Abstract
Energy dependent proteases ensure the timely removal of unwanted proteins in a highly selective fashion. In Caulobacter crescentus, protein degradation by the ClpXP protease is critical for cell cycle progression; however, only a handful of substrates are currently known. Here, we use a trapping approach to identify putative substrates of the ClpP associated proteases in C. crescentus. Biochemical validation of several of these targets reveals specific protease recognition motifs and suggests a need for ClpXP specific degradation beyond degradation of known cell cycle regulators. We focus on a particular instance of regulated proteolysis in Caulobacter by exploring the role of ClpXP in degrading the stalk synthesis transcription factor TacA. We show that TacA degradation is controlled during the cell cycle dependent on the ClpXP regulator CpdR and that stabilization of TacA increases degradation of another ClpXP substrate, CtrA, while restoring deficiencies associated with prolific CpdR activity. Together, our work reveals a number of new validated ClpXP substrates, clarifies rules of protease substrate selection, and demonstrates how regulated protein degradation is critical for Caulobacter development and cell cycle progression.
Introduction
The proteome is maintained by the synthesis and removal of cellular proteins at specific times and places in response to internal and external signals (Goley et al., 2009; Sauer and Baker, 2011). Proteolysis is a robust process that ensures the elimination of proteins when they are no longer needed; however, the irreversible nature of protein degradation demands stringent selectivity in substrate choice. In bacteria, regulated protein degradation is often governed by energy dependent proteases, also known as AAA+ proteases. One member of this family is ClpXP, an oligomeric machine formed from the ClpX unfoldase and ClpP peptidase. ClpP alone is incapable of degrading folded proteins and requires associated unfoldases, such as ClpX, that engage substrates via binding of short peptide sequences (recognition motifs). Targets are actively unfolded and translocated into the ClpP chamber where they are processed to peptides. The ClpP system has been best characterized in E. coli where the Clp family proteases regulate many pathways such as protein quality control, exit from stationary phase and response to damaging agents (Sauer and Baker, 2011).
Caulobacter crescentus undergoes an obligate morphological differentiation from non-replicative swarmer cells to replication competent stalked cells every cell-cycle. This remodeling depends on proper coordination of regulatory proteolysis and synthesis. For example, ClpX and ClpP are essential in Caulobacter (Jenal and Fuchs, 1998) and regulate proteins important for cell development, cell-cycle progression (Biondi et al., 2006a; Domian et al., 1997; Iniesta et al., 2006; Radhakrishnan et al., 2010), chemotaxis (Potocka et al., 2002; Tsai and Alley, 2001), and replication (Gorbatyuk and Marczynski, 2005; Lesley and Shapiro, 2008). Although several ClpXP substrates have been identified in Caulobacter, expression of mutant nondegradable alleles of these proteins or blocking degradation of these substrates does not mirror the many effects that arise when ClpXP is lost (Domian et al., 1997; Iniesta et al., 2006; Radhakrishnan et al., 2010). Thus the ClpXP dependent changes in development and cell cycle progression must rely on degradation of other as yet unknown substrates.
Here, we present a list of potential substrates of the ClpP protease system in Caulobacter that includes known substrates, those documented from other bacteria, and previously unknown candidates. In vitro biochemical experiments validate a number of these targets and confirm the preservation of particular ClpXP recognition motifs across species. Next, we identify a role for the zinc-binding N-terminal domain of ClpX for Caulobacter viability and endogenous substrate selection. Finally, we focus on the stalked cell regulator TacA, a candidate substrate found in our trap. We show that TacA is proteolysed by ClpXP in vitro and that TacA is selectively degraded during cell-cycle progression dependent on the ClpXP regulator CpdR. Interestingly, stabilization of TacA also affects the degradation of CtrA, another CpdR dependent ClpXP substrate, suggesting a linkage between TacA, CpdR and CtrA. Finally, we find that prolific TacA degradation contributes to the stalkless phenotype of cells lacking the PleC phosphatase as stabilization of TacA partially rescues stalked cell formation in this mutant.
Results
Construction of a ClpP trap in Caulobacter crescentus
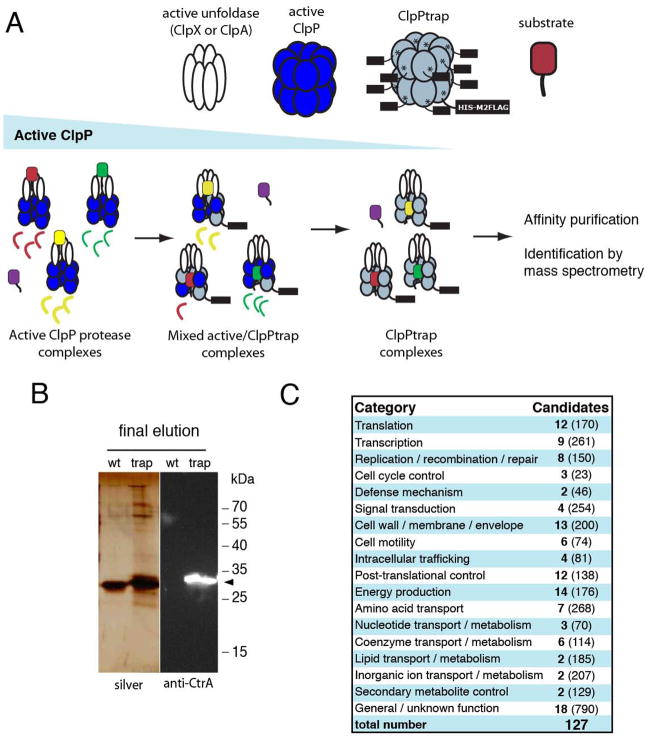
We identified novel substrates of ClpXP in C. crescentus using the strategy outlined in Figure 1 similar to that performed previously in E. coli (Flynn et al., 2003). Briefly, we mutated the active site serine of ClpP and appended a C-terminal tandem affinity tag to generate ClpPtrap (Figure S1). Substrate proteins are recognized by the native active ClpX or ClpA unfoldases and translocated into the inactive chamber of ClpPtrap (Flynn et al., 2003; Gottesman et al., 1993; Katayama et al., 1988). The substrate-containing ClpPtrap complexes are then rapidly purified through successive rounds of affinity chromography. Our initial efforts in wildtype cells were unsuccessful, likely due to incorporation of active subunits in the ClpPtrap oligomer; however, depletion of the chromosomally encoded ClpP while expressing ClpPtrap improved the recovery of candidates (Figure 1). Known substrates of ClpXP, such as the master regulator CtrA, were specifically enriched in the trapped substrate pool (Figure 1 and S1) and subsequent mass spectrometry identified 127 unique proteins in the eluted fraction (Figure 1; Table S1). We pruned this list with high stringency requirements (see Supplemental Information) to generate a candidate list of 32 proteins (Table 1). We note that likely ClpP substrates (such as LexA and others shown below) fail to meet these more stringent requirements, but are clearly identified in our trap (Table S1); therefore, these lists should not be considered exhaustive, but reflect a high confidence subset of ClpP substrates.
Figure 1.
ClpP trapping strategy and substrate characterization. A. Inactive oligomers of ClpP can capture substrates delivered to them by active unfoldases. Depletion of endogenous, active ClpP enriches for inactive ClpPtrap oligomers. Affinity purification followed by mass spectrometry identifies candidate substrates. B. Substrates are co-purified with ClpPtrap, but are absent when an affinity tagged active ClpP is used. Western blotting with antibodies recognizing the known ClpXP substrate CtrA (black markers) confirms trapping procedure. Confirmation of additional substrates by Western blotting is shown in Figure S1. C. Candidate substrates are widely distributed across many functional categories as annotated by COG groups (NCBI), total numbers of proteins in each category shown in parentheses.
Table 1.
Subset (32/127) trapped candidates that satisfy high stringency requirements of >5 unique peptides in replicate trapping experiments, removing proteins that copurifed with active ClpP, and eliminating proteins that are overly abundant in whole cell extracts (see Supplemental Information).
| CC / CCNA | gene | reference |
|---|---|---|
|
| ||
| CC_0008 | dnaA | 2, 6 |
| CC_0011 | dnaJ | 2, 5, 6 |
| CC_0042 | infB | |
| CC_0160 | gyrB | 2, 5 |
| CC_0267 | dnaX | |
| CC_0360 | ||
| CC_0435 | cheR | 1, 2 |
| CC_0695 | mutL | |
| CC_0808 | ||
| CC_1005 | ||
| CC_1087 | recA | 2, 6 |
| CC_1118 | ||
| CC_1961 | clpX | 5 |
| CC_2102 | sspB | 2 |
| CC_2258 | ibpA | 2, 4 |
| CC_2468 | clpA | 2 |
| CC_2540 | ftsZ | 1, 2, 5, 6 |
| CC_2541 | ftsA | 2, 5 |
| CC_2546 | murC | 6 |
| CC_2700 | 2 | |
| CC_3035 | ctrA | 1,2, 4, 6 |
| CC_3145 | mcpJ | 2 |
| CC_3192 | ||
| CC_3315 | tacA | 2 |
| CC_3448 | atpG | |
| CC_3492 | nrdA | 6 |
| CC_3494 | ||
| CC_3527 | sdhA | 3 |
| CC_3691 | 2 | |
| CCNA_00466 | rfaG | |
| CCNA_00469 | ||
| CCNA_00999 | 3, 4 | |
Candidates are listed by CC numbers from CB15 (GenBank ID AE005673) or CCNA numbers from NA1000 (GenBank ID CP001340). Where known, gene names are also listed. References are: (1) proteins known to be unstable during cell cycle progression (Grunenfelder et al., 2001). (2) genes known to be transcriptionally regulated during cell cycle (Laub et al., 2000). (3) proteins known to be tagged by the trans-translation pathway (Hong et al., 2007). (4) proteins that end in either AA or SA at their extreme C-terminus. (5) putative ClpP substrates identified in other bacteria (Flynn et al., 2003; Gerth et al., 2008; Neher et al., 2006). (6) proteins whose levels decrease during carbon starvation(Britos et al., 2011). An expanded version of this table with all ClpP candidates (127) can be found in Supplemental Information (Table S1).
The ClpP candidate pool shown in Tables 1 and S1 is distributed across a wide range of functional categories. Mass spectrometry results identified two previously known Caulobacter ClpP substrates (CtrA and SspB (Chien et al., 2007b; Domian et al., 1997; Jenal and Fuchs, 1998)) and Western blot analysis showed the capture of other known ClpXP substrates such as PdeA (Abel et al., 2011; Rood et al., 2012) that were not detected by mass spectrometry (Figure S1). Interestingly, a number of the candidates found by mass spectrometry had been identified in other proteomic surveys as potential ClpP regulated targets. For example, several are annotated in Caulobacter as substrates of the trans-translation pathway (Hong et al., 2007), wherein polypeptides are cotranslationally appended with degradation tags that target them to either ClpXP or ClpAP. It is possible that some of these candidates are only targets of ClpP when tagged by the ssrA peptide. We also find conservation of ClpP substrates among widely divergent organisms as several candidates are known to be degraded in a ClpP dependent fashion in either E. coli or B. subtilis (Flynn et al., 2003; Gerth et al., 2008; Kock et al., 2004; Neher et al., 2006). Finally, we identify a number of substrates previously shown to be unstable in Caulobacter whose regulation can now be assigned to ClpP (Britos et al., 2011; Grunenfelder et al., 2001).
Recognition motifs for ClpXP are preserved across species
We next used a biochemical approach to validate putative ClpP substrates. We initially focused on candidates with known ClpXP recognition motifs classified by previous studies in E. coli by recombinantly expressing, purifying and biochemically validating several “C-terminal class I” family substrates (distinguished by the presence of Ala-Ala at the extreme C-terminus) (Flynn et al., 2003). Of the six candidates of this class that we tested, four (FlaF, IbpA, CtrA, CC2882) were recognized by ClpXP in vitro (Chien et al., 2007b) (Figure 2 and S2). Furthermore, the ClpXP dependent degradation of CC2323, which contains SA rather than AA, agrees with the known tolerance of this motif (Figure S2A,B). Mutating the C-terminal residues of FlaF and IbpA to Asp-Asp eliminates recognition, revealing the importance of the C-terminal dipeptide for specific recognition by ClpXP (Figure 2A,B). The validated ClpXP substrates do not share any obvious common functional or sequence features that distinguish them from nondegraded substrates (DnaK and CC0321). Thus, our data show, as would be predicted, that while conserved protease recognition motifs can dictate targeting by a protease, the simple presence of a particular motif does not ensure that a protein will be directly recognized by a particular protease.
Figure 2.
Trapped substrates reveal conserved motif requirements for ClpXP proteolysis. A. Select substrates were cloned, expressed recombinantly and purified, then assayed for in vitro degradation by ClpXP. Table lists CC annotation / gene name, sequence of the C-terminal six residues, and whether the candidate substrate was degraded by ClpXP in standard conditions (see Methods and Figure S2). B. IbpA and FlaF are both degraded by ClpXP and mutation of their C-terminal Ala-Ala motif eliminates degradation. DnaK is not degraded by ClpXP in vitro.
The N-terminal domain of ClpX is critical for its in vivo function
We identified CC0360, a putative ornithine decarboxylase, as a likely ClpP substrate using our most stringent requirements (Table 1) and found that it was rapidly degraded by ClpXP in vitro (Figure 3A). The lack of a C-terminal Ala-Ala motif in CC0360 (Figure 2A) prompted us to explore how ClpXP was recognizing this substrate. The N-terminal zinc-binding domain of ClpX is unique to the ClpX family of unfoldases and is required for recognition of some substrates, such as MuA, lambdaO and FtsZ for the E. coli ClpX (Abdelhakim et al., 2008; Wojtyra et al., 2003; Camberg et al., 2009); but dispensible for degradation of others, such as CtrA and ssrA-tagged proteins for the C. crescentus ClpX (Figure 3A,B and Figure S2) (Chien et al., 2007a; Chien et al., 2007b). As in E. coli, the N-terminal domain of the C. crescentus ClpX is also critical for adaptor binding, as seen in the case of SspB (Chien et al., 2007a). Interestingly, we found that CC0360 was exclusively degraded by the full-length ClpXP complex and not by a version of ClpX lacking the N-terminal domain (ΔNClpXP; Figure 3A). A similar, but less pronounced effect was seen with CC2882 (Figure S2D), while other substrates, such as FlaF was recognized by ΔNClpXP with rates similar to full-length ClpXP (Figure S2D). Because ClpX is essential in Caulobacter, we tested the importance of the N-terminal domain of ClpX in vivo and found that ΔNClpX could not support viability (Figure 3C). We interpret this to mean that at least one important role for the full-length ClpXP in vivo is the degradation of substrates which rely on the N-terminal domain of ClpX (such as CC0360). This, of course, does not rule out other crucial roles for the N-terminal domain of ClpX, such as adaptor binding or localization.
Figure 3.
The N-terminal domain of ClpX is a critical modulator of protease specificity. A. CC0360 degradation relies on the N-terminal domain of ClpX even though ssrA tagged substrates (GFP-ssrA) are degraded readily by both constructs. B. CtrA is recognized by both full length and ΔNClpX. C. The N-terminal domain of ClpX is essential for viability. Cells expressing a xylose inducible copy of clpX as the sole chromosomal copy and plasmids constitutively expressing either full length (WT) or ΔNClpX variants plated on inducing (xylose) or noninducing (no xylose) media. D. Western blot analysis using antibodies specific to C. crescentus ClpX confirm the constitutive expression of ΔNClpX from the plasmid in both inducing (xylose) and noninducing (glucose) conditions. Blots are registered so that upper bands (full length ClpX) are aligned.
TacA is degraded in a regulated fashion by ClpXP during the cell cycle
Stalk synthesis is a programmed developmental step in the life cycle of Caulobacter and is controlled transcriptionally by the σ54 dependent response regulator TacA (Biondi et al., 2006b; Marques et al., 1997; Radhakrishnan et al., 2008). TacA was identified in our trapping study and we found that the purified protein was degraded in vitro by ClpXP (Figure 4A). ClpXP recognizes the TacA via its C-terminal residues (ending in MKEAG-cooh) as mutation of the C-terminal dipeptide AG to DD prevents degradation (Figure 4A). An active, epitope-tagged variant of TacA (Biondi et al., 2006b) was degraded in vivo and stabilized by the same mutations that eliminate ClpXP recognition in vitro (Figure 4B and S4B). The regulator CpdR is required for the polar localization of ClpXP during the cell cycle (Iniesta et al., 2006) and is critical for the in vivo degradation of all known ClpXP substrates to date (Abel et al., 2011; Biondi et al., 2006a; Iniesta et al., 2006). As predicted from these observations, and in accordance with its ability to be degraded by ClpXP in vitro (Figure 4A), we saw that the stability of TacA was dependent on CpdR in vivo (Figure 4B), further supporting its identification as a true ClpXP substrate. Stabilization of TacA results in smaller colonies when cells are inoculated into low-percentage agar media, suggesting that TacA degradation may impact normal growth, differentiation or motility (Figure S4D,E).
Figure 4.
TacA is degraded by ClpXP. A. TacA is recognized by ClpXP in vitro and mutating C-terminal residues to Asp-Asp inhibits proteolysis. In these gels, overlapping bands corresponding to ClpX and creatine kinase are marked along with the purified TacA proteins. B. M2-FLAG epitope tagged TacA (M2-TacA) expressed from an inducible plasmid is degraded in vivo in a CpdR-dependent fashion following shift to a noninducing media. Representative western blot is shown here; quantification of replicates can be found in Figure S4. C. Western blots against the M2FLAG epitope and CtrA in synchronized population of wildtype cells shows that M2-TacA is degraded in a cell-cycle dependent fashion, while M2-TacADD is not degraded. Additional replicate illustrating M2-TacA degradation is shown in Figure S4. D. Loss of CtrA after antibiotic mediated shutoff of synthesis in cells expressing M2-TacADD compared to cells expressing M2-TacA. Upper panel shows a representative western blot, lower graph represents averages of biological replicates (n=4). Error bars are standard errors of the mean.
A constitutively expressed M2-TacA was degraded at the G1-S transition in a CpdR-dependent fashion (Figure 4C and S4C),similar to that seen for CtrA (Domian et al., 1997; Iniesta et al., 2006). Interestingly, cells expressing the nondegradable M2-TacA-DD show a delay in CtrA accumulation when compared to cells expressing M2-TacA (Figure 4C). Because CtrA positively regulates its own expression late in the cell cycle (Domian et al., 1999), this result suggests that stabilized TacA results in lower CtrA activity. Our data support a posttranslational role for this lower CtrA activity as CtrA is degraded more rapidly in cells expressing the nonproteolyzed M2-TacA-DD compared with cells expressing the properly degraded M2-TacA (Figure 4D).
Stabilization of TacA restores stalk formation in pleC mutants
Our results suggest proteolysis of TacA is integrated with changes in CpdR activity which in turn are dependent on the changing status of DivK phosphorylation during the cell cycle (Curtis and Brun, 2010). DivK phosphorylation by DivJ is countered by the PleC phosphatase and cells depleted of PleC have reduced CckA kinase activity resulting in lower levels of phosphorylated CpdR (depicted schematically in Figure 5A) (Tsokos et al., 2011). Based on these observations, we hypothesized that the defects in stalk formation of pleC mutant cells (Sommer and Newton, 1989) may arise in part from the rapid turnover of TacA driven by persistent dephosphorylation of CpdR. If our model is correct, then expression of a nondegradable TacA should restore stalk formation in ΔpleC cells. We tested our hypothesis by replacing the endogenous allele of TacA in ΔpleC cells with a nondegradable TacA-DD variant. Because stalked and swarmer cells are difficult to distinguish by size alone, we counted the number of predivisional cells in a mixed population with visible stalks (as illustrated in Figure 5B). Over 90% of wildtype predivisional cells had visible stalks, while 2–3% of predivisional ΔpleC cells expressing normal TacA have stalks (Figure 5C). Expressing a stabilized variant of TacA in ΔpleC cells increased the fraction of stalked predivisional cells 5-fold (Figure 5C) . Consistent with these findings, stalk elongation in ΔpleC during phosphate limitation is also increased when TacA is stabilized (Figure S5). Collectively, our results show how CpdR activity, which is normally regulated during cell cycle, can affect the developmental program of stalk formation via degradation of the novel ClpXP substrate TacA.
Figure 5.
Stabilization of TacA partially rescues stalk formation in ΔpleC cells. A. Cartoon of PleC dependent TacA degradation. PleC dephosphorylates DivK. Dephosphorylated DivK inhibits CpdR dephosphorylation indirectly through CckA/ChpT (not illustrated here). Dephosphorylated CpdR promotes ClpXP degradation of TacA. Thus, loss of PleC would result in more dephosphorylated CpdR and faster TacA degradation. B. Representative images of wildtype cells (upper) with stalks marked with white arrows (predivisional cell) or black arrows (stalked cell) and stalkless ΔpleC predivisional cells (lower). C. Stalk formation is partially recovered in ΔpleC cells expressing stabilized TacA. Quantification of predivisional wildtype cells, ΔpleC strains expressing TacA as the sole variant, or ΔpleC strains expressing TacA-DD as the sole variant. Error bars are standard errors, p-value is calculated from a two-tailed Welch’s t-test. Stalk elongation during phosphate limitation is also more pronounced in ΔpleC cells expressing stabilized TacA (Figure S5).
Discussion
Regulated proteolysis is essential for biological processes in all organisms and ClpP associated proteases contribute to a large fraction of protein degradation in bacteria (Sauer and Baker, 2011). In C. crescentus, control of proteolysis during cell cycle is critical for timing of development, DNA replication, and cell division (Curtis and Brun, 2010). By using a ClpP trapping approach, we now report a number of potential ClpP candidates that include known substrates (such as the master regulator CtrA), factors known to be ClpP substrates in other bacteria (such as LexA and FtsZ), and several novel ClpXP dependent proteolysis targets (such as TacA and IbpA). Some of these validated Caulobacter ClpXP substrates are targeted for degradation in other bacteria by other energy-dependent proteases (e.g., IbpA is degraded by Lon in E. coli (Bissonnette et al., 2010)). Interestingly, CC0360, a putative ornithine decarboxylase, is degraded rapidly by full-length ClpX from Caulobacter and the eukaryotic ornithine decarboxylase is a rapidly degraded ubiquitin independent substrate of the 26S proteasome (Murakami et al., 1992). Biological networks rely on rapidly changing protein levels for effective signaling and the response in a protein’s concentration given changes in its synthesis rate is more rapid when the half-life of that protein is short (Rosenfeld et al., 2002). Thus, our work suggests that some proteins may be rapidly degraded regardless of the organism or proteolytic pathway involved because there is a universal need for their dynamic responses.
ClpXP mediated degradation of protein substrates often requires small peptide motifs that are directly recognized by the protease (Sauer and Baker, 2011) and a classification of these motifs has been proposed (Flynn et al., 2003). We tested the conservation of these protease recognition motifs in our candidate pool and found that while motifs can direct degradation of some substrates, the simple presence of a motif is insufficient to predict direct recognition by ClpXP. We also found that, unlike E. coli, Caulobacter viability requires a function unique to the full-length ClpX protein. Although a variant of ClpX lacking its N-terminal zinc-binding domain can readily degrade substrates such as ssrA-tagged proteins and CtrA, this variant is incapable of supporting viability in Caulobacter and poorly degrades some of the validated ClpXP substrates presented in this work. Together, these biochemical observations demonstrate the utility and limitations of recognition motifs for substrate recognition and reveal a crucial role for the N-terminal domain of the Caulobacter ClpXP in substrate recognition and in vivo function.
The characteristic swarmer to stalked cell transition in Caulobacter requires a substantial reorganization of the proteome and we identified a number of cell stage specific factors in our trapping studies including the σ54-dependent response regulator TacA, several chemotaxis proteins (CheR, CheW, CheD, and McpJ), and flagellar regulators (FlaF and FliM). Our results show that TacA is directly recognized by ClpXP in vitro but degradation in vivo requires CpdR. CpdR is needed for degradation of all known ClpXP substrates in vivo and dephosphorylation of CpdR increases degradation of these substrates (Abel et al., 2011; Biondi et al., 2006a; Iniesta et al., 2006). Phosphorylation of CpdR occurs via the CckA/ChpT phosphotransfer pathway (Biondi et al., 2006a) and CckA is repressed by phosphorylated DivK (Tsokos et al., 2011). The PleC phosphatase dephosphorylates DivK, and as a consequence, loss of pleC results in more dephosphorylated CpdR (Tsokos et al., 2011) and presumably would increase degradation of ClpXP substrates, such as TacA. Because ΔpleC cells lack stalks (Sommer and Newton, 1989) and as TacA is critical for stalk formation (Biondi et al., 2006b), it is tempting to speculate that prolific degradation of TacA is responsible for this absence of stalked cells. In support of this model, we found that stabilization of TacA partially restored stalked cell formation (Figure 5), although the lack of complete restoration implicates the role of additional factors as well.
Why does stabilization of TacA cause increased degradation of CtrA? TacA influences asymmetric cell division in Caulobacter in part through synthesis of SpmX which is needed for the proper accumulation and activation of the histidine kinase DivJ that phosphorylates DivK (Radhakrishnan et al., 2008). As mentioned above, DivK promotes CpdR and CtrA dephosphorylation by inhibiting the kinase activity of CckA (Tsokos et al., 2011), unleashing CpdR’s ability to activate ClpXP (Abel et al., 2011; Biondi et al., 2006a; Iniesta et al., 2006) but blocking CtrA’s activity as a transcription factor (Biondi et al., 2006a; Quon et al., 1996). One intriguing possibility is that TacA promotes CpdR dephosphorylation, perhaps through SpmX, effectively stimulating its own degradation and that of CtrA. In this type of negative feedback loop, increasing TacA through stabilization may result in prolific CpdR activation that could underlie the increased degradation of CtrA.
Material and Methods
Plasmids and strain construction
Standard techniques were used to manipulate C. crescentus. All C. crescentus strains were grown at 30°C in PYE with the appropriate antibiotics and sugars (Skerker et al., 2005). Briefly, we generated the proteolytic inactive ClpPtrap by mutating the active site serine to alanine (S98A) and appending a tandem affinity tag (His6-TEV-M2FLAG) to the C-terminus of ClpP. Low copy plasmids expressing ClpPtrap or wildtype affinity tagged ClpP were transformed into a strain whose sole, genomic copy of ClpP was driven by the xylose promoter (Jenal and Fuchs, 1998) to generate trapping or control strains. ClpP substrate candidates were cloned with appropriate primers into either pET23SUMO plasmids for recombinant protein expression in E. coli (Wang et al., 2007) or into pENTR plasmids for Gateway-based cloning into expression plasmids for either E. coli or C. crescentus (Skerker et al., 2005). ΔNClpX (removing residues 1-58) and full length ClpX were expressed in ClpX depletion strains (Jenal and Fuchs, 1998) using low copy plasmids downstream of the clpX promoter(Osteras et al., 1999). Caulobacter strains harboring alleles of TacA-DD were generated by transforming wildtype or ΔpdeA:tet (Skerker et al., 2005) with pENTR TacA-DD. Because these promoter-less plasmids cannot replicate in Caulobacter, antibiotic resistance is gained only if they are integrated. Subsequent screening by PCR verified that plasmids were inserted at the correct locus downstream of the native tacA promoter.
Purification of ClpP trap and candidate identification
Detailed procedures for ClpPtrap expression and purification are given in the Supplemental Information. Briefly, sequential affinity columns were used to purify ClpPtrap after depletion of endogenous ClpP, preliminary validition of the trap was confirmed by western analysis against the known substrate CtrA. Trapped substrates were separated by 15% SDS-PAGE, gel slices (~1–5 mm) were excised, digested with trypsin and analyzed by mass spectrometry (Proteomics and Mass Spectrometry Facility, University of Massachusetts, Worcester).
Protein purification and degradation assays
Detailed information is given in Supplemental Information. Untagged C. crescentus ClpX and ΔNClpX were purified using a similar protocol as that described for E. coli ClpX (Flynn et al., 2003). Recombinant his-tagged C. crescentus ClpP was purified as described (Chien et al., 2007b). Candidates from the ClpP trap were expressed either as his-tagged constructs (Skerker et al., 2005) or as his-SUMO-tagged constructs (Wang et al., 2007) and purified accordingly. Degradation reactions were performed in H-buffer (20 mM HEPES, 100 mM KCl, 10 mM MgCl2, 10% glycerol, pH 7.5; and an creatine kinase based ATP regeneration mix) at 30°C (Chien et al., 2007b). In a typical timecourse, aliquots at specific times were added to SDS-PAGE loading dye to quench the reaction and snap frozen on dry ice. Samples were heated at 95°C for 5 min or 65°C for 10 min and resolved by 12% or 15% SDS/PAGE gels. Coomassie stained gels were quantified by measuring intensity of substrate bands using ImageJ (NIH).
In vivo stability and synchrony experiments
For asynchronous in vivo degradation assays, cells with plasmids expressing epitope tagged TacA from xylose promoters were grown in PYE with xylose (0.1%) and spectinomycin (25μg/μl) overnight. In the morning the cultures were back diluted into the same media, grown until exponential growth, after which cells were harvested, washed twice in fresh PYE medium and resuspended in PYE-glucose to initiate the timecourse. Protein levels were assayed by Western blotting using antibodies against the M2-FLAG epitope (Sigma-Aldrich). For synchronized growth, Caulobacter were grown to an OD600 of 0.3–0.5 in PYE medium with the appropriate antibiotic, swarmer cells were isolated by Percoll density centrifugation, and cells were released into the same media. For CtrA stability experiments, swarmer cells were isolated and resuspended in pre-warmed PYE media containing 30 μg/ml chloramphenicol to shut off synthesis.
Supplementary Material
Acknowledgments
We thank U. Jenal, J. Gober, and Y. Brun for providing reagents and strains; and L. Francis, M. Laub for comments. N.B. and P.C. were funded by NIH grant R00GM084157. P.S. was also supported by a HHMI Summer Research Fellowship for Undergraduates. We especially thank K. Green (University of Massachusetts, Worcester) for all of her advice and aid in mass spectrometry and protein identification.
References
- Abel S, Chien P, Wassmann P, Schirmer T, Kaever V, Laub MT, Baker TA, Jenal U. Regulatory cohesion of cell cycle and cell differentiation through interlinked phosphorylation and second messenger networks. Molecular cell. 2011;43:550–560. doi: 10.1016/j.molcel.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, Laub MT. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006a;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- Biondi EG, Skerker JM, Arif M, Prasol MS, Perchuk BS, Laub MT. A phosphorelay system controls stalk biogenesis during cell cycle progression in Caulobacter crescentus. Molecular microbiology. 2006b;59:386–401. doi: 10.1111/j.1365-2958.2005.04970.x. [DOI] [PubMed] [Google Scholar]
- Bissonnette SA, Rivera-Rivera I, Sauer RT, Baker TA. The IbpA and IbpB small heat-shock proteins are substrates of the AAA+ Lon protease. Molecular microbiology. 2010;75:1539–1549. doi: 10.1111/j.1365-2958.2010.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britos L, Abeliuk E, Taverner T, Lipton M, McAdams H, Shapiro L. Regulatory response to carbon starvation in Caulobacter crescentus. PloS one. 2011;6:e18179. doi: 10.1371/journal.pone.0018179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberg JL, Hoskins JR, Wickner S. ClpXP protease degrades the cytoskeletal protein, FtsZ, and modulates FtsZ polymer dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10614–10619. doi: 10.1073/pnas.0904886106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien P, Grant RA, Sauer RT, Baker TA. Structure and substrate specificity of an SspB ortholog: design implications for AAA+ adaptors. Structure. 2007a;15:1296–1305. doi: 10.1016/j.str.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Chien P, Perchuk BS, Laub MT, Sauer RT, Baker TA. Direct and adaptor-mediated substrate recognition by an essential AAA+ protease. Proceedings of the National Academy of Sciences of the United States of America. 2007b;104:6590–6595. doi: 10.1073/pnas.0701776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis PD, Brun YV. Getting in the loop: regulation of development in Caulobacter crescentus. Microbiology and molecular biology reviews : MMBR. 2010;74:13–41. doi: 10.1128/MMBR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domian IJ, Quon KC, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Reisenauer A, Shapiro L. Feedback control of a master bacterial cell-cycle regulator. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6648–6653. doi: 10.1073/pnas.96.12.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Molecular cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Gerth U, Kock H, Kusters I, Michalik S, Switzer RL, Hecker M. Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. Journal of bacteriology. 2008;190:321–331. doi: 10.1128/JB.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED, Toro E, McAdams HH, Shapiro L. Dynamic chromosome organization and protein localization coordinate the regulatory circuitry that drives the bacterial cell cycle. Cold Spring Harbor symposia on quantitative biology. 2009;74:55–64. doi: 10.1101/sqb.2009.74.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk B, Marczynski GT. Regulated degradation of chromosome replication proteins DnaA and CtrA in Caulobacter crescentus. Molecular microbiology. 2005;55:1233–1245. doi: 10.1111/j.1365-2958.2004.04459.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S, Clark WP, de Crecy-Lagard V, Maurizi MR. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. The Journal of biological chemistry. 1993;268:22618–22626. [PubMed] [Google Scholar]
- Grunenfelder B, Rummel G, Vohradsky J, Roder D, Langen H, Jenal U. Proteomic analysis of the bacterial cell cycle. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4681–4686. doi: 10.1073/pnas.071538098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Lessner FH, Mahen EM, Keiler KC. Proteomic identification of tmRNA substrates. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17128–17133. doi: 10.1073/pnas.0707671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10935–10940. doi: 10.1073/pnas.0604554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U, Fuchs T. An essential protease involved in bacterial cell-cycle control. The EMBO journal. 1998;17:5658–5669. doi: 10.1093/emboj/17.19.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Gottesman S, Pumphrey J, Rudikoff S, Clark WP, Maurizi MR. The two-component, ATP-dependent Clp protease of Escherichia coli. Purification, cloning, and mutational analysis of the ATP-binding component. The Journal of biological chemistry. 1988;263:15226–15236. [PubMed] [Google Scholar]
- Kock H, Gerth U, Hecker M. The ClpP peptidase is the major determinant of bulk protein turnover in Bacillus subtilis. Journal of bacteriology. 2004;186:5856–5864. doi: 10.1128/JB.186.17.5856-5864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, McAdams HH, Feldblyum T, Fraser CM, Shapiro L. Global analysis of the genetic network controlling a bacterial cell cycle. Science. 2000;290:2144–2148. doi: 10.1126/science.290.5499.2144. [DOI] [PubMed] [Google Scholar]
- Lesley JA, Shapiro L. SpoT regulates DnaA stability and initiation of DNA replication in carbon-starved Caulobacter crescentus. Journal of bacteriology. 2008;190:6867–6880. doi: 10.1128/JB.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MV, Gomes SL, Gober JW. A gene coding for a putative sigma 54 activator is developmentally regulated in Caulobacter crescentus. Journal of bacteriology. 1997;179:5502–5510. doi: 10.1128/jb.179.17.5502-5510.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Matsufuji S, Kameji T, Hayashi S, Igarashi K, Tamura T, Tanaka K, Ichihara A. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- Neher SB, Villen J, Oakes EC, Bakalarski CE, Sauer RT, Gygi SP, Baker TA. Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Molecular cell. 2006;22:193–204. doi: 10.1016/j.molcel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Osteras M, Stotz A, Schmid Nuoffer S, Jenal U. Identification and transcriptional control of the genes encoding the Caulobacter crescentus ClpXP protease. Journal of bacteriology. 1999;181:3039–3050. doi: 10.1128/jb.181.10.3039-3050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocka I, Thein M, MOS, Jenal U, Alley MR. Degradation of a Caulobacter soluble cytoplasmic chemoreceptor is ClpX dependent. Journal of bacteriology. 2002;184:6635–6641. doi: 10.1128/JB.184.23.6635-6641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Pritchard S, Viollier PH. Coupling prokaryotic cell fate and division control with a bifunctional and oscillating oxidoreductase homolog. Developmental cell. 2010;18:90–101. doi: 10.1016/j.devcel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Thanbichler M, Viollier PH. The dynamic interplay between a cell fate determinant and a lysozyme homolog drives the asymmetric division cycle of Caulobacter crescentus. Genes & development. 2008;22:212–225. doi: 10.1101/gad.1601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood KL, Clark NE, Stoddard PR, Garman SC, Chien P. Adaptor-dependent degradation of a cell-cycle regulator uses a unique substrate architecture. Structure. 2012;20:1223–1232. doi: 10.1016/j.str.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld N, Elowitz MB, Alon U. Negative autoregulation speeds the response times of transcription networks. Journal of molecular biology. 2002;323:785–793. doi: 10.1016/s0022-2836(02)00994-4. [DOI] [PubMed] [Google Scholar]
- Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annual review of biochemistry. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. PLoS biology. 2005;3:e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JM, Newton A. Turning off flagellum rotation requires the pleiotropic gene pleD: pleA, pleC, and pleD define two morphogenic pathways in Caulobacter crescentus. Journal of bacteriology. 1989;171:392–401. doi: 10.1128/jb.171.1.392-401.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Alley MR. Proteolysis of the Caulobacter McpA chemoreceptor is cell cycle regulated by a ClpX-dependent pathway. Journal of bacteriology. 2001;183:5001–5007. doi: 10.1128/JB.183.17.5001-5007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos CG, Perchuk BS, Laub MT. A dynamic complex of signaling proteins uses polar localization to regulate cell-fate asymmetry in Caulobacter crescentus. Developmental cell. 2011;20:329–341. doi: 10.1016/j.devcel.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Sauer RT, Baker TA. ClpS modulates but is not essential for bacterial N-end rule degradation. Genes & development. 2007;21:403–408. doi: 10.1101/gad.1511907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.