Abstract
Parasympathetic tone is a dominant neural regulator for basal heart rate. Glutamate transporters (EAAT) via their glutamate uptake functions regulate glutamate neurotransmission in the central nervous system. We showed that EAAT type 3 (EAAT3) knockout mice had a slower heart rate than wild-type mice when they were anesthetized. We design this study to determine whether non-anesthetized EAAT3 knockout mice have a slower heart rate and, if so, what may be the mechanism for this effect. Young adult EAAT3 knockout mice had slower heart rates than those of their littermate wild-type mice no matter whether they were awake or anesthetized. This difference was abolished by atropine, a parasympatholytic drug. Carbamylcholine chloride, a parasympathomimetic drug, equally effectively reduced the heart rates of wild-type and EAAT3 knockout mice. Positive immunostaining for EAAT3 was found in the area of nuclei deriving fibers for vagus nerve. There was no positive staining for the EAATs in the sinoatrial node. These results suggest that EAAT3 knockout mice have a slower heart rate at rest. This effect may be caused by an increased parasympathetic tone possibly due to increased glutamate neurotransmission in the central nervous system. These findings indicate that regulation of heart rate, a vital sign, is one of the EAAT biological functions.
Keywords: glutamate transporter, heart rate, mice, parasympathetic tone
Introduction
Heart rate is a vital sign that is closely regulated by neurohumoral factors to meet the need of animal’s activity. Among the factors, parasympathetic nerve stimulation reduces the heat rate and sympathetic nerve stimulation increases it. The heart rate may be dominantly controlled by parasympathetic nerve under resting condition (Griffioen et al. 2007), although dominant role of sympathetic tone in determining the heart rates of mice at rest has been suggested by studies (Gehrmann et al. 2000; Janssen and Smits 2002). The parasympathetic nerve that innervates the heart is vagus nerve.
Vagus nerve is a cranial nerve that has axons from nucleus ambiguus, nucleus of vagus and solitary nucleus in the medulla oblongata. Injection of glutamate into these areas causes a bradycardic response (Vela et al. 2010), suggesting that extracellular glutamate may increase the vagus nerve tone. Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS). No extracellular enzyme has been identified for glutamate. One major mechanism to regulate its extracellular concentrations is via binding and taking-up by glutamate transporters (also called excitatory amino acid transporters, EAATs) (Danbolt 2001).
There are five EAATs (EAAT1 - 5) in mammals. EAAT1 - 3 are broadly expressed in the CNS including the cerebrum, hippocampus and cerebellum; whereas EAAT4 and EAAT5 are largely restricted to the cerebellar molecular cell layer and retina, respectively (Danbolt 2001; Rothstein et al. 1994). In rodents, EAAT1 and EAAT2 are mostly found in glial cells. On the other hand, EAAT3 and EAAT4 are mainly seen in neurons (Arriza et al. 1997; Lehre et al. 1995; Rothstein et al. 1994). EATT2 and EAAT3 are considered the predominant glial and neuronal EAATs, respectively, based on the functional and expressional data (Danbolt 2001; Rothstein et al. 1996; Tanaka et al. 1997).
We have shown that EAAT3 knockout mice have a slower heart rate than the wild-type mice under isoflurane anesthesia (Li and Zuo 2011). We did not investigate this issue further in our previous study because the study was focused on determining brain ischemic tolerance in these two types of mice. We designed this study to determine whether EAAT3 knockout mice had slower heart rates than wild-type mice when they are awake and, if so, what the mechanism may be.
Methods and Methods
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA).
Animals
Eight- to twelve-week old mice were used in this study. The EAAT3 knockout mice were descendants from mice used in previous studies, in which no expression of EAAT3 proteins in the central nervous system was confirmed (Li and Zuo 2011; Peghini et al. 1997). These mice were generated by conventional gene knockout technique to disrupt the exon1 of eaat3 gene (Peghini et al. 1997). These mice have a CD-1 gene background. Wild-type mice used in this study were littermates of EAAT3 knockout mice.
Blood pressure and heart rate measurement of waking mice
Non-invasive blood pressure and heart rate measurement was performed by using CODA monitor (Kent Scientific Corporation, Torrington, CT). Briefly, awake mice were warmed on a blanket and then placed in the monitor for 10 min for them to be accommodated to the environment. Their blood pressure and heart rates were measured every 30 s for 20 times. The data were averaged to reflect the blood pressure and heart rate of a particular mouse.
Heart rate measurement of anesthetized mice
Heart rates of isoflurane-anesthetized mice (1.3%) were continuously non-invasively monitored using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA). Heart rate of each mouse at ~ 30 min after the onset of anesthesia was recorded.
Continuous heart rate monitoring of awake, free moving mice and drug application
Non-invasive heart rate monitoring was performed by using the MouseOX Murine Plus Oximeter System. Briefly, the mouse was put into a cage with a blank collar clip for 1 h for them to get used to the device. The blank collar clip then was replaced by a recording collar clip. The continuous monitoring of heart rates was started 10 min later. Heart rate was recorded every 1 min for consecutive 9 min. The mouse then was given atropine (2 mg/kg, Sigma-Aldrich, St Louis, MO) or its vehicle, normal saline, intramuscularly (gluteus maximus) at a volume of 0.1 ml with a 30 gauge needle and returned to the same cage. Heart rate data were collected every min during the period of 2 – 40 min after the injection.
Three days later, the heart rate response to intraperitoneal injection of 0.1 mg/kg carbamyocholine chloride (CCh) at a volume of 0.1 ml was tested in the same method as described above.
Tissue collection
One week after all physiological records were accomplished, animals were deeply anesthetized with isoflurane and perfused with ice-cold normal saline. The medulla oblongata of 4 wild-type and 4 EAAT3 knockout mice was collected for Western blotting study. Another 4 animals in each group were perfused with ice-cold saline followed by 20 ml of refrigerated 4 % paraformaldehyde in phosphate buffered saline (pH 7.4). The brain, medulla oblongata and hearts then were collected and post-fixed in 4% paraformaldehyde overnight at 4°C. In the following day, sinoatrial node (SAN) region was dissected from the heart under a microscope according to previous reports (Cifelli et al. 2008; Verheijck et al. 2001). In brief, after removal of the ventricle, a thin glass tube was inserted from the right ventricular cut into the superior vena cava through the right atrium. The right atrium was opened along the back line of the glass tube to expose the crista terminalis, the intercaval area and the interatrial septum. The SAN area is located in the intercaval region adjacent to the crista terminalis. Tissue was trimmed to keep the SAN region and some surrounding atrial tissues. The brain, medulla oblongata and SAN region were gradually dehydrated and embedded in paraffin for immunofluorescent staining.
Western blot
Membrane protein from frozen medulla oblongata was prepared as we described previously (Li and Zuo 2011). Briefly, tissues were homogenized in 1:10 (w:v) of a lysis buffer (200 mM mannitol, 80 mM HEPES and 41 mM KOH, pH7.4) containing 200 μM phenylmethylsulfonyl fluoride, 1 mM EDTA and protease inhibitor cocktail (Sigma-Aldrich). The tissue lysates were then incubated on ice for 10 min and centrifuged at 1000 g for 10 min at 4°C. The supernatants were removed into an ultracentrifuge tube and centrifuged again at 100,000 g for 1 h at 4°C. The pellet was resuspended in the lysis buffer thoroughly with sonication (1 s burst, 1 s interval, 5 times). Protein concentration was measured with Bradford assay. Thirty micrograms of proteins from each sample were separated in a 10% polyacrylamide gel. The proteins were transferred onto a polyvinylidene fluoride membrane and incubated with a primary antibody overnight at 4°C. The primary antibodies used were the rabbit polyclonal anti-EAAT1 antibody, anti-EAAT2 antibody (1:500 dilution; Cell Signaling Technology, Inc., MA), anti-EAAT3 antibody (1:500 dilution; Alpha Diagnostic International Inc., TX), or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000; Sigma Aldrich). In the following day, membranes were washed and incubated with goat anti-rabbit IgG-horseradish peroxidase (1:5000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 h. Protein bands were visualized with the enhanced chemiluminescence method. The densities of EAAT protein bands were normalized to those of GAPDH from the same sample.
Immunofluorescent Study
Eight-micron SAN paraffin sections were stained with a rabbit polyclonal anti- hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN-4) antibody (1:100 dilution; Alomone Labs, Jerusalem, Israel) in 1% bovine serum albumin. HCN-4 is a marker for SAN cells (Cifelli et al. 2008; Liu et al. 2007). Adjacent sections were incubated with the EAAT1, EAAT2 or EAAT3 antibodies (1:500). Medullary cross sections or brain coronary sections (8 μm thickness) were stained with mouse monoclonal anti-NeuN (1:100 dilution; Millipore, Billerica, MA) and the anti-EAAT antibodies (1:500). Primary antibodies of all sections were incubated overnight at 4 °C. They were then incubated with a fluorescein isothiocyanate-conjugated goat anti-mouse IgG secondary antibody and a Cy3 goat anti-rabbit IgG secondary antibody (1:200 dilution; R&D System, Minneapolis, MN) for 1 h at room temperature in the dark. The SAN tissue sections were counterstained with Hoechst 33342 (1:10000 dilution; Thermo Fisher Scientific Inc, Waltham, MA) for a brief 5 min, washed with Tris buffered saline for 3×5 min and then mounted with 50% glycerol sodium carbonate solution. They were observed under a fluorescent microscope (Olympus BX51).
Statistic analysis
Results are presented as means ± S.D. (n ≥ 6). The results of continuous heart rates were analyzed by two-way (CD-1 wild-type mice vs. EAAT3 knockout mice) repeated measures analysis of variance followed by the Tukey test. The comparisons of heart rates at different time points in the same group of animals were performed by one-way repeated measures analysis of variance followed by the Tukey test. All other results were tested by t-test. A P ≤ 0.05 was accepted as significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
Results
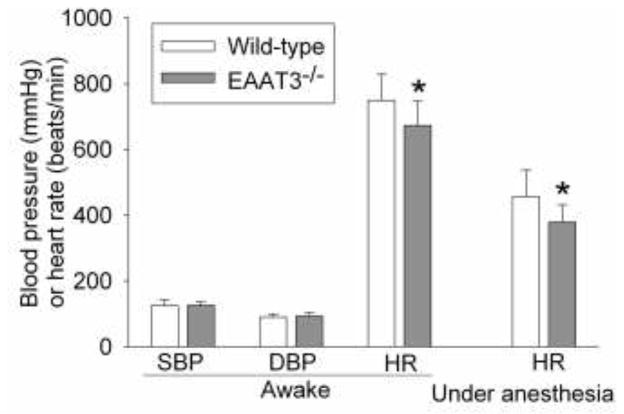
The EAAT3 knockout mice and their littermate wild-type mice had similar body weights (28.1 ± 1.4 and 27.1 ± 1.9 g, respectively, n = 8, P > 0.05). The systolic and diastolic blood pressures were not different between the EAAT3 knockout and wild-type mice. However, the heart rates of EAAT3 knockout mice were significantly slower than those of wild-type mice when they were awake (P = 0.018) or under isoflurane anesthesia (P = 0.003) (Fig. 1). The heart rate under consciousness or isoflurane anesthesia condition was decreased by 10% and 17%, respectively, in EAAT3 knockout mice compared with wild-type mice.
Fig. 1. Slower heart rates in EAAT3 knockout (EAAT3−/−) mice.
Results are mean ± S.D. (n = 12 – 16). * P < 0.05 compared with wild-type mice.
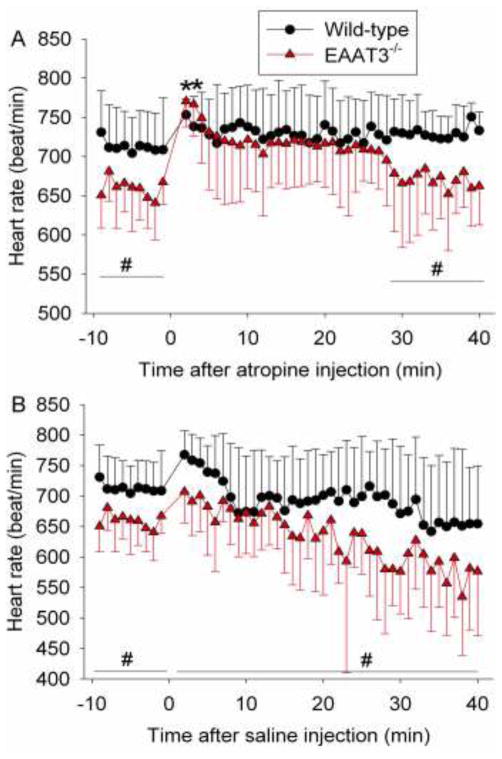
Consistent with the above results, the effects of mouse type (wild-type vs. EAAT3 knockout) on basal heart rates during the 9-min observation time before the injection of atropine were significant (P = 0.011). These effects disappeared after atropine injection if the data during the following 40-min observation time were analyzed as a whole. However, there were significant effects of mouse type on heart rates if the data from the last 12 min of the 40-min observation time were analyzed (P = 0.02). These results suggest that atropine increases the heart rates of EAAT3 knockout mice to the same level as those of wild-type mice for 28 min. In supporting this finding, the heart rates of EAAT3 knockout mice at 2 or 3 min after atropine injection were significantly higher than those immediately before atropine injection. Although the heart rates after atropine injection in the wild-type mice appeared to be increased, none of the heart rates at any time points was statistically significantly different from those immediately before the injection. Also, the effects of times on heart rates were significant (P < 0.001) when the data of the wild-type or EAAT3 knockout mice in the 40-min observation time after atropine injection were analyzed as a whole (Fig. 2A).
Fig. 2. Heart rate regulation by atropine or saline injection in wild-type or EAAT3 knockout (EAAT3−/−) mice.
Awake, freely moving mice received injection of atropine (panel A) or saline (panel B) at time 0. Results are mean ± S.D. (n = 6 – 8). * P < 0.05 compared with corresponding value at time −1 (immediately before atropine injection) from the same animals. # P < 0.05 for the effects of mouse type (wild-type vs. EAAT3 knockout) on heart rates over the indicated time period.
There were significant effects of time on the heart rates when the data of the wild-type or EAAT3 knockout mice in the 40-min observation time after saline injection were analyzed (P < 0.001). Although the heart rates in the wild-type and EAAT3 knockout mice appeared to be faster in the first few minutes after saline injection than those just before the injection, none of them was statistically significantly higher than those just before saline injection. In addition, the effects of mouse type on heart rates were significant (P = 0.045) when the data from the 40-min observation time were analyzed as a whole (Fig. 2B). These results suggest that no difference in the heart rates between the wild-type and EAAT3 knockout mice after atropine injection is caused by drug effects and is not caused by stress due to animal handling and pain associated with injection.
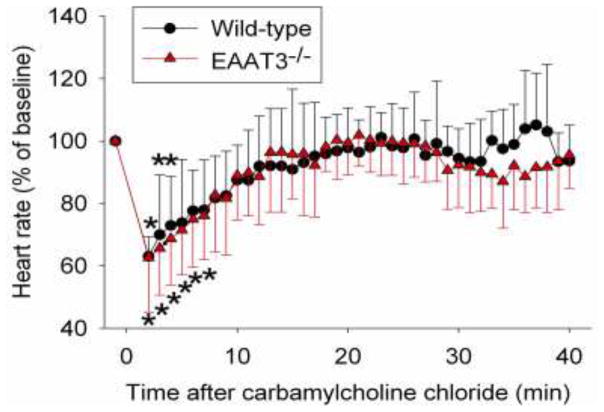
The heart rates within 4 min or 7 min after CCh injection in the wild-type and EAAT3 knockout mice, respectively, were significantly slower than those just before CCh injection (All Ps < 0.05). The traces of heart rates of the wild-type and EAAT3 knockout mice after CCh injection overlapped very well (Fig. 3), suggesting that CCh is equally effective in reducing heart rates in the wild-type and EAAT3 knockout mice.
Fig. 3. Heart rate regulation by carbamylcholine chloride injection in wild-type or EAAT3 knockout (EAAT3−/−) mice.
Awake, freely moving mice received injection of carbamylcholine chloride at time 0. Results are mean ± S.D. (n = 7). * P < 0.05 compared with corresponding value at time −1 (immediately before carbamylcholine chloride injection) from the same animals.
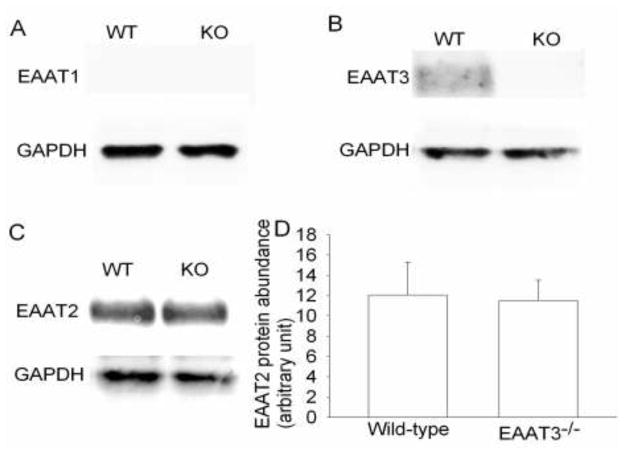
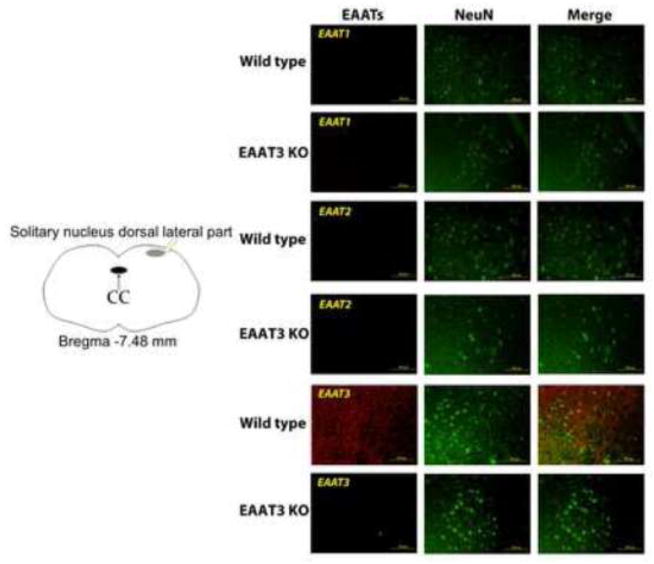
There was a significant amount of EAAT2 but apparently lack of EAAT1 expression in the medulla oblongata. The expression of EAAT2 was not different between wild-type and EAAT3 knockout mice (Fig. 4). No apparent immunostaining for EAAT2 and EAAT1 was identified in the area of solitary nucleus. However, there was immunostaining for EAAT3 in this area. This immunostaining appeared in the cells that were positively stained by the anti-NeuN antibody (Fig. 5), suggesting that this EAAT3 staining is in the neurons. Similarly, EAAT3 positive staining was found in the nucleus ambiguus, gigantocellular reticular nucleus and nucleus of the solitary tract of wild-type mice. This positive staining was in the cells that were also positively stained for NeuN. There was no significant staining for EAAT3 in these areas of the EAAT3 knockout mice (Fig. 6).
Fig. 4. Expression of glutamate transporters (EAATs) in mouse medulla oblongata.
A representative Western blot is shown in panels A, B and C, and the graphic presentation of EAAT2 protein abundance quantified by integrating the volume of autoradiograms from 4 wild-type (WT) or EAAT3 knockout (KO) mice is shown in panel D. In panel D, the results of EAAT2 were normalized by the corresponding data of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Fig. 5. Immunostaining of glutamate transporters (EAATs) in medulla oblongata.
Medulla oblongata from wild-type or EAAT3 knockout (EAAT3 KO) mice was harvested for immunofluorescent staining of EAATs (red) or NeuN (green). NeuN is proteins expressed in neurons. The left panel is a diagram to further indicate the location of the other panels. The black oval in the diagram indicates central cannel (CC). The arrows and squares are used to clearly indicate the locations of the other images. Images are representatives from three wild-type and three EAAT3 KO mice. Scale bar in the right panels represents 100 μm.
Fig. 6. Immunostaining of glutamate transporter type 3 (EAAT3) in the brain and medulla oblongata.
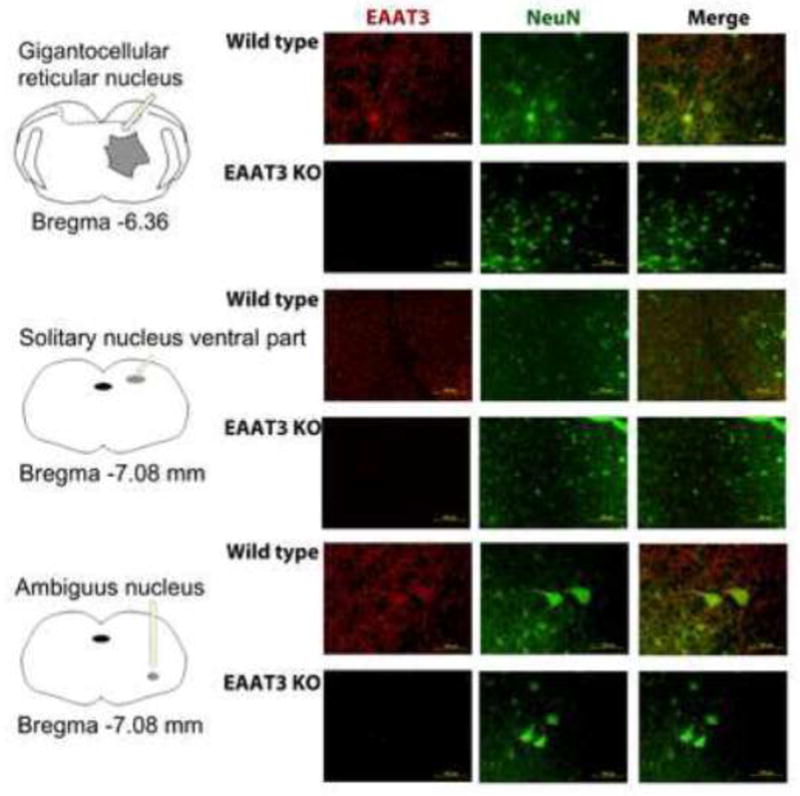
Brain and medulla oblongata from wild-type (WT) or EAAT3 knockout (KO) mice were harvested for immunofluorescent staining of EAAT3 (red) or NeuN (green). NeuN is proteins expressed in neurons. The level for staining gigantocellular reticular nucleus, solitary nucleus and nucleus ambiguous was at ~bregma −6.36,−-7.08 and −7.08 mm, respectively. The left panels are diagrams to indicate the locations of these nuclei. Scale bar in the right panels represents 100 μm.
There was abundant immunostaining for HCN-4 in the area of SAN. However, there was no apparent immunostaining for EAAT1, EAAT2, EAAT3 and NeuN in this area (Fig. 7).
Fig. 7. Immunostaining of glutamate transporters (EAATs) and hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN-4) in the area of sinoatrial node.
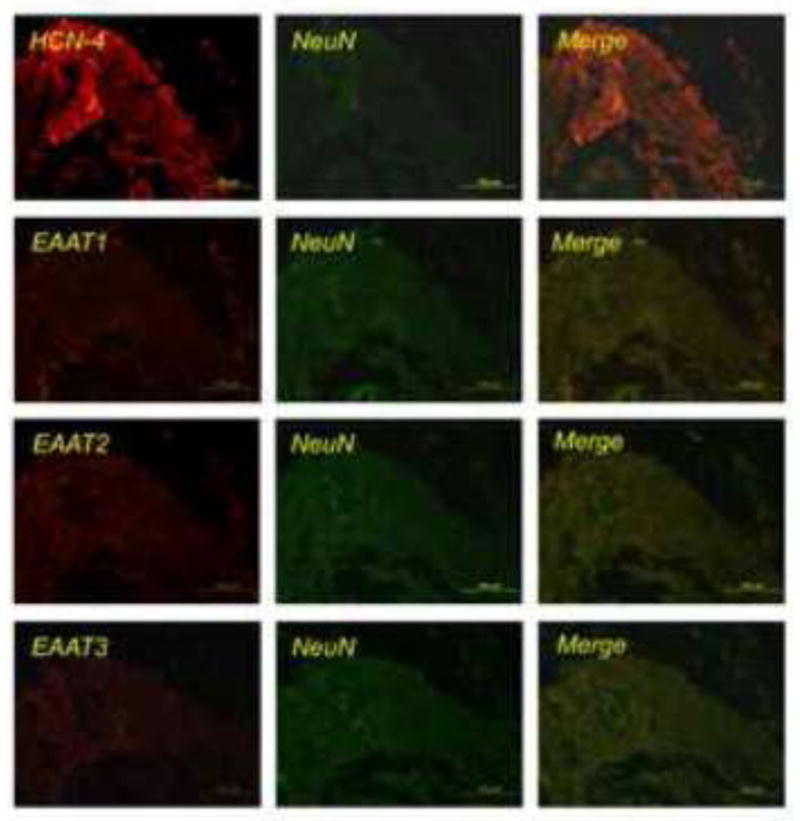
Sections were immunofluorescently stained for EAATs (red), HCN-4 (red), or NeuN (green). NeuN and HCN-4 are proteins expressed in neurons and cells of the sinoatrial node, respectively. Scale bar in each panel represents 100 μm.
Discussion
Our results clearly showed that EAAT3 knockout mice had slower heart rates than wild-type mice no matter whether animals were awake or anesthetized. However, there was no difference in blood pressure between these two types of mice. Parasympathetic modulation is a major neural regulation for heart rate under normal condition (Griffioen et al. 2007). The parasympathetic nerve for the heart, i.e. vagus nerve, mainly innervates SAN and atrioventricular node, and does not innervate the ventricular myocardium. Thus, the slower heart rate in the EAAT3 knockout mice may be due to a higher vagus tone. Consistent with this idea, atropine, a parasympatholytic drug, abolished the difference in heart rate between the wild-type and EAAT3 knockout mice. Also, CCh, a parasympathomimetic drug, equally efficiently reduced the heart rates of the wild-type and EAAT3 knockout mice, suggesting that the SAN in these two types of mice have similar sensitivity to parasympathetic stimulation.
The increased vagus tone in EAAT3 knockout mice is caused most likely in the CNS. In addition to neural tissues, EAATs can be found in other tissues. For example, EAAT3 is expressed in the epithelial cells of the kidneys and many other cells in the autocrine and paracrine system (Bailey et al. 2011; Hinoi et al. 2004; Kanai and Hediger 1992). There was no significant immunostaining of EAAT1 – 3 in the SAN of wild-type mice, although it has been reported that EAATs are expressed in the myocardium (Kanai and Hediger 1992). Vagus nerve fibers that innervate the heart are from the nucleus ambiguus (Machado and Brody 1988). Our results showed that neurons in this area expressed a significant amount of EAAT3. EAATs are known to regulate synaptic transmission. Specifically, EAAT3 can limit the spillover of glutamate to neighboring synapses (Diamond 2001) and, therefore, prevent the activation of these synapses. Importantly, EAAT3 in the presynaptic membrane can provide a negative feedback to glutamate release from presynaptic termini (Kondratskaya et al. 2010; Veruki et al. 2006), meaning that released glutamate activates presynaptic EAAT3, which then reduces glutamate release. These EAAT3 effects may help limit the level of glutamate neurotransmission. This function may be lost in the EAAT3 knockout mice.
The lost function of EAAT3 in limiting the glutamate neurotransmission may play a role in the reduced heart rates of the EAAT3 knockout mice. There is significant glutamatergic input to the neurons in the nucleus ambiguus (Jameson et al. 2008; Wang et al. 2001). Also, injection of glutamate to the area of vagus nerve nuclei reduces heart rates of animals (Vela et al. 2010).
There was a small but non-significant increase of heart rate in animals receiving saline injection. This increase was transient. This may be caused by stress due to holding the animals for intramuscular injection and the slight pain from the injection. Although we did not observe a significantly different response to saline injection between wild-type and EAAT3 knockout mice, this finding can not exclude the possibility that EAAT3 knockout mice may respond to stress differently from wild-type mice. The EAAT3 knockout animals had increased heart rates for ~ 28 min after atropine injection. This duration seems to be short since the elimination half-life of atropine from blood in mice is about 100 min (Palmer et al. 1981). This situation is similar to that in humans. The half-life of atropine in the blood is about 2 h in humans. The increase in heart rates after intravenous injection of 0.5 mg atropine to adult volunteers lasts for about 30 min (Meyer et al. 1988). In addition to the elimination half-life, many other factors, such as dosage, may determine the duration of a pharmacological effect of the drug in a particular situation.
We used EAAT3 knockout mice that were generated by conventional gene knockout technique. Consistent with the anticipation of knocking out EAAT3 expression in all tissues by this technique, EAAT3 expression was knocked out in all brain regions/nuclei that were examined. A significant limitation with using conventional gene knockout mice is the compensatory changes in expression of the genes that are functionally related to the modified gene. We showed here that the expression of EAAT1 and EAAT2 in the medulla oblongata was not different between the wild-type and EAAT3 knockout mice. The expression pattern of EAAT1 and EAAT2 in the area of nucleus of vagus also was not different. These results suggest that there are no significant compensatory changes of EAAT1 and EAAT2 in the EAAT3 knockout mice. Thus, the slower heart rates in these mice may not be caused by the compensatory change of EAAT1 and EAAT2. The slower heart rates of EAAT3 knockout mice may be due to compensatory changes in glutamate receptors. However, our previous study using ketamine (Lee et al. 2010), a glutamate receptor antagonist, suggests that significant glutamate receptor changes in the EAAT3 knockout mice are unlikely.
Our Western blot analysis showed that a significant amount of EAAT2 was expressed in the medulla oblongata but there was no EAAT2-positive immunofluorescent staining in the solitary nucleus. Solitary nucleus is a small component of the medulla oblongata. Our results suggest that solitary nucleus is not the major structure that expresses EAAT2 in the medulla oblongata. Also, we observed that EAAT3- and NeuN-positive staining was not overlapped perfectly in the brain sections. Since NeuN is mainly in the nuclei of neurons and EAAT3 can be in the cytoplasm and plasma membrane of neurons, EAAT3-positive staining may appear in areas that are not NeuN-positive.
EAATs via their glutamate uptake functions have been found to play an important role in various biological functions, such as reducing excitotoxicity and possibly mediating learning and memory (Danbolt 2001; Lee et al. 2011; Levenson et al. 2002). Our findings suggest that EAAT3 contributes to the regulation of basal heart rates, a vital sign. These findings expand the spectrum of biological functions of EAATs.
In summary, we have shown that EAAT3 knockout mice have significantly lower heart rates than wild-type mice. This effect may be due to increased parasympathetic tone from increased glutamate neurotransmission in the CNS.
Acknowledgments
This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, the Robert M. Epstein Professorship endowment, University of Virginia.
Footnotes
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA22908, USA.
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, Auray-Blais C, Vandenberg RJ, Broer S, Rasko JE. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest. 2011;121(1):446–453. doi: 10.1172/JCI44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103(5):527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21(21):8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279(2):H733–740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Gorini C, Jameson H, Mendelowitz D. Purinergic P2X receptors mediate excitatory transmission to cardiac vagal neurons in the nucleus ambiguus after hypoxia. Hypertension. 2007;50(1):75–81. doi: 10.1161/HYPERTENSIONAHA.106.086140. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y. Glutamate signaling in peripheral tissues. Eur J Biochem. 2004;271(1):1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- Jameson HS, Pinol RA, Kamendi H, Mendelowitz D. ATP facilitates glutamatergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2008;1201:88–92. doi: 10.1016/j.brainres.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Smits JF. Autonomic control of blood pressure in mice: basic physiology and effects of genetic modification. Am J Physiol Regul Integr Comp Physiol. 2002;282(6):R1545–1564. doi: 10.1152/ajpregu.00714.2001. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kondratskaya E, Shin MC, Akaike N. Neuronal glutamate transporters regulate synaptic transmission in single synapses on CA1 hippocampal neurons. Brain Res Bull. 2010;81(1):53–60. doi: 10.1016/j.brainresbull.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Lee S, Park S, Zuo Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. J Pharm Pharmacol. 2012;64(2):302–307. doi: 10.1111/j.2042-7158.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neuroscience. 2010;171(3):788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31(5):1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res. 2007;73(4):729–738. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Machado BH, Brody MJ. Role of the nucleus ambiguus in the regulation of heart rate and arterial pressure. Hypertension. 1988;11(6 Pt 2):602–607. doi: 10.1161/01.hyp.11.6.602. [DOI] [PubMed] [Google Scholar]
- Meyer EC, Sommers DK, Schoeman HS, Avenant JC. The effect of atropine on heart-rate: a comparison between two ethnic groups. Br J Clin Pharmacol. 1988;25(6):776–777. doi: 10.1111/j.1365-2125.1988.tb05269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L, Edgar J, Lundgren G, Karlen B, Hermansson J. Atropine in mouse brain and plasma quantified by mass fragmentography. Acta Pharmacol Toxicol (Copenh) 1981;49(1):72–76. doi: 10.1111/j.1600-0773.1981.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16(13):3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Vela C, Diaz-Cabiale Z, Parrado C, Narvaez M, Covenas R, Narvaez JA. Involvement of oxytocin in the nucleus tractus solitarii on central cardiovascular control: interactions with glutamate. J Physiol Pharmacol. 2010;61(1):59–65. [PubMed] [Google Scholar]
- Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001;52(1):40–50. doi: 10.1016/s0008-6363(01)00364-9. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9(11):1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]