Abstract
OBJECTIVE
The objective was to estimate the effect of vaginal childbirth and other obstetric exposures on pelvic muscle strength 6–11 years after delivery and to investigate the relationship between pelvic muscle strength and pelvic floor disorders.
METHODS
Among 666 parous women, pelvic muscle strength was measured with a perineometer 6–11 years after delivery. Obstetric exposures were classified by review of hospital records. Pelvic floor outcomes, including stress incontinence, overactive bladder, anal incontinence, and prolapse symptoms, were assessed with a validated questionnaire. Pelvic organ support was assessed using the Pelvic Organ Prolapse Quantification system. Kruskal-Wallis tests were used to estimate the univariable associations of obstetric exposures and pelvic floor outcomes with peak muscle strength. Stepwise multivariable linear regression models were used to estimate the association between obstetric exposures and muscle strength.
RESULTS
In comparison with women who delivered all of their children by cesarean, peak muscle strength and duration of contraction were reduced among women with a history of vaginal delivery (39 compared with 29 cm H2O, P<.001). Pelvic muscle strength was further reduced after history of forceps delivery (17 cm H2O, P<.001). After vaginal delivery, reduced pelvic muscle strength was associated with symptoms of anal incontinence (P=.028) and pelvic organ prolapse on examination (P=.025); these associations were not observed among those who had delivered exclusively by cesarean.
CONCLUSION
Pelvic muscle strength almost a decade after childbirth is affected by vaginal delivery and by forceps delivery. Although statistically significant, some of the differences observed were small in magnitude.
Pelvic muscle strength decreases after childbirth.,1–3 Moreover, several small studies of postpartum women have shown that pelvic muscle strength is lower after vaginal than after cesarean delivery.4–6 The influence of other aspects of childbirth has not been investigated. Also, the influence of childbirth on pelvic muscle function has not been studied beyond the immediate postpartum period.
Pelvic muscle function is a potentially important outcome of childbirth, because muscle weakness is associated with pelvic floor disorders. For example, pelvic muscle strengthening is recommended to reduce urinary incontinence in the postpartum period and later in life.7 Also, cross-sectional studies suggest an association between urinary incontinence and pelvic muscle weakness,2,6,8 although anal incontinence does not appear to be associated with pelvic muscle weakness.9 Investigations of pelvic muscle strength and pelvic organ prolapse (POP) have reached conflicting conclusions.10–12 A computer simulation model13 predicts that pelvic muscle weakness would result in progressive uterovaginal prolapse. Although this prediction is intriguing, this model has not been validated in vivo.
The current study was undertaken to estimate the effect of vaginal delivery and other obstetric exposures on pelvic muscle strength, measured 6–11 years after first delivery. In the setting of a longitudinal cohort study of maternal health after childbirth,14 we measured pelvic muscle strength among parous women. Our goal was to identify obstetric exposures associated with pelvic muscle strength. We also sought to investigate the relationship between pelvic muscle strength and pelvic floor disorders in this population 6–11 years after a woman’s first delivery.
MATERIALS AND METHODS
This is a supplementary study of the Mothers’ Outcomes after Delivery study, a prospective cohort study of pelvic floor outcomes in women recruited 5–10 years after delivery of their first child.14 Recruitment methods have been described in detail previously.14 Enrolled participants return annually for assessment of pelvic floor disorders and other time-varying exposures.
Institutional review board approval for this supplementary study was obtained from the Johns Hopkins Institutional Review Board. All participants provided written informed consent. Pelvic muscle strength was measured at the second annual study visit (eg, 6–11 years after first delivery) using the Peritron perineometer. The Peritron consists of a 28-mm–diameter compressible probe connected to a handheld microprocessor. The probe is inserted into the vagina. When the probe is compressed, pressure is displayed, in centimeters of water. Previous research has demonstrated that measurements obtained with the Peritron are reproducible and reliable.15,16
Perineometry was performed by the investigators, each of whom demonstrated competency in performing the assessment in a standardized fashion. The assessor of pelvic floor muscle strength was unaware of the participant’s obstetric history and pelvic floor symptoms. Because the Peritron tubing contains latex, women reporting latex allergy were excluded from this study.
Before insertion of the Peritron probe, the participant was instructed in the technique of pelvic muscle contraction, using this script: “Please squeeze your pelvic muscles, as although you were trying to hold in gas.” Digital palpation was then used to confirm correct technique and to exclude accessory contraction of the abdominal or gluteal muscles. The participant was instructed to contract the pelvic floor once as forcefully as possible, to maintain the contraction as long as possible, and to allow the pelvic muscles to relax when the contraction could no longer be maintained.
After the correct technique was confirmed, the Peritron probe was inserted into the vagina and two contractions were measured, with a 10-second rest interval. We recorded peak pressure (in centimeters of water) and contraction duration (in seconds). Peak contraction pressure was averaged over the two recorded contractions. We additionally considered contraction duration, averaged over the two recorded contractions. Ten participants (2%) were unable to perform a pelvic floor contraction despite coaching. Their pelvic muscle pressure was assigned a value of 2 cm H2O, because the lower limit of precision for the Peritron is 5 cm H2O.
The obstetric exposures of interest were derived from abstraction of all delivery records for each participant. Each hospital chart was reviewed by a member of our research team who was also an obstetrician. If delivery records were unavailable (n=61 of 1,285 total deliveries), we substituted maternal recall of delivery events.
Women were classified into five obstetric categories, based on a classification system used in our earlier research.14 The first three groups comprised women who had delivered all of their children by cesarean: women who had delivered all of their children by unlabored cesarean, those who had at least one cesarean delivery during active labor, and those who had at least one cesarean delivery after complete cervical dilation. The other two groups comprised women who had experienced at least one vaginal delivery: those who had only spontaneous vaginal deliveries and those who had experienced at least one operative vaginal delivery. On the basis our previous findings suggesting a strong association between forceps delivery and pelvic floor disorders,17 the operative delivery group was further subdivided into those who had a history of vacuum delivery (but no forceps delivery) and those who had a history of at least one forceps delivery. Among women who delivered vaginally, other variables of potential interest included episiotomy, spontaneous perineal laceration, thirdor fourth-degree perineal laceration, and vaginal delivery of at least one macrosomic neonate (neonatal birth weight of 4,000 g or more). Prolonged second stage of labor (greater than 120 minutes) was determined for all women who reached full cervical dilation.
In addition to obstetric exposures, we considered the following potential confounders: maternal age at the time when muscle strength was quantified, primary race, maternal age greater than 35 years at first delivery, parity, and obesity. Race and parity were self-reported at study enrollment. Each participant’s weight and height were measured, and obesity was defined as a body mass index (calculated as weight (kg)/[height (m)]2) of 30 or greater.
Pelvic floor disorders were assessed at the second study visit (eg, 6–11 years after first delivery). Methods for classifying pelvic floor disorders have been previously reported.14 We used the Epidemiology of Prolapse and Incontinence Questionnaire18 to identify women with bothersome symptoms of pelvic floor disorders. We used the published thresholds from this questionnaire18 to distinguish women with and without each disorder. In addition, pelvic organ support was assessed with the Pelvic Organ Prolapse Quantification examination system.19 Women were classified as having objective evidence of POP if the most dependent point of the vaginal wall or the cervix extended to or beyond the hymen.14,17
Fisher’s exact and Kruskal-Wallis tests were used to estimate the strength of the association between delivery group and maternal characteristics (for categorical variables and continuous variables, respectively). Percentile plots were generated to depict the distribution of peak contraction pressure by delivery group. Kruskal-Wallis tests were used to estimate univariable associations of peak contraction pressure and contraction duration with obstetric exposures and maternal characteristics. Because the univariable analysis revealed substantial reduction of peak pressure in women with history of vaginal deliveries, two stepwise multivariable linear regression models were used: one for women who have delivered only by cesarean and the other for women who have had at least one vaginal delivery. All maternal characteristics, obstetric exposures, and delivery interventions were considered in a stepwise model with a 0.15 significance level for inclusion in the model. The stepwise model for women who underwent cesarean delivery used the unlabored cesarean group as the reference and included indicator variables for active-labor cesarean, cesarean after complete dilation, prolonged second stage, and demographic variables (enrollment age greater than 40 years, African-American race, maternal age at first delivery greater than 35, multiparity, and obesity). The stepwise model for women with at least one vaginal delivery used the spontaneous vaginal delivery group as the reference and included indicator variables for vacuum delivery, forceps delivery, prolonged second stage, vaginal macrosomia, perineal laceration, episiotomy, anal sphincter laceration, three or more vaginal deliveries, and the demographic variables. Years from first delivery (a continuous variable) was also considered in the stepwise models.
To estimate the effect of pelvic muscle strength on pelvic floor disorders, peak strength was compared between women with and without each of the pelvic floor disorders, stratified by delivery type (cesarean only or at least one vaginal delivery). All P values comparing the medians of perineometric results were obtained using a Kruskal-Wallis test.
All analyses were performed using SAS 9.2 statistical software. The percentile plots were created using S-Plus 8.0 statistical software. Statistical significance for all analysis was defined at the 5% significance level.
RESULTS
The second examination was completed by 755 of the 938 women who were due for a second annual examination (80%). There were differences between those who did or did not attend the second visit by age, race, and obesity. Specifically, 77% of women younger than 40 years of age compared with 82% of older women attended the second visit (P=.048). Retention was 73% for African-American women and 81% for all others (P=.040). Last, 82% of women with body mass indexes less than 30 completed the examination compared with 74% for obese women (P=.18). There was no difference in retention by delivery group. Eighty-two women attended the second visit but did not participate in the measurement of pelvic muscle strength (24 declined, 29 reported latex allergy, 2 found the measurement uncomfortable, and 27 did not participate for other reasons). For this analysis, we further excluded seven women because at least one key obstetric variable could not be classified (eg, missing data). Thus, 666 women composed the study population. Maternal characteristics of the six delivery groups of interest are described in Table 1.
Table 1.
Maternal Characteristics of Participants (N=666) by Delivery Group
| Characteristic* | Unlabored Cesarean (n=131) |
Cesarean in Labor (n=150) |
Cesarean After Complete Dilation (n=81) |
Spontaneous Vaginal Delivery (n=225) |
Vacuum Delivery (n=30) |
Forceps Delivery (n=49) |
P† |
|---|---|---|---|---|---|---|---|
| Age (y) | 41.5 (38.3–45.2) | 40.3 (36.9–43.5) | 40.7 (37.5–45.3) | 40.8 (37.3–44.1) | 40.0 (38.3–44.1) | 42.6 (40.5–45.2) | .022 |
| Years from first delivery | 8.2 (7.4–9.8) | 8.5 (7.5–10.0) | 8.3 (7.0–10.2) | 8.6 (7.6–10.4) | 9.5 (7.9–10.9) | 8.7 (7.7–10.3) | .047 |
| African-American race | 12 (9) | 28 (19) | 3 (4) | 23 (10) | 3 (10) | 3 (6) | .009 |
| Maternal age older than 35 y at first delivery | 48 (37) | 36 (24) | 26 (32) | 65 (29) | 5 (17) | 19 (39) | .076 |
| Multiparous | 93 (71) | 107 (71) | 61 (75) | 176 (78) | 24 (80) | 32 (65) | .325 |
| Body mass index 30 kg/m2 or higher | 38 (29) | 49 (33) | 16 (20) | 48 (21) | 5 (17) | 7 (14) | .025 |
Data are median (interquartile range) or n (%) unless otherwise specified.
Measured at the time of muscle strength test unless otherwise noted.
Kruskal-Wallis test for continuous variables; χ2 tests for categorical variables.
Peak contraction pressure (range: 2–124 cm H2O) and duration (range: 0–203 seconds) are compared across maternal and obstetric characteristics in Table 2. There were no significant differences in these outcomes by age, race, parity, or obesity. Significant differences were noted in peak contraction pressure and duration across the six delivery groups (P<.001). A prolonged second stage was associated with significantly reduced peak contraction pressure (P=.009) but duration was not appreciably affected (P=.113). A significant reduction in both strength and duration were associated with macrosomia, perineal laceration, episiotomy, anal sphincter laceration, and number of vaginal deliveries. Only 27 women (4%) reported participating in a program of Kegel exercises. Of those, only 2 (less than 1%) reported a treatment program supervised by a nurse or therapist. Thus, we did not consider previous pelvic muscle therapy as a confounder in these analyses. Because the associations with contraction duration were practically identical to those with peak contraction pressure, analyses hereafter use only the peak pressure.
Table 2.
Perineometry Results for 666 Women by Maternal and Obstetric Characteristics
| Peak Pressure (cm H2O), Averaged Over Two Contractions |
Duration (sec)†, Averaged Over Two Contractions |
||||
|---|---|---|---|---|---|
| Characteristic* | n | Median (IQR) | P‡ | Median (IQR) | P‡ |
| Age older than 40 y | .937 | .754 | |||
| Yes | 380 | 30 (19–45) | 9 (5–15) | ||
| No | 286 | 30 (18–46) | 9 (5–16) | ||
| Primary race | .118 | .121 | |||
| White or other | 594 | 31 (19–46) | 9 (5–16) | ||
| African American | 72 | 28 (16–37) | 8 (4–13) | ||
| Maternal age older than 35 y at first delivery | .340 | .068 | |||
| Yes | 199 | 28 (18–43) | 8 (4–13) | ||
| No | 467 | 31 (19–46) | 10 (5–17) | ||
| Multiparous | .590 | .389 | |||
| Yes | 493 | 31 (18–46) | 9 (5–15) | ||
| No | 173 | 29 (18–44) | 9 (5–16) | ||
| Body mass index 30 kg/m2 or higher | .175 | .892 | |||
| Yes | 163 | 31 (21–48) | 9 (5–15) | ||
| No | 503 | 30 (18–45) | 9 (5–16) | ||
| Obstetric exposure group | <.001 | <.001 | |||
| Unlabored cesarean | 131 | 39 (24–50) | 10 (6–16) | ||
| Active-labor cesarean | 150 | 36 (23–52) | 10 (6–21) | ||
| Cesarean after complete dilation | 81 | 35 (23–48) | 10 (6–19) | ||
| Spontaneous vaginal delivery | 225 | 27 (17–39) | 7 (4–14) | ||
| Vacuum delivery | 30 | 25 (14–31) | 8 (5–14) | ||
| Forceps delivery | 49 | 14 (9–23) | 4 (3–11) | ||
| Prolonged second stage§ | .009 | .113 | |||
| Yes | 158 | 27 (15–42) | 8 (4–13) | ||
| No | 508 | 31 (19–46) | 9 (5–16) | ||
| Macrosomia at vaginal delivery‖ | <.001 | .001 | |||
| Yes | 47 | 20 (11–31) | 6 (3–11) | ||
| No | 619 | 31 (19–46) | 9 (5–16) | ||
| Perineal laceration‖ | <.001 | .017 | |||
| Yes | 183 | 25 (14–37) | 7 (4–14) | ||
| No | 483 | 33 (21–48) | 9 (5–16) | ||
| Episiotomy‖ | <.001 | <.001 | |||
| Yes | 171 | 23 (13–34) | 6 (3–12) | ||
| No | 495 | 33 (21–49) | 10 (5–17) | ||
| Anal sphincter laceration‖ | <.001 | .039 | |||
| Yes | 57 | 21 (12–30) | 7 (4–12) | ||
| No | 609 | 31 (20–46) | 9 (5–16) | ||
| No. of vaginal deliveries | <.001 | <.001 | |||
| 0 | 362 | 36 (24–51) | 10 (6–18) | ||
| 1 | 107 | 26 (15–38) | 8 (4–15) | ||
| 2 | 147 | 25 (14–37) | 7 (4–13) | ||
| 3 or more | 50 | 21 (13–31) | 6 (3–12) | ||
IQR, interquartile range.
As measured at the time of muscle strength test, unless otherwise noted.
Duration data were missing for four women.
Kruskal-Wallis test.
Among those who experienced the second stage of labor at least once.
Among those who experienced at least one vaginal delivery.
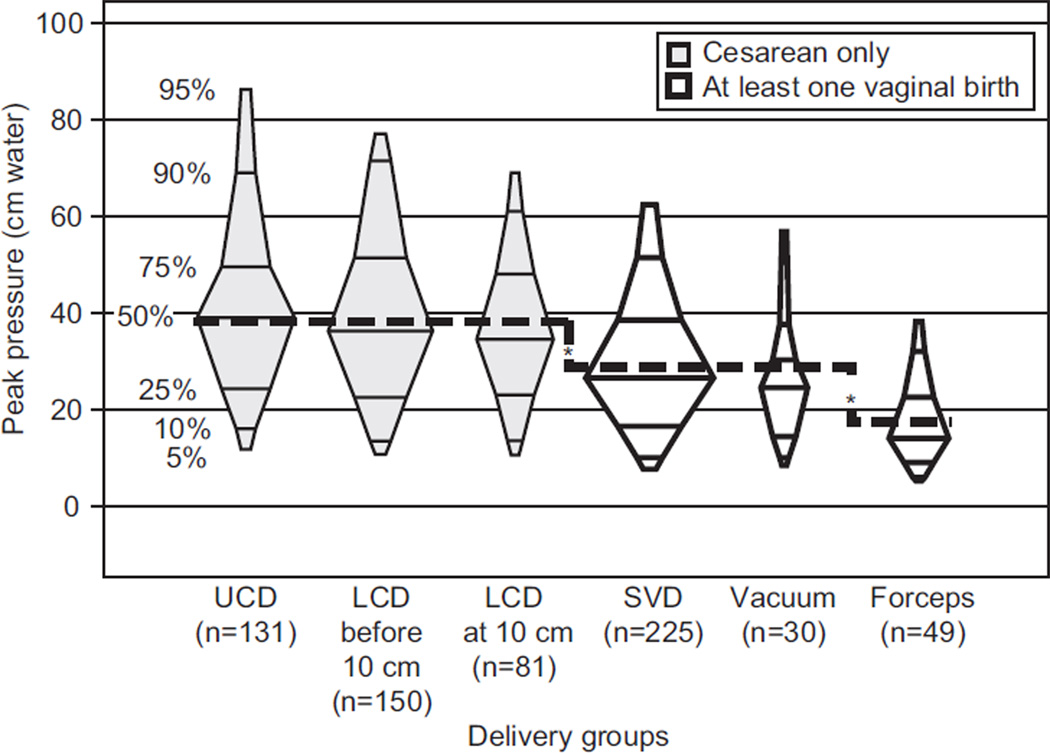
Figure 1 depicts pelvic muscle strength across delivery groups. Linear regression analysis was performed, with peak pressure as the dependent variable and five indicator variables for the six obstetric history groups. With unlabored cesarean delivery as the reference category, there was no significant difference in peak pressure for the other two cesarean delivery groups (P=.551 for cesarean in labor and P=.120 for cesarean after complete cervical dilation). In contrast, the three vaginal groups showed significant reduction of peak pressure (P<.001). With spontaneous vaginal delivery as the reference category, peak pressure was not lower in the vacuum delivery group (P=.306), but there was a significant reduction in this outcome for forceps delivery (P<.001). The mean peak pressure for the three cesarean groups was 39 cm H2O, the mean for spontaneous vaginal delivery and vacuum delivery was 29 cm H2O (P<.001), and the mean for forceps delivery was 17 cm H2O (P<.001).
Fig. 1.
Peak contraction pressure, in centimeters of water, for six delivery groups. The middle width of each box is proportional to the number of women in that delivery group. The dashed line shows the mean peak pressure for the delivery groups based on a standard linear regression with five indicators. The mean peak pressure for the three cesarean groups is 39 cm H2O, the mean for spontaneous vaginal delivery and vacuum delivery is 29 cm H2O (P<.001), and the mean for forceps delivery is 17 cm H2O (P<.001). UCD, unlabored cesarean delivery; LCD before 10 cm, labored cesarean delivery performed in active labor but before complete cervical dilation; LCD at 10 cm, labored cesarean delivery performed after complete cervical dilation; SVD, spontaneous vaginal delivery. *P<.001.
Friedman. Pelvic Muscle Strength After Childbirth. Obstet Gynecol 2012.
In a multivariable stepwise linear-regression model, we considered separately women who had delivered all their neonates by cesarean and those who had experienced at least one vaginal delivery. Among women who delivered exclusively by cesarean, pelvic muscle strength was significantly was associated with race. Specifically, African-American women had a peak pressure 8.8±3.4 cm H2O lower than that of women of other races (P=.010). In a model controlling for race, the only other variable that approached significance was cesarean delivery after complete cervical dilation: women who experienced cesarean delivery after complete cervical dilation had a peak pressure that was 4.4±2.6 cm H2O lower than those in the unlabored cesarean group, but this difference was not statistically significant (P=.097).
Among women who experienced at least one vaginal delivery, peak contraction pressure was reduced 10.7±2.5 cm H2O among women who had delivered by forceps compared with women without a forceps delivery (P<.001). Women with three or more vaginal deliveries had peak pressure of 5.1±2.5 cm H2O lower than did women with one or two vaginal deliveries (P=.042). Pelvic muscle strength was very similar between women with one compared with two vaginal deliveries. In a model controlling for forceps delivery and vaginal parity, the only other variable that approached significance (P=.122) was episiotomy, but the reduction was small: 2.9±1.9 cm H2O.
Analysis of the association between pelvic muscle strength and pelvic floor disorders was stratified by delivery type, considering separately women who had delivered all of their neonates by cesarean and those who had experienced at least one vaginal delivery (Table 3). Among women who delivered exclusively by cesarean, peak contraction strength was not associated with pelvic floor disorders, except that POP was associated with higher peak pressure (P=.031). In contrast, among women with at least one vaginal delivery, strength was significantly poorer among women with anal incontinence (P=.028), symptoms of prolapse (P=.016), and prolapse on examination (P=.025). Also, among women with at least one vaginal delivery, pelvic muscle strength was significantly lower among 113 women with at least one pelvic floor disorder compared with 191 women without any pelvic floor disorder (P=.012).
Table 3.
Mean Peak Pressure (in cm H2O) According to Presence of Pelvic Floor Disorders, Stratified by Delivery Type
| Pelvic Floor Disorders* | n (%) | Cesarean Delivery Only (n=362) |
P† | At Least One Vaginal Delivery (n=304) |
P† |
|---|---|---|---|---|---|
| Stress urinary incontinence | .761 | .996 | |||
| Yes | 53 (8) | 38 (26–49) [18] | 21 (15–41) [35] | ||
| No | 613 (92) | 36 (23–51) [344] | 25 (14–35) [269] | ||
| Overactive bladder | .976 | .739 | |||
| Yes | 26 (4) | 35 (26–45) [9] | 21 (12–39) [17] | ||
| No | 640 (96) | 37 (24–51) [353] | 24 (14–35) [287] | ||
| Anal incontinence | .952 | .028 | |||
| Yes | 70 (11) | 36 (24–49) [36] | 19 (8–34) [34] | ||
| No | 596 (89) | 37 (24–51) [326] | 25 (15–36) [270] | ||
| Prolapse symptoms | .315 | .016 | |||
| Yes | 16 (2) | 46 (32–66) [4] | 14 (9–21) [12] | ||
| No | 650 (98) | 36 (24–51) [358] | 25 (15–36) [292] | ||
| Prolapse on examination | .031 | .025 | |||
| Yes | 72 (11) | 44 (36–57) [17] | 20 (10–31) [55] | ||
| No | 594 (89) | 36 (23–50) [345] | 25 (15–36) [249] | ||
| Any pelvic floor disorder | .149 | .012 | |||
| Yes | 177 (27) | 39 (30–51) [64] | 21 (11–33) [113] | ||
| No | 489 (73) | 36 (23–50) [298] | 26 (16–36) [191] |
Data are median (interquartile range) [n] unless otherwise specified.
Women with pelvic floor disorders included two who had undergone previous physical therapy, four who were currently receiving medications for urinary incontinence, and two who had undergone surgery for treatment of incontinence or prolapse.
Kruskal-Wallis test.
DISCUSSION
These data demonstrate a statistically significant reduction in pelvic muscle strength associated with vaginal compared with cesarean delivery 6–11 years after childbirth. Most notable is the reduction in strength after forceps delivery. Previous studies3–5 have suggested a reduction in pelvic muscle strength after vaginal compared with cesarean delivery but have typically not been blinded (the examiner was aware of delivery events) and have been limited to the first 6 months after delivery. Our data suggest that childbirth has a durable effect on pelvic muscle function almost a decade after childbirth.
The observed reduction in pelvic muscle strength is important because poor pelvic muscle strength is associated with pelvic floor disorders. In our cohort, anal incontinence and POP were associated with reduced pelvic muscle strength after vaginal childbirth. A similar pattern was not observed after cesarean delivery. Thus, our results raise the question of whether the mechanism for the development of pelvic floor disorders may differ after vaginal compared with cesarean delivery. However, the present results pertain only to a single point in time, 6–11 years after a first delivery. This might be an explanation, for example, for the apparent lack of association between muscle weakness and urinary incontinence. Also, although the differences reported here were statistically significant, some of these differences were small in magnitude and we cannot say with certainty whether these differences are clinically significant. Follow-up of this study cohort will establish whether women with weaker pelvic muscles are more likely to develop pelvic floor symptoms in the future and whether the differences seen here will increase over time. Further longitudinal follow-up of this cohort will help to establish whether pelvic muscle weakness is central to the biological pathways leading to pelvic floor disorders.
A limitation of this research is that we could not account for all aspects of a woman’s obstetric history (eg, intervals between deliveries and sequence effects). We also do not have information about pelvic muscle strength before delivery. Thus, we cannot exclude the possibility that the observed differences in pelvic muscle strength preceded childbirth. In addition, we do not have information about overall fitness or athletic conditioning, which could play a role in maintaining pelvic muscle strength. We also do not know the pelvic muscle strength for women who declined or were unable to participate in this evaluation. Finally, because muscle strength and pelvic floor disorders were assessed at the same point in time, we do not know the temporal relationship between these conditions. Therefore, we cannot conclude with certainty that pelvic muscle weakness preceded the development of anal incontinence or POP.
Some of these findings were unexpected. Specifically, in the cesarean group, strength was decreased among African-American women and increased among those with prolapse on examination. These are both unexpected and may be a result of alpha error (eg, apparent statistical association when in truth none exists). Also, the sample size for these subgroups was small (43 African-American women in the cesarean group and 17 women with prolapse in the cesarean group), limiting our ability to explore potential confounders. Further study is necessary to establish the importance of these observations regarding the possible causes of pelvic muscle weakness and its purported role of the development of prolapse.
Strengths of our study include the large sample size, the period of time examined after an index delivery (6–11 years), multiple obstetric exposures considered, and the assessment of pelvic muscle strength with a validated and reliable tool.15,16 In addition, the use of a validated questionnaire and quantitative measure of POP provides the unique opportunity to correlate muscle strength with both subjective and objective measures of pelvic floor disorders.
Given the high prevalence of pelvic floor disorders, prevention is critical to reduce the public health burden of pelvic floor disorders among U.S. women. Research on the relationship between pelvic floor disorders and pelvic muscle weakness may provide a novel target for secondary prevention after vaginal childbirth. We acknowledge that the study was not designed to determine the effects of age on pelvic muscle strength. However, because this study is longitudinal, we potentially have the opportunity to reassess the relationship of age on pelvic muscle strength. Pelvic muscle strength almost a decade after childbirth is affected by vaginal birth and by forceps delivery. Although statistically significant, some of the differences observed were small in magnitude.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD056275).
Footnotes
Presented at the American Urogynecologic Society meeting, October 4, 2012, Chicago, Illinois.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Valeton CT, do Amaral VF. Evaluation of urinary incontinence in pregnancy and postpartum in Curitiba Mothers Program: a prospective study. Int Urogynecol J. 2011;22:813–818. doi: 10.1007/s00192-011-1365-8. [DOI] [PubMed] [Google Scholar]
- 2.Sampselle CM. Changes in pelvic muscle strength and stress urinary incontinence associated with childbirth. J Obstet Gynecol Neonatal Nurs. 1990;19:371–377. doi: 10.1111/j.1552-6909.1990.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdardottir T, Steingrimsdottir T, Arnason A, Bø K. Pelvic floor muscle function before and after first childbirth. Int Urogynecol J. 2011;22:1497–1503. doi: 10.1007/s00192-011-1518-9. [DOI] [PubMed] [Google Scholar]
- 4.Baytur YB, Deveci A, Uyar Y, Ozcakir HT, Kizilkaya S, Caglar H. Mode of delivery and pelvic floor muscle strength and sexual function after childbirth. Int J Gynaecol Obstet. 2005;88:276–280. doi: 10.1016/j.ijgo.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Peschers UM, Schaer GN, DeLancey JO, Schuessler B. Levator ani function before and after childbirth. Br J Obstet Gynaecol. 1997;104:1004–1008. doi: 10.1111/j.1471-0528.1997.tb12057.x. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson E, Victor A, Svärdsudd K. Determinants of urinary incontinence in a population of young and middle-aged women. Acta Obstet Gynecol Scand. 2000;79:208–215. [PubMed] [Google Scholar]
- 7.Landefeld CS, Bowers BJ, Feld AD, Hartmann KE, Hoffman E, Ingber MJ, et al. National Institutes of Health State-of-the-Science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449–458. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JA, O’Sullivan PB, Briffa NK, Neumann P. Assessment of voluntary pelvic floor muscle contraction in continent and incontinent women using transperineal ultrasound, manual muscle testing and vaginal squeeze pressure measurements. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:624–630. doi: 10.1007/s00192-006-0081-2. [DOI] [PubMed] [Google Scholar]
- 9.King VG, Boyles SH, Worstell TR, Zia J, Clark AL, Gregory WT. Using the Brink score to predict postpartum anal incontinence. Am J Obstet Gynecol. 2010;203:486.e1–486.e5. doi: 10.1016/j.ajog.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Borello-France DF, Handa VL, Brown MB, Goode P, Kreder K, Scheufele LL, et al. Pelvic Floor Disorders Network. Pelvic-floor muscle function in women with pelvic organ prolapse. Phys Ther. 2007;87:399–407. doi: 10.2522/ptj.20060160. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 12.Ghetti C, Gregory WT, Edwards SR, Otto LN, Clark AL. Severity of pelvic organ prolapse associated with measurements of pelvic floor function. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:432–436. doi: 10.1007/s00192-004-1274-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108:324–332. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol. 2011;118:777–784. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hundley AF, Wu JM, Visco AG. A comparison of perineometer to brink score for assessment of pelvic floor muscle strength. Obstet Gynecol. 2005;192:1583–1591. doi: 10.1016/j.ajog.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Frawley HC, Galea MP, Phillips BA, Sherburn M, Bø K. Reliability of pelvic floor muscle strength assessment using different test positions and tools. Neurourol Urodyn. 2006;25:236–242. doi: 10.1002/nau.20201. [DOI] [PubMed] [Google Scholar]
- 17.Handa VL, Blomquist JL, McDermott KC, Friedman S, Muñoz A. Pelvic floor disorders after vaginal birth: effect of episiotomy, perineal laceration, and operative birth. Obstet Gynecol. 2012;119:233–239. doi: 10.1097/AOG.0b013e318240df4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:272–284. doi: 10.1007/s00192-005-1314-5. [DOI] [PubMed] [Google Scholar]
- 19.Bump RC, Mattiasson A, Bø K, DeLancey JO, Klarskov P, Shull BL, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]