Abstract
Functional loss of melanocortin-4 receptor (MC4R) activity leads to hyperphagia and an obese, glucose intolerant phenotype. We have previously established that inhibition of angiotensin-converting enzyme (ACE) reduces food intake, body weight and glucose homeostasis in diet-induced obesity. The current study assessed the effect of ACE inhibitor treatment in MC4R-deficient female rats on body weight, adiposity and glucose tolerance. Rats homozygous (HOM) for a loss of function Mc4r mutation had an obese phenotype relative to their wildtype (WT) littermates. Inhibition of ACE for 8 weeks produced reductions in body weight gain in both HOM and WT rats, however, food intake was only reduced in HOM rats. Weight loss following ACE inhibitor treatment was specific to fat mass while lean mass was unaffected. HOM rats were severely glucose intolerant and insensitive to exogenous insulin injection, and treatment with an ACE inhibitor improved both glucose tolerance and insulin sensitivity in HOM rats although not fully to the level of WT rats. The current study indicates that HOM rats are sensitive to the anorectic effects of ACE inhibition, unlike their WT littermates. This resulted in a more rapid reduction in body weight gain and a more substantial loss of adipose mass in HOM animals, relative to WT animals, treated with an ACE inhibitor. Overall, these data demonstrate that MC4R signaling is not required for weight loss following treatment with an ACE inhibitor.
Keywords: Melanocortin, MC4R, ACE inhibitor, renin-angiotensin system, captopril
Introduction
The melanocortin-4 receptor (MC4R) is an important regulator of energy homeostasis [1–3] and functional loss of MC4R activity produces an obese phenotype in both animal models [4, 5] and humans [6, 7]. The obesity resulting from loss of MC4R function is associated with increased food intake, decreased energy expenditure, increased body weight and adipose tissue mass, and glucose intolerance/insulin resistance in both male and female animals [1, 4–6, 8]. MC4Rs are a part of the well-described peptide-signaling system that helps regulate energy balance. In particular, leptin and insulin act at their receptors in the arcuate nucleus of the hypothalamus (ARC) stimulating proopiomelanocortin (POMC) neurons that release α-melanocyte-stimulating hormone (αMSH), which in turn stimulates melanocortin receptors in several hypothalamic and other brain areas to reduce food intake and increase energy expenditure [9]. Leptin and insulin also act in the ARC to reduce activity of agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons [10]. Both AgRP and NPY are anabolic peptides. AgRP is an endogenous antagonist of MC4R and NPY is an agonist at Y1 and Y5 receptors, and both peptides cause increased food intake and body fat/weight [11].
The renin-angiotensin system (RAS) has recently been recognized as an important factor in the regulation of energy balance and glucose homeostasis [12–15]. Angiotensin-converting enzyme (ACE) inhibition [16–18], angiotensin receptor blockade [19–21], and genetic ablation of the RAS [22–24] each results in a phenotype of reduced body weight and improved glucose tolerance. Conversely, increased expression of components of the RAS can lead to increased adiposity and impaired glucose tolerance and insulin sensitivity [25–27]. The metabolic effects of the RAS appear to be independent of energy balance circuits relying on melanocortin or NPY/AgRP signaling [28]. The current study asked whether ACE inhibition reduces body weight in MC4R-deficient rats. Given that ACE inhibition acts independently of melanocortin and NPY/AgRP signaling, it was hypothesized that ACE inhibition would reduce body weight, adiposity and improve glucose tolerance in MC4R deficient rats.
Methods
Animals
Female littermate offspring (n = 36) resulting from mating of rats heterozygous for a nonfunctional mutation in Mc4r (Mc4rK314X) and that had been outcrossed for at least 7 generations [5]. Genotyping was performed by KBiosciences (Hoddesdon, UK) using the KASPar SNP Genotyping system, as we have previously described [29]. Wildtype (Mc4r+/+ [WT]) and homozygous (Mc4r−/− [HOM]) animals were weaned at postnatal day 21 and group-housed (2–3 per cage) until 8 weeks of age, after which they were housed individually. Rats were maintained at the Metabolic Diseases Institute of the University of Cincinnati on a 12/12-h light/dark cycle at 25 ± 2°C in an AAALAC-accredited facility. All rats had ad libitum access to water and a pelleted high-fat diet (HFD; D03082706, 4.54 kcal/g AFE, 15% calories protein, 46% calories carbohydrate, and 40% calories fat, Open Source Diets, New Brunswick, NJ) starting at 10 weeks of age. Rats had access to enrichment in their home-cages (red rat retreat; Bioserve, MD, USA). The University of Cincinnati Institutional Animal Care and Use Committee approved all procedures for animal use.
Groups and treatment
At 10 weeks of age, half the animals of each genotype were continued on normal water (WT, n=9; HOM, n=9) and the other half were provided with drinking water containing the ACE inhibitor captopril (Sigma-Aldrich, St. Louis, MO) at a dose of 0.2 mg/mL (WT+, n=9; HOM+, n=9). Rats were maintained on this regimen for 8 weeks.
Food intake and body weight
Food intake and body weight of rats were measured daily for the first 21 days of the experiment (only weekly data depicted). Subsequently, body weight and food intake were measured weekly for the remainder of the 8-week experiment.
Food intake following fasting
Following 5 weeks of control or ACE-inhibitor treatment, animals were fasted for 24 h at the beginning of the dark phase. Food intake was assessed on a baseline day and after re-feeding at the 1-, 2-, 4- and 24-h time-points.
Body composition
Body composition (fat and lean mass) was assessed using nuclear magnetic resonance (NMR) technology (Echo NMR, Waco, TX) in conscious rats. This was performed prior to the commencement of ACE-inhibitor treatment and again at the completion of the experiment.
Intraperitoneal glucose- and insulin-tolerance tests
Glucose-tolerance was assessed after 6 weeks of treatment. Following a 14-h fast, rats were given an intraperitoneal (i.p.) injection of 50% dextrose (1 g/kg). Blood glucose was assessed at baseline, 30, 60, 90 and 120 min (Accuchek; Roche Diagnostics, Indianapolis, IN). For an insulin tolerance test performed a week later, fed-state animals were given an i.p. injection of human insulin (Humalin R, 0.5 U/kg). Blood glucose was assessed at baseline, 15, 30 and 60 min.
Statistical Analyses
All data are displayed as mean ± S.E.M. Data were analyzed using Statistica 7 (StatSoft, Tulsa, OK, USA). Data were analyzed using two-way (genotype x drug) analysis of variance (ANOVA), or three-way with repeated-measures where appropriate. All ANOVAs were followed by Fisher’s least significant differences (LSD) post hoc test if significant overall interactions were observed. The null hypothesis was rejected at the 0.05 level.
Results
Body weight
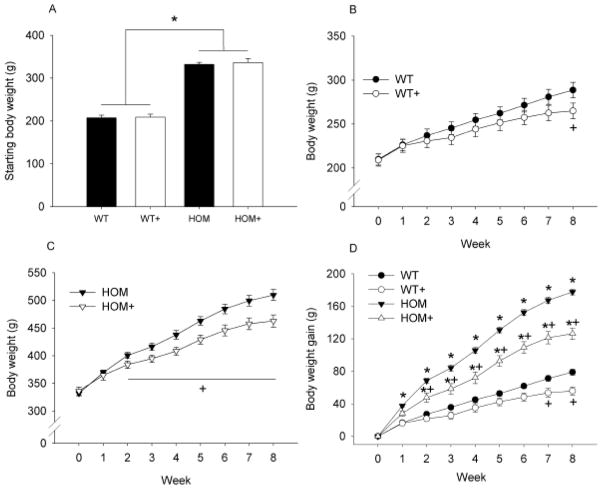
Deficiency of MC4R signaling produced an obese phenotype in female rats compared with their WT controls; body weight immediately prior to the commencement of the experiment was ~60% higher in HOM (334.2 +/− 5.89) relative to WT (209.4 +/− 6.6) rats (see Figure 1A; p<0.05). ACE inhibition reduced body weight in HOM+ rats relative to HOM rats by Week 2 of treatment (p<0.05), and this persisted throughout the experiment (see Figure 1C). The parallel difference in body weight between WT+ and WT rats occurred later than in HOM rats, with a body weight reduction observed only after 8 weeks (see Figure 1B; p<0.05). Over the course of the experiment body weight gain was significantly greater in HOM rats than in all other groups (p<0.05), HOM+ rats gained more weight than both WT groups (p<0.05), and WT rats gained more weight than WT+ animals (see Figure 1D; p<0.05).
Figure 1. The effect of MC4R deficiency and ACE inhibition on body weight in female rats.
MC4R deficiency produced an obese phenotype in female rats compared with their WT littermates (A), 8-weeks of ACE inhibition reduced body weight in both WT (B) and HOM (C) rats. Body weight gain was greater in HOM rats regardless of ACE inhibitor treatment and was reduced in both genotypes by ACE inhibition (D). WT (wildtype), WT+ (WT+ACE inhibitor), HOM (homozygous), HOM+ (homozygous+ACE inhibitor). * = difference from WT control (p<0.05) + = difference between ACE inhibitor and control (p<0.05).
Food and water intake
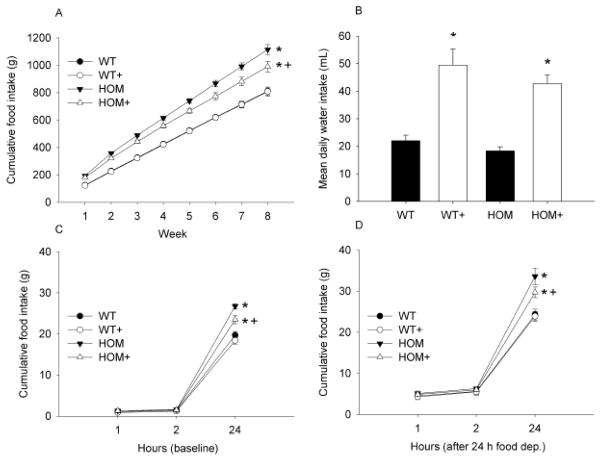
Cumulative food intake was greater in HOM rats relative to WT rats irrespective of treatment with the ACE inhibitor (p<0.05). There was a significant reduction in food intake by HOM+ relative to HOM animals (p<0.05). Over the 8-week experimental period there were no differences observed in cumulative food intake between WT and WT+ groups (see Figure 2A). Mean daily water intake was greater in ACE inhibitor-treated animals than CON animals regardless of genotype (see Figure 2B; p<0.05).
Figure 2. The effect of MC4R deficiency and ACE inhibition on ingestive behaviors.
Cumulative food intake was greater in HOM compared with WT groups and was reduced by 8-weeks of ACE inhibitor treatment only in HOM rats (A). Mean water intake was elevated by ACE inhibition (B). Food intake following fasting was elevated in all groups, and this effect was independent of genotype or treatment (C vs. D). WT (wildtype), WT+ (WT+ACE inhibitor), HOM (homozygous), HOM+ (homozygous+ACE inhibitor). * = difference from WT control (p<0.05) + = difference between ACE inhibitor and control (p<0.05).
Food intake following fasting
Following a 24-h fast, all animals ate more than at baseline (see Figures 2C and 2D; p<0.05). No significant differences in food intake were observed based on ACE inhibitor treatment or genotype after 2 or 4 h, but by 24 h after food return differences between genotypes were observed (p<0.05); ACE inhibition reduced 24-h intake in HOM but not WT animals (p<0.05).
Body composition
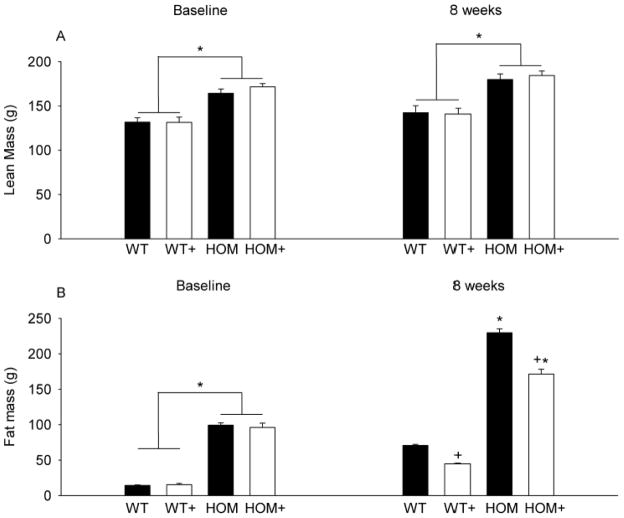
At the onset of the experiment there was significantly greater fat mass in HOM rats compared with WT rats (see Figure 3A; p<0.05). Similarly, lean mass was elevated in HOM rats compared with WT rats (see Figure 3B; p<0.05). After 8 weeks of ACE inhibitor treatment fat mass was significantly reduced in both WT+ and HOM+ animals relative to the respective CON groups (p<0.05). ACE inhibitor treatment did not affect lean mass in either genotype.
Figure 3. The effect of MC4R deficiency and ACE inhibition on body composition.
Lean mass was greater in HOM than WT groups and was unaffected by 8-weeks of ACE inhibition (A). Fat mass was greater in HOM than WT rats and was reduced by 8-weeks of treatment with an ACE inhibitor (B). WT (wildtype), WT+ (WT+ACE inhibitor), HOM (homozygous), HOM+ (homozygous+ACE inhibitor). * = difference from WT control (p<0.05) + = difference between ACE inhibitor and control (p<0.05).
Intraperitoneal glucose- and insulin-tolerance tests
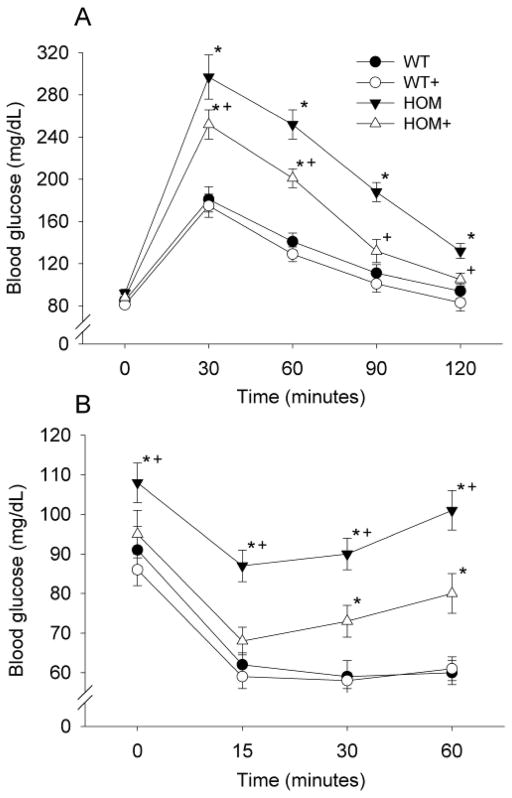
There was no significant difference in fasting blood glucose among the groups. Glucose tolerance was impaired in HOM rats relative to WT rats, with elevated blood glucose at all time-points following glucose injection (p<0.05). ACE inhibition resulted in improved glucose tolerance of HOM+ rats (p<0.05) but not in WT+ rats relative to WT controls (see Figure 4A).
Figure 4. The effect of MC4R deficiency and ACE inhibition glucose tolerance and insulin sensitivity.
Both glucose tolerance (A) and insulin sensitivity (B) were impaired in HOM rats, and this was significantly improved by ACE inhibitor treatment, at 6- and 7-weeks respectively. WT (wildtype), WT+ (WT+ACE inhibitor), HOM (homozygous), HOM+ (homozygous+ACE inhibitor). * = difference from WT control (p<0.05) + = difference between ACE inhibitor and control (p<0.05).
There was a small but significant elevation in fed-state blood glucose in HOM rats compared with all other groups (see Figure 4B; p<0.05). The glucose lowering response to IP insulin injection was markedly lower in HOM animals compared with all other groups at 15, 30 and 60 min (See Figure 4B; p<0.05). There was no significant difference between the responses of HOM+, WT and WT+ rats at 15 min, but by 30 and 60 min, HOM+ animals had elevated glucose relative to the WT and WT+ groups (p<0.05).
Discussion
ACE inhibition decreased weight gain in both WT and HOM rats; however, there was a greater suppression of weight gain in HOM than in WT rats on the ACE inhibitor. The increased efficacy of ACE inhibition on HOM rats may be explained by differential food intake, given that no difference in food intake was observed between WT and WT+ animals whereas there was a substantial decrease in food intake of HOM+ rats relative to that in the HOM group. This occurred despite HOM+ rats receiving a lower dose of captopril relative to body weight. Initial lean mass, fat mass and fat percentage differences reflected the elevated body weight of HOM relative to WT rats. These data are consistent with previous reports in male rats with functional ablation of the MC4R [5, 29] and in both male and female MC4R−/− mice [4].
Acute food-intake (1 and 2-h) following 24-h deprivation was not affected by either genotype or ACE inhibition. By 24 hours, however, intake reflected genotype with HOM groups eating significantly more than WT groups; additionally, ACE inhibitor-treated rats ate less in response to deprivation only at the 24-hour time point. These data may suggest that the total daily intake is a better indication of basal intake rather than being a function of the restriction. Similar restriction studies in captopril-treated male rats have also demonstrated no effect of ACE inhibition on acute feeding [28].
Glucose tolerance was impaired in female MC4R-deficient rats relative to their wildtype controls. Comparable results have previously been reported in male MC4R-deficient rats [29] and mice [4]. ACE inhibition improved the glucose tolerance and insulin sensitivity of MC4R rats, and this was not observed in wildtype animals. Numerous prior studies have demonstrated improved responses to a glucose load following ACE inhibition [13, 18, 28] or genetic knockout of the RAS [22, 30]. Given that ACE inhibition did not alter glucose tolerance in the WT animals in the current study, this may indicate sex differences in the weight-loss response to ACE inhibition. However, it is possible that the differences observed are more a function of differences in body weight and adiposity among groups, for example the difference in body weight between WT animals and WT+ animals was small and not significant until after the glucose tolerance and insulin sensitivity tests had been performed.
It has previously been suggested that an increase in angiotensin signaling in the brain may be the cause of weight loss associated with ACE inhibitor treatment [13, 28], with central angiotensin increasing energy expenditure and decreasing body weight [31]. However, this central angiotensin hypothesis cannot completely explain the effects of RAS blockade on adiposity given that whole body ACE [22], angiotensin type-1 [30] and angiotensin type-2 [32] receptor deletion also result in reduced body weight and adiposity despite presumably no or reduced central signaling. Central angiotensin signaling increases CRH in the rat hypothalamus [33], and this may account for the reduced food intake [30]. Given the differences observed following ACE inhibition on food intake in WT and HOM animals, our data may indicate that MC4R-deficient animals have increased sensitivity to the anorectic effects of CRH; however, this remains to be established.
In this study estrus cycling was not monitored and this may be considered a limitation. ACE inhibitors have no effect on estrus cycle [34]; however, MC4R deficiency results in reproductive dysfunction in mice that is dependent on body weight [35]. It may be of interest to examine whether ACE inhibition restores normal cyclicity in MC4R-deficient animals in future experiments. It may also be of interest to examine sex differences of the effects of ACE inhibition on body weight and food intake to assess interaction with gonadal hormones.
In summary, the current study indicates that female rats with a loss of function mutation in Mc4r are sensitive to the anorectic effects of ACE inhibition, unlike their wildtype littermates. This leads to an earlier onset of body weight differences and a greater change in body composition in HOM animals relative to WT animals treated with an ACE inhibitor. Overall, the data demonstrate that MC4R signaling is not required for the weight loss associated with ACE inhibitor treatment.
Research Highlights.
ACE inhibition reduced weight gain in both MC4R deficient and wildtype rats
Food intake following ACE inhibition was only reduced in MC4R deficient rats
MC4R deficient rats were glucose intolerant this was improved by ACE inhibition
Acknowledgments
This work was supported by NIH DK17844, DPB is supported by an NHMRC Early Career Fellowship. This report is based on a presentation during the 2012 Annual Meeting of the Society for the Study of Ingestive Behavior, July 10–14, 2012, made possible in part by generous donations from Novo Nordisk A/S, Research Diets, Inc., Sanofi, Inc., and TSE, Inc.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 3.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 4.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 5.Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, et al. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 2012;20:612–21. doi: 10.1038/oby.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 7.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–2. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 8.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–43. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begg DP, Woods SC. The central insulin system and energy balance. Handb Exp Pharmacol. 2012:111–29. doi: 10.1007/978-3-642-24716-3_5. [DOI] [PubMed] [Google Scholar]
- 11.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes (Lond) 2001;25:S56–62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100:525–34. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisinger RS, Begg DP, Jois M. Antagonists of the renin-angiotensin system and the prevention of obesity. Curr Opin Investig Drugs. 2009;10:1069–77. [PubMed] [Google Scholar]
- 14.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–30. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisinger RS, Begg DP, Chen N, Jois M, Mathai ML, Sinclair AJ. The problem of obesity: is there a role for antagonists of the renin-angiotensin system? Asia Pac J Clin Nutr. 2007;16 (Suppl 1):359–67. [PubMed] [Google Scholar]
- 16.Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, et al. Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav. 2008;93:820–5. doi: 10.1016/j.physbeh.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol Behav. 2009;98:192–7. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Premaratna SD, Manickam E, Begg DP, Rayment DJ, Hafandi A, Jois M, et al. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int J Obes (Lond) 2012;36:233–43. doi: 10.1038/ijo.2011.95. [DOI] [PubMed] [Google Scholar]
- 19.Miesel A, Muller-Fielitz H, Johren O, Vogt FM, Raasch W. Double blockade of angiotensin II (AT(1) )-receptors and ACE does not improve weight gain and glucose homeostasis better than single-drug treatments in obese rats. Br J Pharmacol. 2012;165:2721–35. doi: 10.1111/j.1476-5381.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller-Fielitz H, Landolt J, Heidbreder M, Werth S, Vogt FM, Johren O, et al. Improved insulin sensitivity after long-term treatment with AT1 blockers is not associated with PPARgamma target gene regulation. Endocrinology. 2012;153:1103–15. doi: 10.1210/en.2011-0183. [DOI] [PubMed] [Google Scholar]
- 21.Fujisaka S, Usui I, Kanatani Y, Ikutani M, Takasaki I, Tsuneyama K, et al. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology. 2011;152:1789–99. doi: 10.1210/en.2010-1312. [DOI] [PubMed] [Google Scholar]
- 22.Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, et al. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci U S A. 2008;105:6531–6. doi: 10.1073/pnas.0802690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 24.Yvan-Charvet L, Massiera F, Lamande N, Ailhaud G, Teboul M, Moustaid-Moussa N, et al. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology. 2009;150:1421–8. doi: 10.1210/en.2008-1120. [DOI] [PubMed] [Google Scholar]
- 25.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–9. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 26.Kalupahana NS, Massiera F, Quignard-Boulange A, Ailhaud G, Voy BH, Wasserman DH, et al. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity (Silver Spring) 2012;20:48–56. doi: 10.1038/oby.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Soltani-Bejnood M, Quignard-Boulange A, Massiera F, Teboul M, Ailhaud G, et al. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J Biomed Biotechnol. 2006;2006:27012. doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kloet AD, Krause EG, Kim DH, Sakai RR, Seeley RJ, Woods SC. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009;150:4114–23. doi: 10.1210/en.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mul JD, Begg DP, Alsters SI, Haaften G, Duran KJ, D’Alessio DA, et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab. 2012;303:E103–10. doi: 10.1152/ajpendo.00159.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto R, Akazawa H, Fujihara H, Ozasa Y, Yasuda N, Ito K, et al. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J Biol Chem. 2011;286:21458–65. doi: 10.1074/jbc.M110.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, et al. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–42. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–9. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 33.de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, et al. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E1081–91. doi: 10.1152/ajpendo.00307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson CM, Morioka N, Zhu C, Ryan JW, LeMaire WJ. Angiotensin-converting enzyme inhibitors have no effect on ovulation and ovarian steroidogenesis in the perfused rat ovary. Reprod Toxicol. 1993;7:131–5. doi: 10.1016/0890-6238(93)90247-5. [DOI] [PubMed] [Google Scholar]
- 35.Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun. 2005;326:638–44. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]