Abstract
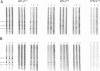
To determine the serologic cross-reactivity between human T-cell lymphotropic virus type I (HTLV-I) and parasite antigens, we measured antibody responses against HTLV-I, Plasmodium falciparum, Plasmodium vivax, and Brugia malayi in serum specimens obtained from regions where malaria (n = 482) and filariasis (n = 101) are endemic. Analysis of immune reactivity to HTLV-I antigens showed that specimens from regions where malaria is endemic had significantly higher rates of enzyme immunoassay (EIA) reactivity (76 of 482 [15.8%] than those from regions where filariasis is endemic (0 of 101 [0%]). Western blot (immunoblot) analysis of the HTLV-I EIA-reactive specimens demonstrated predominant Gag reactivity (HTLV-Iind). Only two specimens each from Indonesia and Brazil and four specimens from Papua New Guinea had Env reactivity by radioimmunoprecipitation analysis. Furthermore, a positive correlation between HTLV-EIA and titers of antibody to the blood stage of P. falciparum (rs = 0.24, P < 0.005) was discerned; no correlation was observed between antibodies to the blood stage or the circumsporozoite protein of P. vivax and the circumsporozoite protein of P. falciparum. In addition, P. falciparum-infected erythrocyte lysate specifically abrogated binding of Gag-specific antibodies in HTLV-Iind specimens from regions where malaria is endemic without affecting binding in HTLV-I-seropositive specimens, suggesting that the immunologic cross-reactivity between HTLV Gag proteins and malaria parasites is restricted to the blood-stage antigens of plasmodia in specimens from regions where malaria is endemic. However, HTLV-seroindeterminate specimens from the United States did not demonstrate serologic cross-reactivity, suggesting that antigenic mimicry of HTLV proteins extends to other nonplasmodial antigens as well.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher D. M., Goudsmit J., Pomeroy K. L., Garruto R. M., Bakker M., Ono S. G., Elliot N., Harris K., Askins H., Eldadah Z. Antibodies to HTLV-I in populations of the southwestern Pacific. J Med Virol. 1988 Dec;26(4):339–351. doi: 10.1002/jmv.1890260402. [DOI] [PubMed] [Google Scholar]
- Banki K., Maceda J., Hurley E., Ablonczy E., Mattson D. H., Szegedy L., Hung C., Perl A. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar R. J., Gigase P. L., Melbye M., Kestens L., Sarin P. S., Bodner A. J., Demedts P., Stevens W. J., Paluku L., Delacollette C. ELISA HTLV retrovirus antibody reactivity associated with malaria and immune complexes in healthy Africans. Lancet. 1985 Sep 7;2(8454):520–523. doi: 10.1016/s0140-6736(85)90461-1. [DOI] [PubMed] [Google Scholar]
- Clarke M. D., Carney W. P., Cross J. H., Hadidjaja P., Oemijati S., Joesoef A. Schistosomiasis and other human parasitoses of Lake Lindu in central Sulawesi (Celebes), Indonesia. Am J Trop Med Hyg. 1974 May;23(3):385–392. doi: 10.4269/ajtmh.1974.23.385. [DOI] [PubMed] [Google Scholar]
- Delaporte E., Peeters M., Simoni M., Piot P. HTLV-I infection in western equatorial Africa. Lancet. 1989 Nov 18;2(8673):1226–1226. doi: 10.1016/s0140-6736(89)91840-0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Gessain A., Boeri E., Yanagihara R., Gallo R. C., Franchini G. Complete nucleotide sequence of a highly divergent human T-cell leukemia (lymphotropic) virus type I (HTLV-I) variant from melanesia: genetic and phylogenetic relationship to HTLV-I strains from other geographical regions. J Virol. 1993 Feb;67(2):1015–1023. doi: 10.1128/jvi.67.2.1015-1023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes C. G., Burans J. P., Oberst R. B. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis. 1991 Feb;163(2):257–262. doi: 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Robert-Guroff M., Metzgar R. S., Franchini G., Kalyanaraman V. S., Palker T. J., Gallo R. C. Monoclonal antibody against human T cell leukemia virus p19 defines a human thymic epithelial antigen acquired during ontogeny. J Exp Med. 1983 Mar 1;157(3):907–920. doi: 10.1084/jem.157.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe J. M., Ferrari B., Andriamananpisoa R., Pavia A. A. Malaria invasion of human erythrocytes. Synthesis of peptides relevant to glycophorin A and evaluation of their inhibitory properties. Int J Pept Protein Res. 1988 Aug;32(2):104–116. [PubMed] [Google Scholar]
- Lal R. B., Ottesen E. A. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988 Nov;158(5):1034–1037. doi: 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Rudolph D. L., Coligan J. E., Brodine S. K., Roberts C. R. Failure to detect evidence of human T-lymphotropic virus (HTLV) type I and type II in blood donors with isolated gag antibodies to HTLV-I/II. Blood. 1992 Jul 15;80(2):544–550. [PubMed] [Google Scholar]
- Lal R. B., Rudolph D. L., Griffis K. P., Kitamura K., Honda M., Coligan J. E., Folks T. M. Characterization of immunodominant epitopes of gag and pol gene-encoded proteins of human T-cell lymphotropic virus type I. J Virol. 1991 Apr;65(4):1870–1876. doi: 10.1128/jvi.65.4.1870-1876.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. B., Rudolph D. L., Nerurkar V. R., Yanagihara R. Humoral responses to the immunodominant gag and env epitopes of human T-lymphotropic virus type I among Melanesians. Viral Immunol. 1992 Winter;5(4):265–272. doi: 10.1089/vim.1992.5.265. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Freeman J. D. Human endogenous retroviruslike genome with type C pol sequences and gag sequences related to human T-cell lymphotropic viruses. J Virol. 1987 Dec;61(12):4060–4066. doi: 10.1128/jvi.61.12.4060-4066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns A., Blattner W. A. The epidemiology of the human T-cell lymphotrophic virus type I and type II: etiologic role in human disease. Transfusion. 1991 Jan;31(1):67–75. doi: 10.1046/j.1537-2995.1991.31191096189.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin G. L., Benedik M. J., Campbell G. H. Repeated immunogenic amino acid sequences of Plasmodium species share sequence homologies with proteins from humans and human viruses. Am J Trop Med Hyg. 1987 Sep;37(2):258–262. doi: 10.4269/ajtmh.1987.37.258. [DOI] [PubMed] [Google Scholar]
- Nerurkar V. R., Miller M. A., Leon-Monzon M. E., Ajdukiewicz A. B., Jenkins C. L., Sanders R. C., Godec M. S., Garruto R. M., Yanagihara R. Failure to isolate human T cell lymphotropic virus type I and to detect variant-specific genomic sequences by polymerase chain reaction in Melanesians with indeterminate western immunoblot. J Gen Virol. 1992 Jul;73(Pt 7):1805–1810. doi: 10.1099/0022-1317-73-7-1805. [DOI] [PubMed] [Google Scholar]
- Re M. C., Tommaseo M., Furlini G., La Placa M. High prevalence of serum antibody against human T cell leukemia virus type I (HTLV-I) among the Bismam Asmat population (Indonesian New Guinea). AIDS Res Hum Retroviruses. 1989 Oct;5(5):551–554. doi: 10.1089/aid.1989.5.551. [DOI] [PubMed] [Google Scholar]
- Renan M. J. Sequence homologies in the control regions of c-myc, c-fos, HTLV and the interleukin-2 receptor. Cancer Lett. 1985 Aug;28(1):69–76. doi: 10.1016/0304-3835(85)90094-1. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler S. G., Fang C., Williams A. Retroviral infections transmitted by blood transfusion. Yale J Biol Med. 1990 Sep-Oct;63(5):353–360. [PMC free article] [PubMed] [Google Scholar]
- Sato A., Isaka Y., Morita F., Ishii A., Goto Y., Imai J., Igarashi H., Yoshie O., Hinuma Y. Human sera from varicella-zoster virus (VZV) infections cross-react with human T cell leukaemia virus type 1 (HTLV-1): common epitopes in VZV gene 22 protein and HTLV-1 p19 gag protein. J Gen Virol. 1992 Nov;73(Pt 11):2969–2973. doi: 10.1099/0022-1317-73-11-2969. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Jameson B. A., Lopalco L., Siccardi A. G., Weiss R. A., Moore J. P. Conserved structural features in the interaction between retroviral surface and transmembrane glycoproteins? AIDS Res Hum Retroviruses. 1992 Sep;8(9):1571–1580. doi: 10.1089/aid.1992.8.1571. [DOI] [PubMed] [Google Scholar]
- Shattles W. G., Brookes S. M., Venables P. J., Clark D. A., Maini R. N. Expression of antigen reactive with a monoclonal antibody to HTLV-1 P19 in salivary glands in Sjögren's syndrome. Clin Exp Immunol. 1992 Jul;89(1):46–51. doi: 10.1111/j.1365-2249.1992.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. P., Udhayakumar V., Alpers M. P., Povoa M. M., Oloo A. J., Ruebush T. K., 2nd, Lal A. A. Natural antibody responses against the non-repeat-sequence-based B-cell epitopes of the Plasmodium falciparum circumsporozoite protein. Infect Immun. 1993 Jun;61(6):2425–2433. doi: 10.1128/iai.61.6.2425-2433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suni J., Närvänen A., Wahlström T., Lehtovirta P., Vaheri A. Monoclonal antibody to human T-cell leukemia virus p19 defines polypeptide antigen in human choriocarcinoma cells and syncytiotrophoblasts of first-trimester placentas. Int J Cancer. 1984 Mar 15;33(3):293–298. doi: 10.1002/ijc.2910330303. [DOI] [PubMed] [Google Scholar]
- Tajima K., Fujita K., Tsukidate S., Oda T., Tominaga S., Suchi T., Hinuma Y. Seroepidemiological studies on the effects of filarial parasites on infestation of adult T-cell leukemia virus in the Goto Islands, Japan. Gan. 1983 Apr;74(2):188–191. [PubMed] [Google Scholar]
- Verdier M., Leonard G., Sangare A., Prince-David M., Bonis J., Barin F., Denis F. Confirmatory tests for human T-cell leukemia virus type 1 (HTLV-1): western blot compared with RIPA on African sera. J Virol Methods. 1990 Dec;30(3):283–289. doi: 10.1016/0166-0934(90)90070-v. [DOI] [PubMed] [Google Scholar]
- Whetstone C. A., Sayre K. R., Dock N. L., VanDerMaaten M. J., Miller J. M., Lillehoj E., Alexander S. S. Examination of whether persistently indeterminate human immunodeficiency virus type 1 Western immunoblot reactions are due to serological reactivity with bovine immunodeficiency-like virus. J Clin Microbiol. 1992 Apr;30(4):764–770. doi: 10.1128/jcm.30.4.764-770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R., Hefner C., Ajdukiewicz A. B. Human T-lymphotropic virus type I infection among blood donors in the Solomon Islands. Transfusion. 1992 Jan;32(1):89–89. doi: 10.1046/j.1537-2995.1992.32192116444.x. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., Nerukar V. R., Ajdukiewicz A. B. Comparison between strains of human T lymphotropic virus type I isolated from inhabitants of the Solomon Islands and Papua New Guinea. J Infect Dis. 1991 Sep;164(3):443–449. doi: 10.1093/infdis/164.3.443. [DOI] [PubMed] [Google Scholar]