Abstract
The purpose of this study was to evaluate how psychological stress, gender and cortisol response to stress relate to risk behavior among 132 14–18 year old adolescents. Participants completed a laboratory based risk task prior to and immediately after a computerized psychological stress task, and salivary cortisol was collected from pre-stress to 60 minutes following initial stress exposure. Results indicate that adolescent boys (n = 59) and girls (n = 73) demonstrate different patterns of risk taking (RT) in response to stress, such that boys evidenced an increase in RT following stress exposure, whereas girls evidenced a decrease in RT. In addition, a gender by cortisol interaction demonstrated that for boys, both a smaller total cortisol output (AUCg) and peak cortisol response to stress (PC) was associated with greater stress-induced RT. Both cortisol measures were unrelated to stress-induced RT among girls. Taken together, data suggest that among boys, a blunted cortisol response to stress underlies an increase in risk taking in the context of psychological stress. Further research with an additional behavioral stress task is needed prior to drawing conclusions regarding the relation between female gender, cortisol response to stress, and risk taking in the context of psychological stress.
Keywords: Risk Taking, Gender, Cortisol, Stress, Adolescence
Introduction
Middle adolescence, which spans the ages of 13 to 17 (Frojd, Missinen, Pelkonen, Marttunen, Koivisto et al., 2008), is a developmental period marked by the emergence and escalation of risk behavior, including substance use, unsafe sexual behavior and delinquency (Scott & Steinberg, 2008; Smith-Khuri et al., 2004). Although some degree of risk taking is considered developmentally appropriate, a subset of youth will experience serious negative consequences or progress to more problematic involvement in risk behavior, increasing their risk of morbidity and mortality over the course of adolescence and adulthood (Brook et al., 2004; Colman, Croudace, Wadsworth & Jones, 2008; Scott & Steinberg, 2008; Sourander et al., 2007). Although there is extensive evidence of the incidence of risk behavior during adolescence, as well as associated negative outcomes, there is a continued need to clarify the processes underlying risk behavior.
The role of positive reinforcement in risk behavior initiation and maintenance during adolescence is well established, with previous research identifying excitement-seeking, mood enhancement and peer acceptance as powerful determinants of risk behavior during middle adolescence (Cooper, Wood, Orcutt & Albino, 2003; Kuntsche, Knibbe, Gmel & Engels, 2005; Scott & Steinberg, 2008). However, less is known about the role of affective distress in maintaining risk behavior during this developmental stage, despite evidence indicating that adolescents frequently endorse emotion regulation and coping functions for a range of risky behaviors (Kuntsche et al., 2005; Nock & Prinstein, 2005; Simons, Gaher, Correia, Hansen & Christopher, 2005; Windle & Windle, 1997). However, not everyone who experiences affective distress responds by engaging in risk behavior and research suggests that individual difference factors may influence whether an individual responds to affective distress with risk behavior (Cooper, Agocha & Sheldon, 2000).
Gender is one factor that may influence engagement in risk behavior in the context of affective distress. Moreover, the influence of gender may be particularly salient in adolescence given the increased frequency of psychological stress experienced during this developmental period (Compas & Wagner, 1991; Ge, Lorenz, Conger, Elder & Simons, 1994; Larson & Ham, 1993). Although boys and girls report comparable subjective stress levels during adolescence, (Gore, Aseltine & Colton, 1992; Hankin, Mermelstein & Roesch, 2007; Kim, Conger, Elder & Lorenz, 2003), evidence suggests that there may be important gender differences in response to stress. For instance, adolescent girls’ response to stress is characterized by negative self-evaluation, rumination, and withdrawal (Daughters et al., 2009; Galaif, Sussman, Chou & Wills, 2003; Gjerde, Block & Block, 1988; Hankin & Abramson, 2001; Horwitz & White, 1987; Nolen-Hoeksema & Corte, 2004; Piko, 2001), whereas adolescent boys’ response to stress more frequently takes the form of risk behavior such as substance use, delinquency, and disagreeable, aggressive or antagonistic behavior (Achenbach, 1991; Gjerde et al., 1988; Hankin et al., 2007). In other words, adolescent girls seem to be more internalizing when experiencing stress, whereas adolescent boys become disinhibited.
Another factor that may contribute to engagement in risk behavior is the body’s stress response system, mediated in part by the hypothalamic-pituitary-ardrenal (HPA) axis. Exposure to a stressor, whether physiological (e.g., pain) or psychological (e.g., fear, frustration), triggers a cascade of activity within the HPA axis, ending with the secretion of glucocorticoids (e.g., cortisol) by the adrenal glands (Dickerson & Kemeny, 2004). In the very short term (i.e., within seconds to minutes), cortisol prepares the body to respond to environmental challenges. Over the course of an hour or more, cortisol also triggers a negative feedback loop within the HPA axis, acting upon the hypothalamus and pituitary glands to inhibit further secretion of cortisol (Sapolsky, Romero & Munck, 2000). The short-term stimulatory effects of cortisol prepare the organism to fight or flee in the presence of a stressor, such that greater cortisol output reflects greater sensitivity and reactivity to environmental stressors (Boyce & Ellis, 2005; Gordis, Granger, Susman & Trickett, 2006; Natsuaki et al., 2009).
Adolescent girls and boys generally show similar patterns of cortisol production in response to stress, with some studies suggesting that boys exhibit slightly greater reactivity than girls (e.g., Del Guidice, Ellis, & Shirtcliff, 2011; Kirschbaum, Wüst, Faig & Hellhammer, 1992; Klimes-Dougan et al., 2001). Further, there are no clear gender differences in the relation between HPA axis functioning and engagement in risk behavior. Findings consistently suggest that high cortisol reactivity is associated with increased inhibition regardless of gender; that is, individuals with stronger physiological responses to stress are more likely to behave in a more cautious and inhibited manner (Fowles, 1980; Gray, 1994; Klimes-Dougan et al., 2001; Moss, Vanyukov & Martin, 1995), whereas those with hypoactive cortisol responses may perceive stressors as less threatening, leading to more disinhibited, risky behavior (e.g., aggression, violent behavior, conduct problems; Brewer-Smyth, Wolbert Burgees & Shults, 2004; Oosterlaan, Geurts, Knol & Sergeant, 2005; Raine, 2005; Shirtcliff, Granger, Booth & Johnson, 2005). Taken together, although data indicate that adolescent boys and girls may differ in their behavioral response to stress, research examining the biological response to stress have not consistently reported these gender differences and it is still unclear how gender interacts with HPA axis functioning to influence risk taking behavior.
Accordingly, the aim of this study was to use an established laboratory-based risk task to evaluate how psychological stress, gender and HPA axis functioning relate to adolescent risk behavior. We hypothesized that there would be a main effect of gender on stress-induced risk behavior, such that boys would demonstrate an increase in risk taking and girls would demonstrate a decrease in risk taking following exposure to a psychological stressor. Further, we hypothesized that this relation would be moderated by HPA axis response to stress, measured via salivary cortisol, such that the lowest levels of risk behavior would be observed among highly reactive adolescents, regardless of gender.
Method
Participants
A total of 150 adolescents and their primary caregiver were recruited via newspaper advertisements and letters sent to guardians of all high school students in the local county asking for adolescents and their primary caregiver to participate in a study examining the relationship between adolescence and stress. Eighteen adolescents were excluded from analyses due to either the use of corticosteroids (n = 14) or regular smoking in the past 30 days (n = 4) which are both known to effect salivary cortisol levels. Therefore, the final sample included 132 adolescents (55.3% female, n = 73) and their primary caregiver (84.8% biological mother, 8.3% biological father, 6.8% female guardian). Participants ranged in age from 14 to 18 (M = 16.1, SD = 1.0). The racial/ethnic background of participants were in line with the US Census Bureau statistics for Prince George’s County, Maryland (U.S. Bureau of the Census, 2010), and included 53.0% African American, 31.8% White, 6.1% Hispanic/Latino, 1.5% Native American, 6.1% Asian, and 1.5% reported ‘Other’. The mean annual household income was $85,800 (SD = 47,600) a year.
Procedure
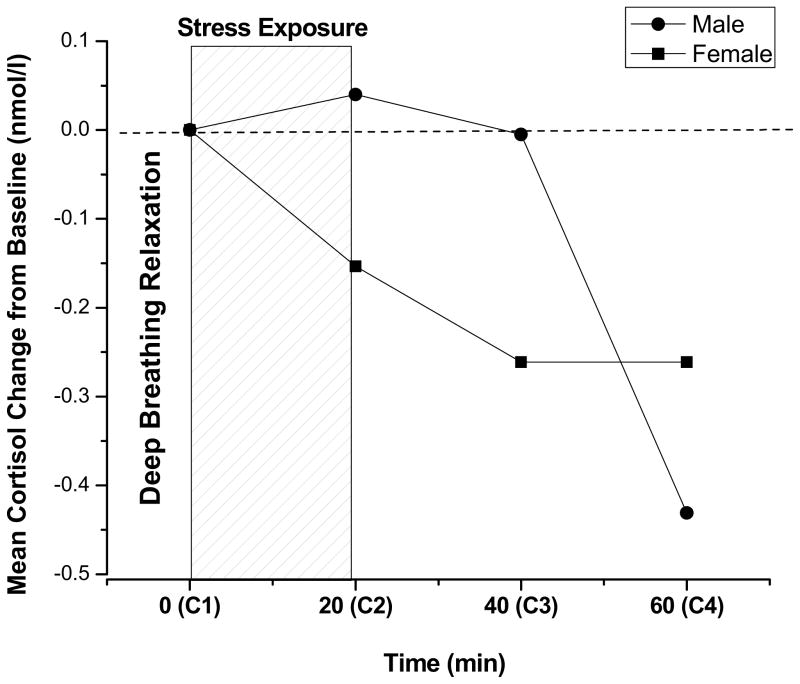
Upon arrival to the testing session, guardians and adolescents provided written informed consent and assent, respectively. Experimental sessions took place on weekdays from 3–5pm to control for the effects of circadian variation in cortisol levels. All aspects of the study and the consent form were approved by the University Institutional Review Board. Following informed consent, participants completed the Automatic Balloon Analogue Risk Task (BART-Auto; Pleskac, Wallsten, Wang, & Lejuez, 2008) to assess baseline levels of risk taking (RT). Afterwards, participants completed a ten minute deep breathing exercise. The first cortisol sample (C1) was collected approximately 10 minutes after the relaxation exercise. Following the first cortisol collection, participants were exposed to a 15-minute psychological stressor, The Behavioral Indicator of Resiliency to Distress (BIRD; Lejuez, Daughters, Danielson & Ruggiero, 2006). A second cortisol sample (C2) was collected immediately following stress exposure (i.e., 20 minutes after C1 collection), and then the BART was re-administered to capture stress induced risk taking (RT-Stress). Participants then completed a battery of self-report measures while providing two additional saliva samples (C3 and C4) at 20 minute intervals. The study timeline is displayed within the context of Figure 1. Compensation for participation consisted of $25 cash for parental guardians and $25 worth of gift cards for adolescents.
Figure 1.
Mean Salivary Cortisol Change for Males and Females in Response to a Psychological Stress Task.
Self-Report Measures
Demographic information was collected from both the adolescent and the parental guardian, including age, gender, race, the nature of the parent-child relationship (e.g., biological mother), and annual family income. Adolescents also completed a self-report measure to account for variables known to influence salivary cortisol levels. Specifically, time (minutes) since last dairy product, time (minutes) since last exercised or engaged in physical activity, use of birth control, and the start date of the female’s last menses. Current menstrual phase was calculated using the last menses start date with females within the first 14 days of their cycle considered in the follicular phase (n =25) and those beyond the 14th day of their cycle considered in the luteal phase (n =39) (Bouma et al., 2009). Eleven (17.2%) females indicated they were using birth control and nine (12.3%) females indicated that they were not menstruating.
HPA Axis Response to Stress
HPA axis response to stress was measured via salivary cortisol which is highly correlated with serum cortisol concentrations in both adults and adolescents (Goodyer et al., 1996; Kirschbaum & Hellhammer, 1994). Saliva was collected from participants using the Salimetrics Oral Swab (SOS) technique (Salimetrics LLC, 2007). Participants were instructed to place a non-toxic, synthetic swab (i.e., SOS) underneath their tongue for approximately two minutes, allowing the swab to collect a significant amount of salvia. Afterwards, participants placed the SOS into a sterile centrifuge tube and all samples were frozen at approximately −20°C within one hour of collection. Samples were analyzed professionally off-site using salivary enzyme-immunoassay (EIA) technology by The University of Trier, Germany cortisol laboratory. Inter-assay coefficients within the current study ranged from 4.9% to 6.1%.
Psychological Stress Task
The Behavior Indicator of Resiliency to Distress (BIRD; Lejuez et al., 2006)
The BIRD task is an adolescent psychological stress task which has demonstrated effectiveness in increasing stress and negative affect among adolescents. Described in greater detail elsewhere (Danielson et al., 2010; Daughters et al., 2009), participants engage in a task with the goal of selecting correct numbered boxes at an increasingly difficult speed in order to free a bird from its cage. When the participant successfully clicks on the correct box, a bird is released from its cage and they earn one point. However, if the participant is unsuccessful (i.e., too slow), they do not earn a point, the bird remains in its cage, and they hear an aversive loud noise. The BIRD has three levels which increase in difficulty. During the second and third levels, the average latency between dot presentations is reduced beyond participants’ individual skill level, resulting in constant forced failure and aversive noise. Adolescents are told that their performance on the task influences the amount of their prize at the end of the session. Participants rate their level of anxiety, frustration, difficulty concentrating, stress, physical discomfort, and irritability on a scale from 1 indicating “none” to 100 indicating “extreme,” both pre and post stressor to assess changes in psychological distress. In addition to the individual scales, ratings are averaged to create a composite pre and post affective distress score (i.e., total distress).
Risk Taking
Automatic Balloon Analogue Risk Task (BART-Auto; Pleskac et al., 2008)
The BART (Lejuez et al., 2002) is a widely used computerized behavioral measure of risk taking that has repeatedly demonstrated an association with real-world risk taking behaviors (Aklin, Lejuez, Zvolensky, Kahler, & Gwadz, 2005; Lejuez, Aklin, Zvolensky, & Pedulla, 2003). A more recent version of the task, the BART-Auto (Pleskac et al., 2008), presents participants with 30 balloons on a computer screen, one at a time. For each balloon, participants are instructed to use the computer’s keyboard to type in the total number of desired pumps to inflate the balloon and then click on a button labeled “Collect”. Participants receive two-cents for each “pump” and thus, the higher the number of entered pumps, the greater amount of potential earnings from each balloon. However, participants are told that the balloons may explode at any given pump (between 1 and 128 pumps) and they are unaware of the explosion point for each balloon. If the participant types in a number which exceeds the balloon’s explosion point, the balloon on the screen will explode and the participant does not receive any money for that balloon. If the number they enter does not exceed the explosion point for that balloon, the balloon on the screen inflates and the money they earn is deposited into their “bank account” located at the bottom right hand corner of the screen. In addition, for all trials, the explosion point for the previous balloon is displayed in the left hand corner of the screen as well as a reminder that the total number of pumps must be between 1 and 128. Finally, participants are told that the amount of their compensation (i.e., “prize”) at the end of the session is dependent on the amount of money they accumulate throughout the task.
Data Analysis Plan
Analyses were conducted with stress inducted risk taking (RTP-Stress) as the primary dependent variable. Primary analyses began with validation of the stress manipulation via subjective report of change in distress. Descriptives for the entire sample are then presented across the dependent variable of RTP-Stress, the independent variables of gender and cortisol response to stress. Demographics and cortisol specific variables were examined as potential covariates. We utilized two methods of assessing HPA axis activity during the testing session. First, we measured total cortisol output from baseline through the post stress period by calculating area under the curve with respect to ground (AUCg; Pruessner, Kirschbaum, Meinlschmid & Hellhammer, 2003) which represents the combined effect of the response to the stressor and declining cortisol levels due to the diurnal cycle. Second, we measured reactivity to the stress task via peak change in cortisol relative to baseline (PC) (Lee, Hempel, TenHarmsel, Liu, Mathe, & Klock, 2012; Ramsey and Lewis, 2003; Stroud, Papandonatos, Williamson, & Dahl). The primary analyses included two hierarchical linear regressions to examine the interactive effects of gender and cortisol (PC and AUCg in separate regressions) on RTP-Stress. Covariates, cortisol, and gender were included in the first step, and the interaction of cortisol and gender in the second step. Procedures outlined by Aiken and West (1991) were then used to decompose significant interaction terms.
Results
Stress manipulation
Participants were exposed to the BIRD psychological stress task for 15 minutes. As reported in Table 1, a 2 (gender) × 2 (pre-post affect ratings) repeated measures ANOVA indicated a main effect of the BIRD task on anxiety, difficulty concentrating, frustration, physical discomfort, irritability, and the composite total distress score, indicating that the task did induce subjective distress. The time x gender interactions indicated that females had a significantly greater increase in irritability, frustration, and the composite total distress score after the BIRD task as compared to males.
Table 1.
Gender Differences in Salivary Cortisol, Risk Taking, and Subjective Responses to a Psychological Stress Task.
| Male (n = 59) | Female (n = 73) | F Statistic | |||||
|---|---|---|---|---|---|---|---|
| Salivary Cortisol Response to Stress | |||||||
| Baseline Cortisol | 3.22 (2.13) | 2.30 (1.38) | 8.90** (ηp2=.07) | ||||
| Peak Cortisol Response (PC)s | 0.74 (1.15) | 0.66 (0.91) | 5.72*(ηp2=.05) | ||||
| Area Under the Curve (AUCg) | 33.51 (23.55) | 27.09 (37.71) | 0.89 | ||||
|
| |||||||
| Pre Stress | Post Stress | Pre Stress | Post Stress | ME of Time | ME of Gender | Interaction | |
|
| |||||||
| Risk Taking | |||||||
| BART Total Pumps | 1489.63 (72.09) | 1585.09 (80.90) | 1374.73 (64.81) | 1359.19 (72.72) | 2.13 | 2.94 | 4.12*(ηp2=.04) |
| Subjective Response to Stress | |||||||
| Anxiety | 10.5 (16.2) | 13.6 (17.0) | 9.3 (15.1) | 14.7 (21.6) | 6.86**(ηp2=.05) | 2.49 | 0.54 |
| Difficulty concentrating | 9.3 (13.1) | 12.4 (17.8) | 11.9 (17.9) | 19.6 (23.5) | 11.18***(ηp2=.08) | 1.81 | 2.13 |
| Frustration | 8.8 (18.9) | 27.3 (26.7) | 7.2 (9.7) | 37.5 (33.7) | 91.15***(ηp2=.41) | 1.81 | 6.73*(ηp2=.05) |
| Physical discomfort | 11.6 (18.4) | 12.6 (20.0) | 8.9 (15.1) | 13.1 (20.3) | 5.36*(ηp2=.04) | 2.64 | 2.01 |
| Irritability | 7.9 (17.5) | 20.2 (24.1) | 10.3 (14.9) | 29.9 (30.7) | 59.09***(ηp2=.31) | 1.53 | 4.85*(ηp2=.04) |
| Total distress | 9.6 (12.4) | 17.2 (15.3) | 9.5 (11.1) | 23.5 (20.1) | 78.60***(ηp2=.38) | 1.65 | 6.83**(ηp2=.05) |
Note.
p < .05,
p < .01,
p < .001; Raw cortisol values are presented yet log transformed variables were used in all analyses. ME = Main effect.
Change in Risk Taking
Risk taking (RT) on the BART-Auto was calculated as the total number of balloon pumps across 30 balloons. Participants averaged 1426.1 (SD = 554.6) pumps at baseline (RT) and 1460.2 (SD = 629.2) pumps post stress (RT-Stress). There was no difference between males and females [F(1,131) = 1.4, p > .05] on baseline RT. Bivariate correlations and ANOVA statistics confirmed that neither age (r = .00, p > .05), ethnicity [F(4,127) = 1.32, p > .05], or income (r = .09, p > .05), were associated with RT.
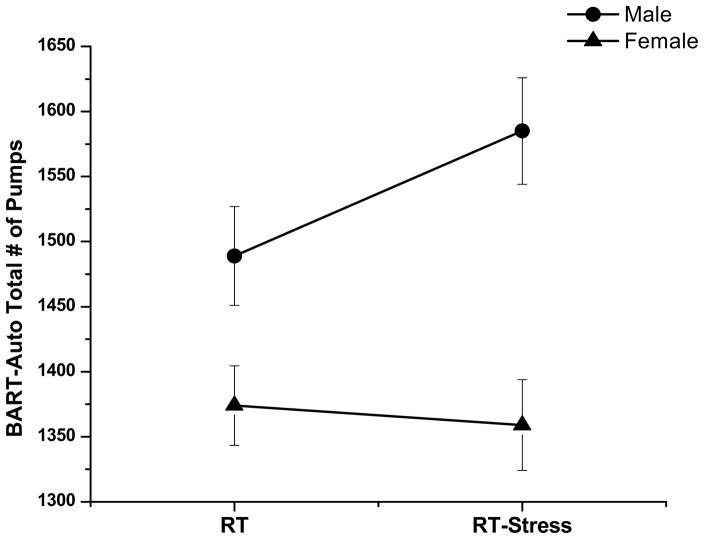
The effects of stress exposure on risk taking was analyzed using a using a 2 (gender) × 2 (RT, RT-Stress) repeated measures ANOVA. As indicated in Table 1, there was no main effect of time or gender, yet there was a significant gender x time interaction. Separate repeated-measures ANOVAs were conducted for each gender to further explore this interaction. As displayed in Figure 2, males demonstrated an increase in risk taking following stress exposure [F(1, 58) = 4.5, p < .05, ηp2 = 0.08] whereas females demonstrated a slight but insignificant decline in risk taking [F(1, 72) = 0.30, p > .05].
Figure 2.
Male and Female Risk Taking Pre (RT) and Post (RT-Stress) Stress Exposure.
Salivary Cortisol Response to Stress
Raw cortisol values were positively skewed and normalized using a log transformation for all analyses; however, raw data are presented in Tables and Figures to facilitate interpretation. As indicated in Table 1, males had significantly greater baseline cortisol levels. As such, baseline cortisol was included as a covariate in subsequent analyses. Bivariate correlations and ANOVA statistics confirmed that neither age (r = .10, p > .05), ethnicity [F(4,127) = 0.67, p > .05], income (r = −.03, p > .05), time since last dairy product (r = .11, p > .05), time since last exercised (r = .07, p > .05), female menstrual cycle [F(1,62) = 0.09, p > .05], or use of birth control [F(1,62) = 1.03, p > .05] were associated with baseline cortisol levels.
Gender differences in cortisol reactivity to the stress task are also displayed in Table 1. Males demonstrated a significantly greater peak cortisol (PC) response to stress. There were no gender differences in total cortisol output (AUCg). Figure 1 displays gender differences in the mean salivary cortisol change from baseline (pre-stress) through the three post-stress assessment time points.
Stress Induced Risk Taking, Gender, and Peak Cortisol (PC) Response to Stress
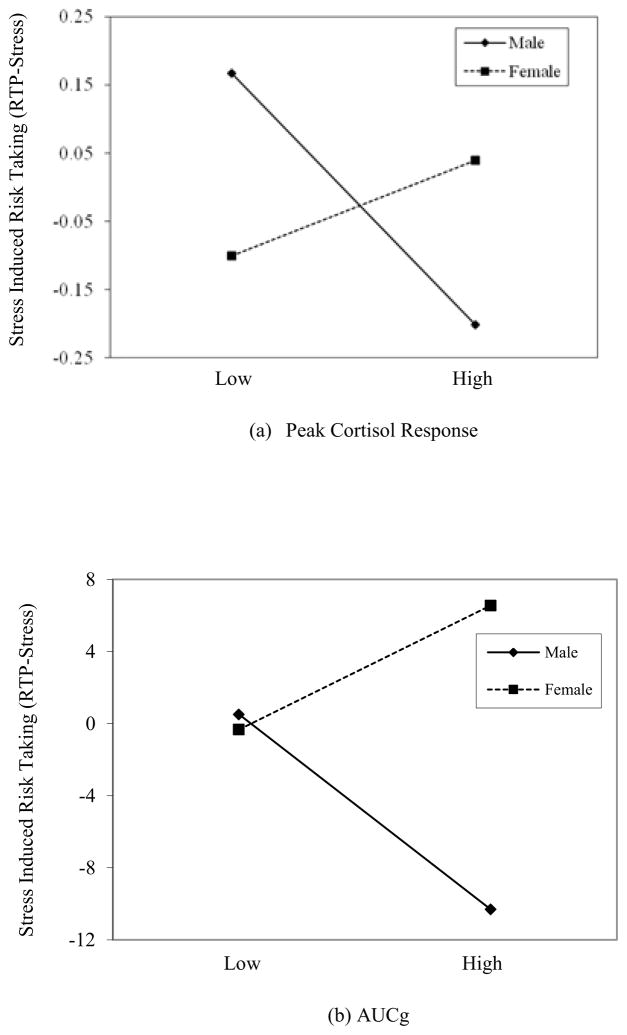
The unique and interactive effect of peak cortisol response to stress (PC) and gender on stress-induced risk taking (RT-Stress) was analyzed using hierarchical linear regression analysis. Baseline RT and baseline cortisol were entered in Step 1 (ΔR2 = 0.75, ΔF = 195.00, p < .001), PC and gender were entered in Step 2 (ΔR2 = 0.01, ΔF = 3.25, p < .05), and the PC x gender interaction variable was entered in a third and final step. After entering the interaction term, Step 3 (ΔR2 = 0.02, ΔF = 8.84, p < .01) was significant, indicating that the PC x gender interaction provided a significant increase in the variance in stress-induced risk taking explained by the model. In the final model [F (5, 126) = 88.84, p < .001], baseline RT (b = 0.86, t = 20.4, p < .001), gender (b = 0.22, t = 2.5, p < .05), and the PC x Gender interaction term (b = −0.25, t = −3.0, p < .01) were significantly associated with RT-Stress. As illustrated in Figure 3a, post-hoc analysis of the simple slopes as outlined by Aiken & West (1991) indicated that adolescent males demonstrated a significant inverse relation between PC and RT-Stress (b = −0.18, t = −3.29, p = .001), whereas the relation between PC and RT-Stress among female adolescents did not reach significance (b = 0.07, t = 1.12, p = .27).
Figure 3. Interaction Effect of Gender with (a) Peak Cortisol Response and (b) Total Cortisol Output (AUCg) on Stress Induced Risk Taking (RT-Stress).
Note. All values are standardized. Low and high values are ± 1 standard deviation from the mean.
Stress Induced Risk Taking, Gender, and Total Cortisol Output (AUCg)
The unique and interactive effect of total cortisol output (AUCg) and gender on stress-induced risk taking (RT-Stress) was analyzed using hierarchical linear regression analysis. Baseline RT and baseline cortisol were entered in Step 1 (ΔR2 = 0.75, ΔF = 195.00, p < .001), AUCg and gender were entered in Step 2 (ΔR2 = 0.02, ΔF = 5.07, p < .01), and the AUCg x gender interaction variable was entered in a third and final step. After entering the interaction term, Step 3 (ΔR2 = 0.01, ΔF = 4.6, p < .05) was significant, indicating that the AUC x gender interaction provided a significant increase in the variance in stress-induced risk taking explained by the model. In the final model [F (5, 126) = 88.3, p < .001], baseline RT (b = 0.87, t = 20.4, p < .001), gender (b = 0.22, t = 2.5, p < .05), and the AUC x Gender interaction term (b = −0.28, t = −2.14, p < .05) were significantly associated with RT-Stress. As illustrated in Figure 3b, post-hoc analysis of the simple slopes as outlined by Aiken & West (1991) indicate that adolescent males demonstrated a significant inverse relation between AUCg and RT-Stress (b = −0.27, t = −2.96, p = .004), whereas the relation between AUCg and RT-Stress among female adolescents did not reach significance (b = 0.08, t = 1.79, p = .08).
Discussion
Although the incidence of risk behavior during adolescence is well-established, less is known about the factors that contribute to the initiation and maintenance of risk behavior during this developmental period. Whereas earlier research emphasized the appetitive (i.e., reward-oriented) aspects of adolescent risk behavior, there is increasing recognition that adolescent risk behavior often occurs in the context of psychological stress (Kuntsche et al., 2005; MacPherson et al., 2010; Nock & Prinstein, 2005). In addition, prior investigations examining the biological response to stress have yielded inconsistent gender differences and it is unclear how these processes relate to risk taking behaviors. As such, the present investigation examined gender and cortisol response to stress as two determinants of engagement in stress-induced risk behavior in a community sample of adolescents. A number of important findings emerged.
First, adolescent boys and girls demonstrated different patterns of risk taking in response to stress, such that boys evidenced an increase in RT from pre- to post-stressor, whereas girls evidenced a decrease in RT. In other words, boys, but not girls, became more disinhibited and reward-oriented in response to stress. In addition to these gender differences, our results reveal a gender by cortisol response to stress interaction in predicting stress-induced RT. For boys, both peak cortisol response to stress and total cortisol output from pre to post stress predicted stress-induced risk behavior; specifically, a blunted cortisol response and lower total cortisol output was associated with greater stress-induced RT, whereas greater cortisol reactivity was associated with less stress-induced RT. In contrast, among the girls, peak cortisol response and total cortisol output was unrelated to stress-induced RT.
Similar to our findings, a relation between cortisol hypoactivity and externalizing problems in boys has been well-documented (Shirtcliff et al, 2005). It has been suggested that individuals with HPA hypoarousal are characterized by an absence of anxiety or fear in situations where such a response would be appropriate. As a result, they may be more prone to engage in risky or reckless reward-oriented behavior, because they do not experience the affective states that trigger withdrawal and avoidance (Raine, 2002). Further, individuals with HPA hypoarousal may seek to up-regulate chronically low arousal by seeking novelty and excitement in their environments (Shirtcliff et al., 2005). This effect has been documented using both basal cortisol and cortisol response to stress. On the other hand, our finding that elevated cortisol output in response to a stressor is associated with diminished RT in boys is consistent with a handful of previous studies which have found that elevated basal cortisol and heightened cortisol reactivity is associated with greater inhibition, shyness and internalizing psychopathology in boys (Klimes-Dougan et al., 2001; Moss et al., 1995; Smider et al., 2002). One possible interpretation is that boys with greater cortisol reactivity may experience stressful situations as more threatening, resulting in more conservative, inhibited behavior in response to stress (Granger, Weisz, McCracken & Ikeda, 1996; Klimes-Dougan et al., 2001).
One theoretical context to consider in interpreting these results is negative reinforcement theory, which emphasizes that the motivational basis of behavior is the reduction or avoidance of affective distress (Baker, Piper, McCarthy, Majeskie & Fiore, 2004; Cox & Klinger, 1988; Khantzian, 1985; Solomon, 1977). Along these lines, it is possible that risk taking serves to alleviate affective distress among boys with a hypoactive cortisol response. Although our findings, along with the studies reviewed above, provide suggestive support of this theory, it is important to note that we did not directly assess the motivational basis for risk behavior. In particular, our data do not indicate that engagement in risk behavior served to alleviate negative affect or enhance positive mood, or whether negative reinforcement expectancies moderate the relation between stress exposure and engagement in risk behavior. As such, future research is needed to explore this theory by examining the subjective and physiological indicators of mood repair following engagement in risk behavior.
While the effects of cortisol on risk behavior have been replicated across a spectrum of clinical severity in boys, findings for girls are less consistent. Among girls, the relation between the physiological stress response and behavior is often weak, or is moderated by contextual and individual difference factors (Shirtcliff, 2005; Susman et al., 2010; Taylor et al., 2000). For instance, the relation between cortisol reactivity and problem behavior is stronger among girls with genetic, physiological and environmental risk factors (e.g., history of maltreatment, early pubertal timing or psychopathology; Benjet, Borges & Medina-Mora, 2010; Powers, Battle, Dorta & Welsh, 2010; Vigil, Geary, Granger & Flinn, 2010) relative to girls without these risk factors. Even when these moderators are identified, girls’ cortisol reactivity shows a weak relation with overall adjustment or global symptom severity (Graber, Nichols & Brooks-Gunn, 2010). Further, girls’ abnormal cortisol reactivity predicts problems in specific social contexts (e.g., school effort, family context) but not cross-situationally (Graber et al., 2010). Further, it is noteworthy that, unlike the boys, the girls in our sample failed to exhibit the expected increase in cortisol in response to the stressor, instead showing a slight decrease in cortisol output. This finding may reflect the stressor used in our study, which was a performance-oriented challenge in which success was nearly impossible. Previous research suggests that men have a stronger cortisol response to achievement-based and evaluative challenges, whether in the laboratory or the real world (Kirschbaum et al., 1992; Kirschbaum, Kudielka, Gaab, Schommer & Hellhammer, 1999; Kudielka et al., 1998), whereas women show greater cortisol response to stressors that involve social rejection (Stroud, Salovey & Epel, 2002). Although our stressor did not elicit the expected cortisol response, girls did report a significant increase in subjective distress following stress exposure. The discrepancy between subjective and physiological indicators of stress may serve as further evidence that girls’ emotional responding is determined by a variety of factors, and may be less directly associated with physiological responses. Yet, it will be critical to examine whether cortisol response to context-specific stressors (i.e., social evaluation) may have greater predictive utility of girls’ stress-induced behavior prior to drawing the conclusion that HPA axis response to stress is unrelated to risk behavior among girls.
The findings of this study should be interpreted in light of its limitations. First, the current design did not include experimental conditions to control for natural recovery from negative affect (i.e., the extent to which negative affect diminishes even if participants are not allowed to engage in risk behavior). Nor did the current study include a no-stress control condition, which would have confirmed that the observed patterns of cortisol output reflected the effects of the stressor, rather than the influence of another, unmeasured variable. In addition, generalizability of our results is limited given that adolescent participants were recruited via local newspapers and letters to parents.
Despite these limitations, the present study has a number of strengths that warrant mention. This is one of the first studies to demonstrate the gender-specific effects of stress and cortisol reactivity on risk behavior using both a laboratory-based psychological stressor and behavioral assessment of risk taking. This type of behavioral assessment eliminates many of the limitations associated with self-report (e.g., difficulty recalling the circumstances under which a behavior occurred; unwillingness to disclose previous involvement in risk behavior), and allows for concurrent assessment of biological and behavioral responses to a psychological stressor. Finally, these findings highlight the potential benefit of addressing affective distress in the context of risk prevention and intervention programs, especially among adolescent boys. Drawing on treatments specifically designed to improve one’s ability to identify and cope effectively with negative emotions, such as acceptance-based behavioral treatments (e.g., Acceptance and Commitment Therapy, ACT (Hayes et al., 1999) or dialectical behavior therapy for adolescents (DBT-A; Miller, Rathus, DuBose, Dexter-Mazza, & Goldklang, 2007) may be particularly useful in this regard.
Acknowledgments
This work was conducted at the University of Maryland, College Park and was supported by National Institute of Drug Abuse Grant R21DA022741 (PI: Daughters). We thank C.W. Lejuez for his consultation on this study. We also thank Ria Travers, Jimeka Leonard, and Kara Smith for their assistance with data collection.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy. 2005;43(2):215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Benjet C, Borges G, Medina-Mora M. Chronic childhood adversity and onset of psychopathology during three life stages: Childhood, adolescence and adulthood. Journal of Psychiatric Research. 2010;44(11):732–740. doi: 10.1016/j.jpsychires.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34(6):884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Boyce W, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brewer-Smyth K, Wolbert Burgess A, Shults J. Physical and sexual abuse, salivary cortisol, and neurologic correlates of violent criminal behavior in female prison inmates. Biological Psychiatry. 2004;55(1):21–31. doi: 10.1016/S0006-3223(03)00705-4. [DOI] [PubMed] [Google Scholar]
- Brook JS, Adams RE, Balka EB, Whiteman M, Zhang C, Sugerman R. Illicit drug use and risky sexual behavior among african american and puerto rican urban adolescents: The longitudinal links. The Journal of Genetic Psychology. 2004;165(2):203–220. doi: 10.3200/GNTP.165.3.310-323. [DOI] [PubMed] [Google Scholar]
- Colman I, Croudace TJ, Wadsworth MJ, Jones PB. Factors associated with antidepressant, anxiolytic and hypnotic use over 17 years in a national cohort. Journal of Affective Disorders. 2008;110(3):234–240. doi: 10.1016/j.jad.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Wagner BM. Psychosocial stress during adolescence: Intrapersonal and interpersonal processes. In: Gore S, Colton ME, editors. Adolescence, stress, and coping. New York: Aldine de Gruyter; 1991. pp. 67–85. [Google Scholar]
- Cooper M, Agocha V, Sheldon MS. A motivational perspective on risky behaviors: The role of personality and affect regulatory processes. Journal of Personality. 2000;68(6):1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Cooper M, Wood PK, Orcutt HK, Albino A. Personality and the predisposition to engage in risky or problem behaviors during adolescence. Journal of Personality and Social Psychology. 2003;84(2):390–410. doi: 10.1037/0022-3514.84.2.390. [DOI] [PubMed] [Google Scholar]
- Cox W, Klinger E. A motivational model of alcohol use. Journal of Abnormal Psychology. 1988;97(2):168–180. doi: 10.1037/0021-843X.97.2.168. [DOI] [PubMed] [Google Scholar]
- Danielson CK, Ruggiero KJ, Daughters SB, Lejuez CW. Distress tolerance, risk-taking propensity, and PTSD symptoms in trauma-exposed youth: Pilot study. The Behavior Therapist. 2010;33:28–34. [Google Scholar]
- Daughters SB, Reynolds EK, MacPherson L, Kahler CW, Danielson CK, Zvolensky M, Lejuez CW. Distress tolerance and early adolescent externalizing and internalizing symptoms: The moderating role of gender and ethnicity. Behaviour Research and Therapy. 2009;47(3):198–205. doi: 10.1016/j.brat.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calabration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: Implications of Gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17(2):87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Fröjd SA, Nissinen ES, Pelkonen MI, Marttunen MJ, Koivisto A, Kaltiala-Heino R. Depression and school performance in middle adolescent boys and girls. Journal of Adolescence. 2008;31(4):485–498. doi: 10.1016/j.adolescence.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Galaif ER, Sussman S, Chou C, Wills TA. Longitudinal relations among depression, stress, and coping in high risk youth. Journal of Youth and Adolescence. 2003;32(4):243–258. doi: 10.1023/A:1023028809718. [DOI] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30(4):467–483. doi: 10.1037/0012-1649.30.4.467. [DOI] [Google Scholar]
- Gjerde PF, Block J, Block JH. Depressive symptoms and personality during late adolescence: Gender differences in the externalization–internalization of symptom expression. Journal of Abnormal Psychology. 1988;97:475–486. doi: 10.1037//0021-843x.97.4.475. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert JJ, Altham PE, Pearson JJ, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 1996;26(2):245–256. doi: 10.1017/S0033291700034644. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gore S, Aseltine RH, Colton ME. Social structure, life stress and depressive symptoms in a high school-aged population. Journal of Health and Social Behavior. 1992;33(2):97–113. doi: 10.2307/2137249. [DOI] [PubMed] [Google Scholar]
- Graber JA, Nichols TR, Brooks-Gunn J. Putting pubertal timing in developmental context: Implications for prevention. Developmental Psychobiology. 2010;52(3):254–262. doi: 10.1002/dev.20438. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67(6):3250–3262. doi: 10.2307/1131777. [DOI] [PubMed] [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 243–247. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability–transactional stress theory. Psychological Bulletin. 2001;127(6):773–796. doi: 10.1037/0033-2909.127.6.77. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Development. 2007;78(1):279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: An experiential approach to behavior change. Guilford Press; New York: 1999. [Google Scholar]
- Horwitz AV, White HR. Gender role orientations and styles of pathology among adolescents. Journal of Health and Social Behavior. 1987;28(2):158–170. doi: 10.2307/2137129. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kim K, Conger RD, Elder Gr, Lorenz FO. Reciprocal influences between stressful life events and adolescent internalizing and externalizing problems. Child Development. 2003;74(1):127–143. doi: 10.1111/1467-8624.00525. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrin Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/S0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke KM, Varadi E, Pilz J, Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. Journal of Clinical Endocrinology and Metabolism. 1998;83:1756–1761. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clinical Psychology Review. 2005;25(7):841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Larson R, Ham M. Stress and ‘storm and stress’ in early adolescence: The relationship of negative events with dysphoric affect. Developmental Psychology. 1993;29(1):130–140. doi: 10.1037/0012-1649.29.1.130. [DOI] [Google Scholar]
- Leadbeater BJ, Blatt SJ, Quinlan DM. Gender-linked vulnerabilities to depressive symptoms, stress, and problem behaviors in adolescents. Journal of Research on Adolescence. 1995;5(1):1–29. doi: 10.1207/s15327795jra0501_1. [DOI] [Google Scholar]
- Lee RJ, Hempel J, TenHarmsel A, Liu T, Aleksander AM, Klock A. The neuroendocrinology of childhood trauma in personality disorder. Psychoneuroendocrinology. 2012;37:78–86. doi: 10.1016/j.psyneuen.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26(4):475–479. doi: 10.1016/S0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Daughters SB, Danielson CW, Ruggiero K. The Behavioral Indicator of Resiliency to Distress (BIRD) 2006 Unpublished manual. [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75–84. doi: 10.1037/1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS ONE. 2009;4:e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Reynolds EK, Daughters SB, Wang F, Cassidy J, Mayes LC, Lejuez CW. Positive and negative reinforcement underlying risk behavior in early adolescents. Prevention Science. 2010;11(3):331–342. doi: 10.1007/s11121-010-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Malone PS, Dodge KA. Developmental trajectories of boys’ and girls’ delinquency: Sex differences and links to later adolescent outcomes. Journal of Abnormal Child Psychology: An official publication of the International Society for Research in Child and Adolescent Psychopathology. 2010;38(7):1021–1032. doi: 10.1007/s10802-010-9430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Rathus JH, DuBose AP, Dexter-Mazza ET, Goldklang AR. Dialectical behavior therapy for adolescents. In: Dimeff LA, Koerner K, editors. Dialectical behavior therapy in clinical practice: Applications across disorders and settings. New York, NY, US: Guilford Press; 2007. pp. 245–263. [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38(8):546–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child and Adolescent Psychology. 2009;38(4):513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Prinstein MJ. Contextual features and behavioral functions of self-mutilation among adolescents. Journal of Abnormal Psychology. 2005;114(1):140–146. doi: 10.1037/0021-843X.114.1.140. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Corte C. Gender and self-regulation. In: Baumeister RF, Vohs KD, Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York, NY US: Guilford Press; 2004. pp. 411–421. [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Research. 2005;134(1):1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Piko B. Gender differences and similarities on adolescents’ ways of coping. The Psychological Record. 2001;51(2):223–235. [Google Scholar]
- Pleskac TJ, Wallsten TS, Wang P, Lejuez CW. Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Experimental and Clinical Psychopharmacology. 2008;16(6):555–564. doi: 10.1037/a0014245. [DOI] [PubMed] [Google Scholar]
- Powers SI, Battle CL, Dorta K, Welsh DP. Adolescents’ submission and conflict behaviors with mothers predicts current and future internalizing problems. Research in Human Development. 2010;7(4):257–273. doi: 10.1080/15427609.2010.526522. [DOI] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavior response to stress. Child Development. 2003;74:456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43(4):417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Raine A. The Interaction of Biological and Social Measures in the Explanation of Antisocial and Violent Behavior. In: Stoff DM, Susman EJ, Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. New York, NY US: Cambridge University Press; 2005. pp. 13–42. [DOI] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scott ES, Steinberg L. Adolescent development and the regulation of youth crime. The Future of Children. 2008;18(2):15–33. doi: 10.1353/foc.0.0011. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(1):167–184. doi: 10.1017/S0954579405050091. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gaher RM, Correia CJ, Hansen CL, Christopher MS. An affective-motivational model of marijuana and alcohol problems among college students. Psychology of Addictive Behaviors. 2005;19(3):326–334. doi: 10.1037/0893-164X.19.3.326. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smith-Khuri E, Iachan R, Scheidt CP, Overpeck DM, Saoirse G, Pickett W, Harel Y. A cross-national study of violence-related behaviors in adolescents. Arch Pediatr Adolesc Med. 2004;158:539–54. doi: 10.1001/archpedi.158.6.539. [DOI] [PubMed] [Google Scholar]
- Sourander A, Jensen P, Davies M, Niemelä S, Elonheimo H, Ristkari T, Almqvist F. Who is at greatest risk for adverse long-term outcomes? The Finnish From a Boy to a Man study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(9):1148–1161. doi: 10.1097/chi.0b013e31809861e9. [DOI] [PubMed] [Google Scholar]
- Solomon RL. Addiction: An opponent-process theory of acquired motivation: The affective dynamics of addiction. In: Maser JD, Seligman MP, Maser JD, Seligman MP, editors. Psychopathology: Experimental models. New York, NY US: W H Freeman/Times Books/Henry Holt & Co; 1977. pp. 66–103. [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. The influence of gender and pubertal development on response to CRH challenge: The Pittsburgh Psychobiologic Studies. Psychoneuroendocrinology. 2011;36:1226–38. doi: 10.1016/j.psyneuen.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/S0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, Dorn LD. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35(4):557–569. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein L, Lewis BP, Gruenewald TL, Gurung RR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107(3):411–429. doi: 10.1037/0033-295X.107.3.411. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of the Census. County Population Estimates by Demographic Characteristics - Age, Sex, Race, and Hispanic Origin; updated annually for states and counties. http://www.census.gov/popest/counties/asrh/. 2010 Census of Population and Housing for places; updated every 10 years. http://factfinder2.census.gov.
- Vigil JM, Geary DC, Granger DA, Flinn MV. Sex differences in salivary cortisol, alpha-amylase, and psychological functioning following Hurricane Katrina. Child Development. 2010;81(4):1228–1240. doi: 10.1111/j.1467-8624.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task (BART) Experimental and Clinical Psychopharmacology. 2008;16(6):565–570. doi: 10.1037/a0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RC, Windle M. An investigation of adolescents’ substance use behaviors, depressed affect, and suicidal behaviors. Journal of Child Psychology and Psychiatry. 1997;38(8):921–929. doi: 10.1111/j.1469-7610.1997.tb01611.x. [DOI] [PubMed] [Google Scholar]