentage of cells showing short processes (shorter than one cell body) was significantly increased in the cells expressing GFP-γAddC38 compared with GFP-expressing cells (44.6± 8.5% vs. 21.41±3.2%, respectively, p<0.02) (Fig. 3f). This result indicates that the C-terminal 38 amino acids of γ-adducin is involved in process elongation in AtT20 cells.
Fig. 3. Effects of adducin on cell morphology in AtT-20 cells.
a The expression of endogenous α-, β-, and γ-adducin and β-actin in 20 µg of proteins from AtT20 cell lysate was detected by Western blotting. b A composite confocal image of AtT20 cells immunostained with antibodies to γ-adducin (green) and α-adducin (red), and its magnified images (i) showed intense staining of γ-adducin in peri-Golgi area and ubiquitous staining of α-adducin throughout the cell. c A confocal image of AtT20 cells that were stained for γ-adducin (green) and a Golgi marker, p115 (red). Magnified image (ii) shows high levels of γ-adducin accumulated around the Golgi area. d Confocal images of GFP-expressing AtT20 cells (green) immunostained for POMC/ACTH (blue) and (e) confocal images of POMC/ACTH vesicles (blue) in GFP-γ-AddC38-expressing AtT20 cells (green). f Bar graphs showing the percentage of the cells with different lengths of processes in cells expressing GFP-γ-AddC38 (C38, n=616) or GFP (n=341) from three experiments. The lengths of processes are categorized as (>1): longer than 1.2 cell body length, (≈1): from 0.9 to 1.1 cell body length, or (<1): shorter than one cell body length. Scale bar=20 µm
C-terminal γ-Adducin Domain Inhibits POMC/ACTH Exit from the Golgi Complex
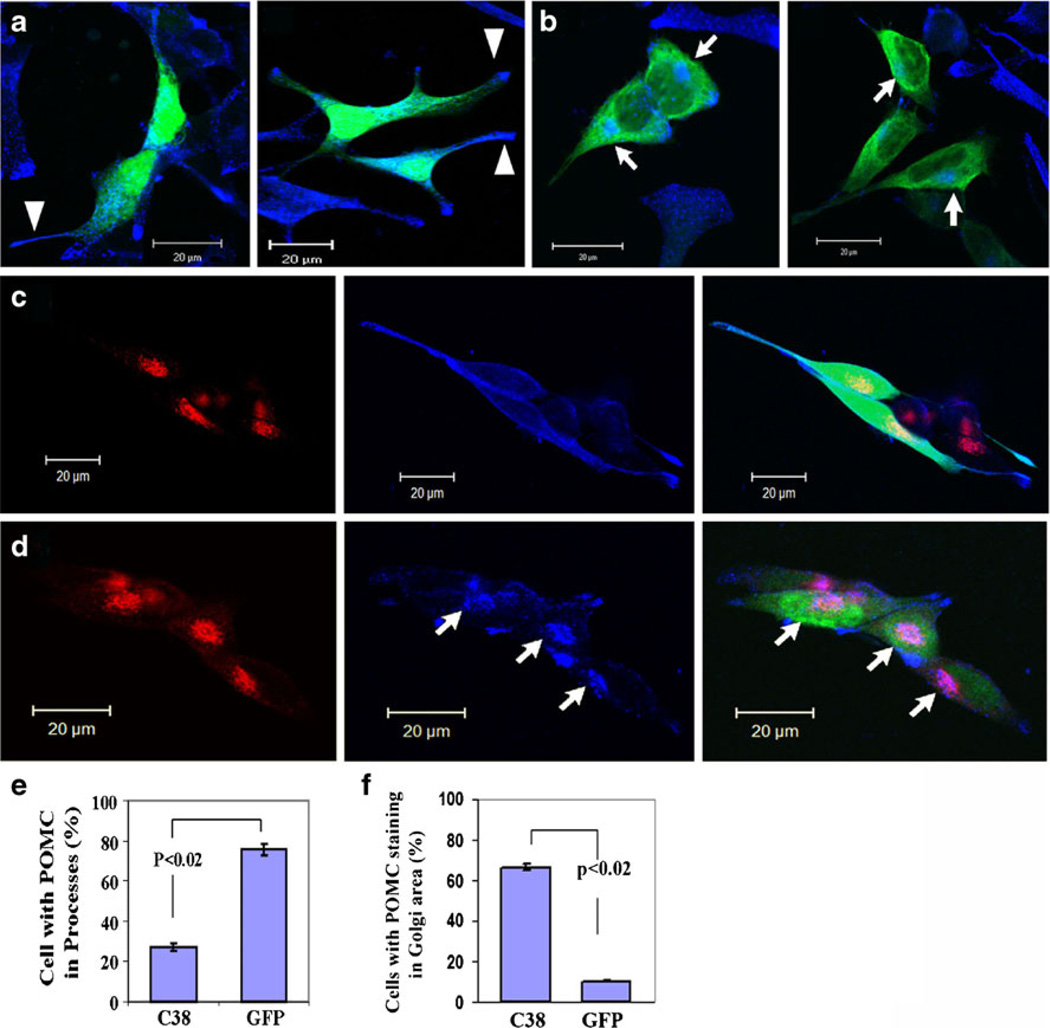
In addition to inhibiting process elongation, expression of GFP-γAddC38 appeared to cause accumulation of POMC/ACTH in the Golgi complex (Fig. 3e).We further investigated the effect of expression ofGFP-γAddC38 on the distribution of POMC/ACTH in AtT20 cells. GFP-expressing cells (n=266) showed distribution of POMC/ACTH vesicles (blue) along and at the tips of the processes (arrow heads) with less in the Golgi region (75.7±3.0%, Fig. 4a, c, e). In contrast, much lower percentage of GFP-γAddC38-expressing cells (n=184) that had short processes showed POMC/ACTH accumulation in the processes (27.4%±1.5 [γC38] vs. 75.7%±2.9 [GFP], p<0.02) (Fig. 4b, e).
Fig. 4. Effect of expression of GFP-γAddC38 on POMC distribution in AtT-20 cells.
a Representative confocal images of GFP-expressing AtT20 cells (green) immunostained for POMC/ACTH (blue). The blue puncti represent POMC/ACTH vesicles accumulated along and at the tips of the processes (see arrowheads). b POMC/ACTH vesicles (blue) in GFP-γAddC38-expressing AtT20 cells (green). Note the lack of POMC/ACTH staining in the processes and the accumulation of POMC/ACTH in the Golgi area (see arrows). c Images of the AtT20 cells immunolabeled for Golgi marker, p115 (red) and POMC/ACTH (blue) in GFP-expressing cells (green). d Colocalization (pink) of p115 (red) and POMC/ACTH (blue) (see arrows) in merged confocal images in GFP-γAddC38-expressing cells (green). e Bar graph showing the percentage of cells with POMC/ACTH in the processes of cells expressing GFP-γAddC38 (C38) (n=184 cells), or GFP (n=266 cells) from three experiments. f Bar graph showing the cells with accumulation of POMC/ACTH in the Golgi area as percent of cells expressing either GFP-γAddC38 (C38, n=101) or GFP (n=83) from two separate experiments. Scale bar=20 µm
Interestingly, GFP-γAddC38-expressing cells showed accumulation of POMC/ACTH mostly in the Golgi complex (labeled with anti-p115 antibody). Most (66.3%%±2.0) of the GFP-γAddC38-expressing cells with processes (n=101) showed significant accumulation of POMC/ACTH in the Golgi complex (arrows) (Fig. 4b, d) but not in the processes. In contrast, only 10.5%±2.0 of GFP-expressing cells (n= 83) showed POMC/ACTH accumulation in the Golgi complex (Fig. 4a, c, f). Figure 4d shows that accumulated POMC/ACTH (blue) (arrows) significantly colocalized (pink) with p115 (red: Golgi) in GFP-γAddC38-expressing cells, but this did not occur in GFP-expressing cells (Fig. 4c). Thus, overexpression of GFP-γAddC38 appears to inhibit vesicular exit of POMC/ACTH from the Golgi complex, resulting in accumulation of POMC/ACTH in the Golgi, with less being transported to the processes.
C-terminal γ-Adducin Domain Perturbs F-actin Organization
Given that γ-adducin is involved in F-actin organization for remodeling of plasma membrane structures, it was possible that introduction of γADDC38 might perturb organization of F-actins for outgrowth of neurite/process (Figs. 2 and 3). In addition, the presence of γ-adducin around the Golgi region (Fig. 3b, c) raised the possibility that it may play a role in actin-dependent vesicular budding of POMC/ACTH from the TGN (Fig. 4).
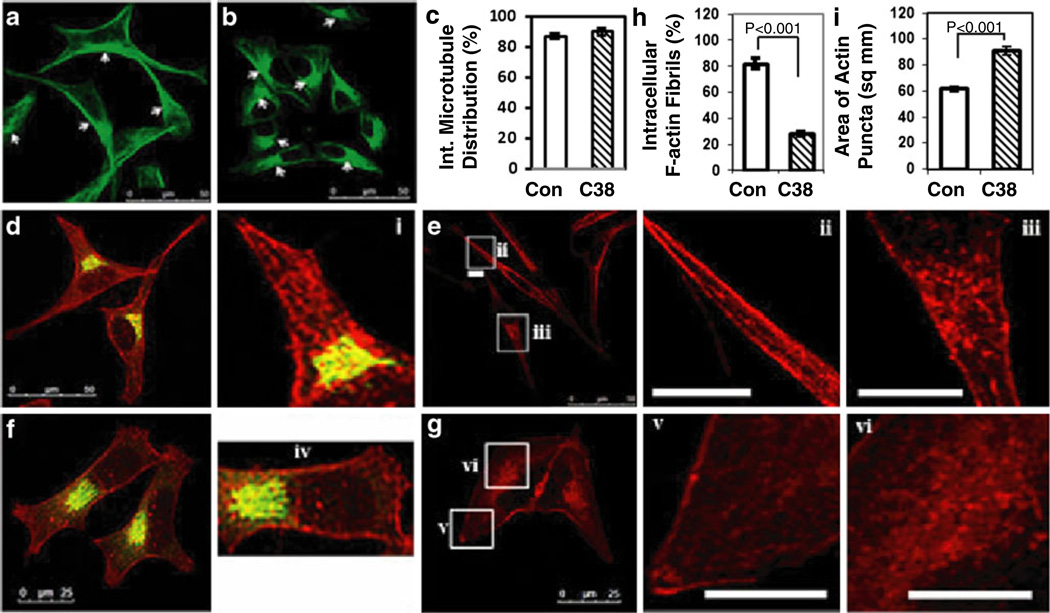
To address this question, we first examined whether γAddC38 expression affects microtubule organization in the stable transfectants. However, we found no significant disruption of interphase microtubule organization in γAddC38 expressing cells compared with control AtT20 cells (Fig. 5a and b, see arrows). The percent of cells showing normal interphase microtubule organization were quantified (control=416 cells and γAddC38=415 cells from three different experiments). Expression of γAddC38 did not perturb the radial arrangement of microtubules from the peri-nuclear region (Control=87%±2, γAddC38=89%±3, p>0.4) (Fig. 5c). This suggests that γAddC38 expression does not affect organization of microtubules.
Fig. 5. Effect of expression of γAddC38 on organization of microtubules and F-actins in AtT-20 cells.
Confocal images of microtubules immunostained with anti-α-tubulin antibody (green) in control AtT20 cells (a), or cells stably expressing γAddC38 (b). c Bar graph showing the percent of cells with normal interphase microtubule arrangements in control cells (Con.) and in cells stably expressing γAddC38 (C38). There is no significant difference between control and γAddC38. d Confocal image of Golgi complex immunostained with anti-p115 antibody (green) and F-actins (rhodamine labeled phalloidin [red]) in control AtT20 cells, or (e) F-actin staining only (red) in control AtT20 cells. f Golgi (green) and F-actin (red) immunostaining in AtT20 cells stably expressing γAddC38. g F-actin-only staining (red) in these cells. The insets i–iii (control) and iv–vi (γAddC38) show the magnified images of F-actin staining (the insets ii and v in the processes and the insets i, iii, iv, and vi in the peri-Golgi area). h Bar graph showing decrease of intracellular F-actin fibrils in the cells stably expressing γAddC38 (C38, n=101) compared with control cells (Con., n=83) (p<0.0001). i Bar graph showing the area of actin puncti distribution around the peri-nuclear region (as square micrometers) in control cells (Con.) and in cells stably expressing γAddC38 (C38, p<0.0001). Scale bar=50 µm (panels a, b, d, e) and 25 µm in panel f. Scale bar in insets=5 µm
To determine the effect of expression of γADDC38 on actin organization along the processes and at the peri-Golgi region, we used stable transfectants of AtT20 cells expressing γAddC38 (γAddC38/pcDNA3.1+) or pcDNA3.1+ alone. AtT20 cells (Con.) transfected with vector alone showed F-actin fibrils along the long processes (Fig. 5d, e and insets i and ii) and F-actin puncti around the Golgi complex (Fig. 5e and inset iii). In contrast, AtT20 cells stably transfected with γAddC38 showed different cell morphology; these cells were more flat in appearance with small pseudo-processes. Actins were dispersed throughout the cytoplasm without forming fibrils (Fig. 5f, g and insets iv–v). These cells showed a reduction in accumulation of F-actin puncti around the Golgi complex (Fig. 5f, g and inset vi). Our quantitative analysis showed that the percent of cells with intracellular F-actin fibrils was significantly decreased in cells expressing γAddC38 compared with control cells (28.2±4.0% in γ-AddC38 vs. 81.9%±4.2% in control, p<0.0001) (Fig. 5h). Moreover, F-actin puncti were not accumulated around the Golgi area in most of the cells expressing γAddC38 (90.95± 3.39 µm−2, p<0.0001) compared with control cells (61.58±2.15 µm−2) (Fig. 5i). However, the average intensity of actins around the Golgi area was not different between control cells (arbitrary units of intensity [AU]=47.68±1.62) and the cells expressing γAddC38 (51.14±1.93 AU, p>0.05). This result suggests that γAddC38 expression affects the organization but not the quantity of actins around the Golgi complex.
The C-terminal Domain of γ-Adducin Interacts with CPE Cytoplasmic Tail
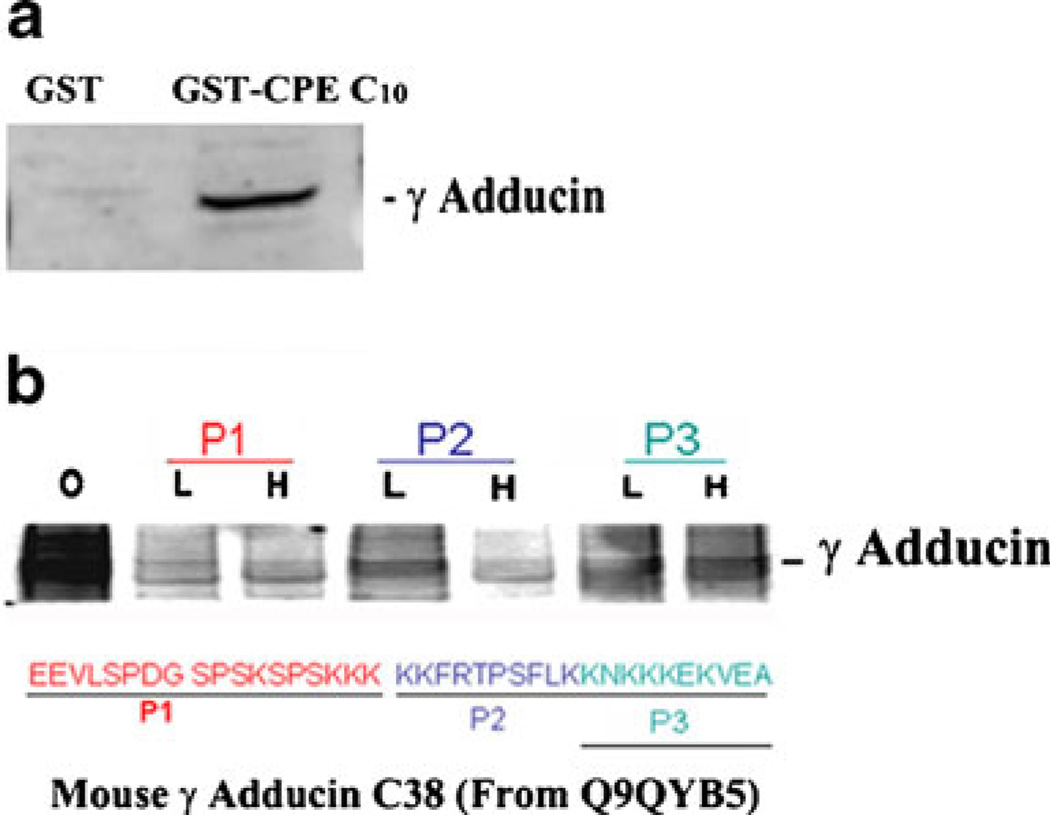
Transmembrane CPE is present in lipid raft domains at the TGN and in secretory vesicles (Dhanvantari and Loh 2000; Dhanvantari et al. 2002). In our previous studies (Lou et al. 2010), we showed that γ-adducin binds directly to the cytoplasmic tail of CPE, in the yeast two-hybrid (γAddC38) and in vitro binding assays (full-length γ-adducin). This prompted us to further investigate the interaction between γ-adducin and the CPE cytoplasmic tail and to identify which part of the C-terminal domain of γ-adducin might be involved. Such an interaction could promote vesicular exit of POMC/ACTH from the TGN. We performed GST pulldown experiments and confirmed that γ-adducin interacted with GST-tagged CPE cytoplasmic tail (GST-CPEC10, Fig. 6a). To identify which region of the C terminus (γAddC38) of γ-adducin interacts with the CPE cytoplasmic tail, three peptides (P1-P3) that corresponded to “669EEVLSPDGSPSKSPSKKK686,” “687KKFRTPSFLK696,” and “697KNKKKEKVEA706” at the C terminus of γ-adducin were used in the in vitro competition assays (Fig. 6b). Same amounts of AtT20 cell cytosol were incubated with GST-CPEC10 in the absence (0) or presence of the three γ-adducin peptides at two different concentrations—10 nM (L) or 100 nM (H). The binding of endogenous full-length γ-adducin to GST-CPEC10 was decreased by all three peptides, but the P1 peptide was the most effective inhibitor of the interaction between γ-adducin and GST-CPEC10. Thus, the P1 peptide-corresponding domain of γ-adducin appears to interact most stringently with the CPE tail.
Fig. 6. Interaction of γ-adducin with CPE cytoplasmic tail in AtT20 cells.
a Western blot of the CPE cytoplasmic tail-bound γ-adducin in AtT20 cell cytosol. AtT20 cytosols were incubated with either GST tag alone or GST-tagged CPE cytoplasmic tail (GST-CPEC10). Proteins bound to GST were examined for γ-adducin by Western blotting. b Competition assay using peptides that correspond to three regions of the γAddC38. Same amount of aliquots (equivalent volume) of AtT20 cell cytosols were incubated with or without 10 nM (L) or 100 nM (H) of the three peptides (P1–P3) before GST pull-down assay using GST-CPEC10. The sequences corresponding to the peptides P1, P2, or P3 are underlined
Discussion
In this study, we have uncovered two new functions of γ-adducin: in promoting process/neurite outgrowth and facilitating vesicular exit of POMC/ACTH from the Golgi complex. Both these functions apparently depend on the final 38 amino acids at the C terminus of γ-adducin (γAddC38), since they are inhibited by expression of γAddC38. The main intracellular cytoskeletal protein that interacts with γ-adducin is F-actin. Hence, F-actins must play a central role in the adducin-mediated process/neurite outgrowth and vesicular exit of POMC/ACTH from the Golgi complex.
F-actins have been demonstrated to help neurite outgrowth at the neurite shaft (Geraldo and Gordon-Weeks 2009). Thus, overexpression of exogenous γ-adducin in COS7 cells, which express very low levels of γ-adducin, may promote reorganization of F-actins to increase microtubule bundling and elongation of processes. On the other hand, overexpression of γAddC38 in N2A and AtT20 cells, both of which express endogenous γ-adducin, inhibited neurite/process outgrowth. Moreover, overexpression of γAddC38 in AtT20 cells caused loss of most filamentous actins in the cytoplasm. This loss of filamentous actins in cells expressing γAddC38 appears to block neurite/process outgrowth. Thus, γ-adducin together with other adducins function in the organization of local actin filaments to promote outgrowth of neurite-like processes. Given that γAddC38 does not significantly affect microtubule organization, the mechanism mediated by γ-adducin for neurite outgrowth appears to be mediated primarily by F-actins.
γ-Adducin also appears to be involved in the vesicular exit of POMC/ACTH from the trans-Golgi network, via interaction with F-actins that cover the cytoplasmic face of the Golgi complex (Godi et al. 1998; Fucini et al. 2000). Cytoskeletal proteins including F-actins have been shown to play a role in vesicle formation/fission and release from the Golgi complex (Fucini et al. 2000; Dubois et al. 2005; De Matteis and Luini 2008; Miserey-Lenkei et al. 2010). A role of γ-adducin in facilitating the exit of POMC/ACTH vesicles from the Golgi is supported by our observation of accumulation of γ-adducin (and α-adducin) around the Golgi complex (Fig. 3). Indeed, overexpression of GFP-γAddC38 caused dispersion of F-actins from the Golgi complex and simultaneous accumulation of POMC in this organelle. We did not observe any abnormal Golgi morphology in GFP-γ-AddC38-expressing cells. Thus, overexpression of γAddC38 may interfere with the interaction between γ-adducin and F-actins required for POMC vesicle budding and release from the TGN. Additionally, we observed that the C terminus of γ-adducin interacted directly and specifically with the cytoplasmic tail of the vesicular protein, CPE, which is present at the TGN of endocrine cells. One could speculate that γ-adducin might act as an intermediary molecule interacting with both the CPE tail, perhaps at a potential budding site on the TGN membrane, and F-actin, to mediate vesicle budding and release. This γ-adducin-mediated link between the transmembrane CPE at the TGN and F-actins could provide a mechanical “pulling force” on the outside of the Golgi membrane to facilitate POMC vesicle formation and exit of the prohormone from the Golgi complex. Conversely, overexpression of γAddC38 which causes disruption of the F-actin network would result in loss of the pulling force for vesicle budding from the TGN and, hence, accumulation of POMC in the Golgi complex. This offers one possible mechanism for the action of γ-adducin in facilitating POMC exit from the Golgi complex.
However, since the C terminus of adducins contains a MARCKS domain that scaffolds protein–protein interactions for F-actin re-organization (Matsuoka et al. 2000), there are other possibilities, for example, the negative effects of overexpression of γAddC38 on neurite/process outgrowth and POMC/ACTH vesicle formation from the TGN may be caused by inhibition of endogenous adducins to scaffold protein–protein interactions for re-organization of F-actins. In addition, overexpression of γ-AddC38 may interrupt F-actin polymerization directly, which was evidenced by loss of F-actin fibrils in stable γAddC38 transfectant, while not in control cells (Fig. 5). These possibilities warrant further study using full-length γ-adducin with the MARCKS domain containing mutations to determine if there is alteration of the interaction of γ-adducin with proteins involved in F-actin organization.
Since there is a high-level of homology in the C-terminal 38 amino acids among γ-adducin, α-adducin, and β-adducin, it is also possible that the γAddC38 dominant negative construct may also affect the function of α-adducin and β-adducin. Therefore, the functions in neurite/process outgrowth and Golgi exit of POMC that we identified in this study may not be limited to γ-adducin. However, the specific localization of γ-adducin (Fig. 3) in the peri-Golgi area supports the hypothesis that γ-adducin may play a major role in facilitating exit of secretory proteins from the Golgi apparatus. The heterogeneity of the composition of heterotetramer consisting of different isoforms (α/β, α/γ) could lead to subtle differences in their function. Future studies using isoform-specific peptides or siRNA will greatly facilitate the identification of the functions of each adducin isoform.
Our study indicates that γ-adducin is involved in two F-actin-based mechanisms: (1) processes/neurite outgrowth for neuronal communication and (2) vesicular exit (or budding) of POMC vesicles/granules from the TGN which may in part be facilitated through interaction with the cytoplasmic tail of transmembrane CPE at the TGN. This latter function of γ-adducin is important for packaging of neuropeptides and hormones in secretory granules for secretion to mediate physiological actions.
Acknowledgments
We thank Dr. Niamh Cawley for helpful discussions (SCN, NICHD). We thank Dr. Vincent Schram (NICHD Microscopy Imaging Core) for technical support. This research was supported by the Intramural Research Program of the NICHD, NIH. Joshua J. Park has been supported by NICHD K22 and ARRA grants.
Contributor Information
Hong Lou, Section on Cellular Neurobiology, Program on Developmental Neuroscience, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bldg 49, Rm 5A22, 49 Convent Drive, Bethesda, MD 20892, USA.
Joshua J. Park, Section on Cellular Neurobiology, Program on Developmental Neuroscience, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bldg 49, Rm 5A22, 49 Convent Drive, Bethesda, MD 20892, USA Department of Neurosciences, University of Toledo, College of Medicine, Toledo, OH 43614, USA.
Andre Phillips, Section on Cellular Neurobiology, Program on Developmental Neuroscience, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bldg 49, Rm 5A22, 49 Convent Drive, Bethesda, MD 20892, USA.
Y. Peng Loh, Email: lohp@mail.nih.gov, Section on Cellular Neurobiology, Program on Developmental Neuroscience, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bldg 49, Rm 5A22, 49 Convent Drive, Bethesda, MD 20892, USA.
References
- Akai J, Storey K. Expression of gamma-adducin is associated with regions of morphogenetic cell movement in the chick embryo. Mech Dev. 2002;119(Suppl 1):S191–S195. doi: 10.1016/s0925-4773(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Barkalow KL, Italiano JE, Jr, Chou DE, Matsuoka Y, Bennett V, Hartwig JH. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek E, Caroni P. beta-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69:1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Burns ME, Sasaki T, Takai Y, Augustine GJ. Rabphilin-3A: a multifunctional regulator of synaptic vesicle traffic. J Gen Physiol. 1998;111:243–255. doi: 10.1085/jgp.111.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuzzello C, Melchionna R, Mangoni A, Tripodi G, Ferrari P, Torielli L, Arcelli D, Helmer-Citterich M, Bianchi G, Capogrossi MC, Napolitano M. Role of rat alpha adducin in angiogenesis: null effect of the F316Y polymorphism. Cardiovasc Res. 2007;75:608–617. doi: 10.1016/j.cardiores.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Chen CL, Hsieh YT, Chen HC. Phosphorylation of adducin by protein kinase Cdelta promotes cell motility. J Cell Sci. 2007;120:1157–1167. doi: 10.1242/jcs.03408. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Loh YP. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem. 2000;275:29887–29893. doi: 10.1074/jbc.M005364200. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 2002;41:52–60. doi: 10.1021/bi015698n. [DOI] [PubMed] [Google Scholar]
- Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, Jaken S. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem. 1995;270:25534–25540. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- Dubois T, Paleotti O, Mironov AA, Fraisier V, Stradal TE, De Matteis MA, Franco M, Chavrier P. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol. 2005;7:353–364. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- Fucini RV, Navarrete A, Vadakkan C, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. Activated ADP-ribosylation factor assembles distinct pools of actin on golgi membranes. J Biol Chem. 2000;275:18824–18829. doi: 10.1074/jbc.M000024200. [DOI] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Santone I, Pertile P, Devarajan P, Stabach PR, Morrow JS, Di Tullio G, Polishchuk R, Petrucci TC, Luini A, De Matteis MA. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc Natl Acad Sci U S A. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Lavaur J, Mineur YS, Picciotto MR. The membrane cytoskeletal protein adducin is phosphorylated by protein kinase C in D1 neurons of the nucleus accumbens and dorsal striatum following cocaine administration. J Neurochem. 2009;111:1129–1137. doi: 10.1111/j.1471-4159.2009.06405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Smith AM, Coates LC, Cawley NX, Loh YP, Birch NP. The transmembrane domain of the prohormone convertase PC3: a key motif for targeting to the regulated secretory pathway. Mol Cell Endocrinol. 2007;267:17–25. doi: 10.1016/j.mce.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Park JJ, Cawley NX, Sarcon A, Sun L, Adams T, Loh YP. Carboxypeptidase E cytoplasmic tail mediates localization of synaptic vesicles to the pre-active zone in hypothalamic presynaptic terminals. J Neurochem. 2010;114:886–896. doi: 10.1111/j.1471-4159.2010.06820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int J Oncol. 2009;35:761–773. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12:645–654. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Shirataki H, Kohno H, Kaibuchi K, Tsugita A, Takai Y. Identification as beta-adducin of a protein interacting with rabphilin-3A in the presence of Ca2+ and phosphatidylserine. Biochem Biophys Res Commun. 1994;205:460–466. doi: 10.1006/bbrc.1994.2688. [DOI] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol. 2008;22:989–1005. doi: 10.1210/me.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro F, Rosato-Siri M, Leone E, Costessi L, Iaconcig A, Tongiorgi E, Muro AF. beta-Adducin (Add2) KO mice show synaptic plasticity, motor coordination and behavioral deficits accompanied by changes in the expression and phosphorylation levels of the alpha- and gamma-adducin subunits. Genes Brain Behav. 2009;9:84–96. doi: 10.1111/j.1601-183X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci. 2005;25:2138–2145. doi: 10.1523/JNEUROSCI.3530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel B, Zuschratter W, Wex H, Garner CC, Gundelfinger ED. Spatial and sub-cellular localization of the membrane cytoskeleton-associated protein alpha-adducin in the rat brain. Brain Res. 1995;700:13–24. doi: 10.1016/0006-8993(95)00962-p. [DOI] [PubMed] [Google Scholar]
- Yang H, Francis SC, Sellers K, DeBarros M, Sun C, Sumners C, Ferrario CM, Katovich MJ, Muro AF, Raizada MK. Hypertension-linked decrease in the expression of brain gamma-adducin. Circ Res. 2002;91:633–639. doi: 10.1161/01.res.0000036749.73316.73. [DOI] [PubMed] [Google Scholar]