Abstract
Background
Combining radioactive colloids and a near-infrared (NIR) fluorophore permit preoperative planning and intraoperative localization of deeply located sentinel lymph nodes (SLNs) with direct optical guidance by a single lymphatic tracer. The aim of this clinical trial was to evaluate and optimize a hybrid NIR fluorescence and radioactive tracer for SLN detection in breast cancer patients.
Method
Patients with breast cancer undergoing SLN biopsy were enrolled. The day before surgery, indocyanine green (ICG)-99mTc-Nanocolloid was injected periareolarly and a lymphoscintigram was acquired. Directly before surgery, blue dye was injected. Intraoperative SLN localization was performed by a gamma probe and the Mini-FLARETM NIR fluorescence imaging system. Patients were divided into two dose groups, with one group receiving twice the particle density of ICG and nanocolloid, but the same dose of radioactive 99mTechnetium.
Results
Thirty-two patients were enrolled in the trial. At least one SLN was identified pre- and intraoperatively. All 48 axillary SLNs could be detected by gamma tracing and NIR fluorescence imaging, but only 42 of them stained blue. NIR fluorescence permitted detection of lymphatic vessels draining to the SLN up to 29 hours after injection. Increasing the particle density by two-fold did not yield a difference in fluorescence intensity, median 255 (range 98 – 542) vs. median 284 (90 – 921; P = 0.590), or signal- to- background ratio, median 5.4 (range 3.0 – 15.4) vs. median 4.9 (3.5 – 16.3; P = 1.000), of the SLN.
Conclusion
The hybrid NIR fluorescence and radioactive tracer ICG-99mTc-Nanocolloid permitted accurate pre- and intraoperative detection of the SLNs in patients with breast cancer.
Keywords: Breast Cancer, Sentinel Lymph Node, Near-Infrared Fluorescence Imaging, Image Guided Surgery, Lymphoscintigraphy
Introduction
Sentinel lymph node (SLN) biopsy is standard of care for nodal staging in breast cancer patients with clinically negative axillary lymph nodes1. To locate the SLNs, a combination of radioactive lymphatic tracers and blue dye staining is often preferred2–5. Radioactive tracers, permit preoperative planning and retention in the first echelon nodes, whereas blue dyes permit direct intraoperative visualization of the lymphatics and SLNs.
Optical imaging using near-infrared (NIR; 700–900 nm) fluorescence has been tested extensively for SLN detection in breast cancer and in other cancers, such as melanoma6–15. The fluorescent tracer indocyanine green (ICG) is used for detection of SLNs up to several millimetres deep in tissue16–18. This tracer has outperformed blue dye staining for SLN identification in multiple clinical trials7;8;18;19. Nevertheless, radioactive colloids are still considered the standard of care for preoperative planning. Radioactive colloids are essential for localization of deeper located SLNs, for example in patients with a higher body mass index (BMI)8;20.
To combine radioactive and NIR fluorescence guidance in one “package”, the hybrid tracer ICG-99mTc-Nanocolloid has been developed21. The fluorescent label ICG and the radioactive label 99mTechnecium (99mTc) are integrated into the same colloidal particle. Unlike “free” ICG, this nanoparticle provides long retention in the SLN22. Feasibility and validity of this tracer has been shown in various tumour types23–25. However, this hybrid tracer has not been studied in SLN biopsy procedures in patients with breast cancer.
The aim of this study was to evaluate the hybrid tracer for SLN detection in patients with breast cancer and to assess nodal accumulation of the tracer and the fluorescent signal intensity in the SLN by increasing the particle density of the tracer.
Methods
This clinical trial was approved by the medical ethics committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. All patients with breast cancer scheduled for SLN biopsy in the period from August 2011 to July 2012 were eligible for participation in the study. Patients had clinically negative axillary nodes as assessed by palpation and ultrasonography. Exclusion criteria were pregnancy, lactation, or an allergy to iodine or ICG. Informed consent was given by all included patients and the acquired data was anonymized.
Tracer preparation
99mTc-Nanocolloid was prepared by adding sodium pertechnetate (approximately 1000 MBq) in 2 mL saline to a vial of 0.5 mg human serum albumin nanocolloid (GE Healthcare, Eindhoven, the Netherlands). After 30 min of incubation at room temperature, 50 µL of 6.4 mM (0.25 mg) ICG (Pulsion Medical Systems, Munich, Germany) was dissolved in water for injection to obtain ICG-99mTc-Nanocolloid at a final ICG concentration of 160 µM and a final pH of 6,0 – 7,024;25. To obtain a two-fold higher concentration ICG-Nanocolloid, 99mTc-Nanocolloid was prepared by adding pertechnetate (approximately 500 MBq) in 1 mL saline to 0.5 mg Nanocolloid, while the amount of ICG (0.25 mg) was kept the same, resulting in a final ICG concentration of 320 µM. This preparation resulted in doubling of the ICG and Nanocolloid concentration, but the same radioactivity dose (approximately 100 MBq). All procedures were performed under current good manufacturing practice and under supervision of the institution’s pharmacist.
Study design
The day before surgery, ICG-99mTc-Nanocolloid was injected intracutaneously periareolar in the quadrant in which the tumour was localized (one deposit; total volume 0.2 mL; 100 MBq). Patients were divided into the low and high ICG-99mTc-Nanocolloid particle density groups; the first consecutive half of the patients were assigned to the low dose, and the second half of the patients to received a two fold higher dose. Anterior, lateral, and anterior oblique planar images were obtained at 15 minutes and approximately three hours after injection, by a detector of a single or two-headed gamma camera (Symbia T6, Siemens, Erlangen, Germany or Toshiba GCA-7200PI/7200DI/7100UI, Toshiba, Tokyo, Japan). The percentage of tracer in the SLN was calculated using the anterior oblique images by dividing the counts in the SLN(s) by the sum of counts in the injection site and all lymph nodes. Simultaneously with the gamma camera images, percutaneous NIR fluorescence images were acquired using the Mini-FLARETM NIR fluorescence imaging system, as described previously26. A SLN was defined as a lymph node on a direct lymphatic drainage pathway from the primary tumour as detected by lymphoscintigraphy27. Intraoperatively, lymph nodes with a gamma count of 10 per cent or more compared to the most radioactive SLN, were also designated as SLNs28.
Directly before surgery, 1 mL total of patent blue (Bleu Patenté V, Guerbet, Brussels, Belgium) was injected periareolarly in multiple deposits. Gentle pressure was applied to the injection site for 1 min. The surgical field was illuminated using the white light luminary of the Mini-FLARETM imaging system. During surgical exploration, the combined radioactive and fluorescent signature of the preoperatively defined SLNs were visualized by a hand-held gamma probe (Europrobe, Euromedical Instruments, Le Chesnay, France) and the Mini-FLARE™.
The primary endpoint of the study was the SLN identification rate. Secondary endpoints were the number of SLN identified per patient, the percentage of the tracer accumulated in the SLN based on the scintigraphy, the fluorescent intensity, and the fluorescent signal- to- background ratio (SBR) of the SLNs. The SBR of the SLN was calculated by dividing the fluorescence intensity of the SLN by the fluorescence intensity of the fatty tissue directly surrounding the SLN. Blue dye staining was used to provide additional optical guidance.
SLNs were fixed in formalin and embedded in paraffin for routine hematoxylin and eosin staining and immunohistopathological staining for cytokeratin (AE1/AE3) at three levels, with an interval of 150 to 250 µm, according to the Dutch guidelines for SLN analysis in breast cancer.
Statistical analysis
For statistical analysis, SPSS statistical software package (Version 20.0, Chicago, IL) was used. To compare categorical characteristics between the two groups of patients, the Fisher’s Exact test was used for binary data and the chi-square test for non-binary data. Continuous data was tested for normal distribution using the Shapiro-Wilk test. Normal distributed continuous data (age and tumour size) was tested using the independent-sample t- test and not normally distributed data was tested using the Mann-Whitney test. Continuous data is presented as median and range. P < 0.05 was considered significant.
Results
Thirty-two consecutive patients with breast cancer undergoing SLN biopsy were included in this study (Fig. 1). The median age was 56 (range 34 – 82) years, median BMI was 24 (18 – 39) kg/m2. Of the 32 patients, the first 16 were assigned to the low ICG-99mTc-Nanocolloid dose and the following 16 received the two-fold higher dose. No differences were observed between the two treatment groups concerning patient, tumour, and treatment characteristics (Table 1). The median time between injection of ICG-99mTc-Nanocolloid and surgery were 24 (19 – 29) hours and 22 (20 – 25) hours for the low dose and higher dose treatment groups, respectively. Similar to the use of “free” ICG7;8;26, no adverse reactions associated with the use of ICG-99mTc-Nanocolloid were observed.
Figure 1. Patient enrolment.
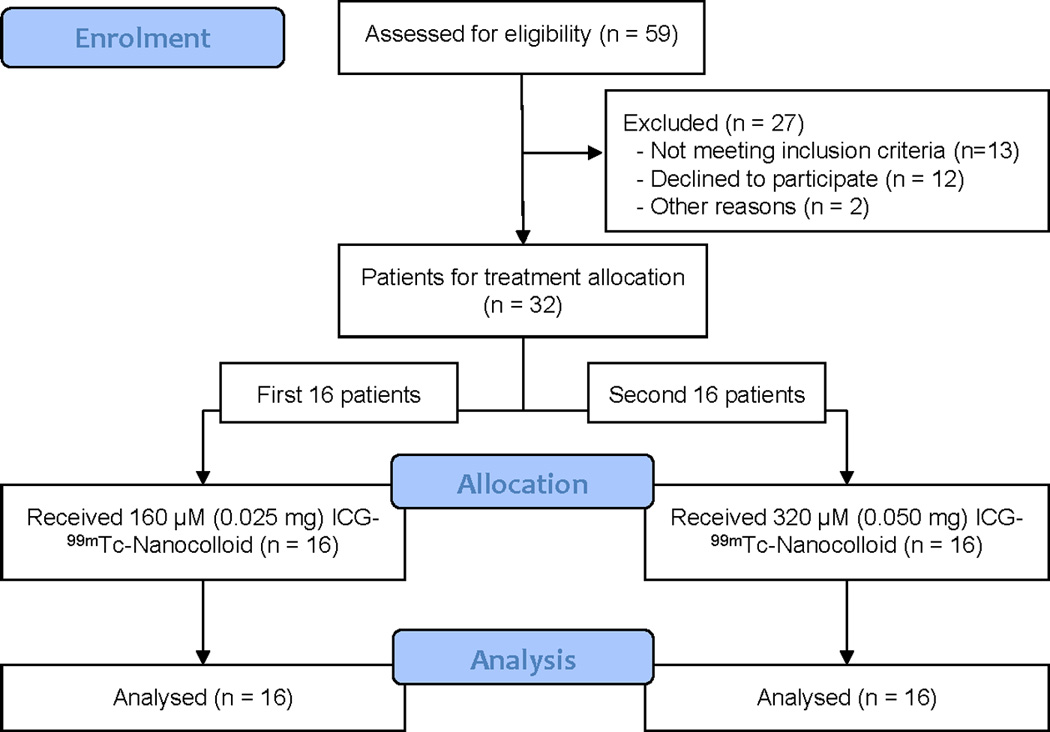
Table 1.
Patient and tumour characteristics in the low- and high dose particle density groups
| Low-dose group (16 patients) |
High-dose group (16 patients) |
||
|---|---|---|---|
| Characteristic | n | n | P |
| Age in years (median; range) | 59 (44 – 82) | 55 (34 – 77) | 0.322 |
| Body mass index, kg/m2 (median; range) | 25 (21 – 39) | 23 (18 – 37) | 0.043 |
| Previous breast procedure | 0.635 | ||
| - Breast augmentation | 1 | 1 | |
| - Lumpectomy | 0 | 1 | |
| - Neoadjuvant hormonal / Chemotherapy | 4 | 2 | |
| - Previous axillary surgery | 0 | 0 | |
| Multifocality | 1 | 5 | 0.172 |
| Type of Operation | 0.458 | ||
| - Mastectomy | 7 | 4 | |
| - Wide local excision | 9 | 12 | |
| Hours between injection and surgery (median; range) | 24 (19 – 29) | 22 (20 – 25) | 0.196 |
| Pathological tumour size in mm (median; range) | 14 (3 – 34) | 15 (4 – 30) | 0.681 |
| Histological type | 0.717 | ||
| - Infiltrating ductal adenocarcinoma | 13 | 13 | |
| - Infiltrating lobular adenocarcinoma | 2 | 1 | |
| - Ductal Carcinoma In Situ (DCIS) | 1 | 2 | |
| Histological grade | 0.058 | ||
| - I | 2 | 3 | |
| - II | 10 | 4 | |
| - III | 1 | 7 | |
| - No grading possible (DCIS) | 3 | 2 |
Sentinel lymph node detection
Preoperative scintigraphy identified at least one SLN in all patients, with a median of one (1 – 2) SLN per patient. All SLNs were located in the axilla. In 22 patients superficial lymph drainage could at least partially be visualized percutaneously by NIR fluorescence at the time of preoperative scintigraphy or prior to surgery (Fig. 2).
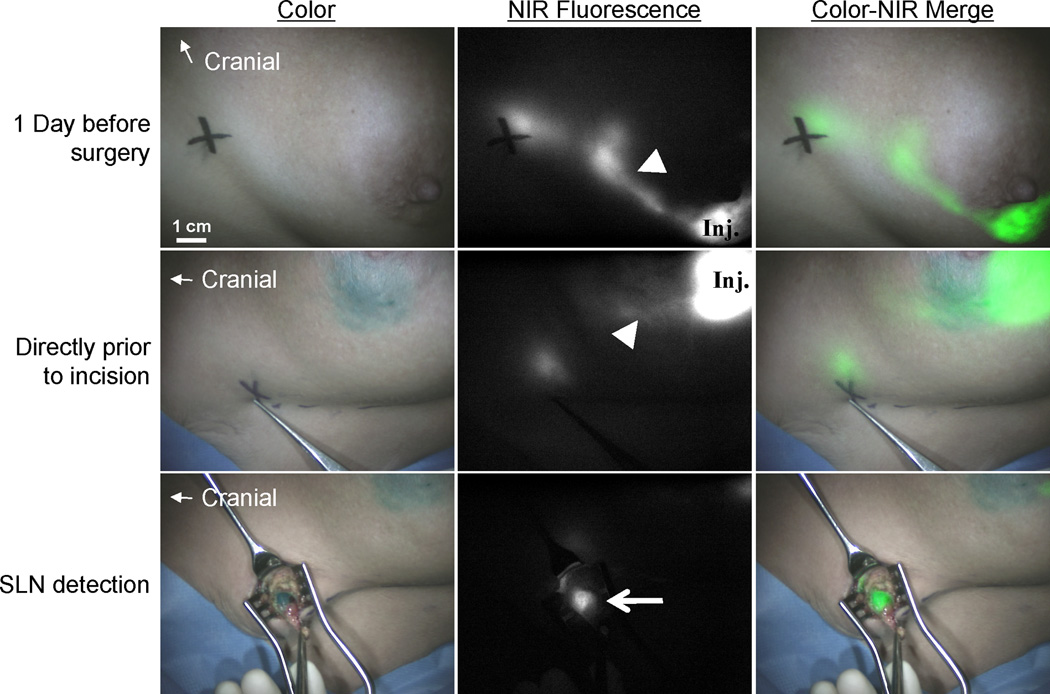
Figure 2. NIR fluorescence imaging during SLN mapping in breast cancer.
The periareolar injection site (inj.) and an afferent lymphatic channel (arrowhead) are clearly visualized the day before surgery (upper panel). Directly before surgery, patent blue is injected periareolarly. The lymphatic channels (arrowhead) can still be visualized percutaneously using NIR fluorescence (middle panel). The sentinel lymph node (SLN) (arrow) is identified by NIR fluorescence and blue staining. The black cross indicates the presumed position of the SLN. Camera exposure times were 100 ms (upper and middle panels) and 40 ms (lower panel).
Surgical excision of the SLNs was performed guided by a combination of radioactivity and fluorescence. After initial guidance by the preoperative scintigraphy and the gamma probe, the axilla was explored using NIR fluorescence imaging. Only in patients where NIR fluorescence did not directly point to the SLN, was the gamma probe used for additional intraoperative guidance. The results of the SLN biopsy procedures are presented in Table 2. The NIR fluorescence based detection of lymphatic vessels that drained to the SLN contributed to their identification (Fig. 3). In all patients, at least one SLN was identified and resected. In addition to the SLNs detected by lymphoscintigraphy, an additional ten lymph nodes were intraoperatively considered as SLN, with a median of one (1 – 3) SLN harvested from each patient. All 48 radioactive SLNs were detected by NIR fluorescence and the gamma probe, but only 42 out of 48 SLNs stained blue. In all patients the NIR fluorescence signal in the SLN was detected before patent blue was visualized.
Table 2.
Results of sentinel lymph node identification
| Total (32 patients) |
Low-dose (16 patients) |
High-dose (16 patients) |
||
|---|---|---|---|---|
| Characteristic | n | n | n | P |
| Preoperative SLN identification | ||||
| -Identification rate | 32 / 32 | 16 / 16 | 16 / 16 | |
| -Number of SLNs detected per patient (median; range) | 1 (1 – 2) | 1 (1 – 2) | 1 (1 – 2) | 0.564 |
| -Percutaneous fluorescence lymph drainage visualization | 22 / 32 | 10 / 16 | 12 / 16 | 0.704 |
| Intraoperative SLN identification | ||||
| -Identification rate | 32 / 32 | 16 / 16 | 16 / 16 | |
| -Total number of SLNs removed | 48 | 21 | 27 | |
| Method of intraoperative detection | ||||
| - Radioactive | 48 / 48 | 21 / 21 | 27 / 27 | |
| - Blue | 42 / 48 | 17 / 21 | 25 / 27 | 0.383 |
| - Fluorescent | 48 / 48 | 21 / 21 | 27 / 27 | |
| Histology sentinel lymph node | 0.686 | |||
| - Negative | 19 / 32 | 10 / 16 | 9 / 16 | |
| - Micrometestases / ITC | 5 / 32 | 3 / 16 | 2 / 16 | |
| - Macrometastases | 8 / 32 | 3 / 16 | 5 / 16 |
ITC; Isolated tumour cells, SLN; Sentinel lymph node
Figure 3. Intraoperative detection of lymphatic vessels using NIR fluorescence.
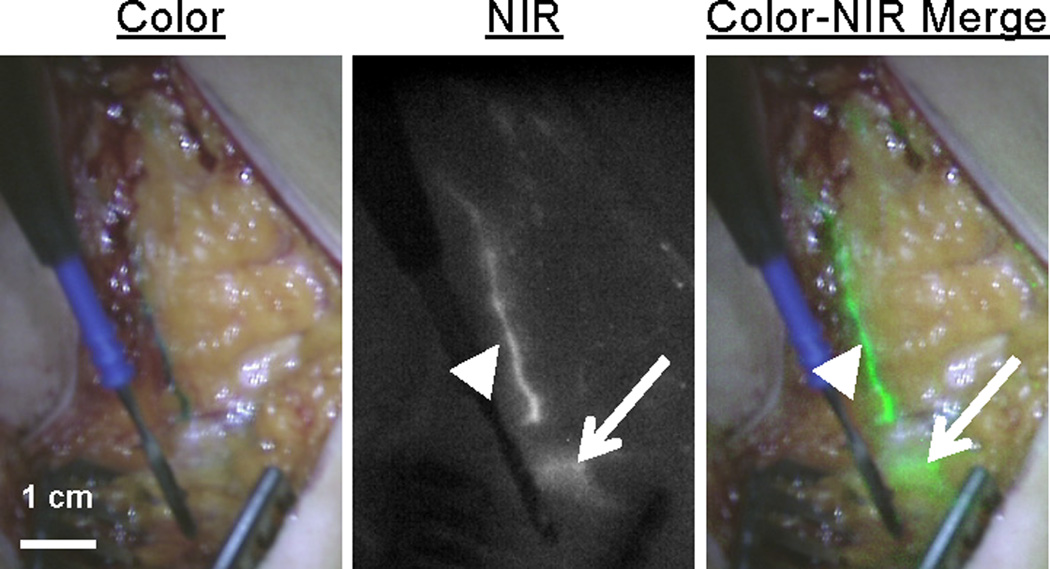
The lymphatic vessel (arrowhead) draining to the sentinel node (arrow) located deeper into the tissue, is clearly identified by NIR fluorescence.
Histological analysis of the SLNs showed lymph node metastases in 13 of 32 patients. Macrometastases (larger than 2 mm) were found in eight patients and isolated tumour cells (ITCs) or micrometastases (equal to or smaller than 2 mm) in five patients.
Comparison between treatment groups
The number of preoperative identified SLNs by radioscintigraphy was not different between the low dose group, median one (1 – 2), and high dose group, median one (1 – 2), P = 0.564. Despite doubling the particle density, the percentage of the amount of injected tracer accumulated in the SLN per patient based on the scintigraphy did not improve, median 1.7 (0.2 – 7.3) per cent vs. median 2.6 (0.4 – 30.4) per cent, P = 0.402. Nevertheless, the higher particle density group had SLNs that showed a higher accumulation of the tracer (Fig. 4). Between the groups no difference was observed in the amount of higher echelon nodes during lymphoscintigraphy.
Figure 4. Influence of ICG-Nanocolloid dose on Radioactivity (A), and Fluorescence (B+C).
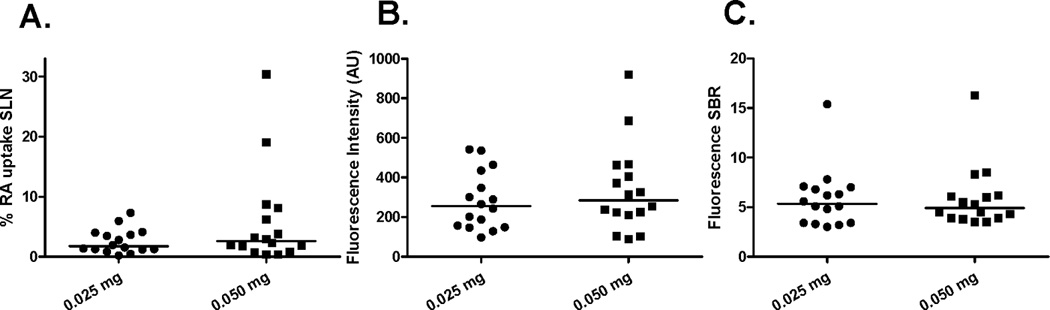
Figure A quantifies the percentage of radioactive (RA) injected dose of the hybrid tracer observed in the sentinel lymph node (SLN) approximately three hours after injection. Figure B quantifies the fluorescence intensity (AU) of the SLN measured during surgery. Figure C quantifies the fluorescence signal- to- background ratio (SBR) of the SLN during surgery. Dots represent individual patient values and the line represents the median.
Intraoperatively, the number of identified SLNs did not differ between the low dose group, median one (1 – 2), and high dose group, median one (1 - 3), P = 0.323. No significant difference was found for the fluorescence intensity, median 255 (98 – 542) vs. median 284 (90 – 921), P = 0.590 or signal-to-background ratio of the SLN, median 5.4 (3.0 – 15.4) vs. median 4.9 (3.5 – 16.3), P = 1.000, between the low dose group and higher dose group, respectively (Fig. 4).
Discussion
The present study shows that the use of ICG-99mTc-Nanocolloid for SLN biopsy is feasible in patients with breast cancer. This tracer permits preoperative imaging and intraoperative guidance. By combining NIR fluorescence and radioactivity in one tracer, discrepancies between the two imaging modalities used for SLN localization are less likely to occur. In the current study all SLNs could be surgically detected by both gamma radiation and NIR fluorescence imaging. Lymphatic vessels draining to the SLN were fluorescent the day after tracer administration and contributed to detection of the SLN.
In an attempt to optimize nodal accumulation of the tracer and the NIR fluorescence signal, a two-fold higher particle density of ICG and Nanocolloid was administered. In the higher dose group, multiple outliers with higher tracer accumulation in the SLN were observed, though not yielding a statistically significant increase in tracer accumulation. The results were similar for the fluorescence based identification (Fig. 4). In contrast to intratumoural tracer deposition29, an increase in particle density does not appear to have a significant influence on the SLN identification when periareolar administrations are used. In the current study the dose of 99mTc was kept the same for both groups (100 MBq). Globally different doses of 99mTc varying from 10 up to 370 MBq are used for SLN detection30. As the lymphatic distribution of ICG-99mTc-Nanocolloid is mainly determined by the large Nanocolloid particles, no significant differences in sensitivity of the fluorescence signal are expected when varying the amount of 99mTc while maintaining the Nanocolloid dose.
In previous studies using ICG alone, 25-times more ICG was injected during surgery7;8. In addition, the volume of these injections was eight times higher, thereby increasing the interstitial fluid pressure and lymphatic drainage. These two factors contributed to a SBR of approximately nine7;8. In this study a lower SBR (approximately six) was observed using ICG-99mTc-Nanocolloid. This decrease is relatively small because the lower dose and smaller injection volume of ICG is likely offset by the longer time between injection and imaging, which aids in the concentration of ICG in the SLN. Importantly, this decrease did not influence surgical guidance, as in all these patients ICG-99mTc-Nanocolloid permitted accurate SLN identification during surgery and lymphatic vessels were still clearly visible.
In the current study 42 out 48 SLNs stained blue, which is comparable to the identification rate of blue dye in large multi-centre studies3. NIR fluorescence identified more SLNs compared to blue dye staining and detected SLNs that were deeper located in the tissue. Similar results were obtained in previous studies7;26, showing no benefit of blue dye when NIR fluorescence imaging was used8. When ICG-99mTc-Nanocolloid is used and blue dye is omitted, no lymphatic tracer has to be injected directly prior to surgery, and in combination with the enhanced detection by NIR fluorescence compared to blue dyes, this can improve logistics and shorten the time of surgery.
Various techniques are being evaluated to improve depth penetration of NIR fluorescence contrast agents31;32 and to study when radioactivity can be omitted8. If in the future depth penetration of NIR fluorescence contrast agents increases, radioactive colloids may possibly be omitted, thereby improving logistics and costs. At present, NIR fluorescence is mainly used in addition to radioactive colloids. Widespread clinical dissemination of this technique requires a cost-effective approach. ICG–99mTc-Nanocolloid is based on a regularly used lymphatic tracer in Europe and only needs addition of a small amount (0.025 mg) of ICG with a cost of approximately 50–80 Euro for 25 mg. A variety of relatively low-cost commercial camera systems are already available for clinical use33.
The hybrid optical/nuclear agent ICG–99mTc-Nanocolloid has shown to be a successful tracer for image-guided SLN biopsy in patients with breast cancer. ICG–99mTc-Nanocolloid provides fully integrated pre- and intraoperative radioactive and NIR fluorescence guidance, no injection directly prior to surgery is necessary and the intraoperative findings are comparable to those when using ICG alone. As no difference was observed between the two ICG-99mTc-Nanocolloid dose groups, a particle density of 160 µM ICG-99mTc-Nanocolloid injected in 200 µL the day before surgery could be recommended.
Acknowledgements
The authors would like to thank the following individuals for their contribution to this study: Gamma Ranke, Elly Krol-Warmerdam (Breast Cancer Unit), Kirsten Schimmel (Central Pharmacy), Hein Putter (Medical Statistics), and David Burrington, Jr. for editing. F.P.R. Verbeek and D.D.D. Rietbergen contributed equally to the study and share second authorship. This work was supported in part by NIH grants R01-CA-115296 and R21-CA-130297, the Dutch Cancer Society grants UL2010-4732 and PGF 2009-4344, and a VIDI grant form the Dutch national research council (NWO-STW-11272). This research was performed within the framework of, the Centre for Translational Molecular Medicine (CTMM), project MUSIS (grant 03O-202). Joost van der Vorst is an MD-medical research trainee funded by The Netherlands Organization for Health Research and Development (grant 92003593).
Footnotes
Disclosures:
FLARE™ technology is owned by Beth Israel Deaconess Medical Centre, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Centre will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has waived post-market royalties, and has decided to donate pre-market proceeds to the FLARE™ Foundation.
Reference List
- 1.Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227(5):645–651. doi: 10.1097/00000658-199805000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer--results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99(2):203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 4.Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del BP, et al. A Randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247(2):207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 5.Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Sentinel Node Identification Rate and Nodal Involvement in the EORTC 10981-22023 AMAROS Trial. Ann Surg Oncol. 2010;17(7):1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Lowik CW, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104(3):323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutteman M, Mieog JS, van der Vorst JR, Liefers GJ, Putter H, Lowik CW, et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127(1):163–170. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, et al. Randomized Comparison of Near-infrared Fluorescence Imaging Using Indocyanine Green and 99(m) Technetium With or Without Patent Blue for the Sentinel Lymph Node Procedure in Breast Cancer Patients. Ann Surg Oncol. 2012;19(13):4104–4111. doi: 10.1245/s10434-012-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe H, Mori T, Umeda T, Tanaka M, Kawai Y, Shimizu T, et al. Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today. 2011;41(2):197–202. doi: 10.1007/s00595-009-4254-8. [DOI] [PubMed] [Google Scholar]
- 10.Kitai T, Kawashima M. Transcutaneous detection and direct approach to the sentinel node using axillary compression technique in ICG fluorescence-navigated sentinel node biopsy for breast cancer. Breast Cancer. 2011;19:343–348. doi: 10.1007/s12282-011-0286-1. [DOI] [PubMed] [Google Scholar]
- 11.Hirche C, Mohr Z, Kneif S, Murawa D, Hunerbein M. High rate of solitary sentinel node metastases identification by fluorescence-guided lymphatic imaging in breast cancer. J Surg Oncol. 2012;105(2):162–166. doi: 10.1002/jso.22075. [DOI] [PubMed] [Google Scholar]
- 12.Polom K, Murawa D, Nowaczyk P, Rho YS, Murawa P. Breast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green--human serum albumin in comparison with gamma emitting radioactive colloid tracer. Eur J Surg Oncol. 2012;38(2):137–142. doi: 10.1016/j.ejso.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Hirano A, Kamimura M, Ogura K, Kim N, Hattori A, Setoguchi Y, et al. A Comparison of Indocyanine Green Fluorescence Imaging Plus Blue Dye and Blue Dye Alone for Sentinel Node Navigation Surgery in Breast Cancer Patients. Ann Surg Oncol. 2012;19:4112–4116. doi: 10.1245/s10434-012-2478-0. [DOI] [PubMed] [Google Scholar]
- 14.Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012;38(8):651–656. doi: 10.1016/j.ejso.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Aoyama K, Kamio T, Ohchi T, Nishizawa M, Kameoka S. Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J Surg Oncol. 2011;9:157. doi: 10.1186/1477-7819-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96(11):1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 17.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7(5):626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012;106(1):41–45. doi: 10.1002/jso.23045. [DOI] [PubMed] [Google Scholar]
- 19.Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19(3):210–213. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Nos C, Freneaux P, Guilbert S, Falcou MC, Salmon RJ, Clough KB. Sentinel lymph node detection for breast cancer: which patients are best suited for the patent blue dye only method of identification? Ann Surg Oncol. 2001;8(5):438–443. doi: 10.1007/s10434-001-0438-1. [DOI] [PubMed] [Google Scholar]
- 21.Buckle T, van Leeuwen AC, Chin PT, Janssen H, Muller SH, Jonkers J, et al. A self-assembled multimodal complex for combined pre- and intraoperative imaging of the sentinel lymph node. Nanotechnology. 2010;21(35):355101. doi: 10.1088/0957-4484/21/35/355101. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen AC, Buckle T, Bendle G, Vermeeren L, Valdes OR, van de Poel HG, et al. Tracer-cocktail injections for combined pre- and intraoperative multimodal imaging of lymph nodes in a spontaneous mouse prostate tumor model. J Biomed Opt. 2011;16(1):016004. doi: 10.1117/1.3528027. [DOI] [PubMed] [Google Scholar]
- 23.van der Poel HG, Buckle T, Brouwer OR, Valdes Olmos RA, van Leeuwen FW. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur Urol. 2011;60:826–833. doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer OR, Buckle T, Vermeeren L, Klop WM, Balm AJ, van der Poel HG, et al. Comparing the Hybrid Fluorescent-Radioactive Tracer Indocyanine Green-99mTc-Nanocolloid with 99mTc-Nanocolloid for Sentinel Node Identification: A Validation Study Using Lymphoscintigraphy and SPECT/CT. J Nucl Med. 2012;53:1034–1040. doi: 10.2967/jnumed.112.103127. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer OR, Klop WM, Buckle T, Vermeeren L, van den Brekel MW, Balm AJ, et al. Feasibility of Sentinel Node Biopsy in Head and Neck Melanoma Using a Hybrid Radioactive and Fluorescent Tracer. Ann Surg Oncol. 2011;19:1988–1994. doi: 10.1245/s10434-011-2180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18(9):2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieweg OE, Tanis PJ, Kroon BB. The definition of a sentinel node. Ann Surg Oncol. 2001;8(6):538–541. doi: 10.1007/s10434-001-0538-y. [DOI] [PubMed] [Google Scholar]
- 28.Chung A, Yu J, Stempel M, Patil S, Cody H, Montgomery L. Is the "10% rule" equally valid for all subsets of sentinel-node-positive breast cancer patients? Ann Surg Oncol. 2008;15(10):2728–2733. doi: 10.1245/s10434-008-0050-8. [DOI] [PubMed] [Google Scholar]
- 29.Valdes Olmos RA, Tanis PJ, Hoefnagel CA, Nieweg OE, Muller SH, Rutgers EJ, et al. Improved sentinel node visualization in breast cancer by optimizing the colloid particle concentration and tracer dosage. Nucl Med Commun. 2001;22(5):579–586. doi: 10.1097/00006231-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Mariani G, Erba P, Villa G, Gipponi M, Manca G, Boni G, et al. Lymphoscintigraphic and intraoperative detection of the sentinel lymph node in breast cancer patients: the nuclear medicine perspective. J Surg Oncol. 2004;85(3):112–122. doi: 10.1002/jso.20023. [DOI] [PubMed] [Google Scholar]
- 31.Kim C, Erpelding TN, Maslov K, Jankovic L, Akers WJ, Song L, et al. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. J Biomed Opt. 2010;15(4):046010. doi: 10.1117/1.3469829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioux S, Mazhar A, Cuccia DJ, Durkin AJ, Tromberg BJ, Frangioni JV. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009;14(3):034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr Opin Urol. 2012;22(2):109–120. doi: 10.1097/MOU.0b013e3283501869. [DOI] [PubMed] [Google Scholar]