Abstract
Background
Higher levels of physical activity are associated with lower colorectal cancer incidence and mortality, perhaps through influencing energy balance, cellular prostaglandin biosynthesis and systemic inflammation. Although evidence suggests interactive effects of energetics, sedentary lifestyle, and tumor CTNNB1 (β-catenin) or CDKN1B (p27) status on colon cancer prognosis, interactive effects of physical activity and tumor PTGS2 (the official symbol for cyclooxygenase-2) status on clinical outcome remain unknown.
Methods
Utilizing molecular pathological epidemiology database of 605 stage I-III colon and rectal cancers in two prospective cohort studies (the Nurse’s Health Study and the Health Professionals Follow-up Study), we examined patient survival according to post-diagnosis physical activity and tumor PTGS2 status (with 382 PTGS2-positive and 223 PTGS2-negative tumors by immunohistochemistry). Cox proportional hazards models were used to calculate colorectal cancer-specific mortality hazard ratio (HR), adjusting for clinical and other tumor variables including microsatellite instability status.
Results
Among PTGS2-positive cases, compared with the least active first quartile, the multivariate HRs (95% confidence interval) were 0.30 (0.14–0.62) for the second, 0.38 (0.20–0.71) for the third, and 0.18 (0.08–0.41) for the fourth quartile of physical activity level (Ptrend=0.0002). In contrast, among PTGS2-negative cases, physical activity level was not significantly associated with survival (Ptrend =0.84; Pinteraction=0.024, between physical activity and tumor PTGS2 status).
Conclusions
Post-diagnosis physical activity is associated with better survival among patients with PTGS2-positive tumors, but not among patients with PTGS2-negative tumors.
Impact
Immunohistochemical PTGS2 expression in colorectal carcinoma may serve as a predictive biomarker in pathology practice, which may predict stronger benefit from exercise.
Keywords: colorectal carcinoma, survivorship, lifestyle, tumor behavior, molecular pathological epidemiology
INTRODUCTION
Higher levels of physical activity are associated with lower risks of not only developing colorectal cancer (1–6), but also dying of the disease (7–19). Accumulating evidence suggests that the potential anti-neoplastic effect of physical activity may be mediated by decreased systemic inflammatory status (20), through a reduction in prostaglandin E2 (PGE2) synthesis (21–23).
PTGS2 (the official symbol for cyclooxygenase-2, or COX-2) and its enzymatic product, PGE2, are key contributors to inflammatory responses, and play important roles in colorectal cancer development and progression (24–27). Regular use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) has been associated with lower risks of colorectal cancer incidence and mortality, at least in part, through inhibition of PTGS2 related pathways (25, 28–31). Because physical activity may also modulate PGE2 synthesis, we hypothesized that the association of physical activity with colorectal cancer survival might be stronger for patients with PTGS2-expressing tumors than for those with PTGS2-nonexpressing tumors.
To test this hypothesis, we conducted a study of 605 colorectal cancer patients within two prospective cohort studies in which we collected validated data on physical activity after diagnosis of colorectal cancer and also assessed status of tumor PTGS2 expression.
MATERIALS AND METHODS
Study group
We utilized data from two prospective cohort studies: the Nurses’ Health Study (NHS, N=121,701 women followed since 1976), and the Health Professionals Follow-up Study (HPFS, N=51,529 men followed since 1986) (32, 33). Biennial questionnaires were used to collect data on dietary and lifestyle factors (including level of physical activity, aspirin use, smoking habits, and alcohol consumption) and family history of colorectal cancer. We also ascertained new cases of colorectal cancers. In total, 1229 men in the HPFS and 3580 women in the NHS were diagnosed as having colorectal cancer (up to 2006). We collected paraffin-embedded tissue blocks from hospitals where colorectal cancer patients had undergone tumor resection. We also collected diagnostic biopsy specimens for rectal cancer patients who had received preoperative treatment. Considering a continuum of pathological and molecular features from rectum to proximal colon (34, 35), we included both colon and rectal cancers in the current study. Tissue sections from all colorectal cancer cases were reviewed by a pathologist (S.O.), and the diagnosis confirmed. Based on the availability of tumor tissue data, post-diagnosis physical activity data, and follow-up data, a total of 605 colorectal cancer cases were included (Table 1). Within the cohort studies, there were no significant differences in demographic features between cases with available tumor tissue specimens and those without (28, 32). Informed consent was obtained from all study subjects. This study was approved by the Harvard School of Public Health and Brigham and Women’s Hospital Institutional Review Boards.
Table 1.
Clinical, pathologic, and molecular characteristics of colorectal cancer cases according to post-diagnosis physical activity quartile
| Clinical, pathologic or molecular feature | Total N | Post-diagnosis physical activity quartile
|
P value | |||
|---|---|---|---|---|---|---|
| Q1 (Lowest) | Q2 (Second) | Q3 (Third) | Q4 (Highest) | |||
| All cases | 605 | 152 | 146 | 158 | 149 | |
| Sex | 0.99 | |||||
| Male (HPFS) | 305 (50%) | 77 (51%) | 75 (51%) | 78 (49%) | 75 (50%) | |
| Female (NHS) | 300 (50%) | 75 (49%) | 71 (49%) | 80 (51%) | 74 (50%) | |
| Mean age (SD) | 67.3 (8.0) | 68.2 (8.5) | 67.8 (8.1) | 66.7 (7.5) | 66.4 (7.6) | 0.025 |
| Body mass index (kg/m2) | 0.017 | |||||
| <30 | 503 (83%) | 116 (76%) | 123 (84%) | 130 (82%) | 134 (90%) | |
| ≥30 | 102 (17%) | 36 (24%) | 23 (16%) | 28 (18%) | 15 (10%) | |
| Family history of colorectal cancer in first degree relative(s) | 0.93 | |||||
| (−) | 482 (80%) | 122 (80%) | 114 (78%) | 128 (81%) | 118 (79%) | |
| (+) | 123 (20%) | 30 (20%) | 32 (22%) | 30 (19%) | 31 (21%) | |
| Year of diagnosis | 0.54 | |||||
| Prior to 1995 | 243 (40%) | 55 (36%) | 65 (45%) | 64 (41%) | 59 (40%) | |
| 1995 to 2006 | 362 (60%) | 97 (64%) | 81 (55%) | 94 (59%) | 90 (60%) | |
| Post-diagnosis aspirin use | 0.80 | |||||
| Non-user | 368 (61%) | 93 (62%) | 85 (58%) | 95 (60%) | 95 (64%) | |
| Aspirin user | 236 (39%) | 58 (38%) | 61 (42%) | 63 (40%) | 54 (36%) | |
| Post-diagnosis smoking status | 0.10 | |||||
| Never | 238 (41%) | 60 (42%) | 58 (41%) | 57 (38%) | 63 (43%) | |
| Former | 305 (53%) | 72 (51%) | 68 (49%) | 84 (55%) | 81 (55%) | |
| Current | 37 (6.4%) | 10 (7.0%) | 14 (10%) | 11 (7.2%) | 2 (1.4%) | |
| Post-diagnosis alcohol consumption | 0.85 | |||||
| None | 229 (39%) | 58 (41%) | 56 (39%) | 62 (40%) | 53 (36%) | |
| Any | 362 (61%) | 85 (59%) | 88 (61%) | 94 (60%) | 95 (64%) | |
| Tumor location | 0.92 | |||||
| Cecum | 108 (18%) | 26 (17%) | 23 (16%) | 33 (21%) | 26 (17%) | |
| Ascending to transverse colon | 156 (26%) | 36 (24%) | 38 (26%) | 43 (28%) | 39 (26%) | |
| Splenic flexure to sigmoid | 190 (32%) | 47 (31%) | 50 (34%) | 44 (28%) | 49 (33%) | |
| Rectosigmoid and rectum | 149 (25%) | 43 (28%) | 35 (24%) | 36 (23%) | 35 (23%) | |
| Disease stage | 0.99 | |||||
| I | 161 (27%) | 38 (25%) | 38 (26%) | 43 (27%) | 42 (28%) | |
| II | 212 (35%) | 51 (34%) | 54 (37%) | 53 (34%) | 54 (36%) | |
| III | 165 (27%) | 45 (30%) | 38 (26%) | 45 (28%) | 37 (25%) | |
| Unknown | 67 (11%) | 18 (12%) | 16 (11%) | 17 (11%) | 16 (11%) | |
| Tumor differentiation | 0.70 | |||||
| Well to moderate | 556 (93%) | 142 (94%) | 136 (94%) | 140 (91%) | 138 (93%) | |
| Poor | 42 (7.0%) | 9 (6.0%) | 9 (6.2%) | 14 (9.1%) | 10 (6.8%) | |
| CIMP status | 0.21 | |||||
| CIMP-low/0 | 483 (84%) | 116 (82%) | 119 (84%) | 123 (81%) | 125 (89%) | |
| CIMP-high | 92 (16%) | 26 (18%) | 22 (16%) | 29 (19%) | 15 (11%) | |
| MSI status | 0.37 | |||||
| MSS | 482 (84%) | 119 (83%) | 118 (85%) | 122 (81%) | 123 (88%) | |
| MSI-high | 90 (16%) | 25 (17%) | 21 (15%) | 28 (19%) | 16 (12%) | |
| LINE-1 methylation level [Mean (SD)] | 61.8 (9.5) | 61.4 (9.9) | 60.8 (10.0) | 62.2 (9.3) | 62.8 (8.9) | 0.12 |
| BRAF mutation | 0.77 | |||||
| (−) | 510 (89%) | 130 (90%) | 124 (89%) | 131 (87%) | 125 (90%) | |
| (+) | 64 (11%) | 14 (9.7%) | 16 (11%) | 20 (13%) | 14 (10%) | |
| KRAS mutation | 0.25 | |||||
| (−) | 364 (63%) | 94 (65%) | 87 (62%) | 104 (68%) | 79 (57%) | |
| (+) | 213 (37%) | 51 (35%) | 53 (38%) | 49 (32%) | 60 (43%) | |
| CDKN1B (p27) expression | 0.36 | |||||
| (−) | 234 (39%) | 52 (34%) | 64 (44%) | 63 (40%) | 55 (37%) | |
| (+) | 371 (61%) | 100 (66%) | 82 (56%) | 95 (60%) | 94 (63%) | |
| Nuclear CTNNB1 (β-catenin) expression | 0.53 | |||||
| (−) | 290 (53%) | 76 (58%) | 72 (54%) | 75 (53%) | 67 (49%) | |
| (+) | 255 (47%) | 56 (42%) | 62 (46%) | 66 (47%) | 71 (51%) | |
| PTGS2 (COX-2) expression | 0.43 | |||||
| (−) | 223 (37%) | 61 (40%) | 58 (40%) | 56 (35%) | 48 (32%) | |
| (+) | 382 (63%) | 91 (60%) | 88 (60%) | 102 (65%) | 101 (68%) | |
(%) indicates the proportion of cases with a specific clinical, pathologic, or molecular feature in a given physical activity quartile. A Chi squared P value is given for comparison across quartiles. ANOVA was used to compare the means of age and LINE-1 methylation. The Bonferroni-corrected P value for significance was P=0.0026 (0.05/19).
CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses’ Health Study; SD, standard deviation.
Assessment of physical activity
Leisure-time physical activity was evaluated every two years in both cohorts, as previously described, and validated against subject diaries (36, 37). Subjects reported the duration of physical activity (ranging from 0 to 11 or more hours/week) engaged in walking (at usual pace), jogging, running, bicycling, swimming laps, racket sports, other aerobic exercises, lower intensity exercise (yoga, toning, stretching), or other vigorous activities (38). Each activity on the questionnaire was assigned a metabolic equivalent task (MET) score (38). MET scores for specific activities represent the activity-related metabolic rate divided and the resting metabolic rate (11, 32). In the present study, values for individual activities were summed to give a total MET-hours/week score. Because we observed differences in the distribution of reported physical activity levels between men and women, we classified physical activity level by generating sex-specific quartiles. To avoid assessment during the period of active oncologic treatment, the first assessment of physical activity was collected at least 1 year, but no more than 4 years after cancer diagnosis (median 17 months) (32). To minimize bias due to declining physical activity in the period around cancer recurrence or death, patients with known metastatic disease (stage IV) were excluded from this analysis, and physical activity was assessed at a single post-diagnosis time point (8, 32).
Assessment of mortality
Ascertainment of deaths was accomplished by reporting from family members, or postal authorities (in the case of non-responders), and by searching for participants in the National Death Index (36). Following medical record review, the cause of death was assigned by study physicians (29, 36).
Sequencing of BRAF and KRAS, and microsatellite instability (MSI) analysis
DNA was extracted from tumor tissue, and PCR and Pyrosequencing targeted for BRAF (codon 600), and KRAS (codons 12 and 13), were performed as previously described (39–41). MSI analysis was performed by PCR using 10 microsatellite markers (BAT25, BAT26, BAT40, D2S123, D5S346, D17S250, D18S55, D18S56, D18S67, and D18S487) (41). MSI-high was defined as the presence of instability in ≥30% of the markers. MSI-low (<30% unstable markers) tumors were grouped with microsatellite stable (MSS) tumors (no unstable markers) because we have previously demonstrated that these two groups show similar features (41).
Methylation analyses for CpG islands and LINE-1
Using real-time PCR (MethyLight) on bisulfite-treated DNA, we quantified DNA methylation in eight CIMP-specific promoters [CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1] (41–43). CIMP-high was defined as the presence of ≥6/8 methylated promoters, and CIMP-low/0 as 0/8-5/8 methylated promoters, according to established criteria (44). In order to quantify LINE-1 methylation, a Pyrosequencing assay was used, as previously described (33, 45, 46).
Immunohistochemical analyses
Immunohistochemical analysis methods for CDKN1B (p27) (47), CTNNB1 (β-catenin) (32), and PTGS2 (cyclooxygenase-2) (28) expression have previously been described, using mouse anti-CDKN1B (Clone 57, BD Transduction Laboratories, Item No. 610242; dilution, 1:200), mouse anti-CTNNB1 (Clone 14, BD Transduction Laboratories, Item No. 610153; dilution, 1:400), and mouse anti-PTGS2 (Clone CX229, Cayman Chemical, Item No. 160112; dilution, 1:300). Appropriate positive and negative controls were included in each run of immunohistochemistry.
Each immunohistochemical marker was interpreted by a pathologist (CDKN1B and PTGS2 by S.O.; CTNNB1 by T.M.) unaware of other data. For agreement studies, a random selection of more than 100 cases for each marker was examined by a second observer (CDKN1B by K.S.; CTNNB1 by S.O.; PTGS2 by T.M.) unaware of other data. The concordance between the 2 observers (all P<0.001) was κ=0.60 for CDKN1B, κ=0.80 for CTNNB1, κ=0.69 for PTGS2, indicating substantial agreement.
Statistical analysis
For all statistical analyses, we used SAS software (Version 9.2, SAS Institute, Cary, NC). All P values were two-sided and statistical significance was set at a P value of 0.05. Our primary hypothesis was that the association of physical activity with survival differed by tumor PTGS2 status. Nonetheless, we interpreted results cautiously, according to the guidelines (48), given that the fundamental study design employed subgroup analyses (in strata of PTGS2 status) to assess clinical outcomes. The subgroups defined, and hypotheses tested in the current study were not planned analyses when the two cohort studies began; rather, our study comprised 5 post-hoc subgroup analyses. To test for differences in the distribution of categorical data, the chi-square test was performed. One-way ANOVA was used to compare mean age and mean LINE-1 methylation level. The statistical significance level for cross-sectional assessment of clinicopathologic and molecular associations was adjusted by Bonferroni correction to P=0.0026 (=0.05/19), given multiple hypothesis testing.
Kaplan-Meier method and log-rank test were used for survival analyses. Patients were observed from the cancer diagnosis, until death or January 1, 2011, whichever came first. For colorectal cancer-specific mortality, deaths from other causes were censored. To control for confounding, we used multivariate Cox proportional hazards regression models. A multivariate model initially included sex, age at diagnosis (continuous), body mass index (BMI) (<30 vs. ≥30 kg/m2), family history of colorectal cancer in a first-degree relative (absent vs. present), year of diagnosis (continuous), post-diagnosis aspirin use (regular user vs. non-user), post-diagnosis smoking status (never vs. former/current smokers), post-diagnosis alcohol consumption (none vs. any), tumor location (proximal vs. distal), tumor differentiation (well to moderate vs. poor), CIMP (low/0 vs. high), MSI (MSS vs. high), LINE-1 methylation (continuous), and BRAF and KRAS mutations. To minimize residual confounding, disease stage (I vs. II vs. III) was used as a stratifying variable using the “strata” option in the SAS “proc phreg” command. For cases with missing information in any of the categorical covariates [post-diagnosis aspirin use (0.2%), post-diagnosis smoking status (4.1%), post-diagnosis alcohol consumption (2.3%), tumor location (0.3%), tumor differentiation (1.2%), CIMP (5.0%), MSI (5.5%), BRAF (5.1%), and KRAS (4.6%)], we included those cases in the majority category of the given covariate. We confirmed that excluding cases with missing information in any of the covariates did not substantially alter results (data not shown). An interaction was assessed by the Wald test on interaction terms that were the cross-products of the variables of interest.
RESULTS
Characteristics of colorectal cancer patients
Characteristics of the 605 participants with stage I-III colorectal cancer in the two prospective cohort studies are summarized according to post-diagnosis physical activity quartile in Table 1. Physically active individuals tended to be younger and leaner than physically inactive individuals.
Among the 605 tumors, 382 (63%) were PTGS2-positive cases, whereas 223 (37%) were negative for PTGS2. Supplementary Table S1 summarizes characteristics of cases according to tumor PTGS2 expression status.
Physical activity and survival of colorectal cancer patients
During follow-up [median, 11.9 (interquartile range, 7.9–15.5) years for censored cases], there were 253 deaths, including 89 colorectal cancer-specific deaths. We initially examined the relation between physical activity (quartiles) and patient survival in each cohort, separately (Table 2). Compared to participants who reported the lowest levels of post-diagnosis physical activity (first quartile, Q1), those reporting higher levels of physical activity experienced lower colorectal cancer-specific mortality in Kaplan-Meier analyses (log-rank P=0.0044 among men in the HPFS, and P=0.027 among women in the NHS). In univariate and multivariate Cox regression analyses, compared with participants in the lowest quartile (Q1), higher levels of physical activity were associated with lower mortality in both men and women (Table 2). There was no significant interaction between post-diagnosis physical activity and sex/cohort (Pinteraction=0.47).
Table 2.
Colorectal cancer mortality by post-diagnosis physical activity quartile
| Post-diagnosis physical activity quartile (MET-hours/week) | # | Colorectal cancer-specific mortality | Overall mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| # of events | Univariate HR (95% CI) | Stage-stratified HR (95% CI) | Multivariate stage-stratified HRa (95% CI) | # of events | Univariate HR (95% CI) | Stage-stratified HR (95% CI) | Multivariate stage-stratified HRa (95% CI) | ||
| Male | |||||||||
| Q1 (<6.4) | 77 | 17 | 1 (referent) | 1 (referent) | 1 (referent) | 42 | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 (6.4–18.4) | 75 | 8 | 0.42 (0.18–0.98) | 0.43 (0.19–1.00) | 0.38 (0.16–0.90) | 39 | 0.87 (0.56–1.34) | 0.87 (0.56–1.36) | 0.80 (0.52–1.25 |
| Q3 (18.6–46.5) | 78 | 13 | 0.64 (0.31–1.31) | 0.61 (0.30–1.27) | 0.69 (0.33–1.44) | 31 | 0.58 (0.36–0.92) | 0.59 (0.37–0.94) | 0.66 (0.41–1.06 |
| Q4 (≥47.1) | 75 | 3 | 0.15 (0.04–0.51) | 0.16 (0.05–0.53) | 0.17 (0.05–0.57) | 30 | 0.60 (0.38–0.96) | 0.62 (0.38–0.99) | 0.63 (0.39–1.02 |
| Ptrendb | 0.0047 | 0.0051 | 0.0099 | 0.035 | 0.044 | 0.086 | |||
| Female | |||||||||
| Q1 (<2.4) | 75 | 20 | 1 (referent) | 1 (referent) | 1 (referent) | 36 | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 (2.4–7.5) | 71 | 9 | 0.44 (0.20–0.96) | 0.46 (0.21–1.01) | 0.43 (0.19–0.94) | 25 | 0.64 (0.38–1.06) | 0.63 (0.38–1.06) | 0.66 (0.39–1.10 |
| Q3 (7.7–17.7) | 80 | 10 | 0.43 (0.20–0.91) | 0.48 (0.22–1.04) | 0.48 (0.22–1.04) | 27 | 0.60 (0.36–0.99) | 0.64 (0.39–1.06) | 0.64 (0.39–1.06 |
| Q4 (≥18.3) | 74 | 9 | 0.41 (0.19–0.90) | 0.42 (0.19–0.93) | 0.40 (0.18–0.89) | 23 | 0.53 (0.31–0.89) | 0.52 (0.31–0.89) | 0.56 (0.33–0.96 |
| Ptrendb | 0.11 | 0.12 | 0.10 | 0.064 | 0.064 | 0.10 | |||
| Combined | |||||||||
| Q1 | 152 | 37 | 1 (referent) | 1 (referent) | 1 (referent) | 78 | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 | 146 | 17 | 0.43 (0.24–0.76) | 0.45 (0.25–0.79) | 0.42 (0.24–0.75) | 64 | 0.76 (0.55–1.06) | 0.77 (0.55–1.08) | 0.76 (0.54–1.06 |
| Q3 | 158 | 23 | 0.52 (0.31–0.88) | 0.52 (0.31–0.88) | 0.54 (0.32–0.91) | 58 | 0.59 (0.42–0.83) | 0.59 (0.42–0.83) | 0.62 (0.44–0.88 |
| Q4 | 149 | 12 | 0.29 (0.15–0.55) | 0.30 (0.16–0.57) | 0.29 (0.15–0.56) | 53 | 0.57 (0.40–0.80) | 0.57 (0.40–0.81) | 0.61 (0.43–0.87 |
| Ptrendb | 0.0011 | 0.0013 | 0.0006 | 0.045 | 0.057 | 0.022 | |||
| Pinteractionc | 0.58 | 0.50 | 0.47 | 0.92 | 0.88 | 0.87 | |||
The multivariate, stage-stratified Cox regression model initially included sex, age, body mass index, family history of colorectal cancer in any first degree relative, year of diagnosis, post-diagnosis aspirin use, post-diagnosis smoking status, post-diagnosis alcohol consumption, tumor location, tumor differentiation, CpG island methylator phenotype, microsatellite instability, LINE-1 methylation, and BRAF and KRAS mutations. A backward elimination with threshold of P=0.05 was used to select variables in the final models.
Tests for linear trend across categories were calculated by using the median value for each quartile of physical activity (MET-hours/week) as a continuous variable in a proportional hazards model.
P for interaction between physical activity quartile and sex/cohort.
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent task.
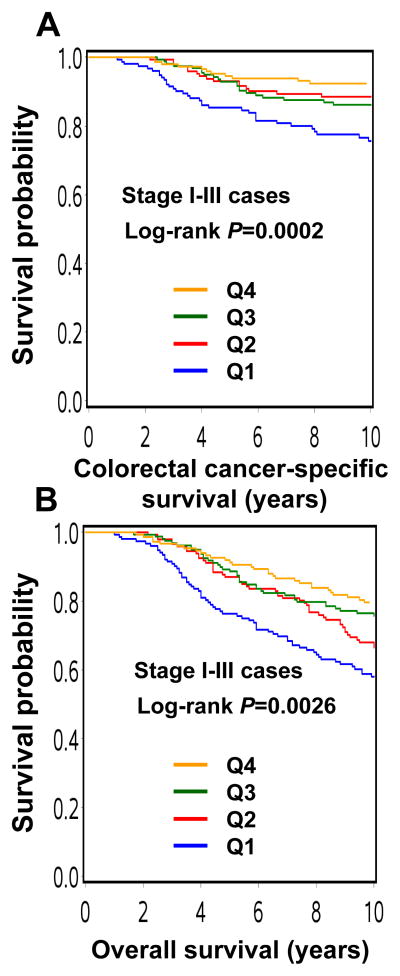
When men and women were combined, compared to participants in Q1, those reporting higher levels of physical activity (Q2-Q4) experienced lower colorectal cancer-specific mortality in Kaplan-Meier analysis (log-rank P=0.0002; Figure 1). In multivariate Cox regression analyses, compared with Q1, the multivariate HR was 0.42 (95% CI, 0.24–0.75) for Q2, 0.54 (95% CI, 0.32–0.91) for Q3, and 0.29 (95% CI, 0.15–0.56) for Q4 (Ptrend =0.0006; Table 2).
Figure 1.
Kaplan-Meier curves for stage I–III colorectal cancer patients.
A. Colorectal cancer-specific survival according to post-diagnosis physical activity quartile.
B. Overall survival according to post-diagnosis physical activity quartile.
Prognostic association of physical activity in strata of PTGS2 status
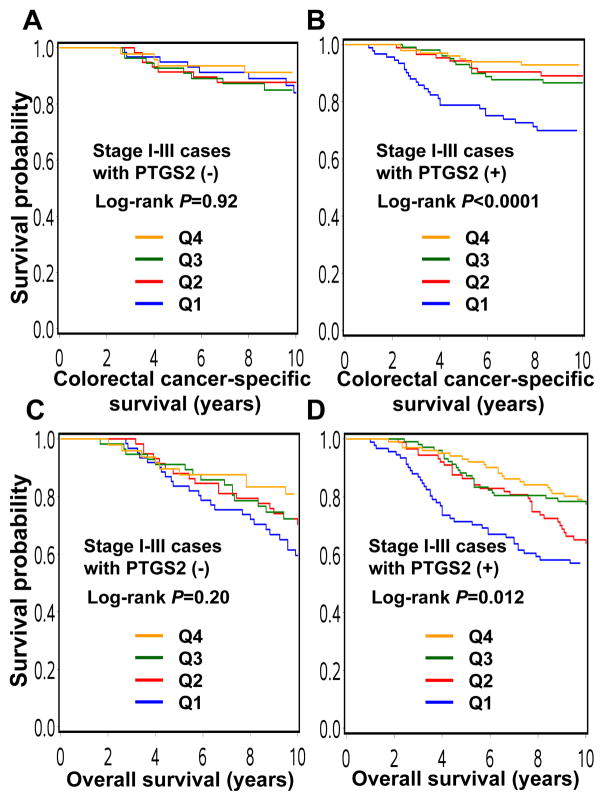
We examined the association between post-diagnosis physical activity quartile and patient survival in strata of tumor PTGS2 status. Notably, for PTGS2-positive cases, compared with the least active participants (Q1), those reporting higher levels of physical activity (Q2-Q4) experienced lower colorectal cancer-specific mortality in Kaplan-Meier analysis (log-rank P<0.0001; Figure 2). In multivariate Cox regression analyses, compared with Q1, the multivariate HR was 0.30 (95% CI, 0.14–0.62) for Q2, 0.38 (95% CI, 0.20–0.71) for Q3, and 0.18 (95% CI, 0.08–0.41) for Q4 (Ptrend =0.0002; Table 3). In contrast, for PTGS2-negative cases, there appeared to be no significant relationship between physical activity and mortality (Figure 2 and Table 3). Furthermore, there was a statistically significant interaction between post-diagnosis physical activity quartile and tumor PTGS2 status (Pinteraction=0.024; Table 3).
Figure 2.
Kaplan-Meier curves for stage I–III colorectal cancer, stratified by tumor PTGS2 status.
A. Colorectal cancer-specific survival according to post-diagnosis physical activity quartile in PTGS2-negative cases.
B. Colorectal cancer-specific survival according to post-diagnosis physical activity quartile in PTGS2-positive cases.
C. Overall survival according to post-diagnosis physical activity quartile in PTGS2-negative cases.
D. Overall survival according to post-diagnosis physical activity quartile in PTGS2-positive cases.
Table 3.
Colorectal cancer mortality by post-diagnosis physical activity quartile, stratified by PTGS2 (COX-2) status
| Post-diagnosis physical activity quartile | # | Colorectal cancer-specific mortality
|
Overall mortality
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| # of events | Univariate HR (95% CI) | Stage-stratified HR (95% CI) | Multivariate stage-stratified HRa (95% CI) | # of events | Univariate HR (95% CI) | Stage-stratified HR (95% CI) | Multivariate stage-stratified HRa (95% CI) | ||
| PTGS2 (COX-2) (−) | |||||||||
| Q1 | 61 | 8 | 1 (referent) | 1 (referent) | 1 (referent) | 28 | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 | 58 | 7 | 0.87 (0.32–2.41) | 0.96 (0.34–2.68) | 0.89 (0.32–2.51) | 24 | 0.83 (0.48–1.43) | 0.86 (0.49–1.49) | 0.87 (0.50–1.52) |
| Q3 | 56 | 8 | 1.07 (0.40–2.85) | 1.10 (0.41–2.94) | 1.14 (0.42–3.08) | 17 | 0.65 (0.36–1.20) | 0.67 (0.37–1.23) | 0.68 (0.37–1.26) |
| Q4 | 48 | 5 | 0.74 (0.24–2.27) | 0.82 (0.26–2.55) | 0.85 (0.27–2.67) | 13 | 0.52 (0.27–1.00) | 0.54 (0.28–1.05) | 0.65 (0.33–1.29) |
| Ptrendb | 0.67 | 0.83 | 0.84 | 0.18 | 0.27 | 0.40 | |||
| PTGS2 (COX-2) (+) | |||||||||
| Q1 | 91 | 29 | 1 (referent) | 1 (referent) | 1 (referent) | 50 | 1 (referent) | 1 (referent) | 1 (referent) |
| Q2 | 88 | 10 | 0.31 (0.15–0.63) | 0.29 (0.14–0.60) | 0.30 (0.14–0.62) | 40 | 0.72 (0.47–1.09) | 0.71 (0.46–1.08) | 0.70 (0.46–1.06) |
| Q3 | 102 | 15 | 0.38 (0.20–0.71) | 0.36 (0.19–0.67) | 0.38 (0.20–0.71) | 41 | 0.54 (0.36–0.82) | 0.54 (0.36–0.82) | 0.60 (0.39–0.91) |
| Q4 | 101 | 7 | 0.18 (0.08–0.41) | 0.18 (0.08–0.41) | 0.18 (0.08–0.41) | 40 | 0.57 (0.37–0.86) | 0.57 (0.37–0.86) | 0.57 (0.38–0.88) |
| Ptrendb | 0.0004 | 0.0004 | 0.0002 | 0.095 | 0.10 | 0.030 | |||
| Pinteractionc | 0.040 | 0.030 | 0.024 | 0.77 | 0.84 | 0.82 | |||
The multivariate, stage-stratified Cox regression model included the same set of covariates selected as in Table 2.
Tests for linear trend across categories were calculated by using the median value for each quartile of physical activity (MET-hours/week) as a continuous variable in a proportional hazards model.
P for interaction between physical activity quartile and tumor PTGS2 status.
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent task.
In the analysis of overall mortality, the difference in the prognostic association of physical activity between PTGS2-positive and PTGS2-negative cases was somewhat attenuated (Figure 2 and Table 3).
Prognostic association of physical activity in strata of PTGS2 and other selected variables
In exploratory analyses, we examined the association between post-diagnosis physical activity quartile and patient survival stratified by tumor PTGS2 status and by other selected variables. Specifically, in order to establish that the association between post-diagnosis physical activity and survival in PTGS2-positive tumors was not attributable to differences in post-diagnosis aspirin use, we performed an analysis limited to post-diagnosis aspirin non-users, and obtained results (Supplementary Table S2) consistent with the primary study findings (Table 3).
We previously reported that the association of post-diagnosis physical activity with cancer-specific survival was modified by tumor CDKN1B (49), and nuclear CTNNB1 status (32). Utilizing physical activity quartile categories we performed analysis stratified by CDKN1B status (Supplementary Table S3), or nuclear CTNNB1 status (Supplementary Table S4). These analyses confirmed our prior associations between post-diagnosis physical activity and mortality among patients with CDKN1B-positive tumors or nuclear CTNNB1-negative tumors (32, 49).
DISCUSSION
We examined the hypothesis that the beneficial prognostic association of physical activity might be stronger in patients with PTGS2-positive colorectal cancer, compared to those with PTGS2-negative tumors. In stage I–III PTGS2-positive colorectal cancer, we found that post-diagnosis physical activity was associated with significantly better colorectal cancer-specific survival, while post-diagnosis physical activity was not significantly associated with survival among PTGS2-negative cases. These results provide evidence for an interactive effect of physical activity and tumor PTGS2 expression in determining tumor behavior, and may give us clues to a role of energy balance in tumor progression and clinical outcome. In addition, tumor PTGS2 status may serve as a predictive biomarker of the beneficial effect of exercise, which can be recommended as part of a program of personalized health care.
Analysis of molecular biomarkers is increasingly important in colorectal and other cancers (50–71). Examining interactions between host factors and tumor markers has emerged as a promising study design in the evolving interdisciplinary field of molecular pathological epidemiology (MPE) (72–75). As an integral part of a more expansive field of “Integrative Epidemiology” (76), MPE specifically addresses molecular and phenotypic heterogeneity of any given disease. MPE integrates molecular pathology and epidemiology to address interactive effects of lifestyle, genetic, and environmental factors, and specific cellular molecular features on disease evolution and progression (72–75). MPE research may be clinically useful and can contribute to personalized medicine, as our current study suggests that tumor PTGS2 status may improve the identification of patients who will benefit most from physical activity.
Prospective observational data suggest that physically active colorectal cancer survivors have lower rates of cancer recurrence and death, compared with physically inactive survivors (7, 8, 10–19). Physical activity is a modifiable lifestyle factor, and thus its beneficial effect on cancer survival has considerable clinical implications (77–81). Identifying predictive biomarkers for clinical interventions is important in cancer research. As with any other oncologic interventions, it is unlikely patients will uniformly derive benefits from exercise, and it would be of great value to be able to identify patient characteristics or tumor molecular features that can predict response to lifestyle interventions. Molecular features of a primary tumor might be different from those of a corresponding recurrent/metastatic tumor. Nonetheless, tumor molecular features have been shown to be generally similar between primary and metastatic tumors (82, 83), and most tumor biomarkers rely on analyses of primary tumor tissues.
Several mechanisms have been postulated to underlie the influence of physical activity on colorectal cancer behavior, including decreased PGE2 activity, reduced gut transit time, and attenuation of hyperinsulinemia (21–23, 84–88). We have previously shown that physical activity appears to be more beneficial in patients with certain subtypes of colorectal cancers, including CTNNB1-negative tumors (32) and CDKN1B (p27)-expressing tumors (49). Nonetheless, colorectal cancer represents a group of complex diseases (89) and additional tumor biomarkers need to be explored. Our current findings suggest a possible effect of post-diagnosis physical activity in attenuating the aggressiveness of PTGS2-positive tumors. In addition, our exploratory data suggest that the beneficial association of post-diagnosis physical activity with colorectal cancer survival is not caused by post-diagnosis aspirin use. Post-diagnosis physical activity and aspirin use may act synergistically to attenuate tumor aggressiveness in patients with PTGS2-positive colorectal cancer. These findings are compatible with our hypothesis that physical activity may improve survival by inhibiting PTGS2 downstream effectors, such as PGE2.
Interestingly, our data imply that even a modest amount of exercise (≥6.4 MET hours/week in men, and ≥2.4 MET hours/week in women) significantly improves colorectal cancer-specific survival among patients with PTGS2-positive tumors. In the previous report (8, 9, 11, 14, 32), the beneficial effects of post-diagnosis physical activity on colorectal cancer survival were apparent in individuals who engaged in much higher levels of exercise. Therefore, our current data may help motivate inactive colorectal cancer survivors to engage in even modest levels of exercise. This apparent discrepancy might be in part because, unlike our current MPE study, the previous studies (8, 9, 11, 14) regarded all colorectal cancer cases (regardless of PTGS2 expression status) as a single disease entity without much consideration of heterogeneity in colorectal cancer biology between cases.
There are some limitations in this study, including limited data on cancer treatment. Nonetheless, it is unlikely that chemotherapy use substantially differed according to tumor PTGS2 status, since this information was unavailable to physicians. In addition, our survival analyses were adjusted for cancer stage, on which treatment decisions are mainly based. Another limitation is that data on cancer recurrence were unavailable. Nonetheless, colorectal cancer-specific mortality was a reasonable surrogate for colorectal cancer-specific outcomes given the long follow-up of those who were censored. We limited our analysis to stage I–III disease for which a vast majority of patients could undergo potentially curative cancer resection and could exercise after recovery from surgery. Thus, it is likely that reverse causation may not be the only explanation for the apparent interactive effect of tumor PTGS2 status and physical activity.
There are advantages in utilizing the data from the two U.S. nationwide prospective cohort studies. Data on anthropometric measurements (such as BMI), cancer staging, and other clinical, pathologic, and tumor molecular variables had been prospectively collected, blinded to patient survival. Cohort participants who were diagnosed with cancer were treated at hospitals throughout the U.S., and are thus more representative of colorectal cancer cases in the general Caucasian population than patients selected from a few academic hospitals. In addition, the comprehensive tumor tissue data enabled us to conduct MPE research (72–75) and assess the interaction between physical activity and tumor PTGS2 status.
In conclusion, our data provide evidence for a possible interactive effect of post-diagnosis physical activity and tumor PTGS2 expression status on colorectal cancer prognosis. Notably, the association between better survival and physical activity was observed only in participants with PTGS2-positive colorectal cancers, whereas no prognostic association was observed for physical activity in PTGS2-negative cases. Our findings not only give insight into the biology of colorectal cancer progression, adding to the expanding literature on energetics and inflammation, but also have the potential to influence clinical recommendations relating to lifestyle modification after a diagnosis of colorectal cancer. Further studies are necessary to confirm our findings, and to elucidate mechanisms that underlie the complex interactions between host energetics, inflammation, and tumor evolution and progression.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Grant Support: This work was supported by National Institute of Health grants [P01 CA87969 (to S.E. Hankinson), P01 CA55075 (to W.C. Willett), 1UM1 CA167552 (to W.C. Willett), P50 CA127003 (to C.S.F.), R01 CA151993 (to S.O.), and R01 CA137178 (to A.T.C.)]. P.L. is a Scottish Government Clinical Academic Fellow and was supported by a Harvard University Knox Memorial Fellowship. A.T.C. is a Damon Runyon Cancer Foundation Clinical Investigator.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- COX-2
cyclooxygenase-2
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- MET
metabolic equivalent task
- MPE
molecular pathological epidemiology
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses’ Health Study
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PGE2
prostaglandin E2
Footnotes
Author Contributions: MY, PL, YI and AK contributed equally. JAM, CSF, ATC and SO contributed equally. ATC and SO contributed to the study concept and design. MY, PL, YI, XL, ZRQ, TM, KS, KW, EG, JAM, CSF, ATC and SO were involved in acquisition of data. All of the authors contributed to the interpretation of the data. MY, AK, RN and SO performed the statistical analysis. MY, PL and SO drafted the manuscript. MY wrote the first draft of manuscript. All of the authors revised the article critically for important intellectual content and approved the final version of the manuscript submitted for publication.
Disclosures: ATC was previously a consultant for Bayer Healthcare, Millennium Pharmaceuticals, and Pfizer Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, or Pfizer Inc. No other conflict of interest exists.
References
- 1.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–52. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 3.Nilsen TI, Romundstad PR, Petersen H, Gunnell D, Vatten LJ. Recreational physical activity and cancer risk in subsites of the colon (the Nord-Trondelag Health Study) Cancer Epidemiol Biomarkers Prev. 2008;17:183–8. doi: 10.1158/1055-9965.EPI-07-0746. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Patel AV, Campbell PT, Jacobs EJ, McCullough ML, Colditz GA, et al. Change in physical activity and colon cancer incidence and mortality. Cancer Epidemiol Biomarkers Prev. 2010;19:3000–4. doi: 10.1158/1055-9965.EPI-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, Energy Balance and Cancer: New Opportunities for Prevention. Cancer Prev Res (Phila) 2012;5:1260–72. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 10.Orsini N, Mantzoros CS, Wolk A. Association of physical activity with cancer incidence, mortality, and survival: a population-based study of men. Br J Cancer. 2008;98:1864–9. doi: 10.1038/sj.bjc.6604354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peel JB, Sui X, Matthews CE, Adams SA, Hebert JR, Hardin JW, et al. Cardiorespiratory fitness and digestive cancer mortality: findings from the aerobics center longitudinal study. Cancer Epidemiol Biomarkers Prev. 2009;18:1111–7. doi: 10.1158/1055-9965.EPI-08-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2010;92:471–90. doi: 10.3945/ajcn.2010.29005. [DOI] [PubMed] [Google Scholar]
- 14.Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20:1410–20. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 15.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 16.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23:1939–48. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of Recreational Physical Activity and Leisure Time Spent Sitting With Colorectal Cancer Survival. J Clin Oncol. 2013;31:876–85. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 20.Friedenreich CM, Neilson HK, Woolcott CG, Wang Q, Stanczyk FZ, McTiernan A, et al. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev Res (Phila) 2012;5:98–108. doi: 10.1158/1940-6207.CAPR-11-0369. [DOI] [PubMed] [Google Scholar]
- 21.Martinez ME, Heddens D, Earnest DL, Bogert CL, Roe D, Einspahr J, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst. 1999;91:950–3. doi: 10.1093/jnci/91.11.950. [DOI] [PubMed] [Google Scholar]
- 22.Sellar CM, Courneya KS. Physical activity and gastrointestinal cancer survivorship. Recent Results Cancer Res. 2011;186:237–53. doi: 10.1007/978-3-642-04231-7_10. [DOI] [PubMed] [Google Scholar]
- 23.Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 2011;4:502–11. doi: 10.1158/1940-6207.CAPR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chell S, Kaidi A, Williams AC, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–19. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–8. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer-reinterpreting paradigms. Nat Rev Clin Oncol. 2012;9:561–70. doi: 10.1038/nrclinonc.2012.137. [DOI] [PubMed] [Google Scholar]
- 27.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224–6. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 29.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–58. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 31.Coghill AE, Phipps AI, Bavry AA, Wactawski-Wende J, Lane DS, LaCroix A, et al. The association between NSAID use and colorectal cancer mortality: results from the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2012;21:1966–73. doi: 10.1158/1055-9965.EPI-12-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305:1685–94. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 37.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, Namgyal C, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–8. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino S, Kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, Spiegelman D, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinoue T, Weisenberger DJ, Lange CP, Shen H, Byun HM, Van Den Berg D, et al. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome research. 2012;22:271–82. doi: 10.1101/gr.117523.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–14. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Chan AT, Schernhammer ES, et al. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst. 2008;100:1734–8. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irahara N, Nosho K, Baba Y, Shima K, Lindeman NI, Hazra A, et al. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagn. 2010;12:177–83. doi: 10.2353/jmoldx.2010.090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogino S, Kawasaki T, Kirkner GJ, Yamaji T, Loda M, Fuchs CS. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 49.Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15:5931–6. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahlin AM, Palmqvist R, Henriksson ML, Jacobsson M, Eklof V, Rutegard J, et al. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845–55. doi: 10.1158/1078-0432.CCR-09-2594. [DOI] [PubMed] [Google Scholar]
- 52.Rozek LS, Herron CM, Greenson JK, Moreno V, Capella G, Rennert G, et al. Smoking, gender, and ethnicity predict somatic BRAF mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:838–43. doi: 10.1158/1055-9965.EPI-09-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nature reviews Gastroenterology & hepatology. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-beta signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:57–69. doi: 10.1158/1055-9965.EPI-10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahearn TU, Shaukat A, Flanders WD, Seabrook ME, Bostick RM. Markers of the APC/beta-catenin signaling pathway as potential treatable, preneoplastic biomarkers of risk for colorectal neoplasms. Cancer Epidemiol Biomarkers Prev. 2012;21:969–79. doi: 10.1158/1055-9965.EPI-12-0126. [DOI] [PubMed] [Google Scholar]
- 56.Dallol A, Al-Maghrabi J, Buhmeida A, Gari MA, Chaudhary AG, Schulten HJ, et al. Methylation of the polycomb group target genes is a possible biomarker for favorable prognosis in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2069–75. doi: 10.1158/1055-9965.EPI-12-0755. [DOI] [PubMed] [Google Scholar]
- 57.Fedirko V, Riboli E, Tjonneland A, Ferrari P, Olsen A, Bueno-de-Mesquita HB, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European ppulations. Cancer Epidemiol Biomarkers Prev. 2012;21:582–93. doi: 10.1158/1055-9965.EPI-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavin PG, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G, et al. Mutation Profiling and Microsatellite Instability in Stage II and III Colon Cancer: An Assessment of Their Prognostic and Oxaliplatin Predictive Value. Clin Cancer Res. 2012;18:6531–41. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hibler EA, Hu C, Jurutka PW, Martinez ME, Jacobs ET. Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2012;21:368–75. doi: 10.1158/1055-9965.EPI-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang WY, Su LJ, Hayes RB, Moore LE, Katki HA, Berndt SI, et al. Prospective study of genomic hypomethylation of leukocyte DNA and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:2014–21. doi: 10.1158/1055-9965.EPI-12-0700-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Limburg PJ, Limsui D, Vierkant RA, Tillmans LS, Wang AH, Lynch CF, et al. Postmenopausal hormone therapy and colorectal cancer risk in relation to somatic KRAS mutation status among older women. Cancer Epidemiol Biomarkers Prev. 2012;21:681–4. doi: 10.1158/1055-9965.EPI-11-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ollberding NJ, Cheng I, Wilkens LR, Henderson BE, Pollak MN, Kolonel LN, et al. Genetic variants, prediagnostic circulating levels of insulin-like growth factors, insulin, and glucose and the risk of colorectal cancer: the Multiethnic Cohort study. Cancer Epidemiol Biomarkers Prev. 2012;21:810–20. doi: 10.1158/1055-9965.EPI-11-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, Passarelli MN, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:1792–8. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thyagarajan B, Wang R, Barcelo H, Koh WP, Yuan JM. Mitochondrial copy number is associated with colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:1574–81. doi: 10.1158/1055-9965.EPI-12-0138-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaught JB, Henderson MK, Compton CC. Biospecimens and biorepositories: from afterthought to science. Cancer Epidemiol Biomarkers Prev. 2012;21:253–5. doi: 10.1158/1055-9965.EPI-11-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing J, Wan S, Zhou F, Qu F, Li B, Myers RE, et al. Genetic polymorphisms in pre-microRNA genes as prognostic markers of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:217–27. doi: 10.1158/1055-9965.EPI-11-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karpinski P, Walter M, Szmida E, Ramsey D, Misiak B, Kozlowska J, et al. Intermediate- and low-methylation epigenotypes do not correspond to CpG island methylator phenotype (low and -zero) in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:201–8. doi: 10.1158/1055-9965.EPI-12-0157. [DOI] [PubMed] [Google Scholar]
- 68.Webster J, Kauffman TL, Feigelson HS, Pawloski PA, Onitilo AA, Potosky AL, et al. KRAS Testing and Epidermal Growth Factor Receptor Inhibitor Treatment for Colorectal Cancer in Community Settings. Cancer Epidemiol Biomarkers Prev. 2013;22:91–101. doi: 10.1158/1055-9965.EPI-12-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buchanan DD, Win AK, Walsh MD, Walters RJ, Clendenning M, Nagler BN, et al. Family History of Colorectal Cancer in BRAF p.V600E mutated Colorectal Cancer Cases. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/055-9965.EPI-12-1211. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Febbo PG, Ladanyi M, Aldape KD, De Marzo AM, Hammond ME, Hayes DF, et al. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9(Suppl 5):S1–32. doi: 10.6004/jnccn.2011.0137. quiz S3. [DOI] [PubMed] [Google Scholar]
- 71.Rosty C, Young JP, Walsh MD, Clendenning M, Walters RJ, Pearson S, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 doi: 10.1038/modpathol.2012.240. in press. [DOI] [PubMed] [Google Scholar]
- 72.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 doi: 10.1038/modpathol.2012.214. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spitz MR, Caporaso NE, Sellers TA. Integrative cancer epidemiology--the next generation. Cancer discovery. 2012;2:1087–90. doi: 10.1158/2159-8290.CD-12-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13:1022–31. [PubMed] [Google Scholar]
- 78.Jones LW, Eves ND, Peppercorn J. Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol. 2010;11:914–6. doi: 10.1016/S1470-2045(10)70184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 80.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 81.Blair SN, Sallis RE, Hutber A, Archer E. Exercise therapy - the public health message. Scand J Med Sci Sports. 2012;22:e24–8. doi: 10.1111/j.1600-0838.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 82.Artale S, Sartore-Bianchi A, Veronese SM, Gambi V, Sarnataro CS, Gambacorta M, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2008;26:4217–9. doi: 10.1200/JCO.2008.18.7286. [DOI] [PubMed] [Google Scholar]
- 83.Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010;16:790–9. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 84.Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev. 2004;28:208–13. doi: 10.1016/j.cdp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–94. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, et al. Voluntary exercise inhibits intestinal tumorigenesis in Apc(Min/+) mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer. 2008;8:316. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008;43:971–8. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- 88.Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013 doi: 10.1136/gutjnl-2011-300776. in press. [DOI] [PubMed] [Google Scholar]
- 89.Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert review of molecular diagnostics. 2012;12:621–8. doi: 10.1586/erm.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.