Abstract
The field of chronic pain medicine is currently facing enormous challenges. The incidence of chronic pain is increasing worldwide, particularly in the developed world. As a result, chronic pain is imposing a growing burden on Western societies in terms of cost of medical care and lost productivity. This burden is exacerbated by the fact that despite research efforts and a huge expenditure on treatment for chronic pain, clinicians have no highly effective treatments or definitive diagnostic measures for patients. The lack of an objective measure for pain impedes basic research into the biological and psychological mechanisms of chronic pain and clinical research into treatment efficacy. The development of objective measurements of pain and ability to predict treatment responses in the individual patient is critical to improving pain management. Finally, pain medicine must embrace the development of a new evidence-based therapeutic model that recognizes the highly individual nature of responsiveness to pain treatments, integrates bio-psycho-behavioral approaches, and requires proof of clinical effectiveness for the various treatments we offer our patients. In the long-term these approaches will contribute to providing better diagnoses and more effective treatments to lessen the current challenges in pain medicine.
Keywords: chronic pain, biomarkers, effective treatments, burden, society, individualized medicine
Introduction
The field of chronic pain medicine is currently at the crossroads of tremendous challenges but also opportunities. The incidence of chronic pain is increasing worldwide, particularly in the developed world, as the population ages and modern Western lifestyles predispose people to chronic pain due to an increase in obesity, decrease in physical activity and rapid changes in the social environment. As a result, chronic pain is imposing a growing burden on Western societies in terms of cost of medical care and lost productivity. This burden is exacerbated by the fact that despite research efforts and a huge expenditure on treatment for chronic pain in some countries, clinicians have few effective treatments or definitive diagnostics for patients, whose suffering often significantly impairs their ability to function. As well as complicating diagnosis and treatment and raising concerns about iatrogenic addiction and drug-seeking behavior, the lack of an objective measure for pain impedes basic research into the biological and psychological mechanisms of chronic pain and clinical research into treatment efficacy. In addition, our current reliance on subjective reporting of pain colors how society views individuals with chronic pain. The development of objective measurements of pain is critical to improving pain management in the long-term to determining modulation of the nervous system that may take place over time. This needs to be taken into context: First, if highly effective treatments are discovered, the issues noted here would be mute. In addition, it is the patient’s own perception of his/her health condition that matters as has recently been shown in a large study in osteoarthritis (OA) patients compared with specific measures of such as joint loading and radiographic evaluation (Shakoor et al., 2012); however, OA is a complex peripheral and central pain condition (Sofat et al., 2011). However, there is much we can do now to improve the sensitivity and reliability of subjective pain measures and optimize our therapeutic approach.
The science of pain has witnessed tremendous advances in the last decade. In particular, studies of pain genetics have identified markers for pain sensitivity and susceptibility to chronic pain or sensitivity to analgesics and functional imaging studies are revealing the complex brain-based mechanisms underlying chronic pain. In addition to changes in CNS sensory circuitry, there are changes to many brain systems, including those that encode reward, aversion and behavioral reinforcement. These changes may reflect emotional/behavioral aspects of chronic pain. Although we are a long way from understanding what triggers chronic pain as a disease or a symptom or from knowing how to cure it, we have an exciting opportunity to use this new knowledge about chronic pain to guide new approaches to both understanding it and treating it more efficiently.
This article discusses current challenges related to chronic non-cancer pain including reasons for the rapid increase in the prevalence of chronic pain in the western world, lack of effective therapies, particularly those that would help all patients, and the resistant nature of chronic pain. Understanding the complex nature of pain, its mechanisms and progression will, however, suggest new solutions. Better definition of the pain pheno-genotype will form the basis for personalized pain management as reasons for some patients responding to treatment while other remain resistant will be better understood. Some recent developments in the field of new therapeutics as well as setting and accepting realistic goals will be discussed as ways to go forward. The article ends by discussing the role of society in general in making a joint effort to improved pain management in the future.
Current Challenges in Pain Medicine
Current clinical practice is by and large devoid of outcome-based measures of efficacy. However, there are reports indicating that multidisciplinary pain management can significantly improve the health related quality of life of chronic pain patients compared with treatment at primary care (Becker et al., 2000) and that the effect can be maintained at least for 3 years after the treatment has ended (Heiskanen 2012). The current standard approach to treating chronic pain is frequently a nearly random trial for each individual, beginning with the least invasive treatment and progressing along an increasing gradient of treatment side effects to find a strategy that provides some measure of relief (see Figure 1). In addition to being inefficient, this ‘trial and error’ approach may create conditions under which subsequent treatments are less or more effective than if administered to naïve patients, so effective solutions may be missed or not fully understood. Evaluation of current treatment options for chronic pain reveals several patterns that cut across the clinical and therapeutic spectrum.
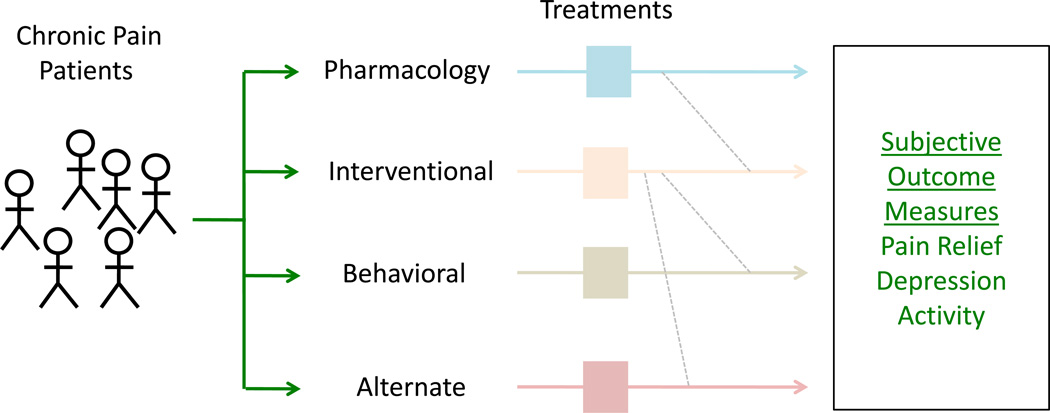
Figure 1. Current Treatment Approaches in Chronic Pain – Treatment Centered.
Current pain treatment is essentially “educated trial and error” where specific treatments are offered, usually within the focus of a clinical domain (e.g., interventional, alternate, behavioral etc.). Even within treatment domains (e.g., pharmacological), medications are tried, response evaluated, and depending on effects and side effects another medication evaluated.
Lack of Therapeutic Efficacy
Although clear improvements may be made to chronic pain care at a population level by increasing access and public education and improving clinician training, even the best treatments available do not provide highly effective treatment to all patients. Placebo-controlled outcome studies for pharmacological approaches to chronic pain reveal that they provide, on average, a meager 30% efficacy (Logothetis and Pfeuffer 2004). The numbers-needed-to-treat (NNT) with the most effective monotherapy to achieve pain reduction of at least 50% are in the range of 2 to 4 (Braune 2004) meaning that on the average one patient in 2 to 4 has his/her pain halved. Recent studies indicate that patients can benefit from combinations of drugs (Gilron and Max 2005); however, few controlled studies of combination therapy have been done, perhaps because there is no clear market incentive. In addition, there are still many drugs for which no good outcome data exist.
Most other treatments for pain have not been subject to the same standards of trial measures and outcomes. Although controlled trials for transcranial stimulation (TMS) have been reported for depression (Berman et al., 2000), few large randomized placebo controlled trials (RTC) have been reported for TMS for chronic pain (Antal and Paulus 2010). Controlled trials for interventional procedures (e.g., epidural steroids) are more difficult to interpret because placebo arms are challenging for ethical reasons. Specifically, performing any interventional “placebo” arm where the risk of serious complications is significant is clearly difficult and an important ethical issue. On the other hand, one can argue that performing high-risk interventions without evidence is also unethical. RCTs have been performed on e.g., epidural steroids (Cucler et al., 1985) and percutaneous intradiscal radiofrequency thermocoagulation. (Kvarstein et al., 2009). Although not ideal, newer trial methods (e.g., n-of-1 trials) or comparison against other treatment options can still provide much needed information about efficacy. N-of-1 trials consider an individual patient in a study investigating the efficacy or side-effect profiles of different interventions using objective data-driven criteria (Lillie et al., 2011). N-of-1 trials may be a useful way to study chronic pain patients as they offer some important benefits including: (a) they potentially offer an effective therapy for the individual; (b) they may be less expensive than standard trials; (c) they can easily define responders and non-responders. However, in the clinic the n-of-1-trials are not performed and reported in a controlled way. Therefore development of standardized easily administered protocols for N-of-1 trials will be required. Some have suggested that an N-of-1 trial may be the ‘ultimate strategy for individualizing medicine” (Lillie et al., 2011). Good examples of the use of oncological drugs include a recent report of cetuximab in neuropathic cancer pain (Kersten and Cameron 2012). Indeed, the use of oncological drugs may provide a new approach to pain treatments (Breivik and Stubhaug 2013) including epidermal growth factor inhibition (Kersten et al., 2013) or KRN-5500, a spicamycin derivative, (Borsook and Edwards 2004; Weinstein et al., 2012).
For some interventions, such as neuromodulation by spinal cord stimulation, there is some evidence of efficacy; the evidence is better for neuropathic pain and complex regional pain syndrome (CRPS) than for chronic back pain. Most of these studies have significant caveats based on problems with blinding, recruitment and assessment (Carter 2004). Similarly, assessment of alternative therapies such as acupuncture is complicated by difficulty with blinding procedures as well as internal validity (Hopton and Macpherson 2011). Another complicating factor is the strong placebo response that is related to trials on analgesia (Dworkin and Turk 2011). Alternative or complementary medicine treatments are very popular despite the fact that, by and large, their effectiveness is poorly characterized or proven ineffective in rigorous controlled trials (Bardia et al., 2006; Cherkin et al., 2003; Linde et al., 1997). A national audit noted that “health care alternatives to be more congruent with their own values, beliefs, and philosophical orientations toward health and life” (Astin 1998). Complimentary and Alternative Medicine (CAM) approaches are commonly used in chronic pain patients (Konvicka et al., 2008). One reason may relate to the relative ineffectiveness and or side effects of pharmacotherapy or interventional approaches. The fact that so many embrace these approaches in the absence of evidence of their efficacy highlights the desperation that makes many patients willing to try any new approach to relieve their pain. While outcome data are clearly needed, the demand from patients will continue to drive these treatments.
Behavioral treatments for chronic back pain have been evaluated using a comparative approach; a recent study found that all behavioral therapies tested were slightly more effective than no treatment or the usual care for short term relief of chronic low back pain, but found no difference in efficacy between the behavioral therapies tested and no effect of any behavioral therapy over the intermediate or long-term (Henschke et al., 2010). Thus, while numerous treatments are commonly offered to patients with chronic pain, not all have met the stringent standards applied to pharmacotherapies. Outcome studies are thus critically needed to allow clinicians to focus resources on processes with known efficacy.
In addition to the limited efficacy of available treatments, there is increasing recognition that some actually create additional problems and may even exacerbate chronic pain. This has been most obvious with opioids. Prescription opioid addiction is a burgeoning problem (Dodrill et al., 2011) and may cause significant changes in the brain (Upadhyay et al., 2010). The emergence of so-called opioid-induced hyperalgesia has become a focus of opioid overuse. While not clinically dangerous nor exceedingly problematic for most patients, it may indicate that the drug is not working and suggests complexities of centralization of pain (see below) that are exacerbated by the drug and potentially more complex but subtle changes in other brain systems (e.g., deficient reward processing) (Upadhyay et al., 2010). An important caveat relates to the practical use of opioids and that that the guidelines for treating cancer pain with opioids do not apply to chronic non-cancer pain as noted many years ago (Foley 1995).
Disease Resistance
If we use the standard of no or even minimal (pain ≤ 2/10) pain as the outcome measure, chronic pain is resistant to most treatments when considering the average response. Even best in class agents rarely provide more than a 30% decrease in pain level (Lunn et al., 2009; Rowbotham et al., 1998; Satoh et al., 2011) and there seems to be little difference in efficacy across classes of such agents (Quilici et al., 2009). This translates into an average decrease of 2 points on a 0–10 point scale. The basis for this ‘resistance’ is currently unknown. It may involve multiple factors such as genetic and epigenetic susceptibility, the complexity of the progressive alterations that occur in brain systems with chronic pain, or simply that currently available treatments do not optimally target the affected systems.
Responders and Non-Responders – The Need for Personalized Pain Medicine
The very best average response that has been reported for any pain treatment is a 50% decrease in reported pain in every other patient. This is clearly insufficient, but averages do not tell the entire story. Meta-analyses based on individual patient data (rather than pooling averages from different studies) indicate that there are patients who respond well to treatment and those who do not and not so many whose responses fall in between (Moore et al., 2010). However, we do not yet know what makes a patient a good vs. a poor responder. Pharmacogenomic studies have identified genetic markers that associate with differences in response to specific analgesics (Chesler et al., 2003; Jannetto and Bratanow 2011; Kasai and Ikeda 2011). Understanding the mechanistic basis for the existence of these distinct responder populations is important for developing targeted individualized treatment strategies and may also suggest new therapeutic targets for drug development. Thus, the ability to differentiate patients in the clinic is a high priority in chronic pain research (Figure 2A, 2B). Recent studies have begun to address this (Scholz et al., 2009).
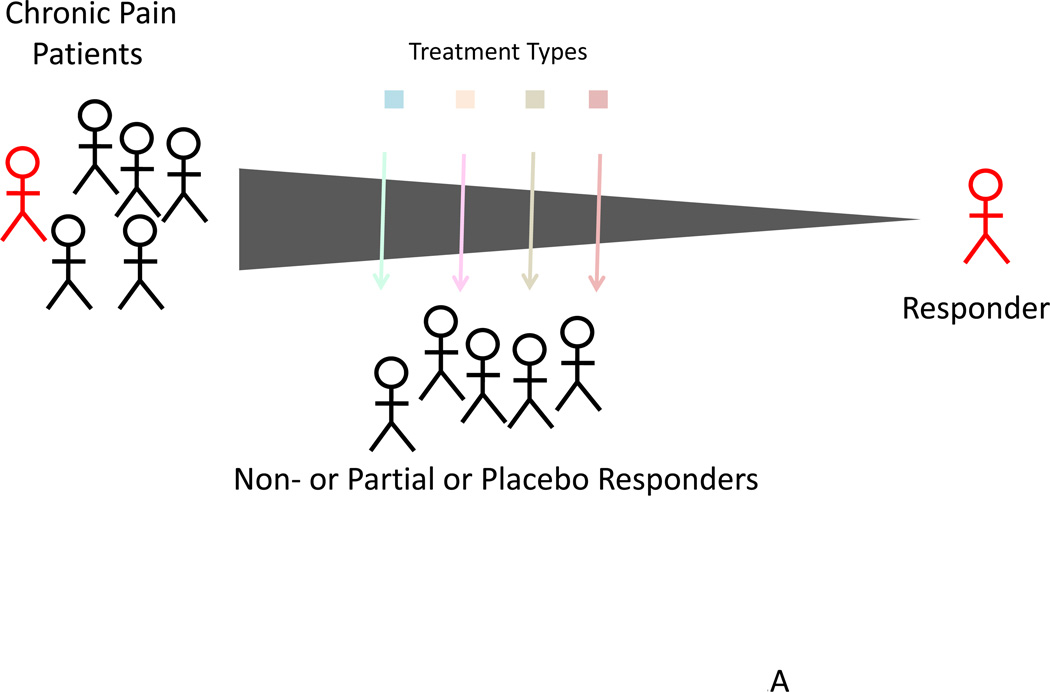
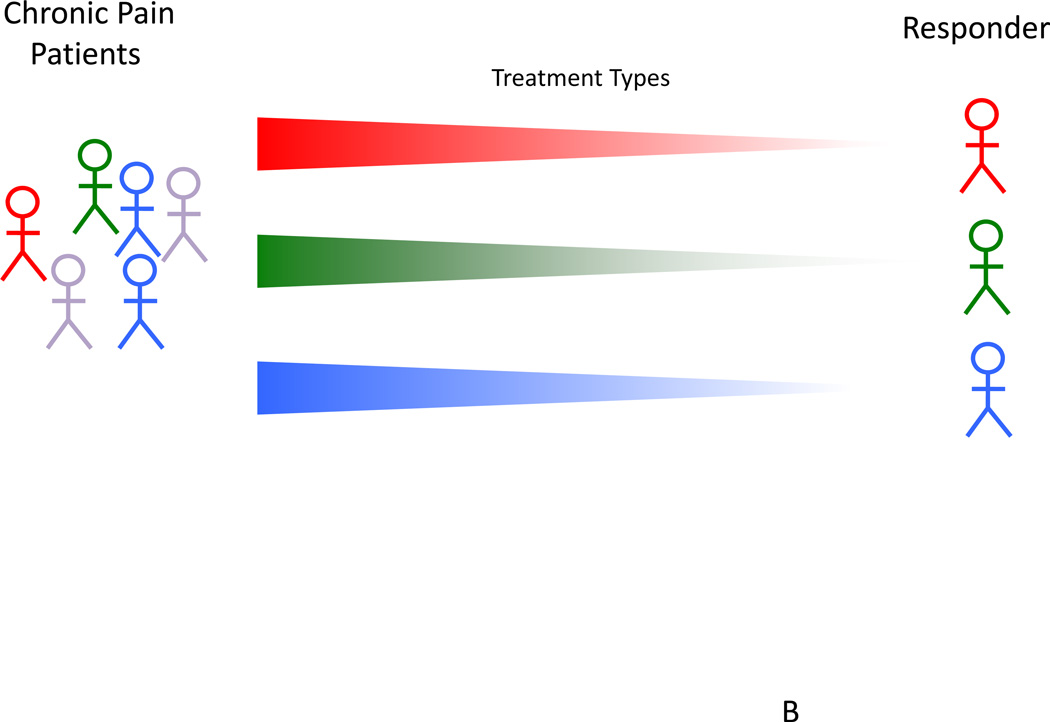
Figure 2. Curating Responders and Non-Responders.
Top: Within every treatment, few patients are responders where the treatment offers more than 50% long-lasting pain relief. In this model, significant numbers of patients are exposed to medications that are not very helpful or unhelpful or even produce side effects.
Bottom: In an ideal therapeutic approach, individualized methods would allow for selection of patients who respond to medications with high efficacy and low side effect profiles.
Disease Progression and Remission
Chronic pain is not static. It may progress or remit. One example of this is pain following trauma: over time, the initial events lead to alterations in sensation, central sensitization and changes in affective and emotional states (Maihofner et al., 2010). Disease progression is another reason for ongoing or worsening pain (e.g., in osteoarthritis). Just as chronic pain may progress, it can also resolve spontaneously. One example of spontaneous remission is pediatric complex regional pain syndrome (CRPS). Unlike adult CRPS, the majority of cases of CRPS in children resolve within a year (Low et al., 2007), presumably reflecting a more ‘plastic’ or adaptive nervous system in children (Stanton-Hicks 2010). Another example of remission is chronic post-amputation phantom pain, which decreases significantly in up to 80% of patients within a year (Houghton et al., 1994; Nikolajsen and Lindvig 2001). Studying remission may thus offer clues to therapy in addition to providing important information for the patient and a basis for preventive studies. Changes may relate to a number of factors including the natural history of the disease as in diabetic neuropathy (Veves et al., 2008), medications that may make the pain worse as is the case for opioids (Eriksen et al., 2006) or treatments for migraine that include excessive use of triptans or opioids that produce chronification of the disease (Bigal 2009). Case reports or uncontrolled studies by their nature make it difficult to account for treatment response, placebo effect or natural variation of the disease.
Co-morbid Conditions
Further complicating our understanding and treatment of pain are co-morbid features including anxiety, depression, and addiction (Bras et al., 2010; Fishbain et al., 1997; McWilliams et al., 2003). Anxiety is closely linked to pain as pain is fundamentally a warning signal (Jordan and Okifuji 2011). Depression usually develops along with chronic pain, but pain may also be a consequence of depression in patients with no prior history of chronic pain (see (Borsook et al., 2007) and (Knaster et al., 2012). These observations highlight the link between pain and emotional/behavioral systems (see (Elman et al., 2011)), reminding us that pain should always be considered in the context of its definition by the International Association for the Study of Pain (IASP) as both a sensory and emotional experience (http://www.iasp-pain.org). Interestingly, functional imaging studies of chronic pain support this link between chronic pain and emotional changes: in patients with chronic pain, there are significant alterations in activity in CNS regions that comprise emotional circuitry (Becerra et al., 2006; Geha et al., 2008). Currently, relatively few physicians are trained to deal with emotional aspects of pain. Aside from enhancing the role of psychology in chronic pain treatment a suggestion for enticing greater involvement of psychiatry into pain has been suggested (see (Elman et al., 2011)), and in including individuals, especially psychologists, trained in this domain in adult and pediatric chronic pain treatment facilities (Carter and Threlkeld 2012; Roditi and Robinson 2011). However, all physicians treating chronic pain patients should be trained in dealing with emotional and cognitive aspects of pain. Indeed, an increasing number of pain physicians, including anesthesiologists, have acquired competence in cognitive behavioral therapy. This is the case at least in Northern Europe.
Centralization of Pain
In chronic pain, initial sensory events following nociception (e.g., following trauma) alter the CNS in a progressive manner resulting in pain that is amplified or occurs in the absence of peripheral nociception. This is termed ‘centralization of pain’ and these CNS alterations may also cause more complex behaviors. Pain may produce alterations in emotional and cognitive processing that result in depression and anxiety; similarly, conditions such as depression may produce or enhance or predispose the development of chronic pain, suggesting these systems are linked in a reciprocal manner. Recent neuroimaging studies have documented significant alterations in brain function, structure and chemistry across a variety of chronic pain conditions including CRPS (Geha et al., 2008; Lebel et al., 2008; Seifert et al., 2009), fibromyalgia (Harris et al., 2009; Napadow et al., 2010), neuropathic pain (Becerra et al., 2006; DaSilva et al., 2008; Lebel et al., 2008), migraine (Borsook and Hargreaves 2010; Prescot et al., 2009), and painful diabetic neuropathy (Cauda et al., 2009). CRPS is a signature example of a pain disease in which a single event results in a cascade of changes. A seemingly trivial injury to a peripheral nerve or tissue that may be hard to define (e.g., stretch injury following twisting of an ankle) results in: (i) progressive pain that incorporates not only the local area, but may spread to involve the limb or contralateral body (van Rijn et al., 2011)); (ii) alterations in autonomic function (Vogel et al., 2010); (iii) potential manifestation of movement disorders or dystonia (van Rijn et al., 2007); (iv) changes in perception such as hemi-neglect (Galer et al., 1995); and (v) anxiety and depression (Rommel et al., 2005). Together, these studies support the notion that pain results in abnormal alterations in different neural networks, including sensory, emotional (notably reward and aversion), cognitive, and modulatory systems. Transformation from a healthy state to one that limits a patient’s ability to work and enjoy life, complicated by the appearance and evolution of co-morbid psychiatric manifestations (e.g., addiction), may result in a negative cascade of failure of systems that worsen the condition, launching a negative feed-forward cycle. Thus, it is important to define the chronification of pain and develop treatments that halt or reverse it at the earliest possible time. In this regard, there are a number of insights garnered from our clinical experience that may be useful in considering how best to improve CRPS treatment. These include (a) early recognition or at least concern that an injury may lead to the condition and to educate the patients and have continued oversight on the potential evolution of the pain condition; (b) to apply early treatments, particularly physical therapy; and (c) to have intensive treatment approaches that target components of the disorder (motor, cognitive, sensory etc.) that have been very successful in the pediatric CRPS population (Logan et al., 2012; Simons et al., 2012) and in adults with chronic back pain (Artner et al., 2012). Clearly, further high quality studies are required.
Channeling Scientific Advances into the Clinic
Most current therapeutic approaches to chronic pain were formulated, informed and put into practice based on clinical observations rather than an understanding of the biology of chronic pain. For the most part, we have little mechanistic understanding of how analgesic interventions work apart from non-steroidal anti-inflammatory analgesics and opioids. For example, little is known about the mechanism of action (MOA) of most CNS-acting drugs used for pain. The use of spinal or central stimulation has no defined mechanistic scientific basis but how pain is actually modulated is still under investigation (Smits et al., 2012). Also acupuncture is widely used, but there is no known MOA. Progress in chronic pain care requires knowledge-based formulations that take into account the biological, biobehavioral, and societal aspects of chronic pain. Novel approaches are being evaluated that utilize current treatment options in new ways. One example is a cumulative clinical approach to back pain, in which treatment escalates with the addition of new interventions to existing ones as pain increases: physical therapy (PT) for mild back pain, PT + pharmacotherapy (P) for moderate back pain, and PT + P + cognitive behavioral therapy (CBT) for more severe back pain. These approaches take into account how chronic pain progresses to affect greater areas of the central nervous system and how more complex treatments may be required to treat a more complex disease state (Hill et al., 2011). Such insights suggest that treatments should be evaluated in studies for disease modification. A potential mechanism for CBT is that it increases frontal lobe activation (involved in a number of pain related processes including pain modulation; interactions on anxiety etc.) (Jensen et al., 2012). One way to evaluate the benefit is how components of different treatments may provide additive or interim benefit for patients to move along the treatment continuum that includes pain control, coping and adapting to normalizing their lifestyle. Given interindividual differences, in response to pain treatments including CBT (Linton and Andersson 2000) As with many treatments, some behavioral therapies may have a differential benefit across different pain conditions and individuals.
A number of important scientific advances have been made that could enhance clinical assessment and treatment of pain. Chronic pain is increasingly acknowledged as a dysfunction of the nervous system whether initiated by peripheral damage (e.g., neuropathic pain, arthritis) or as a manifestation of central changes (e.g., fibromyalgia). Recent discoveries of the transient receptor potential (TRP) channels (Krause et al., 2005) and sodium channelopathies as well as findings from brain imaging studies (Davis et al., 2011; Seifert and Maihofner 2011) have provided insight into chronic pain processing. Mutations in sodium channel genes cause a broad spectrum of effects on pain processing, ranging from complete lack of pain (missense mutations) to the severe paroxysmal pains that occur in erythromelalagia (activating mutations) (Reimann et al., 2010).
Transformation from Acute to Chronic Pain – Mechanisms of Pain Chronification
Understanding the transformation from acute to chronic pain (Voscopoulos and Lema 2010; Woolf 2011) is a current initiative of the National Institutes for Health (http://grants.nih.gov/grants/guide/rfa-files/RFA-DE-12-003.html). Several common clinical syndromes provide models for studying the process of “chronification” of pain including the transition from acute to chronic back pain (Henschke et al., 2008), acute postoperative pain to chronic neuropathic pain (Katz and Seltzer 2009; Kehlet et al., 2006), and episodic migraine to chronic daily headache (Bigal and Lipton 2011). It is particularly important to identify therapeutic targets that might halt the progression to chronic pain state. To date, animal studies have demonstrated effective preemptive analgesia, but most studies in humans have not been successful. It seems likely that the failure to reliably demonstrate pre-emptive analgesia in humans is due to individual variations in epigenetic and genetic factors or in specific changes in brain systems or underlying behavioral phenomena. The importance of behavioral phenotypes in development of chronic pain is strongly suggested by the finding that one of the most accurate predictors of chronic pain following surgery is catastrophizing (Peters et al., 1990). By identifying the risk factors and patients at the highest risk (Sipila et al., 2012) aggressive preventive interventions can be studied/introduced. Usually analgesics only damp done the symptom of pain; for example, in animal studies the TRPA1 antagonists not only relieve DM-related pain but also modify the disease (DM) by preventing toxic metabolites acting on the TRPA1 to cause pain and damage the nerves (and other organs!) (Koivisto et al., 2012).
Responders vs. Non-responders – Defining the Pain Pheno-genotype
As discussed above, meta-analyses of clinical outcome studies reveal that, for most chronic pain therapies studied in a controlled fashion, there are distinct groups of responders and non-responders. The ability to identify responders and non-responders in clinics and in clinical trials should greatly improve treatment outcomes (Baron et al., 2012; Borsook et al., 2011a). Understanding the mechanisms underlying effective treatments should help identify potential markers for responder populations. Recent studies have begun to address this by segregating pain phenotypes (Scholz et al., 2009).
While research in pain genomics has not yet provided a treatment for chronic pain patients, it has begun to identify genes that may participate in the development of post-surgical chronic pain or determine individual responses to drugs. In addition, genetic markers of psychiatric co-morbidities have been defined that are clear enough to be developed for use in the clinic. For example, allelic characterization of GTP cyclohydrolase (GCH1) (Tegeder et al., 2006) and characterization of K-channel alleles (Costigan et al., 2010) may be effective for pre-surgical identification of patients at increased risk for development of chronic post-surgical chronic pain. Genes may predict chronic neuropathic pain after surgery (Nissenbaum et al., 2010; Omair et al., 2012) and there are now increasing insights into other genes that are associated with chronic pain. The latter include genetic variation the association of the Catechol-O-methyltransferase (COMT) gene polymorphisms are found in chronic pain conditions (Fernandez-de-las-Penas et al., 2011; Tammimaki and Mannisto 2012) and acid-changing allele KCNS1 that seems to predict multiple chronic pain states (Costigan et al., 2010). Other genetic markers for chronic pain include altered dopamine receptors in burning mouth syndrome (Hagelberg et al., 2003b) and in facial pain (Hagelberg et al., 2003a); such markers provide evidence for advances in understanding the underlying mechanisms and can provide ideas for future therapies. Finally, use of pharmacogenomics in providing a more focused, individualized treatment can improve responsiveness that has mostly focused on opioid systems (Tremblay and Hamet 2010).
Modulating Brain Systems
The chronic pain disease state causes alterations in brain circuitry that manifest as a behavioral phenotype (i.e., specific symptoms and signs to a class or subclass of chronic pain). Looking for a treatment that can reset these systems to baseline seems a reasonable approach to take in the search for a cure for chronic pain. Effective medications may alter connectivity by modulating synaptic connections, leading to modification of neural networks as may be the case for antidepressants (Castren 2004; Malberg and Blendy 2005). The few studies that have addressed this issue in a robustly controlled manner suggest that this approach is promising and deserves further investigation. High doses of ketamine (Becerra et al., 2009; Finch et al., 2009; Schwartzman et al., 2009; Sigtermans et al., 2009), electroconvulsive therapy (Suda et al., 2008; Suzuki et al., 2009), transcranial magnetic stimulation (TMS) (Sampson et al., 2011) and deep brain stimulation (Bittar et al., 2005) all have dramatic effects on CNS circuitry, which may serve a “reset” function, either by delivering strong and widespread non-specific activation (ECT, TME, deep brain stimulation) or by inhibiting activity in affected systems for long enough to interrupt pathological feed-forward mechanisms that reinforce chronic pain (ketamine).
Natural Rectifiers – The Biology of Endogenous Systems and the Undoing of Pain
Why do many chronic pain conditions either improve over time or resolve spontaneously? Understanding spontaneous resolution could clearly be a game changer for the field. Some recent advances focus on this, including the recent discovery of endogenous pro-resolving entities (resolvins) that reverse and possibly prevent chronic pain (Xu and Ji 2011). Studying the placebo response may identify additional endogenous pathways for analgesia or reversal of pain. The placebo response has a strong biological foundation in pain neurobiology (Benedetti et al., 2011; Carlino et al., 2011; Petrovic et al., 2002). Understanding why some individuals can activate a strong placebo response whereas others cannot, and thus should be an important field of research to understand interindividual variation in therapeutic response. However, eliciting the placebo response in patients as a clinical treatment has been a challenge. The response to any treatment is believed to be a complex integration of the biological effects of the treatment with effects triggered by the individual’s thoughts, feelings, and prior experiences, which may decrease (placebo) or worsen (nocebo) pain. The placebo response provides powerful evidence of how the brain may ‘manipulate’ our responses to pain (Colloca and Benedetti 2007). The therapeutic interaction, an important constituent of the placebo response, should be used as part of all therapies as a positive enhancer. This approach is based on a trusting patient-physician (or other therapist) interaction and minimizing factors that increase anxiety and uncertainly in the patient.
Society and Chronic Pain
Chronic pain affects individuals of all ages across the socioeconomic spectrum (Breivick H 2006; Breivik 2012; Gupta et al., 2010; Phillips and Harper 2011; Sleed et al., 2005). A recent report indicates that 19 percent individuals with chronic pain in Europe are unable to work as a result of their chronic pain. It has become imperative for governments to respond in a more defined manner to manage this epidemic (Langley 2011; Reid et al., 2011). Chronic pain is linked to psychosocial risk factors, low education and poverty. Unhealthy diet, smoking, unemployment, low physical exertion at work, and obesity all increase risk for chronic widespread pain (Vandenkerkhof et al., 2011).
Much has been written about chronic pain and costs to society. Both US and European figures are staggering – in the billions of dollars (Ferrari 1998). A recent Institute of Medicine (IOM) report concluded that the economic toll of chronic pain is estimated at $635 billion a year in medical treatment and productivity in the United States (www.iom.edu/Reports/2011). Similarly high costs have been documented for Europe (Badia et al., 2004; Pradalier et al., 2004). In the United States for example, the cost of pain was greater than the annual costs of heart disease ($309 billion), cancer ($243 billion), and diabetes ($188 billion) (Gaskin and Richard 2012). Furthermore, in a recent report, the ‘direct and indirect costs of patients with a diagnosis related to chronic pain’ was assessed in nearly a million patients in Sweden (Gustavsson et al., 2012). The data show a staggering cost of 32 billion EUR per year (equal to 20% of the total tax burden in Sweden in 2007 or about a tenth of the total Swedish GNP). Similar costs in other European countries have been reported (Lambeek et al., 2011; Raftery et al., 2012). Patients, providers, developers of treatments (e.g., drug companies), and government regulators all play essential roles in the quest to improve care for chronic pain conditions. Integrating the efforts of these groups is important for progress in this area, but currently, there is little cross-fertilization of ideas and findings. It is the severity of the symptom, in this case pain that has an impact on the quality of life or function of the individual rather than the diagnosis itself, e.g. osteoarthritis. Therefore, it is important to classify pain as a significant co-morbidity or symptom in the new ICD-11. Another important factor to be considered is the patient’s capacity to cope with the symptom (pain).
Patient Advocacy - Partners in the search for improved treatment
Perhaps more than in any other disease, our insights into chronic pain come from our patients who can also play a significant role in changing policy, research priorities, and public perception of chronic pain (e.g., American Pain Foundation (www.painfoundation.org); The American Chronic Pain Association (theacpa.org/33/SupportGroups.aspx)). The impact of patient advocacy for AIDS is an excellent example of how a group of patients pushed the political and research system into highly effective action (Cecchi 1986). Independent groups advocating for patients with chronic pain do exist (e.g., CRPS (Reflex Sympathy Dystrophy Syndrome Association (www.rsds.org), Spinal Cord Injury (National Spinal Cord Association (www.spinalcord.org), Fibromyalgia (The National Fibromyalgia & Chronic Pain Association (NFMCPA) (theacpa.org/33/SupportGroups.aspx)), but others such as the Europe Against Pain initiative are at a national process (www.iapsar.org/Europe_against_pain.htm). These groups provide support, lobby government, and in some cases fund research. Advocacy is an important avenue to improve pain treatment (Sessle 2012). It has been shown to be useful at state (Dahl et al., 2002) and consumer levels. In Europe, both EU and national pain advocacy groups are present; for example, a recent alliance, initially representing 11 countries, was formed to lobby for 100 million pain patients (Pain Alliance, Europe; www.pae-eu.eu/). In the USA a number of advocacy groups specific to a disease state (e.g., Reflex Sympathetic Dystrophy Syndrome Association www.rsds.org; National Fibromyalgia Association or www.fibromyalgiahcp.org) or a more widespread program (e.g., American Pain Foundation; www.painfoundation.org/) are active. Other programs such as CHANGEPAIN has been formed “to enhance the understanding of patients who suffer from severe chronic pain and to improve pain management” (Varrassi et al., 2011).
Clinicians –Treating Chronic Pain with a Limited Therapeutic Armamentarium
Pain is the most common symptom in clinical practice. Independent of drivers such as a financial imperative for providers, the challenges of treating chronic pain are enormous for clinicians, whether they are in general practice or specialize in pain or in diseases in which pain is highly prevalent. Chronic pain patients are complex and even evaluation of their condition usually requires more time than is practicable in modern medical practice. Because of this and because of the lack of good treatment options, specialties that have a significant contribution to make, such as psychiatry (Elman et al., 2011) and neurology (Borsook 2011), tend to focus on other areas. Coordination and evaluation of care are confounded when multiple approaches with multiple providers overlap. The psychological impact on clinicians of focusing on chronic pain cannot be ignored. The reward of successful treatment is rare in this field – it is not uncommon for patients to try all available approaches and still lack significant relief, sending them into an orbit of helplessness or defeat. What do clinicians say when we have nothing more to offer? Multidisciplinary approaches may have more to offer, but, as mentioned above, we need more information about individual and combined methods and how they affect individual patients (see Allostatic Load below). Personalized medicine should be considered as a systematic approach to finding the most effective therapy for the individual patient based on evidence, data on interindividual variation and understanding the patient as an entity covering genes, pathophysiology of the disease, mood, coping capacity, motivation, and social aspects. Accepting the limits of current therapies is also beneficial not only for the patient but also for the physician.
The Pharmaceutical Industry
The pharmaceutical industry has been a major player in the development of new and effective therapies. While many large pharmaceutical companies such as Pfizer and Astra Zeneca have dismantled their pain research units, advances in the initial development of new pharmacotherapies will be left to academic centers and biotechnology companies. The risk/benefit ratio has been very high as few CNS drugs, including analgesics, successfully complete phase III trials (Pammolli et al., 2011). This is understandably troubling for biopharmaceutical companies, which are backing away from research into chronic pain. The development of new chemical entities for chronic pain treatment is at risk and is currently being carried by biotechnology. However, without big pharmaceuticals, who will carry the costs of clinical development? New approaches to address the current impasse in drug development have been suggested by industry (Munos and Chin 2011) and academia (Grootendorst 2009; Woolf 2010). These include the establishment of precompetitive, academic or academic-industry consortia, of which the London Pain Consortium (http://www.lpc.ac.uk), the German Research Network on Neuropathic Pain (www.neuro.med.tu-muenchen.de/dfns) and the European Pain Consortium are good working examples. Similar developments are in process in the USA through initiatives such as Analgesic Clinical Trial Innovations, Opportunities, and Networks (ACTION) (Dworkin and Turk 2011), a public-private partnership designed to facilitate the discovery and development of analgesics with improved efficacy, safety, and tolerability for acute and chronic pain conditions (http://www.fda.gov/AboutFDA/PartnershipsCollaborations/PublicPrivatePartnershipProgram/ucm231130.htm). New methods that avoid duplication of efforts and unnecessary exposure of patients to drugs that may have already failed need to be implemented (Norman et al., 2011). Understanding the subpopulations of chronic pain patients (viz., defining the pain patients phenotypes accurately) we may be able to identify therapies that actually work in these cohorts (but not in all). For example, there is hope as some of the new analgesics may not only alleviate pain but also modify the underlying disease as maybe the case regarding TRPA1 antagonists in diabetic neuropathy (Koivisto et al., 2012).
Regulatory Agencies
Governmental regulatory agencies are primarily concerned with the safety of drugs and other therapies. However, therapies also must be shown to be effective in controlled trials. Aside from a shortage of new chemical entities, there are significant issues related to how drugs achieve regulatory acceptance. One of these relates to clinical trials achieving significance. Methodologies for decreasing variance are clearly useful but the following concerns arise. (i) Approval of “me-too” drugs that have similar actions and perhaps slightly improved side effect profiles or may be administered by different routes (e.g., intranasal) are not really helping the field move forward and expose increasing numbers of patients to trials without really enhancing efficacy. The problem with me-too drugs is that they generally do not enhance efficacy compared with currently available treatments. (ii) Approval of drugs with a significant trial outcome, but marginal increased efficacy. The FDA is just beginning to address this with a number of initiatives in the analgesic domain to try to enhance the approval process (Woodcock 2009; Woodcock et al., 2007). Specific changes include improving trial design. Clinician researchers should be active in creating and developing new meaningful trial designs that take into account the advances in pain research and individual response variation. This, however, cannot be possible without significant support from the governments. These new trial designs could be first tested on old, cheap, and effective drugs such as amitriptyline before using them to study new molecules.
Public Policy – Chronic Pain should be a National Agenda
Given the high incidence of chronic pain, the related problems of addiction, the high cost and relative ineffectiveness of treatment, and limited access to health care for many, pain must be considered a public health care issue (Blyth 2008) and addressed as such. New public policies are needed to address the impact of chronic pain on society (Jordan et al., 2008). At the request of the Department of Health and Human Services, National Institutes of Health, the Institute of Medicine (IOM) recently addressed the current state of pain research, care, and education, and explored approaches to advance the field of pain (www.iom.edu/Reports/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research.aspx). We are hopeful that this report, which “offers a blueprint for action in transforming prevention, care, education, and research, with the goal of providing relief for people with pain in America” will provide the nidus of a new and aggressive public health policy in basic and clinical research to bridge the gap that currently exists in the field. A number of pain initiatives have also been undertaken in Europe. The European Federation of IASP Chapters (EFIC) is a multidisciplinary professional organization in the field of pain research and medicine (www.efic.org). In addition, the initiative Pain-in-Europe (paineurope www.painineurope.com) provides all healthcare professionals within Europe with a diverse range of resources to further their knowledge of effective pain management. European initiatives like “Societal Impact of Pain (SIP)”, the “Road Map for Action” that have been proclaimed in the EUParliament by the “European Federation of IASP Chapters (EFIC)”, and the data provided by the published Proceedings of the SIP Symposia 2010, 2011 and 2012 (www.efic.org). Such initiatives have also been very contributory to further developments. For example, the annual European Week against Pain, first launched in 2001, developed into the Global Day against Pain in 2004. Such approaches clearly increase the awareness of the problem.
Going Forward – Towards Targeted Integrated Approaches
Improving pain treatment requires advances at several levels. First, we need a better understanding of the neuroscience underlying chronic pain. Second, we need to develop evidence-based approaches that recognize and target individual variations in pain processing and support them with changes in societal and governmental processes. An important step towards all of this is improved evaluation of existing and new treatments and the pressing need for a better foundation in outcome measures. Objective measures of pain would enable the process. As noted in Figure 3B, multiple factors (biological, behavioral, the position of a patient in the social ecosystem) may contribute to the overall pain state. Systematic evaluation of integrating approaches by combining drugs and cognitive behavioral therapy, lifestyle change etc. would seem a necessary and important opportunity to improve chronic pain in individuals. It is also important not signal overoptimistic goals such as freedom from pain is every patient’s right if that is not realistic.
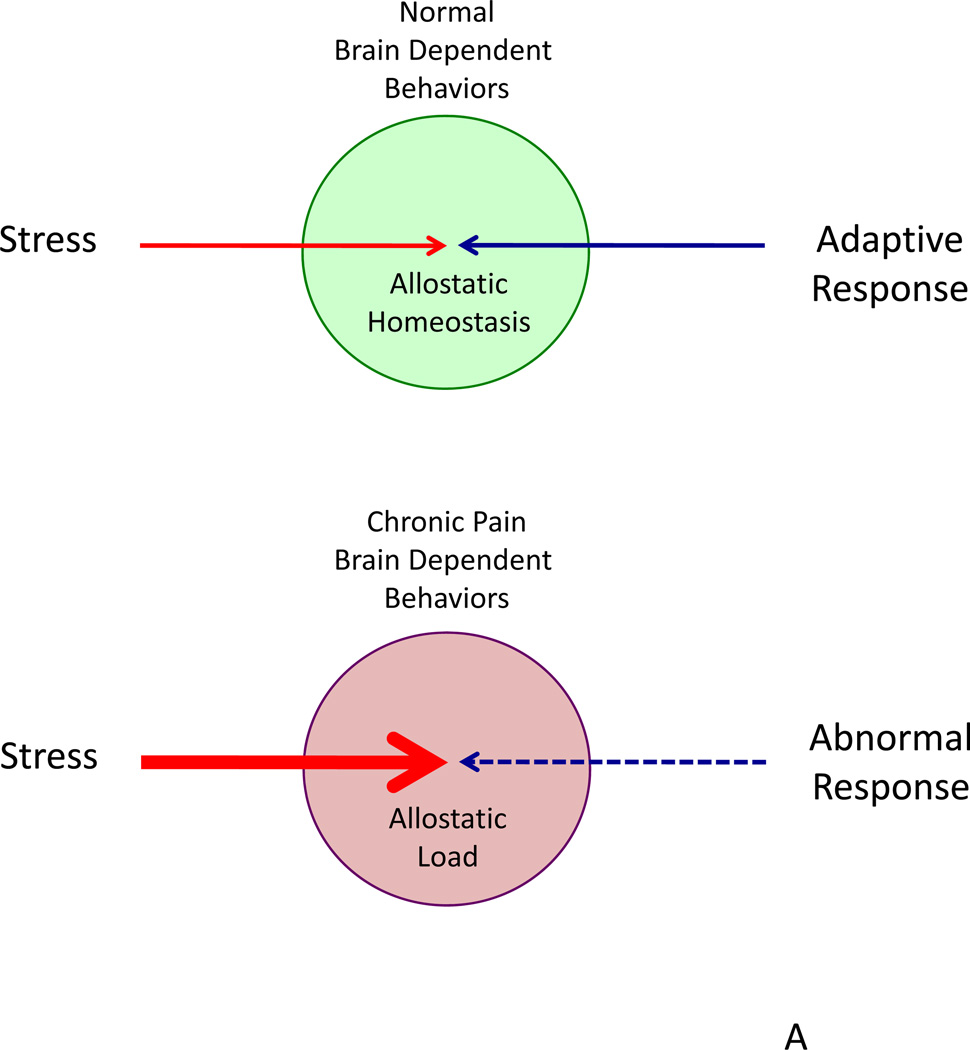
Figure 3. Brain Dependent Changes in Chronic Pain.
Top: A model of stress and allostasis in chronic pain. Under normal conditions, adaptive responses occur to a stressor (e.g., nociceptive pain). However, with stressors that results in ongoing process (e.g., neuropathic pain), responses become abnormal and maladaptive with changes in brain dependent behaviors (e.g., onset of depression or depression induced chronic pain).
Bottom: The figure shows containment and normal adaptive processing to various stressors (noted below in the key); these normal responses are balanced and adaptive (adapt to ‘homeostatic set-point’) over time. In chronic pain responses may be exaggerated (out of ‘homeostatic set-point’) or inhibited. In a multidimensional biological process such as chronic pain each of these stressors may affect an individual differently as represented in the ‘bar-code’ noted on the right.
Objective Measures, Pain Phenotyping and Outcome Studies
Current attempts at providing objective measures or biomarkers for chronic pain (Marchi et al., 2009) include imaging (Borsook et al., 2011a; b), and genetic studies like those defined for migraine subtypes (Chasman et al., 2011; De Vries et al., 2006; Lafreniere et al., 2010) and for specific pain subtypes such as fibromyalgia (Ablin et al., 2009). An important part of defining objective measures and outcomes relates to better phenotyping pain patients (Fourney et al., 2011; Knoop et al., 2011; Mahal et al., 2011; Scholz et al., 2009). This may include molecular (e.g., burning mouth (Hagelberg et al., 2003b)) and genetic profiling (e.g., a recent study demonstrated significant association between an allele within a gene (KCNS1) encoding a potassium channel (Kv9.1) and increased risk for chronic pain (Costigan et al., 2010)). While waiting for these areas to develop the psychosocial profiling of the patient could be very useful regarding expected outcome. Regarding outcomes, pain intensity is clearly insufficient unless combined with measures of quality of life including physical and psychosocial functioning. Evaluation of new approaches to prevention of pain includes modualation of inflammatory cells (e.g., T-lymphoyctes) that may provide and exciting opportunity to inhibit surgically induced neuropathic pain (SNPP) (Costigan et al., 2009).
Physician Education
A majority of primary care physicians continue to be noncompliant with evidence-based guidelines for pain conditions such as chronic back pain (Webster et al., 2005). In addition, decreasing segmentation of care is important to improve outcome for chronic pain patients. The ideal pain clinician would have expertise in pain neurobiology, behavioral medicine (including psychiatry/psychology), pharmacology and interventional procedures. Integrative care should target treatments for both symptom management and disease modification (see TRPA1 above) and be specific to individual patients. In addition, greater cross-fertilization between clinicians and basic researchers would advance care: one of the missing links in current pain treatment is the lack of greater involvement of basic scientists in the clinics at academic centers (Plebani and Marincola 2006; Segal et al., 2011).
Integrating Quality of Life Measures
Most Health Related Quality of Life (HRQoL) studies are epidemiological in nature (Lidal et al., 2008; Loyland et al., 2010; Petersen et al., 2009; Peuckmann et al., 2009; West and Wallberg-Jonsson 2009; Widar et al., 2004). There are important implications of understanding HRQoL outcomes including understanding long-term suffering, implications for prevention and rehabilitation, and social issues pertaining to relatives and dependent individuals. However, it is argued that a minimum set of such HRQoL data is necessary to contribute to informed decisions in clinical practice (Efficace et al., 2003). HRQoL is also increasingly used in outcome research (Becker et al., 2000; Heiskanen 2012; Schaufele and Boden 2003; Working Group on Health Outcomes for Older Persons with Multiple Chronic 2012)
Integrative, Measurable, Treatment Approaches for Personalized Medicine
While initial attempts at personalized medicine for chronic pain may be tailored to the molecular and genomic profile of an individual, other issues including systems biology, psychological manifestations, therapeutic interaction (placebo response) and social issues all contribute to the chronic pain state and need to be considered. The so-called bio-psycho-social model has evolved to try to evaluate and treat chronic pain in this context (Guzman et al., 2006). Future integrative approaches need to be done in the context of defined, quantitative, or objective measures (e.g., phenotype, genetic, imaging). In this way therapies can be directed not just at individual variations in susceptibility but at specific CNS processes in pain. Specific brain-directed therapies may include medications, brain modulation (e.g., TMS), and cognitive training. Understanding factors that set individual risk levels for chronic pain, including genetic markers and psychological phenotypes such as anxiety, fear avoidance (Hasenbring and Verbunt 2010) and catastrophizing (Edwards et al., 2011), is central to improving pain management. The model of allostatic load may be useful for conceptualizing the interplay of these factors for individual patients.
Allostatic Load
Allostasis refers to the process by which systems maintain balance in response to stressors and other inputs. Allostatic load (McEwen and Gianaros 2011) occurs when repeated stressors alter the normal response of physiological systems. In the case of chronic pain, allostatic load may alter brain networks both functionally and structurally (McEwen and Gianaros 2011), resulting in abnormal responses to environmental conditions (psychological or physiological) (Juster et al., 2010; McEwen and Gianaros 2010) (see Figure 3A and 3B). The concept has been applied to other brain centered disease such as mood disorders (McEwen 2003) and migraine (Borsook et al., 2012). The allostatic load model suggests that removing individual components that contribute to overall ‘stress’ may mitigate the severity of other ongoing processes. For example, treating depression may increase activity and decrease pain levels. Clearly, meaningful scales and measures that are not limited to pain intensity should be integrated into evaluation and monitoring of chronic pain. One concrete example of basing practice on neuroscience is using genetic and psychological markers to identify patients with high risk of post-operative chronic pain before surgery. Other examples may include the use of imaging to visualize indicators of important deleterious or beneficial changes to a patient’s condition that may be present ahead of subjective awareness so that objective measures for measuring pain may decrease intraoperative nociception and potentially long-term chronic neuropathic pain. Specific examples include gray matter morphometry (Gustin et al., 2011; Gwilym et al., 2010; Seminowicz et al., 2011) and measures of brain resting state networks (Borsook and Becerra 2011; Cauda et al., 2010; Malinen et al., 2010) compared with normal baseline datasets. Critical to any evaluation of the individual is a full understanding of brain networks that contribute to the complex symptoms in chronic pain including anxiety, depression, catastrophizing, and sleep-related issues. A focus on brain systems in the individual with chronic pain would allow for a rational, targeted, and measured approach to evaluation and treatment options. In addition, as new technologies or discoveries become available, these can be seamlessly integrated into this approach to the individual patient.
Research Funding for Chronic Pain
In the United States, the National Institutes of Health (NIH) and National Science Foundation (NSF) have been the predominant funders of pain research. The incentive for further pain research has been supported by a recent Institute of Medicine report (http://www.iom.edu/Reports/2011). In Europe and the UK, programs such as the Wellcome Trust (www.wellcome.ac.uk) or funding for pain research in Germany and France have provided significant support. Companies still provide investigator-initiated grants for innovative pain research. Perhaps some of the more exciting programs relate to those where translation of pain research to the clinic is a major aim. Research initiatives included the EU 7th Framework Programme for Research (FP7) 2007 (FP7) that aims to “improve the health of the European citizens and increasing the competitiveness of the European health=−related industries and businesses” (http://www.sip-platform.eu/global-pain-news) currently has a special call for pain.
Conclusions
New approaches based on the biopsychosocial model will be critical to success in treating chronic pain and applying modern science to the complex behaviors resulting from chronic pain. A better understanding of how pain systems modulate affective and emotional processing is therefore of paramount importance in advancing therapeutic success. A number of models exist to achieve these goals but we believe the ideal model is a competitive center of excellence that integrates proven multidisciplinary clinical approaches with research.
To improve patient care, the practice of pain medicine must embrace the development of a new evidence-based therapeutic model that recognizes the highly individual nature of responsiveness to pain treatments, integrates bio-psycho-behavioral approaches, and requires proof of clinical effectiveness for the various treatments we offer our patients (Figure 4). Transforming pain medicine will be no easy task since it impacts on research and public policy, and requires significant efforts in outcome data to move the field forward. The challenge is large, but the capacity to define novel approaches through ongoing research is present, the societal commitment is growing, and the opportunity to improve the lot of chronic pain patients has never been so promising. Lets do it.
Figure 4. Integrative Personalized Medicine for Chronic Pain.
For each chronic pain patient the process of Diagnosis, ‘Treatment’ and ‘Follow-up’ need to be integrated in a manner that optimizes specificity of diagnosis (including phenotype, predictors of drug responsiveness etc.) and objective measures of treatment efficacy.
Acknowledgments
Funding Sources: Support was provided by a Grant to DB from NINDS (K24) and the Herlands Fund for Pain Research
References
- Ablin JN, Buskila D, Clauw DJ. Biomarkers in fibromyalgia. Curr Pain Headache Rep. 2009;13:343–349. doi: 10.1007/s11916-009-0056-3. [DOI] [PubMed] [Google Scholar]
- Antal A, Paulus W. Transcranial magnetic and direct current stimulation in the therapy of pain. Schmerz. 2010;24:161–166. doi: 10.1007/s00482-010-0899-x. [DOI] [PubMed] [Google Scholar]
- Artner J, Kurz S, Cakir B, Reichel H, Lattig F. Intensive interdisciplinary outpatient pain management program for chronic back pain: a pilot study. Journal of pain research. 2012;5:209–216. doi: 10.2147/JPR.S31754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin JA. Why patients use alternative medicine: results of a national study. JAMA : the journal of the American Medical Association. 1998;279:1548–1553. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- Badia X, Magaz S, Gutierrez L, Galvan J. The burden of migraine in Spain: beyond direct costs. Pharmacoeconomics. 2004;22:591–603. doi: 10.2165/00019053-200422090-00004. [DOI] [PubMed] [Google Scholar]
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol. 2006;24:5457–5464. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet neurology. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2009 doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- Becker N, Sjogren P, Bech P, Olsen AK, Eriksen J. Treatment outcome of chronic non-malignant pain patients managed in a danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Pain. 2000;84:203–211. doi: 10.1016/s0304-3959(99)00209-2. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, Charney DS, Boutros NN. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- Bigal M. Migraine chronification--concept and risk factors. Discovery medicine. 2009;8:145–150. [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Migraine chronification. Curr Neurol Neurosci Rep. 2011;11:139–148. doi: 10.1007/s11910-010-0175-6. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Blyth FM. Chronic pain--is it a public health problem? Pain. 2008;137:465–466. doi: 10.1016/j.pain.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Borsook D. Neurological diseases and pain. Brain. 2011 doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L. How close are we in utilizing functional neuroimaging in routine clinical diagnosis of neuropathic pain? Curr Pain Headache Rep. 2011;15:223–229. doi: 10.1007/s11916-011-0187-1. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discovery medicine. 2011a;11:197–207. [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: how, where, and what to look for using functional imaging. Discov Med. 2011b;11:209–219. [PubMed] [Google Scholar]
- Borsook D, Edwards AD. Antineuropathic effects of the antibiotic derivative spicamycin KRN5500. Pain medicine. 2004;5:104–108. doi: 10.1111/j.1526-4637.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R. Brain imaging in migraine research. Headache. 2010;50:1523–1527. doi: 10.1111/j.1526-4610.2010.01761.x. [DOI] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding Migraine through the Lens of Maladaptive Stress Responses: A Model Disease of Allostatic Load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bras M, Dordevic V, Gregurek R, Bulajic M. Neurobiological and clinical relationship between psychiatric disorders and chronic pain. Psychiatr Danub. 2010;22:221–226. [PubMed] [Google Scholar]
- Braune S. Evidence-based pharmacotherapy of neuropathic pain syndromes. MMW Fortschr Med. 2004;146:49–51. [PubMed] [Google Scholar]
- Breivick HCB, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Breivik H. A major challenge for a generous welfare system: a heavy socio-economic burden of chronic pain conditions in Sweden--and how to meet this challenge. European journal of pain. 2012;16:167–169. doi: 10.1002/j.1532-2149.2011.00025.x. [DOI] [PubMed] [Google Scholar]
- Breivik H, Stubhaug A. A new treatment principle for neuropathic pain? Approved oncologic drugs: Epidermal growth factor receptor (EGFR) inhibitors dramatically relieve severe neuropathic pain in a case series. Scandinavian Journal of Pain. 2013;4:1–2. doi: 10.1016/j.sjpain.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Carlino E, Pollo A, Benedetti F. Placebo analgesia and beyond: a melting pot of concepts and ideas for neuroscience. Curr Opin Anaesthesiol. 2011;24:540–544. doi: 10.1097/ACO.0b013e328349d0c2. [DOI] [PubMed] [Google Scholar]
- Carter BD, Threlkeld BM. Psychosocial perspectives in the treatment of pediatric chronic pain. Pediatric rheumatology online journal. 2012;10:15. doi: 10.1186/1546-0096-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ML. Spinal cord stimulation in chronic pain: a review of the evidence. Anaesth Intensive Care. 2004;32:11–21. doi: 10.1177/0310057X0403200102. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi RL. Health care advocacy for AIDS patients. QRB Qual Rev Bull. 1986;12:297–303. doi: 10.1016/s0097-5990(16)30062-8. [DOI] [PubMed] [Google Scholar]
- Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AM, Fendrich K, Volzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RY, Kurth T. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med. 2003;138:898–906. doi: 10.7326/0003-4819-138-11-200306030-00011. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu T, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Gershon E, Livneh J, Shen PH, Nikolajsen L, Karppinen J, Mannikko M, Kelempisioti A, Goldman D, Maixner W, Geschwind DH, Max MB, Seltzer Z, Woolf CJ. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain : a journal of neurology. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JL, Bennett ME, Bromley MD, Joranson DE. Success of the state pain initiatives: moving pain management forward. Cancer practice. 2002;10(Suppl 1):S9–S13. doi: 10.1046/j.1523-5394.10.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Anastakis DJ. Nerve injury triggers changes in the brain. Neuroscientist. 2011;17:407–422. doi: 10.1177/1073858410389185. [DOI] [PubMed] [Google Scholar]
- De Vries B, Haan J, Frants RR, Van den Maagdenberg AM, Ferrari MD. Genetic biomarkers for migraine. Headache. 2006;46:1059–1068. doi: 10.1111/j.1526-4610.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- Dodrill CL, Helmer DA, Kosten TR. Prescription pain medication dependence. Am J Psychiatry. 2011;168:466–471. doi: 10.1176/appi.ajp.2010.10020260. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC. Accelerating the development of improved analgesic treatments: the ACTION public-private partnership. Pain Med. 2011;12(Suppl 3):S109–S117. doi: 10.1111/j.1526-4637.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'Haese S, Zurlo A. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials--does HRQOL evaluation in prostate cancer research inform clinical decision making? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Archives of general psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125:172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-las-Penas C, Ambite-Quesada S, Rivas-Martinez I, Ortega-Santiago R, de-la-Llave-Rincon AI, Fernandez-Mayoralas DM, Pareja JA. Genetic contribution of catechol-O-methyltransferase polymorphism (Val158Met) in children with chronic tension-type headache. Pediatric research. 2011;70:395–399. doi: 10.1203/PDR.0b013e318229448a. [DOI] [PubMed] [Google Scholar]
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics. 1998;13:667–676. doi: 10.2165/00019053-199813060-00003. [DOI] [PubMed] [Google Scholar]
- Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: A double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146:18–25. doi: 10.1016/j.pain.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- Fourney DR, Andersson G, Arnold PM, Dettori J, Cahana A, Fehlings MG, Norvell D, Samartzis D, Chapman JR. Chronic low back pain: a heterogeneous condition with challenges for an evidence-based approach. Spine (Phila Pa 1976) 2011;36:S1–S9. doi: 10.1097/BRS.0b013e31822f0a0d. [DOI] [PubMed] [Google Scholar]
- Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (Complex Regional Pain Syndrome-1) J Pain Symptom Manage. 1995;10:385–391. doi: 10.1016/0885-3924(95)00061-3. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. The journal of pain : official journal of the American Pain Society. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev Neurother. 2005;5:823–830. doi: 10.1586/14737175.5.6.823. [DOI] [PubMed] [Google Scholar]
- Grootendorst P. How should we support pharmaceutical innovation? Expert Rev Pharmacoecon Outcomes Res. 2009;9:313–320. doi: 10.1586/erp.09.34. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mehdi A, Duwell M, Sinha A. Evidence-based review of the pharmacoeconomics related to the management of chronic nonmalignant pain. J Pain Palliat Care Pharmacother. 2010;24:152–156. doi: 10.3109/15360281003713826. [DOI] [PubMed] [Google Scholar]
- Gustavsson A, Bjorkman J, Ljungcrantz C, Rhodin A, Rivano-Fischer M, Sjolund KF, Mannheimer C. Socio-economic burden of patients with a diagnosis related to chronic pain--register data of 840,000 Swedish patients. European journal of pain. 2012;16:289–299. doi: 10.1016/j.ejpain.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. WITHDRAWN: Multidisciplinary bio-psycho-social rehabilitation for chronic low-back pain. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD000963.pub2. CD000963. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, Nagren K, Eskola O, Jaaskelainen SK. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003a;106:43–48. doi: 10.1016/s0304-3959(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, Luutonen S, Nagren K, Jaaskelainen S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003b;101:149–154. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbring MI, Verbunt JA. Fear-avoidance and endurance-related responses to pain: new models of behavior and their consequences for clinical practice. Clin J Pain. 2010;26:747–753. doi: 10.1097/AJP.0b013e3181e104f2. [DOI] [PubMed] [Google Scholar]
- Heiskanen TR, R P, Kalso E. Multidisciplinary pain treatment - Which patients do benefit? Scand J Pain. 2012;3:199–240. doi: 10.1016/j.sjpain.2012.05.073. [DOI] [PubMed] [Google Scholar]
- Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke N, Ostelo RW, van Tulder MW, Vlaeyen JW, Morley S, Assendelft WJ, Main CJ. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD002014.pub3. CD002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, Konstantinou K, Main CJ, Mason E, Somerville S, Sowden G, Vohora K, Hay EM. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–1571. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopton AK, Macpherson H. Assessing blinding in randomised controlled trials of acupuncture: challenges and recommendations. Chin J Integr Med. 2011;17:173–176. doi: 10.1007/s11655-011-0663-9. [DOI] [PubMed] [Google Scholar]
- Houghton AD, Nicholls G, Houghton AL, Saadah E, McColl L. Phantom pain: natural history and association with rehabilitation. Ann R Coll Surg Engl. 1994;76:22–25. [PMC free article] [PubMed] [Google Scholar]
- Jannetto PJ, Bratanow NC. Pain management in the 21st century: utilization of pharmacogenomics and therapeutic drug monitoring. Expert Opin Drug Metab Toxicol. 2011;7:745–752. doi: 10.1517/17425255.2011.565051. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Jordan KD, Okifuji A. Anxiety disorders: differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother. 2011;25:231–245. doi: 10.3109/15360288.2011.596922. [DOI] [PubMed] [Google Scholar]
- Jordan KP, Thomas E, Peat G, Wilkie R, Croft P. Social risks for disabling pain in older people: a prospective study of individual and area characteristics. Pain. 2008;137:652–661. doi: 10.1016/j.pain.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kasai S, Ikeda K. Pharmacogenomics of the human micro-opioid receptor. Pharmacogenomics. 2011;12:1305–1320. doi: 10.2217/pgs.11.68. [DOI] [PubMed] [Google Scholar]
- Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–744. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kersten C, Cameron MG. Cetuximab alleviates neuropathic pain despite tumour progression. BMJ case reports. 2012;2012 doi: 10.1136/bcr.12.2011.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten C, Cameron MG, Mjåland S. Epidermal growth factor receptor (EGFR)-148 inhibition for relief of various neuropathic pain syndromes. A case series. Scandinavian Journal of Pain. 2013;4:3–7. doi: 10.1016/j.sjpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Knaster P, Karlsson H, Estlander AM, Kalso E. Psychiatric disorders as assessed with SCID in chronic pain patients: the anxiety disorders precede the onset of pain. Gen Hosp Psychiatry. 2012;34:46–52. doi: 10.1016/j.genhosppsych.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Knoop J, van der Leeden M, Thorstensson CA, Roorda LD, Lems WF, Knol DL, Steultjens MP, Dekker J. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2011;63:1535–1542. doi: 10.1002/acr.20571. [DOI] [PubMed] [Google Scholar]
- Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Akerman KE, Lindstedt K, Pertovaara A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;65:149–158. doi: 10.1016/j.phrs.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Konvicka JJ, Meyer TA, McDavid AJ, Roberson CR. Complementary/alternative medicine use among chronic pain clinic patients. Journal of perianesthesia nursing : official journal of the American Society of PeriAnesthesia Nurses / American Society of PeriAnesthesia Nurses. 2008;23:17–23. doi: 10.1016/j.jopan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Krause JE, Chenard BL, Cortright DN. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Curr Opin Investig Drugs. 2005;6:48–57. [PubMed] [Google Scholar]
- Kvarstein G, Mawe L, Indahl A, Hol PK, Tennoe B, Digernes R, Stubhaug A, Tonnessen TI, Beivik H. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy--a 12-month follow-up. Pain. 2009;145:279–286. doi: 10.1016/j.pain.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Lafreniere RG, Cader MZ, Poulin JF, Andres-Enguix I, Simoneau M, Gupta N, Boisvert K, Lafreniere F, McLaughlan S, Dube MP, Marcinkiewicz MM, Ramagopalan S, Ansorge O, Brais B, Sequeiros J, Pereira-Monteiro JM, Griffiths LR, Tucker SJ, Ebers G, Rouleau GA. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat Med. 2010;16:1157–1160. doi: 10.1038/nm.2216. [DOI] [PubMed] [Google Scholar]
- Lambeek LC, van Tulder MW, Swinkels IC, Koppes LL, Anema JR, van Mechelen W. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine. 2011;36:1050–1058. doi: 10.1097/BRS.0b013e3181e70488. [DOI] [PubMed] [Google Scholar]
- Langley PC. The prevalence, correlates and treatment of pain in the European Union. Curr Med Res Opin. 2011;27:463–480. doi: 10.1185/03007995.2010.542136. [DOI] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- Lidal IB, Veenstra M, Hjeltnes N, Biering-Sorensen F. Health-related quality of life in persons with long-standing spinal cord injury. Spinal cord. 2008;46:710–715. doi: 10.1038/sc.2008.17. [DOI] [PubMed] [Google Scholar]
- Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personalized medicine. 2011;8:161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, Jonas WB. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet. 1997;350:834–843. doi: 10.1016/s0140-6736(97)02293-9. [DOI] [PubMed] [Google Scholar]
- Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive-behavior intervention and two forms of information for patients with spinal pain. Spine. 2000;25:2825–2831. doi: 10.1097/00007632-200011010-00017. discussion 2824. [DOI] [PubMed] [Google Scholar]
- Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A Day-hospital Approach to Treatment of Pediatric Complex Regional Pain Syndrome: Initial Functional Outcomes. The Clinical journal of pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27:567–572. doi: 10.1097/BPO.0b013e318070cc4d. [DOI] [PubMed] [Google Scholar]
- Loyland B, Miaskowski C, Paul SM, Dahl E, Rustoen T. The relationship between chronic pain and health-related quality of life in long-term social assistance recipients in Norway. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19:1457–1465. doi: 10.1007/s11136-010-9707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007115.pub2. CD007115. [DOI] [PubMed] [Google Scholar]
- Mahal BA, Cohen JM, Allsop SA, Moore JB, Bhai SF, Inverso G, Dimitrakoff JD. The role of phenotyping in chronic prostatitis/chronic pelvic pain syndrome. Curr Urol Rep. 2011;12:297–303. doi: 10.1007/s11934-011-0196-y. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Seifert F, Markovic K. Complex regional pain syndromes: new pathophysiological concepts and therapies. Eur J Neurol. 2010;17:649–660. doi: 10.1111/j.1468-1331.2010.02947.x. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi A, Vellucci R, Mameli S, Rita Piredda A, Finco G. Pain biomarkers. Clin Drug Investig. 2009;29(Suppl 1):41–46. doi: 10.2165/0044011-200929001-00006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers-needed-to-treat analyses--do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo-controlled chronic low back pain trials. Pain. 2010;151:592–597. doi: 10.1016/j.pain.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Munos BH, Chin WW. How to revive breakthrough innovation in the pharmaceutical industry. Sci Transl Med. 2011;3:89cm16. doi: 10.1126/scitranslmed.3002273. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajsen L, Lindvig M. Phantom pain after amputation of extremities. Ugeskr Laeger. 2001;163:3338–3341. [PubMed] [Google Scholar]
- Nissenbaum J, Devor M, Seltzer Z, Gebauer M, Michaelis M, Tal M, Dorfman R, Abitbul-Yarkoni M, Lu Y, Elahipanah T, delCanho S, Minert A, Fried K, Persson AK, Shpigler H, Shabo E, Yakir B, Pisante A, Darvasi A. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome research. 2010;20:1180–1190. doi: 10.1101/gr.104976.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman T, Edwards A, Bountra C, Friend S. The precompetitive space: time to move the yardsticks. Sci Transl Med. 2011;3:76cm10. doi: 10.1126/scitranslmed.3002399. [DOI] [PubMed] [Google Scholar]
- Omair A, Lie BA, Reikeras O, Holden M, Brox JI. Genetic contribution of catechol-O-methyltransferase variants in treatment outcome of low back pain: a prospective genetic association study. BMC musculoskeletal disorders. 2012;13:76. doi: 10.1186/1471-2474-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- Peters MS, Thibodeau SN, White JW, Jr, Winkelmann RK. Mycosis fungoides in children and adolescents. J Am Acad Dermatol. 1990;22:1011–1018. doi: 10.1016/0190-9622(90)70143-6. [DOI] [PubMed] [Google Scholar]
- Petersen S, Hagglof BL, Bergstrom EI. Impaired health-related quality of life in children with recurrent pain. Pediatrics. 2009;124:e759–e767. doi: 10.1542/peds.2008-1546. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Moller S, Eriksen J, Sjogren P. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. European journal of pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Phillips CJ, Harper C. The economics associated with persistent pain. Curr Opin Support Palliat Care. 2011;5:127–130. doi: 10.1097/SPC.0b013e3283458fa9. [DOI] [PubMed] [Google Scholar]
- Plebani M, Marincola FM. Research translation: a new frontier for clinical laboratories. Clin Chem Lab Med. 2006;44:1303–1312. doi: 10.1515/CCLM.2006.238. [DOI] [PubMed] [Google Scholar]
- Pradalier A, Auray JP, El Hasnaoui A, Alzahouri K, Dartigues JF, Duru G, Henry P, Lanteri-Minet M, Lucas C, Chazot G, Gaudin AF. Economic impact of migraine and other episodic headaches in France: data from the GRIM2000 study. Pharmacoeconomics. 2004;22:985–999. doi: 10.2165/00019053-200422150-00003. [DOI] [PubMed] [Google Scholar]
- Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, Renshaw P, Burstein R, Borsook D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK, Garcia-Cebrian A, Monz B. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. doi: 10.1186/1471-2377-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery MN, Ryan P, Normand C, Murphy AW, de la Harpe D, McGuire BE. The economic cost of chronic noncancer pain in Ireland: results from the PRIME study, part 2. The journal of pain : official journal of the American Pain Society. 2012;13:139–145. doi: 10.1016/j.jpain.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bekkering GE, Kleijnen J. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449–462. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, Wood L, Wu TX, Karppinen J, Nikolajsen L, Mannikko M, Max MB, Kiselycznyk C, Poddar M, Te Morsche RH, Smith S, Gibson D, Kelempisioti A, Maixner W, Gribble FM, Woods CG. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci U S A. 2010;107:5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roditi D, Robinson ME. The role of psychological interventions in the management of patients with chronic pain. Psychology research and behavior management. 2011;4:41–49. doi: 10.2147/PRBM.S15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel O, Willweber-Strumpf A, Wagner P, Surall D, Malin JP, Zenz M. Psychological abnormalities in patients with complex regional pain syndrome (CRPS) Schmerz. 2005;19:272–284. doi: 10.1007/s00482-004-0337-z. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Sampson SM, Kung S, McAlpine DE, Sandroni P. The use of slow-frequency prefrontal repetitive transcranial magnetic stimulation in refractory neuropathic pain. J ECT. 2011;27:33–37. doi: 10.1097/YCT.0b013e31820c6270. [DOI] [PubMed] [Google Scholar]
- Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T, Shoji S. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28:109–116. doi: 10.1111/j.1464-5491.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- Schaufele MK, Boden SD. Outcome research in patients with chronic low back pain. The Orthopedic clinics of North America. 2003;34:231–237. doi: 10.1016/s0030-5898(03)00030-0. [DOI] [PubMed] [Google Scholar]
- Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, Scoffings D, Phillips A, Guo J, Laing RJ, Abdi S, Decosterd I, Woolf CJ. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000047. e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Segal RL, Lewek MD, McCulloch K, Mercer VS. The necessity for effective interaction between basic scientists and rehabilitation clinicians. Cells Tissues Organs. 2011;193:290–297. doi: 10.1159/000323676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain. 2009;132:788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol. 2011;24:515–523. doi: 10.1097/ACO.0b013e32834a1079. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle B. Pain advocacy: the evolution continues, with further calls for action. Journal of orofacial pain. 2012;26:5. [PubMed] [Google Scholar]
- Shakoor N, Lee KJ, Fogg LF, Wimmer MA, Foucher KC, Mikolaitis RA, Block JA. The relationship of vibratory perception to dynamic joint loading, radiographic severity, and pain in knee osteoarthritis. Arthritis Rheum. 2012;64:181–186. doi: 10.1002/art.30657. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What Does It Take? Comparing Intensive Rehabilitation to Outpatient Treatment for Children With Significant Pain-Related Disability. Journal of pediatric psychology. 2012 doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila R, Estlander AM, Tasmuth T, Kataja M, Kalso E. Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. British journal of cancer. 2012;107:1459–1466. doi: 10.1038/bjc.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleed M, Eccleston C, Beecham J, Knapp M, Jordan A. The economic impact of chronic pain in adolescence: methodological considerations and a preliminary costs-of-illness study. Pain. 2005;119:183–190. doi: 10.1016/j.pain.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Smits H, van Kleef M, Joosten EA. Spinal cord stimulation of dorsal columns in a rat model of neuropathic pain: evidence for a segmental spinal mechanism of pain relief. Pain. 2012;153:177–183. doi: 10.1016/j.pain.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology (Oxford) 2011;50:2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- Stanton-Hicks M. Plasticity of complex regional pain syndrome (CRPS) in children. Pain Med. 2010;11:1216–1223. doi: 10.1111/j.1526-4637.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- Suda S, Takagai S, Inoshima-Takahashi K, Sugihara G, Mori N, Takei N. Electroconvulsive therapy for burning mouth syndrome. Acta Psychiatr Scand. 2008;118:503–504. doi: 10.1111/j.1600-0447.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ebina Y, Shindo T, Takano T, Awata S, Matsuoka H. Repeated electroconvulsive therapy courses improved chronic regional pain with depression caused by failed back syndrome. Med Sci Monit. 2009;15:CS77–CS79. [PubMed] [Google Scholar]
- Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenetics and genomics. 2012;22:673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lotsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- Tremblay J, Hamet P. Genetics of pain, opioids, and opioid responsiveness. Metabolism: clinical and experimental. 2010;59(Suppl 1):S5–S8. doi: 10.1016/j.metabol.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn MA, Marinus J, Putter H, Bosselaar SR, Moseley GL, van Hilten JJ. Spreading of complex regional pain syndrome: not a random process. J Neural Transm. 2011;118:1301–1309. doi: 10.1007/s00702-011-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn MA, Marinus J, Putter H, van Hilten JJ. Onset and progression of dystonia in complex regional pain syndrome. Pain. 2007;130:287–293. doi: 10.1016/j.pain.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Vandenkerkhof EG, Macdonald HM, Jones GT, Power C, Macfarlane GJ. Diet, lifestyle and chronic widespread pain: results from the 1958 British Birth Cohort Study. Pain Res Manag. 2011;16:87–92. doi: 10.1155/2011/727094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrassi G, Collett B, Morlion B, Kalso E, Nicolaou A, Dickenson A, Pergolizzi J, Schafer M, Muller-Schwefe G. Proceedings of the CHANGE PAIN Expert Summit in Rome, June 2010. Current medical research and opinion. 2011;27:2061–2062. doi: 10.1185/03007995.2011.619424. [DOI] [PubMed] [Google Scholar]
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain medicine. 2008;9:660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Vogel T, Gradl G, Ockert B, Pellengahr CS, Schurmann M. Sympathetic dysfunction in long-term complex regional pain syndrome. Clin J Pain. 2010;26:128–131. doi: 10.1097/AJP.0b013e3181b5effc. [DOI] [PubMed] [Google Scholar]
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(Suppl 1):i69–i85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- Webster BS, Courtney TK, Huang YH, Matz S, Christiani DC. Physicians' initial management of acute low back pain versus evidence-based guidelines. Influence of sciatica. J Gen Intern Med. 2005;20:1132–1135. doi: 10.1111/j.1525-1497.2005.0230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Abernethy AP, Spruill SE, Pike IM, True Kelly A, Jett LG. A spicamycin derivative (KRN5500) provides neuropathic pain relief in patients with advanced cancer: a placebo-controlled, proof-of-concept trial. Journal of pain and symptom management. 2012;43:679–693. doi: 10.1016/j.jpainsymman.2011.05.003. [DOI] [PubMed] [Google Scholar]
- West E, Wallberg-Jonsson S. Health-related quality of life in Swedish men and women with early rheumatoid arthritis. Gender medicine. 2009;6:544–554. doi: 10.1016/j.genm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Widar M, Ahlstrom G, Ek AC. Health-related quality of life in persons with long-term pain after a stroke. Journal of clinical nursing. 2004;13:497–505. doi: 10.1046/j.1365-2702.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- Woodcock J. A difficult balance--pain management, drug safety, and the FDA. N Engl J Med. 2009;361:2105–2107. doi: 10.1056/NEJMp0908913. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat Rev Drug Discov. 2007;6:703–710. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Group on Health Outcomes for Older Persons with Multiple Chronic C. Universal Health Outcome Measures for Older Persons with Multiple Chronic Conditions. Journal of the American Geriatrics Society. 2012 doi: 10.1111/j.1532-5415.2012.04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Ji RR. Resolvins are potent analgesics for arthritic pain. Br J Pharmacol. 2011;164:274–277. doi: 10.1111/j.1476-5381.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]