Abstract
Prostate cancer poses a major public health problem in the developed countries. The remarkable biological and clinical heterogeneity of prostate cancer provides unique opportunities as well as challenges for the diagnostic imaging evaluation of this prevalent disease. The disease is characterized by a natural history that ranges from localized slowly-growing hormone-dependent tumor progressing to metastatic hormone-refractory disease. Positron emission tomography (PET) is an ideal imaging tool for noninvasive interrogation of the underlying tumor biology. Fluorine-18 fluorodeoxyglucose (FDG) is the most common PET radiotracer used for oncologic applications which is based upon elevated glucose metabolism in malignant tissue in comparison to normal tissue. FDG uptake in the prostate cancer depends on tumor differentiation with low accumulation in well-differentiated tumors and high uptake in aggressive poorly-differentiated tumors. Cumulative current evidence suggests that FDG PET may be useful in detection of disease in a small fraction of patients with biochemical recurrence, in the imaging evaluation of extent and treatment response in metastatic disease and in prediction of patient outcome.
Keywords: Prostate, Cancer, FDG, PET
Introduction
Prostate cancer is the most common male cancer with a lifetime risk of about 1 in 6 men in the developed countries. During the period 2005–2009, the age-adjusted incidence rate and death rate were 154.8 per 100,000 men per year and 23.6 per 100,000 men per year, respectively, in the United States (1, 2). In the post prostate specific antigen (PSA) screening era, the stage distribution for all races during the period 2000–2008 were 81% localized (primary tumor confined to prostate gland), 12% regional (spread to regional lymph nodes), 4% distant (metastases to other organs), and 3% unstaged (1). While men with localized prostate cancer are treated with the curative intent, approximately 40% of patients will experience a detectable rise in the serum PSA level (biochemical failure) within 10 years from the primary treatment (3). Locally recurrent cancer is eventually detected in about 25–35%, metastatic disease only in about 20–25% and both local recurrence and metastatic disease in 45–55% of men with biochemical failure (4). While most men with metastatic disease respond to androgen deprivation therapy, many will develop castrate-resistant state within 1–3 years with the hallmark of tumor growth despite castrate levels of serum androgens (5, 6). Castrate-resistant metastatic prostate cancer is incurable and the main cause of disease-related morbidity and mortality (7).
Positron emission tomography (PET) is a quantitative tool for noninvasive imaging-based interrogation of the underlying tumor biology. Several promising radiotracers are currently being investigated for the imaging evaluation of prostate cancer including, but not limited to, 18F- or 11C-choline, 18F- or 11C-acetate, 16β-18F-fluoro-5α-dihydrotestosterone, targeted to the androgen receptor, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, a synthetic L-leucine analog, as well as PET radiotracers based on prostate-specific membrane antigen, prostate stem cell antigen, and gastrin-releasing peptide receptor (8, 9). However, the exact diagnostic and prognostic roles of these radiotracers in prostate cancer are undefined and will require additional investigation. 18F-fluorodeoxyglucose (FDG) is the most common PET radiotracer used for oncologic applications. This article briefly reviews the utility and limitations of FDG PET/CT in prostate cancer.
Preclinical Studies
The Warburg effect of elevated glucose metabolism in the malignant tissue in comparison to the normal tissue is the underlying mechanism for the accumulation of FDG in cancer. While the biological detail of this hallmark of cancer is relatively complex, the malignancy-induced hypermetabolism is generally based upon overexpression of cellular membrane glucose transporters (mainly GLUT-1) and enhanced hexokinase enzymatic activity in tumors (10,11).
In-vitro studies have shown that GLUT1 expression is higher in the poorly differentiated cell lines DU145 and PC3 than in the well-differentiated hormone-sensitive LNCaP cell line, suggesting that the level of GLUT1 expression increases with progression of malignancy grade (12). GLUT1 gene expression is also significantly higher in prostate cancer than in benign prostatic hyperplasia (BPH) tissue and is correlated directly with Gleason score (R = .274, p = .026) (13).
Other studies have shown that androgen may affect the level of FDG uptake in the androgen sensitive, androgen receptor positive prostate cell lines (e.g. LNCaP, CWR-22) while no effect is noted in the androgen-independent, androgen receptor negative cell lines (e.g. PC-3) (14, 15). This observation may be due to the modulatory effect of androgens onto the GLUT1 and hexokinase expression levels (16, 17). Kukuk et al reported a statistically significant decrease in FDG uptake of the androgen-sensitive CWR-22 xenograft tumor model from 4.11+1.29 %ID/cm3 before to 2.19+1.45 %ID/cm3 after surgical castration (18). Androgen ablation therapy reduced the Ki67 proliferation index from 60% to 3%, the thymidine kinase 1 expression from 32% to 1% and the expression of GLUT1 from a baseline level to none. These findings support the notion that androgen has significant effect on the biology of hormone-sensitive prostate tumors that may then be assessed noninvasively through molecular imaging.
Diagnosis of Primary Tumor and Initial Staging
The level of FDG accumulation can overlap in normal prostate, BPH and prostate cancer tissues, which often co-exist (19, 20). A recent investigation assessed the level of FDG uptake in the presumed “normal” prostate gland in relation to age and prostate size in 145 men without known or suspected prostate pathology (21). The mean and maximum standardized uptake values (SUVs) for the prostate gland were 1.3 ± 0.4 (range 0.1–2.7) and 1.6 ± 0.4 (range 1.1–3.7), respectively, excluding those prostate glands with visible calcification or urethral urine activity. The mean SUV tended to decrease as the prostate size increased (r = −0.16, P = 0.058), whereas the prostate size tended to increase with increasing age (r = 0.32, P <0.001).
Minamimoto et al evaluated FDG PET/CT for detecting prostate cancer in 50 men with elevated serum PSA level who underwent subsequent prostate biopsy (22). The sensitivity and specificity were 51.9% and 75.7% for the entire prostate gland, 73% and 64% for the peripheral zone, and 22.7% and 85.9% for the central zone, respectively. The positive predictive value of FDG PET/CT was 87% in a subset of men older than 70 years of age and with serum PSA level greater than 12 ng/mL. The authors concluded that FDG PET/CT may be useful for detection of peripheral zone prostate cancer in men at more than intermediate risk. Nevertheless, as shown by the same group of investigators in a review of the Japanese nationwide survey of the FDG-PET cancer screening program in asymptomatic individuals without known history of cancer during 2006–2009, PET demonstrated a low sensitivity of only 37% for detection of prostate cancer (23).
In another retrospective study that compared FDG, 11C-choline, and magnetic resonance imaging (MRI) in patients with suspected prostate cancer, sensitivity for detection of tumor was significantly higher with MRI (88%) and 11C-choline PET (73%) than with FDG PET (31%)(24). Recently, Hwang et al reported incidental prostate hypermetabolism in 1.5% of patients who had undergone FDG PET/CT for a variety of indications excluding those with known prostate cancer or recent prostate procedures (25). The validation was through follow-up evaluation with digital rectal examination, serum PSA level, or biopsy in 65% of these patients. The median serum PSA level was 3.2 and 49.7 ng/mL in the benign and cancer groups, respectively. There was no significant difference in the mean SUVmax between the cancer and the benign groups (5.7±5.1 versus 4.8±2.7, p=0.37). The majority of patients with hypermetabolic prostate cancer (56.5%) had Gleason scores in the range 8–10.
There is little data on use of FDG PET/CT in initial staging of prostate cancer given the general low avidity of FDG for the primary prostate cancer. It is interesting however that in the early analysis of the National Oncologic PET Registry (NOPR) data in the Unites States involving 2042 scans for initial staging of prostate cancer (the most common cancer type in the initial staging subgroup), FDG PET/CT had an impact on clinical management in 32% (95% CI: 30.0%–34.1%) of the cases (26).
In summary, FDG PET cannot generally be recommended in the diagnosis or staging of clinically organ-confined disease owing to overlap of uptake among normal, benign and tumor tissues and because the high excreted radiotracer in the adjacent urinary bladder may mask lesions in the prostate gland that is located in the vicinity (27). False positive results may also occur with prostatitis (28). FDG PET may however be useful in the subset of patients with suspected poorly differentiated primary tumors (Gleason sum score above 7) and higher serum PSA values (29).
Biochemical Recurrence and Restaging
Biochemical failure (PSA relapse) is declared when there is an initial serum PSA of 0.2 ng/mL or higher with a second confirmatory PSA rise in prostatectomized patients or when there is a rise by 2 ng/mL or more above the nadir PSA level in men who had undergone primary radiation treatment (30, 31). Standard imaging (contrast-enhanced CT, 99mTc-based bone scintigraphy, prostate ultrasonography or magnetic resonance imaging) is typically employed to localize the disease in order to direct appropriate treatment (salvage surgery or radiation therapy for local recurrence in the prostate bed or systemic therapy for metastatic disease). More recently, radiolabeled choline (with 11C or 18F) has been reported to be useful in this clinical setting with a diagnostic performance that generally depends on the serum PSA level (32).
FDG PET may be useful in detecting disease in a small number of patients in this clinical setting. In one study, FDG PET was performed before pelvic lymph node dissection in 24 patients with biochemical recurrence and negative pelvis CT (33). The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of FDG PET in detecting metastatic pelvic lymph nodes, were 75.0%, 100%, 83.3%, 100% and 67.7%, respectively. In another retrospective study of 91 patients with PSA relapse following prostatectomy and validation of tumor presence by biopsy or clinical and imaging follow-up, mean serum PSA levels were reported to be higher in FDG PET positive patients than in FDG PET-negative patients (9.5 ± 2.2 ng/ml vs 2.1 ± 3.3 ng/ml) with an overall PET detection rate of 31% (34). However, the unique contribution of PET in this study could not be deciphered since some patients had disease evident on standard imaging and therefore the PET detection rate might have been overestimated. FDG PET has also been found to be advantageous over 111In-capromab pendetide scintigraphy in this clinical setting (35).
We recently reported our findings of a prospective investigation on the potential utility of FDG PET/CT and 18F-NaF PET/CT in detection of occult metastases in 37 men with PSA relapse and negative standard imaging studies (36). FDG PET/CT only was positive in one patient, 18F-NaF PET/CT only was positive in 8 patients, and both were positive in another 2 patients (Fig. 1). Our substantially lower detection rate of 8.1% (3 of 37 patients) for 18F-FDG PET/CT in the setting of biochemical recurrence is probably more realistic than those reported in the prior studies which occasionally employed concurrent positive standard imaging for validation of PET findings, thereby violating the true definition of PSA relapse only condition.
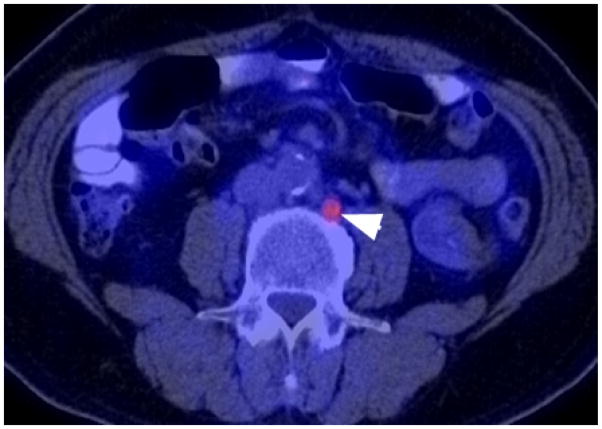
Fig. 1.
Biochemical recurrence of prostate cancer after curative prostatectomy (serum PSA=2.2 ng/mL, initial Gleason score 9). Note the 1 cm hypermetabolic metastatic left paraaortic lymph node (arrowhead) on the axial fused FDG PET/CT image.
Assessment of Treatment Response
Preclinical studies have shown that FDG accumulation in androgen-sensitive prostate tumors declines with androgen withdrawal suggesting that FDG PET might be useful for assessing response and potentially for early detection of castrate-resistant state (14). In this context, one clinical study reported that FDG uptake in the primary prostate cancer and metastatic sites decreased over a period of 1–5 months after initiation of androgen deprivation therapy (37). Nevertheless, another study of prostate cancer in rats showed that the global FDG SUV might remain unchanged after chemotherapy with gemcitabine; however potential relevance to clinical setting remains to be established (38). Our preliminary results show that tumor FDG uptake decreases with successful chemohormonal treatment, although imaging findings may be discordant with those of other manifestations of disease including changes in the levels of serum PSA or circulating tumor cells (39) (Fig. 2). For example, there may be differences in imaging-based assessment based on the response criteria that is employed in the data analysis such as Response Evaluation Criteria In Solid Tumors (RECIST 1.0 and RECIST 1.1), European Organization for Research and Treatment of Cancer (EORTC), or PET Response Criteria in Solid Tumors (PERCIST) (39). Clearly additional studies will be needed to decipher the optimal combination of relevant data that can most accurately reflect the effect of various current and novel therapies.
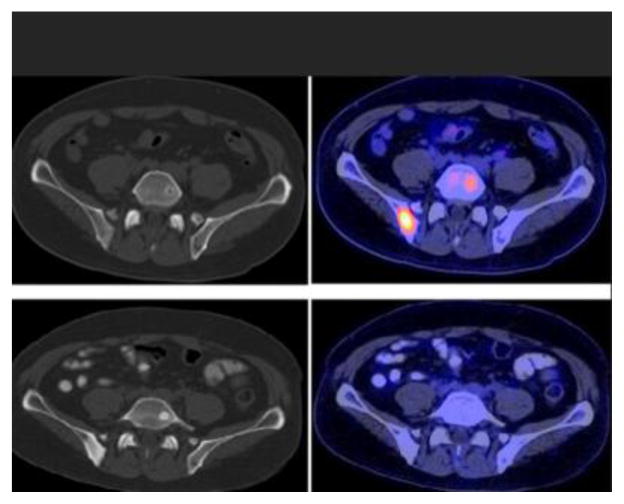
Fig. 2.
Treatment response evaluation with FDG PET/CT. CT at bone window level (left) and fused PET/CT (right) images demonstrate hypermetabolic lesions with little or no structural abnormality involving the right posterior ilium (SUVmax 5.5) and left L5 (SUVmax 3.6) before therapy (top panel, serum PSA=67.4 ng/mL) that become metabolically inactive (SUVmax 1.3) and sclerotic after successful chemotherapy (bottom panel, serum PSA = 0.3 ng/mL).
Prediction of Patient Outcome
An important unmet clinical need is the objective assessment of the comparative effectiveness of various conventional and emerging novel treatment strategies that will provide maximum benefit to individual patient (40). To this end, a quantitative patient-specific imaging-based predictive model of patient outcome can be of significant clinical value.
Few studies have reported on the potential prognostic utility of FDG PET/CT in prostate cancer. The Japanese investigators found that primary prostate tumors with high SUVs had a poorer prognosis in comparison to those with low SUVs (41). Morris et al showed that an increase of over 33% in the average maximum SUV measurement from up to 5 lesions, or the appearance of new lesions, was able to categorize castrate-sensitive metastatic prostate cancer patients treated with antimicrotubule chemotherapy into progressors or nonprogressors (42).
Meirelles and colleagues evaluated the prognostic utility of bone scintigraphy and FDG PET in 39 patients with castrate resistant and 12 patients with castrate-sensitive disease who were followed for at least 5 years or until death (43). The bone scintigraphy studies were analyzed using the bone scan index (44). The SUVmax of the most active bone lesion was used as the outcome measure for the FDG PET studies. Survival was inversely associated with bone scan index and SUVmax. The median survival of 28.2 months for patients with bone scan index of less than 1.27 was significantly longer than 14.7 months for those patients with bone scan index of greater than 1.27. Similarly, median survival of 32.8 months for SUVmax of less than 6.10 was significantly longer than 14.4 months for SUVmax of greater than 6.10. SUVmax was determined to be an independent prognostic factor in the multivariate analysis.
We recently showed in a prospective investigation of men with castrate-resistant metastatic prostate cancer that the sum of maximum SUV of up to 25 metabolically active lesions on FDG PET/CT (proxy for total burden of metabolically active disease) contributes independent prognostic information on overall survival in a multivariate Cox regression analysis adjusting for standard clinical parameters of age, serum PSA and alkaline phosphatase levels, use of pain medication, prior use of chemotherapy and Gleason score at initial diagnosis (45)(Fig. 3).
Fig. 3.
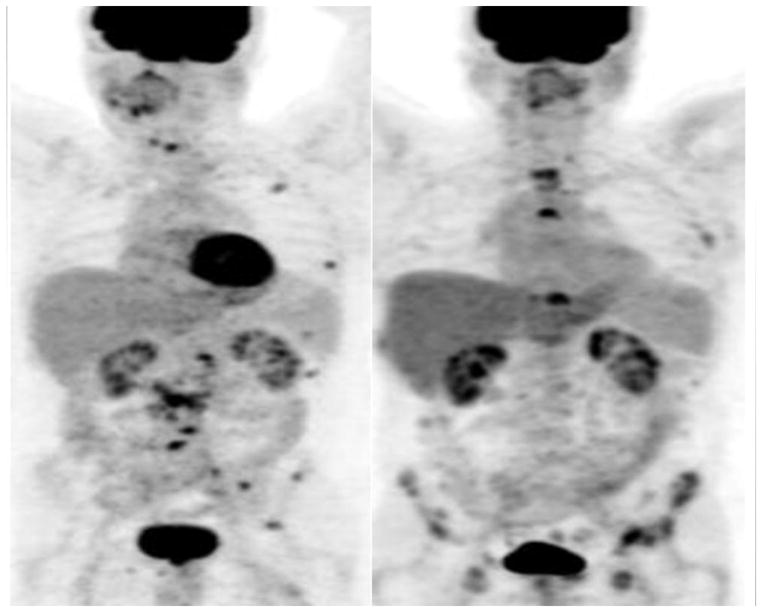
Prognostic utility of FDG PET/CT in metastatic prostate cancer. Maximum intensity projection PET images show clinical states of castrate-sensitive predominantly lymph node disease at baseline (left panel) developing into castrate-resistant predominantly bone metastatic disease after 12 months (right panel). The patient died at 28.5 months after the baseline scan.
Conclusion
FDG PET/CT is limited in the detection and localization of primary prostate cancer and initial staging of disease since most primary tumors are slow-growing, well-differentiated, multiple, and small with tumor uptake level that can overlap with those in normal tissue and benign prostatic hyperplasia. Aggressive poorly-differentiated tumors may however display high uptake although this is nonspecific and may be seen with benign inflammatory conditions. FDG PET/CT may detect occult metastatic disease in a small portion of men who present with biochemical recurrence after primary treatment with curative intent. Detection of local recurrence may however be limited due to overlap of uptake between recurrent tumor and that in post-therapy change tissue as well as the interference from the nearby bladder urine activity. FDG PET/CT may be most useful in detection of aggressive disease, the evaluation of extent and treatment response in metastatic disease and in prognostication of castrate-resistant clinical state.
Acknowledgments
This works was supported by the United States National Institutes of Health, National Cancer Institute, grants R01-CA111613 and R21-CA142426.
Footnotes
Conflict of Interest: The author declares no conflicts of interest.
References
- 1.Surveillance Epidemiology and End Results. National Cancer Institute. [accessed December 17, 2012];Cancer of the Prostate. http://seer.cancer.gov/statfacts/html/prost.html.
- 2.Center MM, Jemal A, Lorter-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Kessler B, Albertsen P. The natural history of prostate cancer. Urol Clin North Am. 2003;30:219–226. doi: 10.1016/s0094-0143(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 4.Carroll P. Rising PSA after a radical treatment. Eur Urol. 2001;40:9–16. doi: 10.1159/000049879. [DOI] [PubMed] [Google Scholar]
- 5.Fox JJ, Morris MJ, Larson SM, Schoder H, Scher HI. Developing imaging startegies for castration resistant prosatte cancer. Acta Oncologica. 2011;50(Suppl 1):39–48. doi: 10.3109/0284186X.2011.572914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marques RB, Erkene-Schutze S, de Ridder CM, et al. Androgen receptor modifications in prostate cancer cells upon long-term androgen ablation and antiandrogen treatment. Int J cancer. 2005;117:221–229. doi: 10.1002/ijc.21201. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadvar H. Molecular imaging of prostate cancer: PET radiotracers. AJR Am J Rontgenol. 2012;199:278–291. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macheda ML, Rogers S, Bets JD. Molecular and cellular regulation of glucose transport (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 11.Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci. 2000;57:170–178. [PubMed] [Google Scholar]
- 12.Effert P, Beniers AJ, Tamimi Y, et al. Expression of glucose transporter 1 (GLUT-1) in cell lines and clinical specimen from human prostate adenocarcinoma. Anticancer Res. 2004;24:3057–3063. [PubMed] [Google Scholar]
- 13.Stewardt GD, Gray K, Pennington CJ, et al. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter 1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–1567. [PubMed] [Google Scholar]
- 14.Jadvar H, Li X, Shahinian A, et al. Glucose metabolism of human prostate cancer mouse xenografts. Mol Imaging. 2005;4:91–97. doi: 10.1162/15353500200505118. [DOI] [PubMed] [Google Scholar]
- 15.Emonds KM, Swinnen JV, van Weerden WM, et al. Do androgens control the uptake of 18F-FDG, 11C-choline and 11C-acetate in human prostate cancer cell lines? Eur J Nucl Med Mol Imaging. 2011;38:1842–1853. doi: 10.1007/s00259-011-1861-6. [DOI] [PubMed] [Google Scholar]
- 16.Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–1632. [PubMed] [Google Scholar]
- 17.Moon JS, Jin WJ, Kwak JH, et al. Androgen stimulates glycolysis for de novo lipid synthesis by increasing activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 in prostate cancer cells. Biochem J. 201(433):225–233. doi: 10.1042/BJ20101104. [DOI] [PubMed] [Google Scholar]
- 18.Kukuk D, Reischl G, Raguin O, et al. Assessment of PET tracer uptake in hormone-independeent and hormone-dependent xenograft prostate cancer mouse models. J Nucl Med. 2011;52:1654–1663. doi: 10.2967/jnumed.110.086702. [DOI] [PubMed] [Google Scholar]
- 19.Salminen E, Hogg A, Binns D, et al. Investigations with FDG PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–429. doi: 10.1080/028418602320405005. [DOI] [PubMed] [Google Scholar]
- 20.Jadvar H. Molecular imaging of prostate cancer with [F-18]-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–323. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadvar H, Ye W, Groshen S, et al. [F-8]-fluorodeoxyglucose PET–CT of the normal prostate gland. Ann Nucl Med. 2008;22:787–793. doi: 10.1007/s12149-008-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamimoto R, Uemura H, Sano F, et al. The potential of FDG PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–27. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 23.Minamimoto R, Senda M, Jinnouchi S, et al. The current status of an FDG-PET cancer screening program in Japan based on a 4-year (2006–2009) nationwide survey. Ann Nucl Med. 2012 doi: 10.1007/s12149-012-0660-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe H, Kanematsu M, Kondo H, et al. Preoperative detection of prostate cancer: a comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET, and MR imaging. J Magn Reson Imaging. 2010;31:1151–1156. doi: 10.1002/jmri.22157. [DOI] [PubMed] [Google Scholar]
- 25.Hwang I, Chong A, Jung SI, et al. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2012 doi: 10.1007/s12149-012-0663-7. [Epub ahead of publication] [DOI] [PubMed] [Google Scholar]
- 26.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med. 2008;49:1926–1935. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 27.Liu IJ, Zafar MB, Lai YH, et al. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108–111. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 28.Kao PF, Chou YH, Lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med. 2008;33:308–310. doi: 10.1097/RLU.0b013e3181662f8b. [DOI] [PubMed] [Google Scholar]
- 29.Oyama N, Akino H, Suzuki Y, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–629. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 30.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localzised prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 31.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiation Oncology Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Radiol. 2011;59:51–60. doi: 10.1016/j.eururo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Chang CH, Wu HC, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by (18)f-2-deoxyglucose positron emission tomography in patients with prostate-specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311–315. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 34.Schoder H, Herrmann K, Gonen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–4769. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 35.Seltzer MA, Barbaric Z, Belldegrun A, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322–1328. [PubMed] [Google Scholar]
- 36.Jadvar H, Desai B, Ji L, Conti PS. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Haberkorn U, Bellemann ME, Altmann A, et al. PET 2-fluoro-2-deoxyglucose uptake in rat prostate adenocarcinoma during chemotherapy with gemcitabine. J Nucl Med. 1997;38:1215–1221. [PubMed] [Google Scholar]
- 39.Jadvar H, Desai B, Quinn D, et al. Treatment Response Assessment of Metastatic Prostate Cancer with FDG PET/CT. J Nucl Med. 2011;52(Suppl 1):431P. [Google Scholar]
- 40.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the PSA Working Group. J Clin Oncol. 1999;17:1–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 41.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 42.Morris MJ, Akhurst T, Larson SM, et al. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11:3210–3216. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meirelles GS, Schoder H, Ravizzini GC, et al. Prognostic value of baseline [18F]fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing prostate cancer. Clin Cancer Res. 2010;16:6093–6099. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imbriaco M, Larson SM, Yeung HW, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the bone scan index. Clin Cancer Res. 1998;4:1765–1772. [PubMed] [Google Scholar]
- 45.Jadvar H, Desai B, Ji L, et al. Prognostic utility of FDG PET/CT in men with castrate-resistant metastatic prostate cancer. J Nucl Med. 2012;53 (Suppl):116. [Google Scholar]