Abstract
Alcohol use and misuse is known to involve structural brain changes. Numerous imaging studies have examined changes in gray matter (GM) volumes in dependent drinkers, but there is little information on whether non-dependent drinking is associated with structural changes and whether these changes are related to psychological factors – such as alcohol expectancy – that influence drinking behavior. We used voxel based morphometry (VBM) to examine whether the global positive scale of alcohol expectancy, as measured by the Alcohol Expectancy Questionnaire AEQ-3, is associated with specific structural markers and whether such markers are associated with drinking behavior in 113 adult non-dependent drinkers (66 women). Alcohol expectancy is positively correlated with GM volume of left precentrral gyrus (PCG) in men and women combined and bilateral superior frontal gyri (SFG) in women, and negatively correlated with GM volume of the right ventral putamen in men. Furthermore, mediation analyses showed that the GM volume of PCG mediate the correlation of alcohol expectancy and the average number of drinks consumed per occasion and monthly total number of drinks in the past year. When recent drinking was directly accounted for in multiple regressions, GM volume of bilateral dorsolateral prefrontal cortices (DLPFC) correlated positively with alcohol expectancy in the combined sample. To our knowledge, these results are the first to identify the structural brain correlates of alcohol expectancy and its mediation of drinking behaviors. These findings suggest that more studies are needed to investigate increased GM volume in the frontal cortices as a neural correlate of alcohol expectancy.
Keywords: cerebral morphometry, frontal cortex, gender difference, positive alcohol expectancy, prefrontal cortex, social drinkers
Introduction
According to the National Epidemiologic Survey on Alcohol and Related Conditions, approximately 70 percent of young adults in the United States consumed alcohol in the year 2004. Many of these 19 million individuals are engaged in heavy and binge drinking and become alcohol dependent. Alcohol dependence involves a wide range of serious medical and non-medical conditions such as alcohol-related liver diseases, violence, and traffic accidents. Understanding the psychological and neural processes leading to heavy, habitual and uncontrollable use of alcohol is an important public health issue.
An important psychological factor that contributes to alcohol misuse is alcohol expectancy. Alcohol expectancy describes expectancy of alcohol effects, as can be measured by the Alcohol Expectancy Questionnaire (AEQ, (Brown et al., 1980)). It is posited that alcohol use behavior is related to individuals’ learning and experience with the outcome of alcohol use (Brown et al., 1985). A belief that “drinking makes it easier to concentrate on the good feelings I have at the time” – a global positive expectancy on the AEQ – is conducive to alcohol consumption.
Clinical and behavioral studies provided evidence validating alcohol expectancy as a psychological construct in relation to alcohol use. For instance, alcohol expectancy mediates attentional bias to alcohol-related cues in social drinkers (Field et al., 2011; Townshend and Duka, 2001). Alcohol expectancy predicted alcohol involvement in adolescents (Cranford et al., 2010; Shell et al., 2010; Urban et al., 2008) as well as alcohol consumption and hazardous drinking in young adults (Supplementary references S1–S8). In young adults, alcohol expectancy mediates the association between reward sensitivity and hazardous alcohol use (Gullo et al., 2010) and may predict or interact with impulsivity, social anxiety, or mood state to predict alcohol use and alcohol-related problems (Fu et al., 2007; McCarthy et al., 2001; Meade Eggleston et al., 2004; Stein et al., 2000; Zamboanga, 2006). Furthermore, challenges to positive alcohol expectancy in multiple behavioral sessions reduced alcohol consumptions in young heavy drinkers (Dunn et al., 2000; Wiers and Kummeling, 2004). Changes in alcohol expectancy over the treatment period predicted outcome in dependent patients undergoing cognitive behavioral therapy (Young et al., 2011). Together, these studies support the conceptual import and utility of alcohol expectancy as a psychological risk factor and predictor of alcohol misuse.
Brain imaging is a powerful tool to unravel the cerebral processes underlying alcohol misuse. Alcohol expectancy seemed to be associated with anterior cingulate activation during working memory and vigilance tasks in adolescents (Gundersen et al., 2008; Pulido et al., 2009). However, to our knowledge, there have not been systematic studies of alcohol expectancy as a neural endophenotype of alcohol misuse (Goldman, 2002). Recently, investigators showed that inter-subject behavioral variability can be predicted by local structure of white and gray matters, as measured by voxel-based morphometry (VBM, (Ashburner and Friston, 2000)). The association between individual behavior and brain anatomy has been observed in a wide range of laboratory tasks involving perception and decision making, as well as general intelligence (Haier et al., 2004) and the Big Five personality traits (Supplementary references S9–S12). Investigation of the cerebral morphometrical correlates of alcohol expectancy may inform an important intermediate phenotype of alcohol use and misuse.
As a step to understanding the structural cerebral basis of alcohol use behaviors, the current study examined the gray matter (GM) volume correlates of alcohol expectancy in non-dependent drinkers. The AEQ underwent several revisions since its introduction by Brown et al. (1980), and in a recent version (AEQ-3,(George et al., 1995)), alcohol expectancy comprises eight subscales. Although the expectancy subcomponents are statistically discernible, the high subscale inter-correlations (ranging from r=0.42 to 0.92, mean=0.78) suggest that the degree of distinctiveness among the subscales is at best modest ((George et al., 1995); see also Results). Thus, in the current study, we focused on the global positive factor as a variable to identify inter-subject variations in the GM volume correlates of alcohol expectancy. Furthermore, because alcohol expectancy is likely to be associated with alcohol use, which is known to affect cerebral structures, we assessed the correlation of the structural changes with recent alcohol use and how these structural alterations mediate the relationship between alcohol expectancy and recent alcohol use. In particular, because men and women show important differences in their drug and alcohol using behaviors and clinical profiles of substance/alcohol use disorders (Beck et al., 1995; Brady and Randall, 1999; Derringer et al., 2010; Greenfield et al., 2010; Hensing and Spak, 2009; Kampov-Polevoy et al., 2004; McGue et al., 1997; Schulte et al., 2009), we explored these structural correlates in a sample combining men and women as well as separately in men and women.
We adopted an exploratory approach in the current study, because, to our knowledge, no studies have examined the structural cerebral bases of alcohol expectancy. On the other hand, although there were no known studies on the structural correlates of alcohol expectancy, a few functional imaging and evoked potential studies may provide useful information for more focused analyses (Anderson et al., 2005; Deckel et al., 1995; Gundersen et al., 2008). In particular, Gundersen and colleagues directly manipulated alcohol expectancy in a working memory task and observed greater activation of precentral cortices when participants expected to receive alcohol (Gundersen et al., 2008). Similarly, Deckel and colleagues demonstrated that alcohol expectancy is related to frontal functioning in an electroencephalographics study (Deckel et al., 1995). Thus, we also focused on the frontal cortex including the precentral gyrus as a specific region of interest, in addition to voxelwise whole brain analyses.
Materials and Methods
Subjects and Assessment
Study participants were recruited from flyers posted in the greater New Haven, Connecticut area. All participants were screened to be free of major medical illness, past or present neurological (e.g., epilepsy, learning impairments, head trauma) and psychiatric illnesses including substance (except nicotine) use disorders (SCID-I for DSM-IV; (Supplementary reference S13), denied current use of illicit substance, and showed negative urine toxicology tests for stimulants, opioids, marijuana, and benzodiazepines at the time of initial screening and fMRI. Individuals who were using any psychotropic medications were not invited to participate in the study. Pregnant or lactating women were also excluded. Participants were further required to be free of MRI-contraindications based on the Yale Magnetic Resonance Research Center’s safety guidelines.
One hundred and thirteen social drinkers (66 women; age 32 ± 14 years; all right-handed) were invited and paid to participate. All participants completed questionnaires regarding their alcohol use over the past year, including average number of days of alcohol use and the average number of drinks consumed per occasion, framed on a monthly basis. Alcohol use data were also collected for the Alcohol Use Disorders Identification Test (AUDIT) scores (S14). AUDIT scores are calculated from the sum of ten self-report questions regarding level of alcohol use, alcohol-related problems, and concern expressed by others for one’s drinking behavior. Each question receives a score ranging from 0 to 4, with higher numbers corresponding to a greater level of risk for having or developing an alcohol use disorder. The mean (±SD) AUDIT scores were 3.0 (±2.5) for women, 4.4 (±3.6) for men, and 3.6 (±3.1) for women and men combined. These AUDIT scores appeared to be typical of non-dependent drinkers and are significantly lower than those reported for alcohol dependent individuals (S15). In an effort to examine the relationship between alcohol expectancy and recent alcohol consumption, we focused our analyses on self-reported monthly drinking measures reported for the previous year. A summary of demographic and clinical measures is presented in Table 1A.
Table 1.
Demographics of participants and correlations between alcohol expectancy (AE) and drinking variables.
| (A) Demographics of participants. | ||||
|---|---|---|---|---|
| Groups | all (n=113) | women (n=66) | men (n=47) | p value* |
| Age | 32.2 ± 13.6 | 30.8 ± 12.6 | 34.2 ± 14.8 | 0.1887 |
| AUDIT | 3.6 ± 3.1 | 3.0 ± 2.5 | 4.4 ± 3.6 | 0.0145 |
| Monthly frequency of drinking (F) | 4.5 ± 5.0 | 3.6 ± 4.0 | 5.9 ± 6.0 | 0.0184 |
| Number of drinks per occasion (N) | 2.2 ± 1.6 | 2.0 ± 1.4 | 2.5 ± 1.8 | 0.1057 |
| Monthly number of drinks (F ×N) | 12.0 ± 14.9 | 9.0 ± 11.9 | 16.1 ± 17.5 | 0.0113 |
| AEQ (GP) | 9.2 ± 3.9 | 9.1 ± 3.8 | 9.4 ± 4.1 | 0.7178 |
| BIS | 59.5 ± 8.9 | 58.6 ± 9.1 | 60.8 ± 8.5 | 0.2035 |
| (B) Correlations between alcohol expectancy (AE) and drinking variables with Spearman regression. | ||||||
|---|---|---|---|---|---|---|
| Groups | all (n=113) | women (n=66) | men (n=47) | |||
| AE correlations | coef (s) | p-value | coef (s) | p-value | coef (s) | p-value |
| Monthly frequency of drinking | 0.4793 | 7.84e-08 | 0.444 | 1.88e-04 | 0.5192 | 1.84e-04 |
| Number of drinks per episode | 0.4634 | 2.36e-07 | 0.5823 | 2.91e-07 | 0.3123 | 0.0326 |
| Monthly number of drinks | 0.5052 | 1.16e-08 | 0.5033 | 1.65e-05 | 0.4877 | 5.07e-04 |
Note:
two-tailed two-sample t test
Participants were assessed with the Alcohol Expectancy Questionnaire (AEQ-3; (S16)) and the Barratt Impulsivity Scale (BIS-11, (S17)). The AEQ-3 consisted of 40 items to address both positive (6 subscales) and negative (2 subscales) alcohol expectancy. Each subscale contains 4 to 6 statements that can be endorsed on a six-point scale, from “disagree strongly (1)” to “agree strongly (6)”. The global positive subscale contains five items and thus ranges from 5 to 30 in total score, with a greater score indicating higher global positive alcohol expectancy. The BIS-11 measured an impulsive personality trait, which has been implicated in alcohol misuse and was included in the analyses as a covariate.
All subjects signed a written informed consent, in accordance to a protocol approved by the Yale Human Investigation Committee.
Imaging protocol
Participants were scanned on a Siemens 3-Tesla scanner (Trio; Siemens AG, Erlangen, Germany). Data for each participant consisted of a single high-resolution T1-weighted gradient-echo scan: 176 slices; 1 mm3 isotropic voxels; field of view = 256 × 256 mm; data acquisition matrix = 256 × 256; TR =2530 ms; TE = 3.66 ms, bandwidth = 181 Hz/pixel; flip angle = 7°.
Voxel-based morphometry (VBM)
The aim of VBM is to identify differences in the local composition of brain tissue and its association with behavioral and cognitive measures, while discounting large scale differences in gross anatomy and position. This can be achieved by spatially normalizing individuals’ structural images to the same stereotactic space, segmenting the normalized images into distinct brain tissues, smoothing the gray-matter images, and performing a statistical test to localize significant associations between anatomical and behavioral measures (Ashburner and Friston, 2000).
Voxel-based morphometry was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) packaged in Statistical Parametric Mapping 8 (Wellcome Department of Imaging Neuroscience, University College London, U.K.). T1-images were first co-registered to the Montreal Neurological Institute or MNI template space (1.5 mm3 isotropic voxels) using a multiple stage affine transformation, during which the 12 parameters were estimated. Co-registration started with a coarse affine registration using mean square differences, followed by a fine affine registration using mutual information. In this step, coefficients of the basis functions that minimize the residual square difference (between individual image and the template) were estimated. Tissue probability maps (TPM) constructed from 471 healthy subjects were used in affine transformation. After affine transformation, T1-images were corrected for intensity bias field (kernel size FWHM = 60mm) and a local means denoising filter (Manjon et al., 2010) with default parameter 1 was applied, to account for intensity variations (inhomogeneity) and noise caused by different positions of cranial structures within MRI coil; and, finally, they were segmented into cerebrospinal fluid, gray and white-matters, using an adaptive maximum a posteriori (MAP) method (S18) with k-means initializations, as implemented in VBM8, generating tissue class maps (which included the grey matter or GM maps). In segmentation, partial volume estimation (PVE) was performed with default parameter 5, with a simplified mixed model of at most two tissue types (S19). Segmented and the initially registered tissue class maps were normalized using Dartel (S20), a fast diffeomorphic image registration algorithm of SPM. As a high-dimensional non-linear spatial normalization method, Dartel generates mathematically consistent inverse spatial transformations. We used the standard Dartel template in MNI space, constructed from 550 healthy subjects of the IXI-database (http://www.brain-development.org/), to drive the Dartel normalization. Normalized GM maps were modulated to obtain the absolute volume of GM tissue corrected for individual brain sizes. Finally, the GM maps were smoothed by convolving with an isotropic Gaussian kernel. Smoothing helps to compensate for the inexact nature of spatial normalization and reduces the number of statistical comparisons (thus making the correction for multiple comparisons less severe); however, it reduces the accuracy of localization. Most VBM studies used a kernel size of FWHM=12mm. We used a smaller kernel size of FWHM=8mm to achieve localization accuracy.
In group analyses, we used two models of multiple regressions, both of which were done for women and men combined, as well as for women and men separately. In the first model, we regressed the GM volumes of the whole brain against the alcohol expectancy (AEQ global positive) score, with the Barratt Impulsivity Scale (BIS-11) score and age as covariates. We included impulsivity score as a covariate because it has been reported to be associated with GM volume differences (S21). Because recent drinking affects GM volumes, we considered the influence of drinking in the past year by including drinking variables in the second model. Thus, in this second multiple regression, we used alcohol expectancy score, impulsivity score, age, and drinking variables, including the average monthly frequency of drinking and the average number of drinks consumed in a single occasion, as regressors.
Mediation analysis
To test whether GM volume variation of the regions of interest mediates the correlation between alcohol expectancy and drinking variables, we performed mediation analyses (S22), using the toolbox M3, developed by Tor Wager and Martin A. Lindquist (http://wagerlab.colorado.edu/tools). Mediation analysis was successfully applied to fMRI of emotion regulation (S23) and functional connectivity analysis (Ide and Li, 2011).
In a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e. X→Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing three regression equations (S22):
where a represents X→M, b represents M→Y (controlling for X), c′ represents X→Y (controlling for M), and c represents X→Y. The constants i1, i2, i3 are the intercepts, and e1, e2, e3 are the residual errors. In the literature, a, b, c and c′ were referred as path coefficients or simply paths (S22, S23). We followed this notation in this work. Variable M is said to be a mediator of connection X→Y, if (c – c′) is significantly different from zero, which is mathematically equivalent to the product of the paths a*b (S22). If the product a*b and the paths a and b are significant, one concludes that X→Y is mediated by M. In addition, if path c′ is not significant, there is no direct connection from X to Y and that X→Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and it should not be confused with the correlation coefficient between Y and M.
Results
Alcohol expectancy and drinking variables
Global positive alcohol expectancy averaged across participants at 9.2 ± 3.9 (mean ± standard deviation), similar to the mean of 9.7 reported earlier for a cohort of 1,260 social drinkers (S16). The global positive subscale was highly correlated with other subscale scores with a rho ranging from 0.683 to 0.849 (all p’s <0.00001, Spearman regression). These results suggest that our cohort is typical of a population of social drinkers in terms of alcohol expectancy and that the global positive subscale can capture much of the variance in alcohol expectancy as assessed by AEQ-3.
Using Spearman regression, we correlated the AEQ-3 global positive score with average monthly frequency of drinking (F), number of drinks consumed per occasion (N), and the average monthly total number of drinks consumed (F × N), in women and men combined as well as separately. The results showed that alcohol expectancy was positively correlated with all drinking measures except the number of drinks per occasion in men (Table 1B).
Voxel-based Morphometry (VBM)
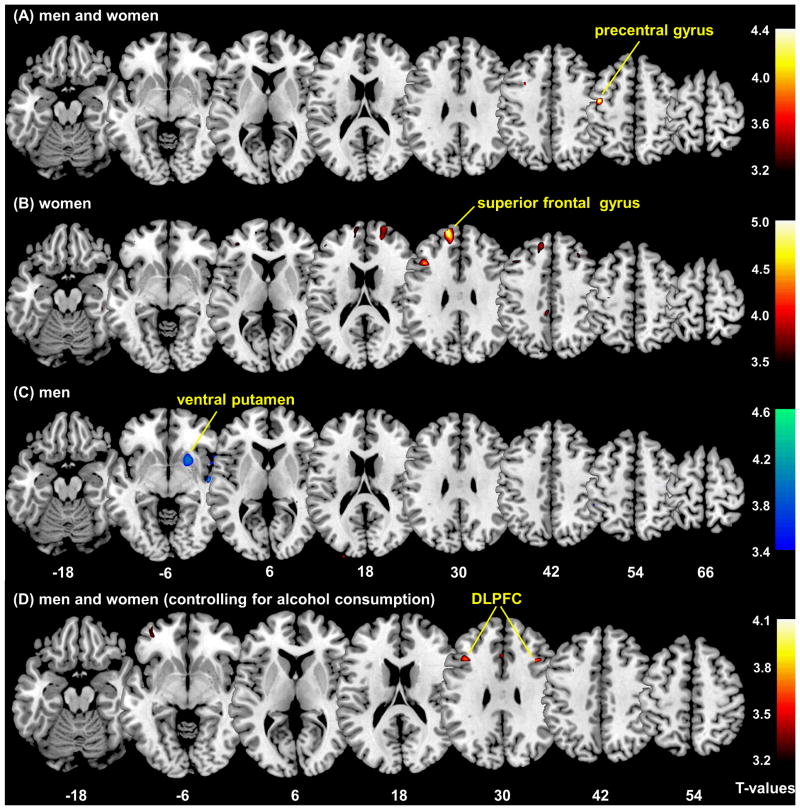
In multiple regressions (first model) based on a threshold of voxel p<0.001, uncorrected and 100 voxels of cluster size, regions that correlated positively with alcohol expectancy included: left precentral gyrus (PCG; 126 voxels, peak voxel MNI coordinate [−41 −6 55], Z=3.85) in men and women combined (Figure 1A); as well as bilateral superior frontal gyri (SFG; left: 1,255 voxels, peak voxel [−14 55 31], Z=4.69; right: 489 voxels, peak voxel [21 60 22], Z=3.89; Figure 1B) in women. Regions that correlated negatively with alcohol expectancy included: right ventral putamen (449 voxels, peak voxel [21 13 −4], Z=3.59; Figure 1C) in men. The finding of the left precentral gyrus in the combined sample was significant at a threshold of p<0.05, corrected for family-wise error of multiple comparison in a region of interest analysis using small volume correction for the middle frontal and primary motor cortices obtained from the Automated Anatomic Labeling atlas (S24).
Figure 1.
Voxel based morphometry: multiple regressions against alcohol expectancy. The clusters show regions with GM volume correlated with alcohol expectancy, p<0.001, uncorrected. (A) men and women combined (n=113): precentral gyrus; (B) women (n=66): superior frontal gyrus; (C) men (n=47): ventral putamen. (D) When alcohol consumption is accounted for as a covariate, the GM volume of bilateral dorsolateral prefrontal cortices (DLPFC) correlated positively with alcohol expectancy in men and women combined (n=113), p<0.001, uncorrected. Color bars represent voxel T value; warm color: positive correlation with AEQ; cool color: negative correlation with AEQ. The numbers at the bottom are z coordinates of axial sections.
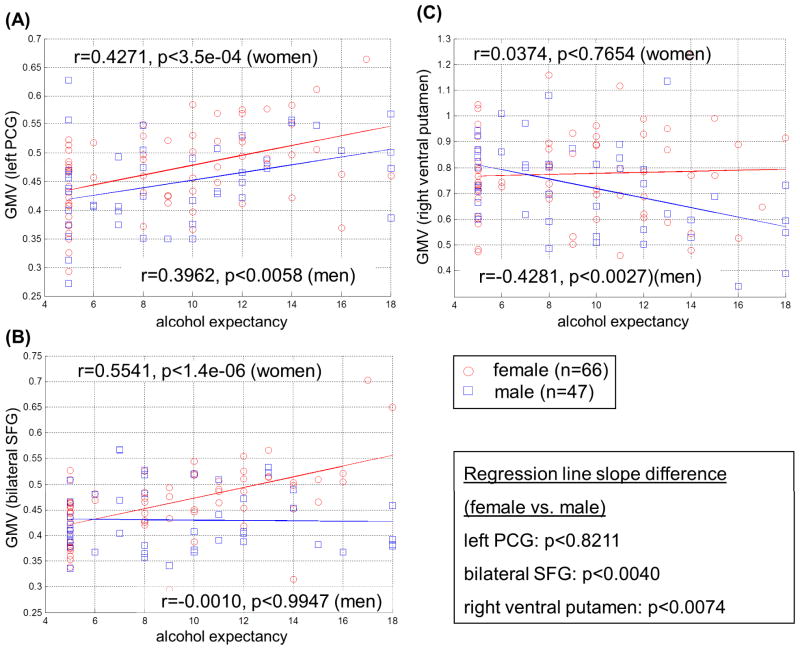
We extracted the effect size of GM volume for these regions of interest (ROIs). Figure 2 shows the correlation between the GM volume of these ROIs and global positive alcohol expectancy in their respective samples. We also compared the GM volume of these ROIs between man and women, with women showing a marginally significant greater GM volume in the bilateral SFG (p<0.0102, two-tailed two-sample t test) but not in the left PCG (p<0.1111) or right ventral striatum (p<0.1731). Most importantly, we assessed gender differences by comparing the regressions of GM volume and alcohol expectancy between men and women for each of the ROIs (S25). A significant difference in the slope indicated that the correlations between GM volume and AEQ were different between men and women. The results showed that men and women differed for the bilateral SFG (t=2.9429, p=0.0040), and right ventral putamen (t=2.7304, p=0.0074) but not left PCG (t=0.2267, p=0.8211).
Figure 2.
Correlation of GM volume of regions of interest (ROIs) with alcohol expectancy plotted for men and women separately: (A) left precentral gyrus; (B) bilateral superior frontal gyri; and (C) right ventral putamen. Inset shows results of differences in the regression slope between men and women for each of the ROIs.
In the second regression model, at a threshold of p<0.001, uncorrected and 50 voxels in the extent of activation, the GM volume of bilateral dorsolateral prefrontal cortices (left: 130 voxels, peak voxel [−39 21 31], Z=3.73; right: 60 voxels, peak voxel [40 21 31], Z=3.79) correlated positively with alcohol expectancy (Figure 1D). In both regression models, we also included smoking status (smoker or not) as a categorical covariate. The results of region of interest analyses showed clusters with identical peak coordinates.
Correlation of GM volumes and drinking variables
With Spearman’s regression we examined the correlations of the four clusters – (left precentral gyrus, right and left superior frontal gyri, and the right ventral putamen) – with the average monthly frequency of drinking, number of drinks per occasion, and monthly total number of drinks, in their respective samples (Table 2A). Correcting for multiple tests, we considered correlations with a p value less than 0.05/12(4 ROI’s and 3 drinking variables)=0.00417 as significant. The GM volumes of left precentral gyrus (left PCG) showed a significant, positive correlation with all drinking variables in the combined sample. The left superior frontal gyrus (left SFG) showed a significant, positive correlation with the number of drinks per occasion but not the frequency of drinking or the total number of drinks per month in women. The right superior frontal gyrus (right SFG) and right ventral putamen did not show any significant correlations with any drinking parameters, and were thus not included in mediation analyses.
Table 2.
ROI analysis of GM volumes and results of mediation analyses.
| (A) Correlations between GM volumes and drinking variables: Spearman regression. | |||
|---|---|---|---|
| ROI | Frequency/month | Drinks/time | Drinks/month |
| Left PCG (n=113) | r = 0.29 (p<0.0017) | r = 0.39 (p<1.6e-05) | r = 0.35 (p<0.0001) |
| Left SFG (n=66) | r = 0.27 (p<0.0286) | r = 0.35 (p<0.0040) | r = 0.32 (p<0.0081) |
| Right SFG (n=66) | r = 0.01 (p<0.9594) | r = 0.15 (p<0.2414) | r = 0.04 (p<0.7598) |
| Right ventral putamen (n=47) | r = −0.25 (p<0.0957) | r = 0.08 (p<0.6069) | r = −0.14 (p<0.3627) |
| (B) Mediation analysis results between alcohol expectancy (AE), drinking variables and GM volumes. | |||||
|---|---|---|---|---|---|
| Mediation models | Path a (X→M) | Path b (M→Y) | Path c′ (X→Y) | Mediation path a*b | |
| Model 1 (n=113) | β | 0.40 | 0.03 | 0.30 | - |
| X (AE) → Y (Freq./month) mediated by M (left PCG) | p | 0.0005* | 0.7656 | 0.0007* | 0.7297 |
|
| |||||
| Model 2 (n=113) | β | 0.40 | 0.26 | 0.25 | - |
| X (AE) → Y (Drinks/time) mediated by M (left PCG) | p | 0.0006* | 0.0088* | 0.0107* | 0.0060* |
|
| |||||
| Model 3 (n=113) | β | 0.29 | 0.34 | 0.24 | - |
| X (AE) → Y (Drinks/month) mediated by M (left PCG) | p | 0.0028* | 0.0008* | 0.0085* | 0.0010* |
|
| |||||
| Model 4 (n=66) | β | 0.53 | 0.04 | 0.54 | - |
| X (AE) → Y (Drinks/time) mediated by M (left SFG) | p | 0.0000* | 0.5754 | 0.0064* | 0.5171 |
β denotes the regression coefficients and p values are uncorrected. The mark “*” indicates p<0.05, Bonferroni corrected (p=0.05/4=0.0125).
Mediation analysis
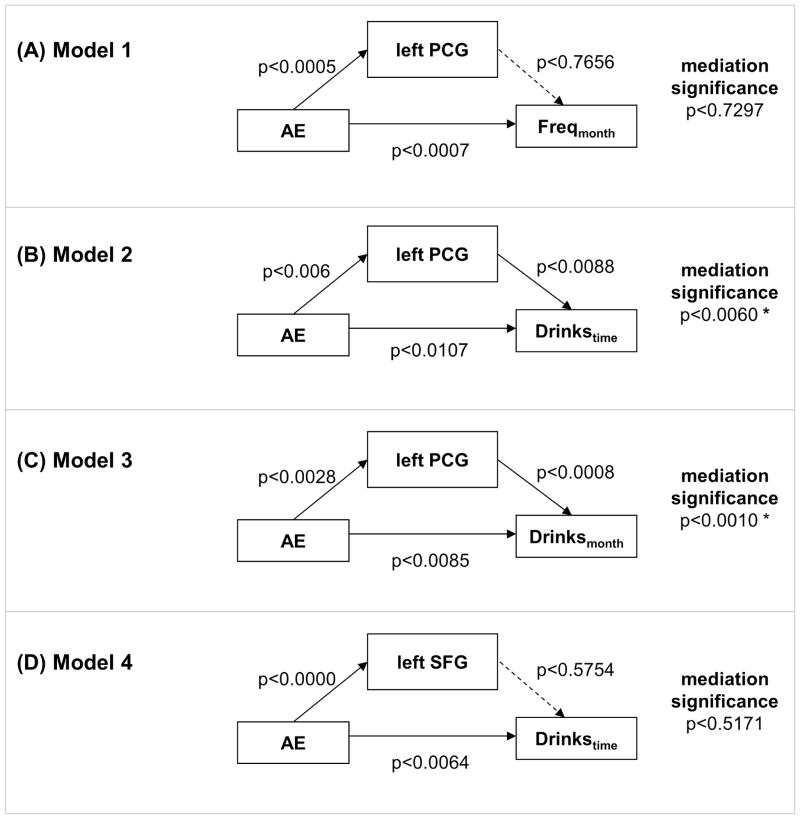
We performed mediation analysis to test whether the association between alcohol expectancy and drinking variables were mediated by GM volumes of the left precentral gyrus (PCG), in women and men combined, and the left superior frontal gyrus (SFG), in women. We did not consider the right superior frontal gyrus or the right ventral putamen, since their GM volumes were not correlated with drinking measures. We derived for individual subjects the average GM volume for each of the regions of interest, and performed a single-level mediation analysis. We tested whether the association between alcohol expectancy (X) and each of the three drinking measures was mediated by GM volumes (M) of left PCG in women and men combined (n=113). Similarly, we tested whether the association between alcohol expectancy and the number of drinks per occasion was mediated by the GM volume of the left SFGC in women alone (n=66) (Figure 3 and Table 2B). The results showed that the correlation between alcohol expectancy and the number of drinks consumed per occasion and total number of drinks consumed per month, but not monthly frequency of drinking, were mediated by GM volume of the left PCG in the combined sample. In contrast, the left SFG did not mediate the correlation between alcohol expectancy and the number of drinks consumed per occasion in women.
Figure 3.
Single-level mediation analysis of alcohol expectancy (AE), drinking variables, and GM volumes. Dashed arrows indicate the lack of a significant connectivity at p<0.05, Bonferroni corrected (p=0.05/4=0.0125). The mark “*” indicates significant mediation at p<0.05, corrected.
Discussion
Structural brain correlates of alcohol expectancy
The current results suggest that global positive alcohol expectancy is associated with increased gray matter (GM) volume of the left precentral gyrus (PCG) in men and women as well as the superior frontal gyri in women, and with less GM volume of the ventral putamen in men. Furthermore, the left PCG GM volume mediates the association between alcohol expectancy and the quantity of drinking per episode and per month. These results link alcohol expectancy to structural brain differences and suggest how these differences may account for drinking behaviors in non-dependent adult drinkers. When recent drinking is accounted for, GM volumes of bilateral dorsolateral prefrontal cortices (DLFPC) correlated positively with alcohol expectancy. An increased DLPFC GM volume may reflect a correlate of alcohol expectancy independent of recent drinking.
A functional interpretation for the finding of greater GM volume in the PCG may be that alcohol expectancy is associated with sensation or novelty seeking and the intent to initiate exploratory behaviors. Interestingly, the personality trait of novelty seeking is associated with increased GM volume in the precentral cortex (Gardini et al., 2009; Van Schuerbeek et al., 2011), in support of this interpretation. The dorsolateral prefrontal cortex, in contrast, is more involved in working memory, maintenance of a mental set, and response selection, rather than directly involved in movement preparation (S26–S28). Thus, a prefrontal correlate may reflect the cognitive or premotor rather than motor processes of alcohol expectancy in these adult drinkers.
Although previous imaging studies have not addressed the structural correlates of alcohol expectancy, a number of functional studies should be discussed with respect to the current findings. In an fMRI study of working memory, participants consumed alcoholic or non-alcoholic drinks before the scanning session, with half of them in each group misinformed about their drink (Gundersen et al., 2008). By comparing participants who ingested non-alcoholic drinks and thought they were ingesting alcoholic drinks and those who ingested non-alcoholic drinks and were correctly informed, the investigators examined regional activations of alcohol expectancy during working memory. The results showed that alcohol expectancy is associated with greater activation in a number of cortical structures including the left middle frontal cortex (x=−27, y=6, z=60; as compared to the left PCG at x=−41, y=−6, z=55, in the current study). Gundersen and colleagues suggested that alcohol expectancy may sensitize cognitive control in participants anticipating to be intoxicated (Marczinski and Fillmore, 2005). An earlier electro-encephalographic (EEG) study suggested frontal but not parietal EEG power as a predictor of alcohol expectancy (Deckel et al., 1995). The latter study also implicated performance in a number of prefrontal neuropsychological tests in association with alcohol expectancy, although the direction of association did not appear to be consistent across testing batteries. Thus, along with these previous studies, the current findings suggest precentral frontal cortices as a potential cerebral correlate of alcohol expectancy for both men and women.
A recent review highlighted alterations of neurochemical signaling in the striatum in association with alcohol craving and consumption in animals and humans (Chen et al., 2011). In animal models, neuronal synaptic and morphological changes occurred in the putamen (but not caudate) of alcohol-drinking monkeys, compared to non-drinkers (Cuzon Carlson et al., 2011). Alcohol cue elicited significant and consistent activation of the right striatum including putamen in alcohol dependent individuals (Schacht et al., 2011). In our previous fMRI study of the stop signal task, we demonstrated decreased activation in subcortical structures including the right putamen during risk-taking in alcohol abusing individuals (Li et al., 2009a). These results can be broadly related to the current finding of a negative correlation between ventral putamen GM volume and alcohol expectancy in men. However, the mechanistic link remains to be explored.
Comparison with structural brain changes in dependent drinking
The current findings in nondependent drinkers do not appear to mirror structural brain changes in association with alcohol dependence. Numerous studies have described GM volume loss in dependent drinkers. A recent review noted that GM volume loss is widespread, age related, and most noted in the frontal cortex, perisylvian region and the cerebellum (Buhler and Mann, 2011). On the other hand, studies of non-dependent drinkers are fewer and presented less consistent results, with some showing changes in GM volume in the frontal cortices, amygdala, and hippocampus, but not in others (Medina et al., 2007; Sachdev et al., 2008; Sasaki et al., 2009; Taki et al., 2006). Thus, the current findings of increased frontal GM volume in nondependent drinkers seem unlikely to be attributable to the effects of greater alcohol consumption in individuals with higher alcohol expectancy. Rather, as the results of mediation analyses suggested, these altered cerebral structural correlates in association with alcohol expectancy contributed to increased alcohol use in this non-dependent population.
An additional consideration is that increased GM volume is seemingly contradictory to the idea of impaired cognitive function as a result of chronic alcohol misuse. First, our participants are a cohort of non-dependent social drinkers, which, unlike dependent drinkers, did not appear to demonstrate overt deficits in cognitive functions (Bednarski et al., 2012; Li et al., 2009b) or, as discussed above, consistent changes in cerebral GM volumes. Furthermore, impairment in cognitive functions is not necessarily associated with decrease in cerebral GM volumes in substance abusers. For instance, individuals with cocaine dependence are known to have deficits in multiple domains of cognitive functions, in association with decreased GM volumes in cortical regions. However, studies have also reported increased GM volume in other cerebral structures in individuals with chronic cocaine misuse (Ersche et al., 2011). Thus, these considerations along with our results of mediation analyses suggest interpretations of the current findings beyond a simple, linear association between alcohol drinking, decreased GM volume, and impaired cognitive functioning.
Alcohol misuse and gender differences
As describe earlier, men and women show important differences in the clinical characteristics of drug and alcohol use behaviors. For instance, men use illicit substances more frequently and in greater quantities than women (S29–S31). Although women substance users typically begin using substances later than do men, they demonstrate an accelerated transition to addiction (S32, S33).
Imaging studies have also suggested important gender differences in cerebral morphometry and regional activations in association with alcohol use and the risk of alcohol misuse (Pfefferbaum et al., 2001). Mann and colleagues showed that, despite lower amounts of alcohol consumption and a shorter duration of alcohol dependence in women, alcohol dependent women and men developed brain atrophy to a comparable extent (S33). In fMRI studies of working memory, female adolescents showed greater differences in brain activations than males, when compared to control participants (Caldwell et al., 2005). Furthermore, female binge drinkers showed less regional activations than female controls, while male bingers exhibited greater responses than male controls (Squeglia et al., 2010). Morphometric analyses showed that male but not female adolescents with a positive family history of alcoholism have larger left hippocampi than those without (Hanson et al., 2010). Female adolescents with alcohol use disorders (AUD) demonstrated smaller prefrontal gray and white matter volumes, while males with AUD had larger prefrontal volumes (Medina et al., 2008). Our recent fMRI study demonstrated decreased cortico-striatal activations during risk taking in both women and men non-dependent drinkers (Bednarski et al., 2012; Yan and Li, 2009). However, the extent of this deficit appeared to be correlated with monthly frequency of drinking only in women.
Thus, the current results of cerebral morphometry add to this growing literature on gender differences in the psychological processes related to alcohol use and in the effects of alcohol use on brain structures and functions.
Limitations of the study and conclusions
There are a few limitations to consider. First, the results of voxelwise analyses were significant only at an uncorrected threshold, which likely reflects the influences of a multitude of factors other than alcohol expectancy that were not considered in the current work. For instance, many other environmental and psychological factors, such as self-efficacy (Atwell et al., 2011; Duka et al., 2011), affect drinking behaviors. None of these variables are thoroughly assessed in the current work. On the other hand, we wish to emphasize that the current findings are congruent with the only two imaging and ERP studies that we know in supporting a role of the precentral gyrus in alcohol expectancy. Second, subcortical structures are notoriously difficult to segment (Cappabianco et al., 2011). Although we did not observe any correlations of alcohol expectancy with GM volumes in subcortical structures other than putamen, future work with algorithms targeting segmentation of subcortical tissues (such as the ventral striatum and thalamic subnuclei) is warranted. Third, our preliminary analyses using smoking status as a covariate revealed similar results. However, smoking behavior may vary between smokers and was not thoroughly assessed for the current cohort. Thus, more studies are required to examine association of drinking and smoking and confirm the current findings are specific to alcohol expectancy. Finally, the directionality of influence between variables is assumed for mediation analyses. Here, our model builds on the assumption that cerebral morphometry is the neural endophenotype and thus a mediator of alcohol expectancy. These results do not rule out the validity of other potential models and experiments (e.g., with a longitudinal design) are warranted to test and disambiguate competing hypotheses. To conclude, we demonstrated structural brain correlates of alcohol expectancy in non-dependent social drinkers. These correlates appear to aggregate in the frontal cortices, vary between men and women, and show gender specific association with drinking behaviors.
Supplementary Material
Acknowledgments
This study was supported by NIH grants R21AA018004 and K02DA026990. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Authors Contribution:
J.S.I. participated in the design of the study, data analyses and writing of the manuscript. S.Z. and S.H. participated in the design of the study and data analyses, D.M., S.R.B., E.M., O.M.F. participated in subject recruitment and assessment as well as running of the fMRI studies, C.-S.R.L. participated in the design of the study, data analyses, and writing of the manuscript.
Financial Disclosures:
We have no financial interests to disclose for the current study.
References
- Anderson KG, Schweinsburg A, Paulus MP, Brown SA, Tapert S. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. Journal of studies on alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Atwell K, Abraham C, Duka T. A parsimonious, integrative model of key psychological correlates of UK university students’ alcohol consumption. Alcohol Alcohol. 2011;46:253–260. doi: 10.1093/alcalc/agr016. [DOI] [PubMed] [Google Scholar]
- Beck KH, Thombs DL, Mahoney CA, Fingar KM. Social context and sensation seeking: gender differences in college student drinking motivations. Int J Addict. 1995;30:1101–1115. doi: 10.3109/10826089509055830. [DOI] [PubMed] [Google Scholar]
- Bednarski S, Zhang S, Luo X, Erdman E, Li C-S. Neural correlates of an indirect analogue of risk taking in non-dependent heavy alcohol drinkers. Alcoh Clin Exp Res. 2012;36:768–779. doi: 10.1111/j.1530-0277.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brown SA, Goldman MS, Christiansen BA. Do alcohol expectancies mediate drinking patterns of adults? J Consult Clin Psychol. 1985;53:512–519. doi: 10.1037//0022-006x.53.4.512. [DOI] [PubMed] [Google Scholar]
- Brown SA, Goldman MS, Inn A, Anderson LR. Expectations of reinforcement from alcohol: their domain and relation to drinking patterns. J Consult Clin Psychol. 1980;48:419–426. doi: 10.1037//0022-006x.48.4.419. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappabianco F, Ide JS, Falcao A, Li C-sR. Automatic subcortical tissue segmentation of MR images using optimum-path forest clustering. Image Processing (ICIP), 2011 18th IEEE International Conference on; 2011. pp. 2653–2656. [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Zucker RA, Jester JM, Puttler LI, Fitzgerald HE. Parental alcohol involvement and adolescent alcohol expectancies predict alcohol involvement in male adolescents. Psychol Addict Behav. 2010;24:386–396. doi: 10.1037/a0019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckel AW, Hesselbrock V, Bauer L. Relationship between alcohol-related expectancies and anterior brain functioning in young men at risk for developing alcoholism. Alcohol Clin Exp Res. 1995;19:476–481. doi: 10.1111/j.1530-0277.1995.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Iacono WG, McGue M. Modeling the impact of age and sex on a dimension of poly-substance use in adolescence: a longitudinal study from 11- to 17-years-old. Drug Alcohol Depend. 2010;110:193–199. doi: 10.1016/j.drugalcdep.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Crombag HS, Stephens DN. Experimental medicine in drug addiction: towards behavioral, cognitive and neurobiological biomarkers. J Psychopharmacol. 2011;25:1235–1255. doi: 10.1177/0269881110388324. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Lau HC, Cruz IY. Changes in activation of alcohol expectancies in memory in relation to changes in alcohol use after participation in an expectancy challenge program. Exp Clin Psychopharmacol. 2000;8:566–575. doi: 10.1037//1064-1297.8.4.566. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Hogarth L, Bleasdale D, Wright P, Fernie G, Christiansen P. Alcohol expectancy moderates attentional bias for alcohol cues in light drinkers. Addiction. 2011;106:1097–1103. doi: 10.1111/j.1360-0443.2011.03412.x. [DOI] [PubMed] [Google Scholar]
- Fu AT, Ko HC, Wu JY, Cherng BL, Cheng CP. Impulsivity and expectancy in risk for alcohol use: comparing male and female college students in Taiwan. Addict Behav. 2007;32:1887–1896. doi: 10.1016/j.addbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- George WH, Frone MR, Cooper ML, Russell M, Skinner JB, Windle M. A revised Alcohol Expectancy Questionnaire: factor structure confirmation, and invariance in a general population sample. Journal of studies on alcohol. 1995;56:177–185. doi: 10.15288/jsa.1995.56.177. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: the unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcohol Clin Exp Res. 2002;26:737–746. [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, O’Malley S, Randall PK, Randall CL. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res. 2010;34:1803–1812. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo MJ, Dawe S, Kambouropoulos N, Staiger PK, Jackson CJ. Alcohol expectancies and drinking refusal self-efficacy mediate the association of impulsivity with alcohol misuse. Alcohol Clin Exp Res. 2010;34:1386–1399. doi: 10.1111/j.1530-0277.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Gruner R, Ersland L, Hugdahl K. Separating the effects of alcohol and expectancy on brain activation: an fMRI working memory study. Neuroimage. 2008;42:1587–1596. doi: 10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am J Drug Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensing G, Spak F. Introduction: gendering socio cultural alcohol and drug research. Alcohol Alcohol. 2009;44:602–606. doi: 10.1093/alcalc/agp073. [DOI] [PubMed] [Google Scholar]
- Ide JS, Li CSR. Error-Related Functional Connectivity of the Habenula in Humans. Frontiers in Human Neuroscience. 2011:5. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28:1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Li CS, Chao HH, Lee TW. Neural correlates of speeded as compared with delayed responses in a stop signal task: An indirect analog of risk taking and association with an anxiety trait. Cerebral cortex. 2009a;19:839–848. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcohol Clin Exp Res. 2009b;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: effects on inhibition and activation of behavior. Psychopharmacology (Berl) 2005;181:337–346. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Miller TL, Smith GT, Smith JA. Disinhibition and expectancy in risk for alcohol use: comparing black and white college samples. Journal of studies on alcohol. 2001;62:313–321. doi: 10.15288/jsa.2001.62.313. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcohol Clin Exp Res. 1997;21:513–520. [PubMed] [Google Scholar]
- Meade Eggleston A, Woolaway-Bickel K, Schmidt NB. Social anxiety and alcohol use: evaluation of the moderating and mediating effects of alcohol expectancies. Journal of Anxiety Disorders. 2004;18:33. doi: 10.1016/j.janxdis.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009;70:87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Chen X, Wen W, Anstey KJ. Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: a voxel-based morphometric study. Psychiatry Res. 2008;163:61–69. doi: 10.1016/j.pscychresns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Abe O, Yamasue H, Fukuda R, Yamada H, Takei K, Suga M, Takao H, Kasai K, Aoki S, Ohtomo K. Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage. 2009;46:505–510. doi: 10.1016/j.neuroimage.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage. 2011;56:61–68. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell DF, Newman IM, Xiaoyi F. The influence of cultural orientation, alcohol expectancies and self-efficacy on adolescent drinking behavior in Beijing. Addiction. 2010;105:1608–1615. doi: 10.1111/j.1360-0443.2010.03006.x. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2010;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Goldman MS, Del Boca FK. The influence of alcohol expectancy priming and mood manipulation on subsequent alcohol consumption. J Abnorm Psychol. 2000;109:106–115. [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Inoue K, Okada K, Ono S, Kawashima R, Fukuda H. Both global gray matter volume and regional gray matter volume negatively correlate with lifetime alcohol intake in non-alcohol-dependent Japanese men: a volumetric analysis and a voxel-based morphometry. Alcohol Clin Exp Res. 2006;30:1045–1050. doi: 10.1111/j.1530-0277.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Urban R, Kokonyei G, Demetrovics Z. Alcohol outcome expectancies and drinking motives mediate the association between sensation seeking and alcohol use among adolescents. Addict Behav. 2008;33:1344–1352. doi: 10.1016/j.addbeh.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R. Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Res. 2011;1371:32–42. doi: 10.1016/j.brainres.2010.11.073. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Kummeling RH. An experimental test of an alcohol expectancy challenge in mixed gender groups of young heavy drinkers. Addict Behav. 2004;29:215–220. doi: 10.1016/s0306-4603(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Yan P, Li CS. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: A preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RM, Connor JP, Feeney GF. Alcohol expectancy changes over a 12-week cognitive-behavioral therapy program are predictive of treatment success. J Subst Abuse Treat. 2011;40:18–25. doi: 10.1016/j.jsat.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Zamboanga BL. From the eyes of the beholder: alcohol expectancies and valuations as predictors of hazardous drinking behaviors among female college students. Am J Drug Alcohol Abuse. 2006;32:599–605. doi: 10.1080/00952990600920573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.