Abstract
This article presents a simple and reliable method for generating polystyrene (PS) yarns composed of bundles of nanofibrils by using a proper combination of solvent and relative humidity. We elucidated the mechanism responsible for the formation of this new morphology by systematically investigating the molecular interactions among the polymer, solvent(s), and water vapor. We demonstrated that vapor-induced phase separation played a pivotal role in generating the yarns with a unique structure. Furthermore, we discovered that the low vapor pressure of N,N-dimethylformamide (DMF) was critical to the evolution of pores in the interiors. On the contrary, the relatively high vapor pressure of tetrahydrofuran (THF) hindered the formation of interior pores but excelled in creating a rough surface. In all cases, our results clearly indicate that the formation of either internal porosity or surface roughness required the presence of water vapor, a nonsolvent of the polymer, at a proper level of relative humidity. The exact morphology or pore structure was dependent on the speed of evaporation for the solvent(s) (DMF, THF, and their mixtures), as well as the inter-diffusion and penetration of the nonsolvent (water) and solvent(s). Our findings can serve as guidelines for the preparation of fibers with desired porosity both internally and externally through electrospinning.
Keywords: Electrospinning, polystyrene yarn, porous structure, vapor-induced phase Separation
INTRODUCTION
Porous materials have found widespread use in a wide variety of applications such as filtration, catalysis, and biomedical research.1–4 Through rational design and controlled fabrication, it is possible to optimize their properties – including, for example, the strength or stiffness-to-density ratio, surface area-to-mass or volume ratio, thermal and chemical resistance, permeability, and insulation capability – to meet the requirements of various applications.5,6 Recent progress in the fabrication strategy and technique has greatly expanded the scope of available morphologies and properties for porous materials, leading to ever-increasing opportunities in new applications such as drug delivery, tissue engineering, and regenerative medicine.7–10 A variety of techniques have been developed for generating porous materials, including electrochemical anodization, freeze drying, sol-gel chemistry, self-assembly, three-dimensional (3-D) printing, and atomic layer deposition.11–17 Most of these method are based upon templating and phase separation, typically combined with dissolution/extraction, etching, and high-temperature calcination or pyrolysis to generate pores via selective removal of the sacrificial component(s).
Generally deemed as a simple and versatile technique for generating continuous fibers with sizes down to the nanoscale, electrospinning also excels in the production of 3-D constructs or membranes with hierarchical porosity by stacking the nanofibers in a random or organized fashion.18,19 Two types of pores can exist in a membrane of electrospun nanofibers: the inter- and intra-fiber pores.20 The inter-fiber pores, a signature feature of fibrous membrane, naturally form in a nonwoven mat of nanofibers during electrospinning and the pore size and shape could be varied by controlling the electrospinning parameters and/or collection method.21 The intra-fiber pores, however, do not exist in most of the electrospun nanofibers due to the solidification of polymer solution during an electrospinning process. When nonwoven mats of electrospun nanofibers are used as filters, gas absorbents, and catalyst supports in a variety of applications, their performance could be greatly improved by increasing their specific surface areas through the introduction of intra-fiber pores.20 To this end, a number of methods have been developed for generating pores in individual electrospun nanofibers,22–30 and they can be broadly divided into two strategies: i) employment of a proper post treatment (e.g., solvent extraction and calcination) and ii) manipulation of polymer-solvent phase separation. In general, the method based upon selective removal of a sacrificial component should be the most straightforward and versatile route to generating porous nanofibers, except that it requires an extra step of post processing. In addition, the harsh conditions used for solvent extraction or high-temperature calcination can cause the fibrous structures to collapse or deform, leading to the formation of a fragmented or nonporous product.31,32 Furthermore, calcination can only be applied to inorganic materials. By carefully selecting suitable parameters for electrospinning (including, for example, the type of polymer, solvent, and relative humidity), porous nanofibers have also been prepared through a phase separation mechanism.23–25,27–29,33,34 However, most of these nanofibers did not contain pores in their interiors; they tended to just show rough surfaces with dents or shallow holes.23–25
In this study, we demonstrate the fabrication of polystyrene (PS) yarns with an inherently porous structure in the interior using a convenient and reliable approach without involving any special collection method or post-spinning treatment. We chose PS because it is commonly used for electrospinning to generate fibers with pores on the surfaces.24,25,35,36 In a few studies, people also managed to produce a small quantity of PS fibers with pores in the interiors but the electrospinning process was unstable and the fiber quality tended to be very low. Moreover, it was a great challenge to identify the exact conditions under which the fabrication process could be made reproducible because a number of parameters were varied simultaneously in these studies.27,29,34,37 The most important finding from the previous explorations is the critical role played by the volatility or vapor pressure of a solvent in the generation of a porous structure. It was generally believed that a volatile solvent was instrumental to the generation of pores on the surfaces of fibers. However, another critical parameter, the relative humidity of the atmosphere, was largely neglected or underestimated in all these studies. It has been suggested that the formation of both internally and externally porous fibers during electrospinning might be a synergetic effect of the solvent evaporation and relative humidity, but this concept has not been validated experimentally.33,34 To simplify the fabrication process and put it under tight control, here we only adjusted the relative humidity once the solvent had been fixed. All other parameters including voltage, feeding rate, needle-collector distance, temperature, polymer concentration, and environmental air flow, were kept essentially the same by using the optimized values and configuration. As a result, we were able to single out the most important factor in determining the generation of a porous structure inside the fiber. Furthermore, we also designed the experiments in such a way to achieve a deep understanding of the mechanism for pore formation during electrospinning by investigating the molecular interactions among the polymer, water vapor (nonsolvent), and solvent(s).
EXPERIMENTAL SECTION
Chemicals and Materials
Polystyrene (PS, Mw≈350,000, Mn≈170,000), anhydrous N,N-dimethylformamide (DMF, 99.8%, Vp=0.36 kPa at 20 °C, Bp=153 °C) and tetrahydrofuran (THF, 99.9%, Vp=19.07 kPa at 20 °C, Bp≈65–67 °C) were obtained from Sigma-Aldrich and used as received without further purification. The water (Vp=2.34 kPa at 20 °C, Bp=100 °C) for regulating relative humidity was purified with a Milli-Q plus purification system (Millipore).
Fabrication of PS yarns
Three PS solutions with the same concentration of 20 wt.% were prepared by dissolving 2 g of PS into 8 g of DMF, THF, and DMF/THF (w:w 1:1), respectively. All the solutions were vigorously stirred for 24 h before further use. In a typical electrospinning experiment, the PS solution was loaded into a 3 mL syringe fitted with a 23 gauge inner diameter and ~2.5 cm long flat metal needle (BD Medical) and delivered at 1 mL/h with a syringe pump (KDS 200, KD Scientific). Each solution was electrospun by applying a constant voltage of 15 kV using a DC power supply (ES 30-0.1 P, Gamma High Voltage Research) connected to the vertically positioned metal needle. The charged jet sprayed into fine fibers that were collected on a piece of aluminum foil (20 cm × 20 cm) placed 25 cm beneath the tip of the needle. The electrospinning was conducted in a plastic box (see Figure S1 for a photograph of the typical setup) with a dimension of 50 cm × 50 cm × 50 cm. The electrospinning setup was put in a fume hood with a controlled air follow of 100 feet/min. All the experiments were carried out at 20 °C under the specific relative humidity. The temperature was controlled by the laboratory central air conditioning system and the relative humidity was determined by the environmental humidity, which could be further tuned with a narrow window (±2%) by using a humidifier/dehumidifier. The as-prepared electrospun fibrous membrane was dried at ambient temperature under vacuum for 24 h prior to the subsequent characterization.
Instrumentation
The surface morphology and the internal porosity at the broken cross section of the as-spun PS yarns were examined using a scanning electron microscope (SEM, Nova 200, FEI) after gold coating for 60 s (Bio-Rad). The images were taken at an accelerating voltage of 15 kV and a working distance of ~5 mm. The diameters of the fibers and the surface pores were measured through the ImageJ software and then statistically analyzed using Origin 8 (OriginLab).
RESULTS AND DISCUSSION
The internally porous PS yarns could be routinely fabricated by electrospinning a 20 wt.% PS solution in N,N-dimethylformamide (DMF) under a set of optimal conditions: voltage (15 kV); feeding rate (1 mL/h); needle-to-collector distance (25 cm); temperature (20 °C); and relative humidity (52%). The spinning proceeded in a highly stable and reliable manner, producing a nonwoven mat of PS yarns 20 cm × 20 cm × 2 mm in dimensions within a few hours. Needle clogging or solution dripping, which are two common issues in electrospinning, especially after charge buildup on the collector due to continuous deposition of nanofibers, were not observed within a period up to three hours. The resultant PS yarns were then examined by SEM and the results are shown in Figure 1. Since the collector used in our experiments had a flat, continuous surface, the yarns were deposited in a randomly stacked fashion (Figure 1A). These PS yarns were uniform in sizes, with an average diameter of 2.2 µm, and they all showed a rather smooth surface without any noticeable porosity (Figure 1B). However, a highly porous interior could be clearly seen at the cross section of a broken yarn (Figure 1C). To confirm this observation, we examined the cross sections of a large number of yarns from different samples and a similar porous structure was resolved for all of them. Moreover, by scrutinizing these cross sections carefully, we noticed that each individual PS yarn was actually consisted of a bundle of distinguishable but possibly entangled nanofibers or fibrils (Figure 1D), similar to the structure of a conventional yarn. Therefore, we refer to this structure as “PS yarn” in our following discussion. The fundamental fibrils, with diameters less than 50 nm, were more or less orientated along the long axis of the PS yarn. A sleek sheath completely wrapped the bundle of fibrils and thus shielded this delicate structure.
Figure 1.
SEM images showing the yarns of PS nanofibers electrospun from a 20 wt.% PS solution in DMF at 20 °C and under relative humidity of 52% (voltage: 15 kV; feeding rate: 1 mL/h; and needle-collector distance: 25 cm). The as-spun yarns showed (A) good uniformity throughout the mat, (B) smooth surfaces on individual yarns, and (C, D) an inherently porous structure in the interior, with nanofibrils being aligned parallel to the long axis of each yarn.
The hierarchically porous structure of a PS yarn is intriguing and its formation mechanism deserves further investigation. Two models for phase separation, namely, thermally induced phase separation and vapor-induced phase separation, have been proposed to account for the formation of nano- and microscale pores in polymer thin films. They have also been used to explain the development of porous polymer fibers during electrospinning.24,27,33,38 In thermally induced phase separation, the solution (or the liquid jet during electrospinning) will pass through the bimodal curve of a phase diagram to enter the metastable region when the solution is cooled, resulting in the phase separation into polymer-rich and solvent-rich regions. Eventually, the polymer-rich phase will then transform into a solid matrix while the solvent-rich phase will evolve into pores after the solvent has evaporated. Although the environmental temperature can be kept constant during electrospinning, the rapid evaporation of solvent can lower the temperature of a liquid jet by absorbing the heat. Therefore, it has been widely claimed that solvents of high volatility or with high vapor pressures, such as acetone, CH2Cl2/CHCl3, and tetrahydrofuran (THF), play a critical role in the formation of porous fibers.23,24,29 This statement not only contradicts our result (as we used low volatile DMF), but also conflicts with the general observation for a variety of other electrospun fibers including polyethylene glycol (PEO), poly(vinyl pyrrolidone) (PVP), and poly(vinyl alcohol) (PVA), which did not become porous even though volatile solvents were used.24 The other model, vapor-induced phase separation, considers the penetration of a nonsolvent from the vapor phase into the polymer solution and thereby cause a phase separation for the polymer. In this case, the nonsolvent precipitates the polymer out of the solution to generate the solid matrix whereas the solvent-rich phase evolves into porous regions. Although this model has been used to explain the formation of pores on the surface of electrospun fibers, it is largely overlooked with regard to the fabrication of electrospun fibers with an internally porous structure. In addition, vapor-induced phase separation seems to disagree with the fact that majority of the hydrophobic polymers are electrospun into nonporous fibers with a solid structure in spite of the penetration of water vapor.
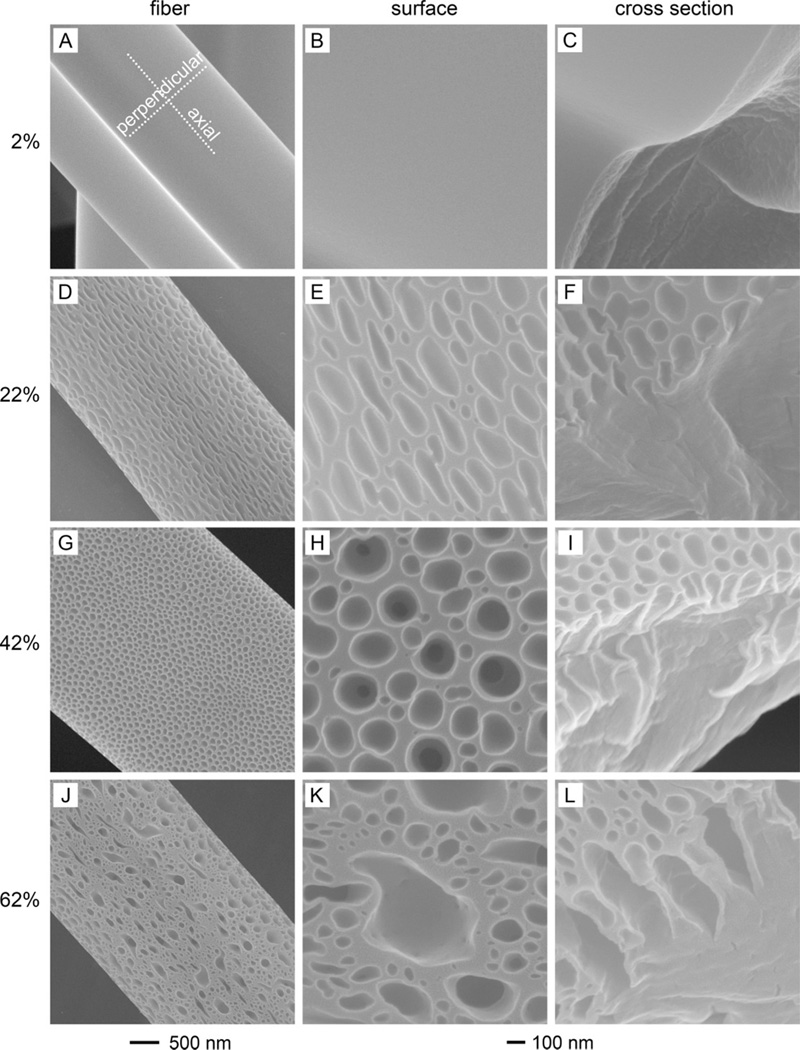
In order to understand the formation mechanism of porous PS yarns as well as to validate the aforementioned two models, we designed three experiments with three solvent systems based on DMF, THF, and DMF-THF mixture while the relative humidity was varied. Both DMF and THF, as well as their mixtures, have been proven to be good solvents for PS.24,39 Furthermore, they are miscible with each other, and with water.[40] In thermally induced phase separation, the determining factor for phase separation can be attributed to the reduction in temperature due to a fast evaporation of the solvent. Two strategies, either decreasing the relative humidity or using a solvent with a high vapor pressure, were used to increase the evaporation rate of the solvent during electrospinning. In a dry atmosphere with relative humidity of 2%, all the PS solutions failed to be electrospun into porous yarns (Figure 2, A and B; Figure 3 and 4, A, B, and C), clearly demonstrating the inadequacy of thermally induced phase separation for the production of porous PS yarns. On the contrary, either internally porous PS yarns (Figure 2, C–H) or PS fibers with surface porosity (Figure 3, D–L) were fabricated by increasing the relative humidity to 22%, manifesting the essential role of water vapor (a nonsolvent for PS in this case). Although the importance of water vapor has been noticed in the previous studies, it was not considered as a decisive factor for the generation of porous fibers, especially for the formation of pores in the interiors. Our finding clearly demonstrated an indispensable role of water vapor and thus relative humidity in producing PS fibers with both internal and external pores.
Figure 2.
Comparison of the surfaces (left panel) and cross sections (right panel) of PS fibers/yarns fabricated by electrospinning a 20 wt.% PS solution in DMF under different relative humidity: (A, B) 2%, (C, D) 22%, (E, F) 42%, and (G, H) 62% (voltage: 15 kV; feeding rate: 1 mL/h; needle-collector distance: 25 cm; and temperature: 20 °C). The scale bar in (H) applies to all other images.
Figure 3.
Representative PS fibers with pores on the surfaces (left panel) and their corresponding surfaces (middle panel) and cross sections (right panel) fabricated by electrospinning a 20 wt.% PS solution in THF under different relative humidity: (A, B, C) 2%, (D, E, F) 22%, (G, H, I) 42%, and (J, K, L) 62% (voltage: 15 kV; feeding rate: 1 mL/h; needle-collector distance: 25 cm; and temperature: 20 °C). The 500 nm scale bar applies to the images in the left panel while the 100 nm scale bar applies to the middle and right panels.
Figure 4.
SEM images showing PS fibers (left panel) and the corresponding surfaces (middle panel) and cross sections (right panel) by electrospinning a 20 wt.% PS solution in DMF/THF (1:1 w:w) at different relative humidity: (A, B, C) 2%, (D, E, F) 22%, (G, H, I) 42%, and (J, K, L) 62% (voltage: 15 kV; feeding rate: 1 mL/h; needle-collector distance: 25 cm; and temperature: 20 °C). The 500 nm scale bar applies to the left panel images and 100 nm to the middle and right panel ones.
One of the greatest challenges in this study was to determine the explicit role played by relative humidity in the generation of various porous structures of PS during electrospinning. In order to achieve this goal, all the experiments were performed strictly under the same conditions to eliminate the effects from other trivial variations such as temperature and circulating airflow. Figure S2 shows an overview of the fibrous mats fabricated by electrospinning 20 wt.% PS in DMF at four different levels of relative humidity (2%, 22%, 42%, and 62%). Beaded fibers were obtained at both low (2%) and high (62%) relative humidity while uniform fibers with smooth surfaces were produced at relative humidity of 22% and 42%. The average diameters of PS fibers increased from 0.62±0.14 µm to 2.76±0.79 µm with the increase of relative humidity from 2% to 62% (Figure S3). When examining the surface morphology and interior structure, we found that quite different features actually existed in the seemingly similar PS fibers (Figure 2). At relative humidity of 2%, both the PS fibers and the beads displayed a smooth surface and a solid interior (Figure 2, A and B; Figure S4). When the relative humidity was increased to 22%, the fiber diameter increased to 0.91±0.12 µm. In addition, tiny, observable nanofibers began to take shape inside the previously solid PS fibers (Figure 2D), but most of them still adhered to each other duo to the confined interior space. These internal nanofibers became well separated when the relative humidity was further increased to 42% and 62%, resulting in a yarn-like structure with a smooth sheath (Figure 2, E–H).
Figure 5 shows a plausible mechanism for the formation of PS yarns. At a low level of relative humidity (2% in this case), DMF evaporated at its maximum speed but still gave enough time for the fibers to be stretched before solidifying (Figure 5B) owning to its low vapor pressure (0.36 kPa at 20 °C). However, the rapid evaporation of solvent led to the formation of some large beads along the fibers, as shown in Figure S2, A. Furthermore, only solid fibers with smooth surfaces were obtained because vapor-induced phase separation was essentially hindered under a dry atmosphere while thermally induced phase separation was inadequate. At high relative humidity (42% and 62%), the mutual diffusion and penetration of DMF and water vapors played a critical role in generating a porous structure in the fibers (Figure 5C). Due to the fact that water (2.34 kPa) has a higher vapor pressure than that of DMF (0.36 kPa) at the experimental temperature of 20 °C, it is reasonable to assume that the water vapor saturated the nearby region (the air side) of the interface between the jet and the air first, followed by its action as a nonsolvent to precipitate a layer of PS on the surface of the liquid jet (Figure 5C, step 1). The solidified PS layer or sheath helped trap the DMF inside and slowed down its evaporation rate, which possibly prevented the water vapor from rapidly accumulating or condensing on the surface to form big droplets. The water vapor passed through the sheath and constantly entered the PS-DMF phase, resulting in a fast phase separation (Figure 5C, step 2). The PS-DMF phase was further transformed into nanofibers due to whipping and stretching instabilities (Figure 5C, step 3). In the water penetration process, the discharge of fibers and the resultant charge of water vapor might have also provided significant drive to successively pump water into the water-miscible DMF reservoir. Moreover, a small portion of the DMF could be mechanically squeezed out of the fiber in the rapidly thinning course, leaving behind visible pores on the surface (Figure 2, C, E, and G). This observation also corroborated well with the proposed entrapment of DMF by the mechanically strong sheath.
Figure 5.
A schematic illustration detailing the structural characteristics at different stages of an electrospinning process: (1) immediately after ejection from the needle, (2) during whipping and stretching, and (3) upon drying on the collector. The electrospinning was conducted at (B) low relative humidity (2%) and (C) high relative humidity (42% and 62%). The blue, green, and gray colors represent water (vapor), DMF, and solidified PS, respectively. The intensity of green color corresponds to the gradually decreased DMF concentrations from center of the yarn to its outer surface while the white and grey drawings indicate no DMF remaining. The arrows indicate the diffusion directions of the liquid. The presence of solid lines at two sides of the yarn (grey) in (C) refers to the formation of a mechanically strong sheath. The drawing only shows the trend of reduction in size for the yarn but not proportional to the actual scales.
As compared to low volatile DMF and water, THF is an extremely volatile solvent with a vapor pressure of 19.07 kPa at 20 °C. Therefore, it is expected to cause some differences to the electrospun PS fibers. When THF was used, the electrospinning process became unstable under the four different levels of relative humidity, during which the fiber was observed to progress in an almost linear fashion instead of the standard spiral route from the needle to the collector. The overall profiles of the fibers, shown in Figure S5, were essentially opposite to those from the DMF system, namely, relatively uniform fibers were generated at relative humidity of 2% and 62% whereas beaded fibers were obtained at relative humidity of 22% and 42%. Additionally, most of the fibers were present in a ribbon-like shape, with an average diameter around 4.5 µm (Figure S6), which can be attributed to a rapid drying followed by collapse of the liquid jet.36 Surface pores were observed on the samples prepared at relative humidity of 22%, 42%, and 62%, but not at 2% (Figure 3), agreed well with the previous reports.24,25,33 For the sample fabricated at relative humidity of 22%, the surface pores were elliptical in shape (Figure 3, D–F), with a long axis diameter of 279±82 nm and a short axis diameter of 78±16 nm (Figure S7, A and B). Furthermore, these elliptical pores were more or less aligned along the long axis of the fiber, which could be ascribed to the stretching of the fibers in the electric field.33 When the relative humidity was increased to 42%, the pores were transformed into a round shape and became more uniform in size (Figure 3, G and H). With a further increase of relative humidity to 62%, the surface pores became less uniform and both big and small pores with irregular shapes coexisted on the surface (Figure 3, J and K). After examining the cross sections of these fibers (Figure 3, F, I, and L), as well as the scattered beads (Figure S8), we confirmed that all of them actually had solid interiors covered by porous outer layers. The cross-sectional images also indicate that the surface pores were quite shallow and were not connected internally with each other. Specifically, the depths of the surface pores, estimated from their cross sections, were 0.04 µm, 0.37 µm and 0.49 µm for the samples prepared at relative humidity of 22%, 42% and 62%, respectively, correlating well with the increased amounts of the available water vapor (Figure S9).
Although PS fibers with porous surfaces have been reported in several previous studies, none of them succeeded in giving an affirmative explanation on the reason why the interior pores were missing when THF was used as a solvent.24,25,29 Furthermore, the breath figure model, which has been widely employed to elucidate the formation mechanism of surface pores on a 2-D system such as a flat film,38 may not be applicable to the 3-D system such as a fiber. In the breath figure model, the gravity serves as the driving force to keep water droplets attached to the film surface. However, the pores were created around the entire surface on a fiber, with a more or less uniform distribution. It was impossible to achieve such a configuration even considering the fiber rotated during traveling a short distance in air. After carefully analyzing our results as well as those from the previous studies, we came up with a plausible mechanism as shown in Figure 6. At low relative humidity (Figure 6A), the extremely volatile THF evaporated immediately after the initiation of liquid jet, leaving behind a solidified fiber with a smooth surface and solid interior, as confirmed by SEM observation (Figure 3, A–C). Since THF has a much higher vapor pressure than that of water, the jet-air interface was always saturated by THF even under high levels of relative humidity (22%, 42%, and 62%), which effectively hindered the formation of a sheath as well as internal pores by preventing the water vapor from penetrating into the jet and inducing phase separation. The evaporation of THF absorbed a great amount of heat and cooled the nearby environment and water vapor began to condense in the vicinity of the jet-air interface. Meanwhile, the positive charges distributed on the surface of fiber polarized and attracted the condensed tiny water droplets, creating numerous nano- or micro-scale water pockets, which then dried to generate water imprints or “pores” on the fiber surface (Figure 6B). Therefore, a fiber with pores on the entire surface could be created through charge-charge interactions instead of the unidirectional gravity.
Figure 6.
A diagram illustrating the changes to structural characteristics at positions of (1) immediately after ejection from the needle, (2) during whipping and stretching, and (3) upon drying on the collector from electrospinning conducted at (A) low relative humidity (2%) and (B) high relative humidity (22%, 42%, and 62%). The blue, red, and grey colors represent water, THF, and solidified PS, respectively. The intensity of red color corresponds to the concentration of THF while the grey drawing indicates no THF remaining. The blue spheres refer to the condensed water vapor in the form of tiny droplets which should not be deemed as the actual size relative to the PS fiber. The arrows indicate the diffusion directions of the liquid. The drawing only shows the trend of reduction in size for the fiber but not proportional to the actual scales.
From the above analysis, it is clear that the low vapor pressure of DMF plays an important role in creating porous internal structures, while the high vapor pressure of THF plays a critical role in patterning the surface with pores but discouraging the formation of internal pores. Both solvents require the presence of water vapor as the nonsolvent for PS. In order to further validate this mechanism, a mixture of DMF and THF with a mass ration of 1:1 was utilized as the solvent for PS. Electrospinning of this solution was as stable as that of PS in DMF alone and produced highly uniform fibers in all the four different levels of relative humidity (Figure S10). Figure S11 shows that the relative humidity has less effect on the fiber size as compared to those electrospun from DMF and THF solutions. It is also worth noting that DMF and THF in the mixture offset each other’s deficiency of generating beaded fibers and get well balanced to yield almost bead-free fibers at proper relative humidity. As expected, solid fibers with smooth surfaces were collected by conducting the electrospinning at low relative humidity (2%) (Figure 4, A–C), further confirming the central role of water vapor in generating porous structures both internally and externally. At relative humidity of 22%, only solid PS fibers were fabricated from the DMF-THF mixture solution (Figure 4, D–F) while the phase separation was quite obvious at the same relative humidity when only DMF was used (Figure 2D), demonstrating the effect of THF in resisting the generation of interior pores. A phase separation was observed at relative humidity of 42% (Figure 4I) and it led to the formation of well-separated fibrils at relative humidity of 62% (Figure 4L), verifying the crucial role of DMF in creating internal pores to generate a yarn-like structure.
Interestingly, instead of forming recessed pores or holes as observed in Figure 3, numerous protruding “nano-pillars” were found to stand on the fiber surface at relative humidity of 22%, 42%, and 62%. Figure 7 gives an example of the nano-pillars, produced at the relative humidity of 22%, whose average height was measured to be 30.1±4.3 nm. For the solid fibers with smooth surfaces generated at low relative humidity (2%), the formation mechanism for DMF and THF systems should also be applicable for the mixture solvent except for subtle fiber diameter changes. In order to explain the formation of the surface nano-pillars, we only need to combine the DMF and THF models together, as shown in Figure 8. At high relative humidity, the competitive penetration and diffusion of mixture solvent had three major effects on the fibers: i) formation of the mechanically strong sheath due to the interface precipitation; ii) internal phase separation caused by the penetration of water vapor into mixture solvents; and iii) accumulation of condensed water onto the surface of the fibers resulting from the evaporative cooling and charge-charge interaction. Because a mechanically strong sheath was created first, it was able to withstand the attack of accumulated water droplets, leaving no pores as seen from the THF system (Figure 3). Furthermore, a small portion of THF, entrapped in the fiber, desperately wanted to get out of the confinement. The dense PS sheath probably blocked most of the regions and forbade a fast evaporation of THF, but some weak points might still exist, where the rate of the THF evaporation could be very fierce. The erupted PS THF solution encountered the surface water droplets and precipitated to nano-pillars afterwards. The above hypothesis was well supported by the decreased sizes of the nano-pillars with the increase of relative humidity as seen in Figure 4, E, H, and K, in which the high evaporation rate of THF at lower relative humidity tended to bring out more PS, leading to the formation of bigger nano-pillars.
Figure 7.
SEM images showing (A) a PS fiber with nano-pillars on the surface and (B) enlarged area boxed in (A). The electrospinning was conducted with a 20 wt.% PS solution in DMF/THF (1:1 w:w) under relative humidity of 22% (voltage: 15 kV; feeding rate: 1 mL/h; needle-collector distance: 25 cm; and temperature: 20 °C).
Figure 8.
A schematic showing the formation mechanism of PS yarns with nano-pillars on the surface, as well as structural changes to the jet at different positions: (1) immediately after ejection from the needle, (2) during whipping and stretching, and (3) upon drying on the collector. The electrospinning was conducted with a 20 wt.% PS solution in DMF/THF (w:w 1:1) at high relative humidity (62%). The intensities of the green and red colors correspond to the amounts of DMF and THF inside the liquid jet while the white and grey drawings indicate no residual DMF and THF. The blue spheres refer to the condensed water moisture in the form of tiny droplets which should not be deemed as the actual size relative to the PS yarn. The arrows indicate the diffusion directions of the liquid. The presence of solid lines at two sides of the yarn (grey) refers to the formation of a mechanically strong sheath. The drawing only shows the trend of reduction in size for the yarn but not proportional to the actual scales.
CONCLUSIONS
We have demonstrated a convenient and reliable approach to the fabrication of internally porous PS yarns, which consisted of bundles of nanofibrils and were wrapped by smooth sheaths on the surfaces. In order to understand the mechanism responsible for the formation of PS yarns, we tested three solvent systems (i.e., DMF, THF, and DMF-THF mixture) under four controlled levels of relative humidity. We found that vapor-induced phase separation, rather than the long acclaimed thermally induced phase separation, played an essential role in the generation of yarns with an inherently porous structure. This claim was confirmed by the observation that only solid fibers with smooth surfaces were produced at low relative humidity (2%), where only thermally induced phase separation could occur. On the contrary, both internally and externally porous PS fibers/yarns were obtained by conducting electrospinning at high relative humidity (22%, 42%, and 62%), where vapor-induced phase separation began to play a major role. From these results, we also validated the explicit role of relative humidity or the presence of water vapor, a factor that was largely underestimated in previous studies, in the formation of pores in the interiors of electrospun fibers. In addition, the charge-charge interaction between the charged liquid jet and the polarized vapor and/or its condensates could serve as a driving force for vapor diffusion and penetration, as well as for generating pores on the entire surface of a PS yarn. Furthermore, we discovered that the low vapor pressure of DMF facilitated the formation of internal porosity while the high vapor pressure of THF hindered the formation of interior pores and could only produce surface porosity. The PS yarns, with controlled internal porosity and surface roughness, hold a great potential for a wide variety of applications such as filtration, catalysis, and tissue engineering. Importantly, our new understanding of the mechanism responsible for the formation of pores can provide useful guidelines for the fabrication of nanofibers with controlled porosity and pore structure by electrospinning.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by the NIH (R01 AR058332) and startup funds from Georgia Institute of Technology.
Footnotes
Supporting Information Available: A photograph of the typical setup for electrospinning; SEM images of fibers/yarns electrospun from PS solutions in DMF, THF and DMF-THF, respectively, at different relative humidity and their corresponding size distributions. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Dawson R, Cooper AI, Adams DJ. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012;37:530–563. [Google Scholar]
- 2.Tsioptsias C, Stefopoulos A, Kokkinomalis I, Papadopoulou L, Panayiotou C. Development of micro- and nano-porous composite materials by processing cellulose with ionic liquids and supercritical CO2. Green Chemistry. 2008;10:965–971. [Google Scholar]
- 3.Yang SY, Ryu I, Kim HY, Kim JK, Jang SK, Russell TP. Nanoporous Membranes with Ultrahigh Selectivity and Flux for the Filtration of Viruses. Adv. Mater. 2006;18:709–712. [Google Scholar]
- 4.Cooper AI. Nanoporous Organics Enter the Cage Age. Angew. Chem. Int. d. 2011;50:996–998. doi: 10.1002/anie.201006664. [DOI] [PubMed] [Google Scholar]
- 5.Colombo P. In Praise of Pores. Science. 2008;322:381–383. doi: 10.1126/science.1162962. [DOI] [PubMed] [Google Scholar]
- 6.Theato P, Ungar G. Nanoporous ordered materials: Osmotically shocked. Nature Materials. 2012;11:16–17. doi: 10.1038/nmat3210. [DOI] [PubMed] [Google Scholar]
- 7.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for Direct Fluidic Intracellular Access. Nano Lett. 2012;12:3881–3886. doi: 10.1021/nl204051v. [DOI] [PubMed] [Google Scholar]
- 8.Ali M, Ramirez P, Nguyen HQ, Nasir S, Cervera J, Mafe S, Ensinger W. Single Cigar-Shaped Nanopores Functionalized with Amphoteric Amino Acid Chains: Experimental and Theoretical Characterization. ACS Nano. 2012;6:3631–3640. doi: 10.1021/nn3010119. [DOI] [PubMed] [Google Scholar]
- 9.Ou JZ, Rani RA, Ham M-H, Field MR, Zhang Y, Zheng H, Reece P, Zhuiykov S, Sriram S, Bhaskaran M, Kaner RB, Kalantar-zadeh K. Elevated Temperature Anodized Nb2O5: A Photoanode Material with Exceptionally Large Photoconversion Efficiencies. ACS Nano. 2012;6:4045–4053. doi: 10.1021/nn300408p. [DOI] [PubMed] [Google Scholar]
- 10.Xin HL, Pach EA, Diaz RE, Stach EA, Salmeron M, Zheng H. Revealing Correlation of Valence State with Nanoporous Structure in Cobalt Catalyst Nanoparticles by In Situ Environmental TEM. ACS Nano. 2012;6:4241–4247. doi: 10.1021/nn3007652. [DOI] [PubMed] [Google Scholar]
- 11.Tappan BC, Steiner SA, Luther EP. Nanoporous Metal Foams. Angew. Chem. Int. d. 2010;49:4544–4565. doi: 10.1002/anie.200902994. [DOI] [PubMed] [Google Scholar]
- 12.Boissiere C, Grosso D, Chaumonnot A, Nicole L, Sanchez C. Aerosol Route to Functional Nanostructured Inorganic and Hybrid Porous Materials. Adv. Mater. 2011;23:599–623. doi: 10.1002/adma.201001410. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Lu K. Hierarchically Branched Titania Nanotubes with Tailored Diameters and Branch Numbers. Langmuir. 2012;28:2937–2943. doi: 10.1021/la204154h. [DOI] [PubMed] [Google Scholar]
- 14.Martens JA, Jammaer J, Bajpe S, Aerts A, Lorgouilloux Y, Kirschhock CEA. Simple synthesis recipes of porous materials. Microporous Mesoporous Mater. 2011;140:2–8. [Google Scholar]
- 15.Zhang H, Hussain I, Brust M, Butler MF, Rannard SP, Cooper AI. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nature Materials. 2005;4:787–793. doi: 10.1038/nmat1487. [DOI] [PubMed] [Google Scholar]
- 16.Ryckman JD, Liscidini M, Sipe JE, Weiss SM. Direct Imprinting of Porous Substrates: A Rapid and Low-Cost Approach for Patterning Porous Nanomaterials. Nano Lett. 2010;11:1857–1862. doi: 10.1021/nl1028073. [DOI] [PubMed] [Google Scholar]
- 17.George SM. Atomic Layer Deposition: An Overview. Chem. Rev. 2010;110:111–131. doi: 10.1021/cr900056b. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Wang Y, Xia Y. Electrospinning nanofibers as uniaxially aligned arrays and layerby- layer stacked films. Adv. Mater. 2004;16:361–366. [Google Scholar]
- 19.Li D, Ouyang G, McCann JT, Xia Y. Collecting Electrospun Nanofibers with Patterned Electrodes. Nano Lett. 2005;5:913–916. doi: 10.1021/nl0504235. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Xia Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004;16:1151–1170. [Google Scholar]
- 21.Li D, Wang Y, Xia Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003;3:1167–1171. [Google Scholar]
- 22.Li D, Xia Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004;4:933–938. [Google Scholar]
- 23.Bognitzki M, Czado W, Frese T, Schaper A, Hellwig M, Steinhart M, Greiner A, Wendorff JH. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001;13:70–72. [Google Scholar]
- 24.Megelski S, Stephens JS, Chase DB, Rabolt JF. Micro- and Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromolecules. 2002;35:8456–8466. [Google Scholar]
- 25.Casper CL, Stephens JS, Tassi NG, Chase DB, Rabolt JF. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and Molecular Weight in the Electrospinning Process. Macromolecules. 2004;37:573–578. [Google Scholar]
- 26.McCann JT, Li D, Xia Y. Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J. Mater. Chem. 2005;15:735–738. [Google Scholar]
- 27.McCann JT, Marquez M, Xia Y. Highly porous fibers by electrospinning into a cryogenic liquid. J. Am. Chem. Soc. 2006;128:1436–1437. doi: 10.1021/ja056810y. [DOI] [PubMed] [Google Scholar]
- 28.Kongkhlang T, Kotaki M, Kousaka Y, Umemura T, Nakaya D, Chirachanchai S. Electrospun Polyoxymethylene: Spinning Conditions and Its Consequent Nanoporous Nanofiber. Macromolecules. 2008;41:4746–4752. [Google Scholar]
- 29.Lin J, Ding B, Yu J, Hsieh Y. Direct Fabrication of Highly Nanoporous Polystyrene Fibers via Electrospinning. Acs Applied Materials & Interfaces. 2010;2:521–528. doi: 10.1021/am900736h. [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Huang Q, Liu BD, Bando Y, Hsieh YL, Mukherjee AK. Macroporous Silicon Oxycarbide Fibers with Luffa-like Superhydrophobic Shells. J. Am. Chem. Soc. 2009;131:10346–10347. doi: 10.1021/ja902757a. [DOI] [PubMed] [Google Scholar]
- 31.Rudisill SG, Wang Z, Stein A. Maintaining the Structure of Templated Porous Materials for Reactive and High-Temperature Applications. Langmuir. 2012;28:7310–7324. doi: 10.1021/la300517g. [DOI] [PubMed] [Google Scholar]
- 32.Lu P, Huang Q, Mukherjee A, Hsieh YL. Effects of polymer matrices to the formation of silicon carbide (SiC) nanoporous fibers and nanowires under carbothermal reduction. J. Mater. Chem. 2011;21:1005–1012. [Google Scholar]
- 33.Dayal P, Liu J, Kumar S, Kyu T. Experimental and Theoretical Investigations of Porous Structure Formation in Electrospun Fibers. Macromolecules. 2007;40:7689–7694. [Google Scholar]
- 34.Pai C-L, Boyce MC, Rutledge GC. Morphology of Porous and Wrinkled Fibers of Polystyrene Electrospun from Dimethylformamide. Macromolecules. 2009;42:2102–2114. [Google Scholar]
- 35.Lee KH, Kim HY, Bang HJ, Jung YH, Lee SG. The change of bead morphology formed on electrospun polystyrene fibers. Polymer. 2003;44:4029–4034. [Google Scholar]
- 36.Wang LF, Pai CL, Boyce MC, Rutledge GC. Wrinkled surface topographies of electrospun polymer fibers. Appl. Phys. Lett. 2009;94 [Google Scholar]
- 37.Lin J, Ding B, Yang J, Yu J, Sun G. Subtle regulation of the micro- and nanostructures of electrospun polystyrene fibers and their application in oil absorption. Nanoscale. 2012;4:176–182. doi: 10.1039/c1nr10895f. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasarao M, Collings D, Philips A, Patel S. Three-dimensionally ordered array of air bubbles in a polymer film. Science. 2001;292:79–83. doi: 10.1126/science.1057887. [DOI] [PubMed] [Google Scholar]
- 39.Wannatong L, Sirivat A, Supaphol P. Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym. Int. 2004;53:1851–1859. [Google Scholar]
- 40.Lange NA, Speight JG. Lange's handbook of chemistry. New York: McGraw-Hill; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.