Abstract
Background
Antithymocyte globulin (ATG) has been increasingly used to prevent graft-vs-host disease (GVHD), however, its impact on immune reconstitution is relatively unknown. Here we studied (1) immune reconstitution after ATG-conditioned hematopoietic cell transplantation (HCT), (2) determined factors influencing the reconstitution, and (3) compared it to non-ATG-conditioned HCT.
Methods
Immune cell subset counts were determined at 1–24 months posttransplant in 125 HCT recipients who received ATG during conditioning. The subset counts were also determined in 46 non-ATG-conditioned patients (similarly treated).
Results
(1) Reconstitution after ATG-conditioned HCT was fast for innate immune cells, intermediate for B cells and CD8 T cells, and very slow for CD4 T cells and invariant NKT (iNKT) cells. (2) Faster reconstitution after ATG-conditioned HCT was associated with higher number of cells of the same subset transferred with the graft in case of memory B cells, naïve CD4 T cells, naïve CD8 T cells, iNKT cells and myeloid dendritic cells; lower recipient age in case of naïve CD4 T cells and naïve CD8 T cells; cytomegalovirus recipient seropositivity in case of memory/effector T cells; absence of GVHD in case of naïve B cells; lower ATG serum levels in case of most T cell subsets including iNKT cells, and higher ATG levels in case of NK cells and B cells. (3) Compared to non-ATG-conditioned HCT, reconstitution after ATG-conditioned HCT was slower for CD4 T cells, and faster for NK cells and B cells.
Conclusions
ATG worsens reconstitution of CD4 T cells but improves reconstitution of NK and B cells.
Keywords: Anti-thymocyte globulin (ATG), Hematopoietic stem-cell transplantation, Immune reconstitution, Immune system, Immunity, Lymphocytes
Introduction
Successful immune reconstitution is associated with lower rates of infection, relapse and possibly second malignancy after hematopoietic stem cell transplantation (HCT) (1–9). Rabbit-anti-human T cell globulin (eg, anti-Jurkat T cell line globulin or antithymocyte globulin (ATG)) is a polyclonal IgG that has been used in HCT conditioning to reduce the incidence of graft rejection and graft-vs.-host disease (GVHD). The increasing use of ATG stems from the fact that it appears to reduce GVHD without increasing relapse (10–14).
ATG is composed of antibodies to antigens expressed by many immune cell subsets, i.e., CD1a, CD2, CD3/T cell receptor, CD4, CD5, CD6, CD7, CD8, CD11a, CD11b, CD16, CD19, CD20, CD25, CD28, CD30, CD32, CD38 CD40, CD45, CD54, CD58, CD80, CD86, CD95, CD138, HLA class I/β2M, and HLA class II (15). Thus it targets not only T cells but also B cells, NK cells, granulocytes, monocytes/macrophages and dendritic cells. ATG may kill the targeted immune cells by inducing apoptosis, complement-mediated or NK cell-mediated lysis (16–18). Alternatively, the antibodies may alter immune cell function by inhibiting T cell proliferation, inducing T cell differentiation into regulatory cells, or blocking surface antigens needed for chemotaxis or for interaction with other cells (19–21).
Despite the profound effect of ATG on immune cells, data on immune reconstitution after human ATG-conditioned HCT are scarce (22–24). Moreover, it is not known, whether the same factors that influence immune reconstitution after non-ATG-conditioned HCT (e.g, number of CD34+ cells (25) or immune cells in the graft (26, 27), recipient age (27–29), cytomegalovirus (CMV) serostatus (26, 30, 31), donor match (32) or GVHD (27, 33)) also influence immune reconstitution after ATG-conditioned HCT. Here we present data on immune reconstitution after ATG-conditioned HCT, including factors influencing the reconstitution. To determine the effect of ATG on immune reconstitution, we compare 1. ATG-conditioned patients with high vs. low early posttransplant ATG levels (by evaluating for correlation between ATG levels and immune cell subset counts), and 2. ATG-conditioned vs. non-ATG-conditioned patients.
Methods
Patients and transplantation
Between December 2004 and August 2008, 176 allogeneic HCT recipients in Calgary consented to participate in this Research Ethics Board-approved study. Blood was drawn before starting conditioning and on day 7, 28, 56, 84, 180, 365 and 730 posttransplant. Of the 176 patients, we selected a homogenous group of 125 patients who met the following selection criteria: First allogeneic transplantation, ATG (Thymoglobulin, Genzyme/Sanofi) in conditioning, filgrastim-mobilized blood stem cells as graft source, and availability of immune cell subset count data from at least one of the post transplant time points. Patients were also excluded if they had not engrafted or relapsed or died by day 30. For a diagram of patient selection, see Supplementary Figure 1. Conditioning was with fludarabine, 250 mg/m2, busulfan, approximately 12.8 mg/kg IV (pharmacokinetics-adjusted), and ATG, 4.5 mg/kg (0.5 mg on day -2, 2 mg on days -1 and 0). Total body irradiation (TBI), 4 Gy, was added for most patients with acute leukemia. GVHD prophylaxis was with methotrexate on day 1, 3, 6 and 11 and cyclosporine from day -1 until 6 months posttransplant (longer in the case of cGVHD). Supportive care included prophylactic cotrimoxazole and acyclovir, and preemptive ganciclovir. Blood products were from CMV safe. Grade 2–4 acute GVHD (aGVHD) or clinically significant chronic GVHD (cGVHD) was treated with corticosteroids, supplemented in some patients by other immunosuppressive drugs. Patients were followed until death, relapse or second malignancy, whichever occurred first. For detailed characteristics of the ATG-conditioned patients, who were used to describe the immune reconstitution after ATG-conditioned HCT and the factors influencing the reconstitution, see Table 1, column “ATG conditioned, total”.
Table 1.
| Patient Characteristics | ATG-conditioned, total | ATG-conditioned, matched sibs† | Non-ATG-conditioned, matched sibs | P value* |
|---|---|---|---|---|
| N | 125 | 61 | 46 | |
| Median Patient Age (range) | 45 (19–64) | 47 (20–64) | 44 (18–61) | 0.080 |
| Median Donor Age (range) | 37 (15–67) | 44 (15–67) | 44 (13–63) | 0.303 |
| Patient Sex M/F | 74/51 | 35/26 | 31/15 | 0.229 |
| Donor Sex M/F | 83/42 | 40/21 | 25/21 | 0.318 |
| Diagnosis/disease stage at transplant | 0.516 | |||
| Poor Risk@ | 54 (43) | 24(39) | 25 (54) | |
| Good Risk@ | 71 (57) | 37 (61) | 21 (46) | |
| First transplant | 125 (100) | 61 (100) | 46 (100) | 1.000 |
| Myeloablative Conditioning | 125 (100)** | 61 (100)** | 46 (100)*** | 1.000 |
| Stem Cell Source: Blood Stem Cells | 125 (100) | 61 (100) | 46 (100) | 1.000 |
| Donor Type | 1.000 | |||
| HLA Matched Sibling | 61 (49) | 61 (100) | 46 (100) | |
| Other | 64 (51)$ | 0 (0) | 0 (0) | |
| Donor/Recipient CMV Serotatus | 0.091 | |||
| Positive/Positive | 36 (29) | 19 (31) | 10 (22) | |
| Positive/Negative | 27 (22) | 11 (18) | 13 (28) | |
| Negative/Positive | 10 (8) | 6 (10) | 10 (22) | |
| Negative/Negative | 51 (41) | 25 (41) | 12 (26) | |
| Unknown or Indeterminate | 1 (1) | 0 (0) | 1 (2) | |
| GVHD Prophylaxis with methotrexate (Days 1,3,6,11) and cyclosporine (for 6 months) | 122^ (98) | 61 (100) | 46 (100) | 1.000 |
| Acute GVHD# | 0.001 | |||
| Grade 0–1 | 93 (74) | 49 (80) | 15 (32) | |
| Grade 2–4 | 32 (26) | 12 (20) | 31 (68) | |
| Chronic GVHD by day 365# | 0.003 | |||
| None or Limited | 66 (53) | 43 (57) | 19 (31) | |
| Extensive | 44 (35) | 18 (30) | 27 (59) | |
| Not evaluable | 15 (12) | 8 (13) | 5 (9) | |
| Chimerism Status | 1.000 | |||
| >90% donor by day 90 | 99 (100)^^ | 45 (100)^^ | 40 (100)^^ | |
| Median day of neutrophil engraphment (>0.5 ×109/L × 3 Days) | 14 (10–21) | 15 (11–20) | 16 (11–28) | 0.001 |
| Death without relapse during day 30–365 | 13 (10) | 9 (15) | 4 (9) | 0.388 |
| Relapse between day 30–365 | 15 (12) | 6 (10) | 8 (15) | 0.265 |
| Subsets in Graft (×106/kg recipient body weight) | ||||
| CD34+ Cells | 6.1 (0.8–17.2) | 6.26 (0.9–14.5) | 7.4 (1.0–17.5) | 0.003 |
| B Cells | 49 (2–313) | 64 (2–129) | 73 (6–141) | 0.128 |
| Naïve B cells | 33 (0.2–160) | 48 (0.2–160) | 64 (2–129) | 0.007 |
| Memory B cells | 4 (0.01–95) | 4.5 (0.01–17) | 10 (1–34) | 0.001 |
| CD4 T Cells | 140 (17–346) | 168 (38–314) | 172 (64–414) | 0.292 |
| Naïve CD4 T Cells | 45.5 (5–201) | 46 (4–166) | 61 (19–179) | 0.110 |
| Memory/Effector CD4 T Cells | 89 (6–195) | 102 (34–194) | 117 (1–204) | 0.831 |
| CD8 T Cells | 63 (0.57–242) | 57 (11–198) | 80 (22–254) | 0.003 |
| Naïve CD8 T Cells | 12 (0.1–114) | 8.5 (0.3–114) | 34 (15–85) | 0.001 |
| Memory/Effector CD8 T Cells | 46 (0.47–196) | 45 (10–102) | 33 (7–106) | 0.136 |
| Monocytes | 369 (76–1068) | 377 (174–1068) | 547 (150–2142) | 0.002 |
| NK Cells | 89 (14–303) | 98 (49–203) | 30 (13–71) | 0.001 |
| Median post transplant day when blood was drawn for immune cell subset enumeration (range) | ||||
| 1 month post transplant | 28 (23–57) | 28 (25–57) | 31.5 (25–36) | 0.001 |
| 2 months post transplant | 56 (50–63) | 56 (50–63) | Not applicable | |
| 3 months post transplant | 84 (77–105) | 84 (77–105) | 78 (68–105) | 0.001 |
| 6 months post transplant | 182 (166–200) | 182 (166–200) | 186 (182–236) | 0.116 |
| 12 months post transplant | 363 (334–400) | 363 (350–396) | 371.5 (335–426) | 0.036 |
| 24 months post transplant | 733 (679–775) | 729 (679–757) | Not applicable | |
| Table 1 ContinuedNumber of patients with available immune cell subset counts | ||||
| 1 month post transplant | 83 | 40 | 43 | |
| 2 month post transplant | 83 | 40 | Not applicable | |
| 3 month post transplant | 78 | 43 | 39 | |
| 6 month post transplant | 52 | 26 | 25 | |
| 12 month post transplant | 45 | 27 | 33 | |
| 24 month post transplant | 16 | 13 | Not applicable |
Values represent numbers (percentages) of patients unless otherwise indicated.
GVHD indicates graft-versus-host disease; TBI, total body irradiation; CMV, cytomegalovirus.
These patients are a subcohort (matched sibling transplant recipients) of the total ATG-conditioned (Calgary) cohort. This subcohort was used for all the comparisons with the non-ATG-conditioned (Seattle) cohort, which was composed of only matched sibling transplant recipients.
P values refer to significance of difference between Calgary matched sibs and Seattle matched sibs. Numbers of patients were compared using the Fisher exact test. Ordinal values were compared using the Mann-Whitney rank sum test.
Good risk disease/stage (pretransplant) was defined as chronic myelogenous leukemia in first chronic or accelerated phase, acute leukemia in first remission, myelodysplasia with <5% blasts, or aplastic anemia. Any other disease/disease stage was considered poor risk.
Typically fludarabine 250 mg/m2 + busulfan 12.8 mg/kg ± total body irradiation 4 Gy (Russell JA et al: Biol Blood Marrow Transplant 16:509, 2010).
Typically cyclophosphamide 120 mg/kg + total body irradiation 12 Gy.
10/10 HLA allele-matched unrelated (n=44), 9/10 HLA allele-matched related) (n=1), and 8–9/10 HLA allele-matched unrelated (n=17).
In two patients, GVHD prophylaxis was with cyclosporine only, and in one patient with methotrexate and tacrolimus.
Among patients with known chimerism status. Chimerism status was unknown for 26 Calgary patients (16 matched sibs) and 6 Seattle patients. Chimerism studies were determined on total marrow or peripheral blood cells.
Acute GVHD was graded according to 1994 Consensus Conference (Przepiorka D. et al.: Bone Marrow Transplant 1995, 15:825–828). Extensive cGVHD was defined in Seattle per Shulman criteria (Shulman H.M. et al: American Journal of Medicine. 69(2):204–17, 1980) and in Calgary as cGVHD treated with systemic immunosuppressive drugs (Calgary clinicians treat extensive but not limited cGVHD with systemic immunosuppressive drugs). Patients who were followed till less than 100 days were considered not evaluable for cGVHD (typically due to death or relapse before day 100)
For comparison, we used a historical cohort of 46 non-ATG conditioned blood stem cell transplant recipients undergoing transplantation in Seattle, who had immune cell subset counts determined on days 30, 80, 180 and 365, using a similar flow cytometric technique as the Calgary cohort (26). Conditiong was typically with cyclophosphamide 120 mg/kg plus total body irradiation of 12 Gy. As the donors for the non-ATG cohort were all matched siblings, comparisons of subset counts were made with ATG-conditioned (Calgary) patients who received grafts from matched sibling donors. This subgroup of Calgary patients and the Seattle group were balanced in most demographic/clinical characteristics (Table 1). Exceptions included lower incidence of acute and chronic GVHD in the ATG-conditioned patients (as expected), and lower contents of CD34 cells, monocytes, NK cells, naïve and memory B cells and naïve CD8 T cells in the grafts of the ATG-conditioned patients (probably due to the shorter aphereses in Calgary).
As healthy controls, we used related graft donors of the Calgary patients who consented to having blood drawn before filgrastim mobilization (n=33).
Techniques used in Calgary are described below, whereas those used in Seattle have been described by Storek et al (26).
Immune Cell Subsets
Heparinized blood (200 microliters) or graft (20 microliters) was pipetted into 12 × 75 mm polystyrene tubes and washed by addition of 2 mL of phosphate buffered saline (PBS) and centrifugation. Supernatant was aspirated and florochrome-labeled monoclonal antibody cocktails (Supplementary Table 1) were added to the cell pellet.
Cells were incubated with the antibodies for 15 minutes at room temperature. Erythrocytes were then lysed using an ammonium chloride lysing solution followed by a wash with PBS as above. Cells were resuspended in 0.5 mL of 0.1% formaldehyde in PBS (Polysciences, Warrington, PA, USA). Flow cytometry was performed on the same day, using FC500 flow cytometer (Beckman Coulter, Hialeah, FL, USA). During analysis of blood specimens using Winlist software (Verity, Topsham, ME, USA), mononuclear cells (MNCs) (lymphocytes plus monocytes) were gated on forward vs. side scatter plots. Percentages of cell subsets were determined according to the definitions shown in Table 2. Each absolute cell subset count in blood was calculated as the absolute MNC count multiplied by the subset percentage (among total MNCs) divided by 100. The absolute MNC count represented the sum of the absolute lymphocyte count and the absolute monocyte count determined by a clinical hematology laboratory. For grafts, total nucleated cells were gated on forward × side-scatter plots, and each absolute cell subset count was calculated as the absolute nucleated cell count multiplied by the percentage of the cell subset among total nucleated cells divided by 100. Neutrophils were enumerated by a clinical hematology laboratory.
Table 2.
Definitions of subsets of mononuclear cells*
| Subset | Definition |
|---|---|
| B Cells | CD19 Positive or CD20 Positive |
| CD5+ B cells | CD5 Positive and (CD19 Positive or CD20 Positive) |
| CD5− B cells | CD5 negative and (CD19 Positive or CD20 Positive) |
| Naïve B cells | mIgD positive and CD27 negative and (CD19 Positive or CD20 Positive) |
| Memory B cells | CD27 positive and (CD19 Positive or CD20 Positive) |
| Switched Memory B cells (IgM/D→IgG/A/E switched) | mIgD negative and CD27 positive and (CD19 Positive or CD20 Positive) |
| Non-switched Memory B cells | mIgD positive and CD27 positive and (CD19 Positive or CD20 Positive) |
| CD4 T Cells | CD3 Positive & CD4 Positive & CD8 Negative |
| Naïve CD4 T Cells | CD4 Positive, CD45RA high & CD11A low/negative CD4 T cells & CD3 Positive & & CD8 Negative |
| Memory/Effector CD4 T Cells | Non-naïve CD4 T cells |
| CD8 T Cells | CD3 Positive, CD4 Negative and CD8 Positive |
| Naïve CD8 T Cells | CD8 Positive, CD45RA High and CD11A low/negative & CD3 Positive, and CD4 Negative |
| Memory/Effector CD8 T Cells | Non-naïve CD8 T cells |
| Memory/Effector CD8 T cells, CD45RA+ | Non-naïve CD8 T cells expressing CD45RA |
| Memory/Effector CD8 T cells, CD45RA− | Non-naïve CD8 T cells not expressing CD45RA |
| CD4-CD8- T Cells | CD3 Positive & CD4 Negative and CD8 Negative |
| CD4+CD8+ T Cells | CD3 Positive & CD4 Positive and CD8 Positive |
| Regulatory CD4 T Cells | CD3 Positive & CD4 Positive and CD25 High |
| Non-regulatory CD4 T cells | CD3 Positive & CD4 Positive and CD25 Low/Negative |
| Invariant NKT (iNKT) Cells | CD3 Positive and Valpha24 Positive and Vbeta11 Positive |
| CD4+ iNKT Cells | CD3 Positive, Valpha24 Positive, and Vbeta11 Positive & CD4 Positive, |
| CD4− iNKT Cells | CD3 Positive, Valpha24 Positive, and Vbeta11 Positive & CD4 Negative, |
| Monocytes | CD14 Positive |
| Inflammatory Monocytes | CD14+ CD16− |
| Resident Monocytes | CD14+ CD16+ |
| NK Cells | CD14 Negative, CD3 Negative and [CD16 Positive or CD56 Positive] |
| Regulatory NK Cells | CD16 Negative and CD56 High NK cells |
| Cytolytic NK Cells | CD16 Positive and CD56 Intermediate NK cells |
| Myeloid Dendritic cells (MDC) | HLADR high and Lineage (CD3/14/16/19/56) Negative and CD11c Positive and CD123 Negative |
| Plasmacytoid Dendritic cells (PDC) | HLADR high and Lineage (CD3/14/16/19/56) Negative and CD11c Negative and CD123 Positive |
| Basophils | HLADR Negative, Lineage Negative and CD123 High |
Used in Calgary. Definitions used in Seattle (Storek J et al: Blood 97:3380, 2001) were near identical for most subsets. However, because the subset enumeration was performed in the 1990’s in Seattle vs. in the 2000’s in Calgary, more modern definitions were used in Calgary for naïve and memory B cells, naïve and memory/effector CD4 T cells, and naïve and memory/effector CD8 T cells. Therefore, for the comparison of these subsets between Calgary matched sibling graft recipients and Seattle patients (all of whom were matched sibling graft recipients), an additional flow cytometry analysis of Calgary matched sibling recipient data was performed, using the following definitions (closely matching the Seattle definitions): Naïve B cells were defined as B cells (CD19 or CD20 Positive) that were membrane IgD (mIgD) Positive. Memory B cells were defined as B cells (CD19 or CD20 Positive) that were mIgD Negative. Naïve CD4 T cells were defined as CD3 Positive and CD4 Positive and CD8 Negative cells that were CD45RA High. Memory/effector CD4 T cells were defined as CD3 Positive and CD4 Positive and CD8 Negative cells that were CD45RA Low/Negative. Naïve CD8 T cells were defined as CD3 Positive and CD8 Positive and CD4 Negative cells that were CD11a Low/Negative. Memory/effector CD8 T cells were defined as CD3 Positive and CD8 Positive and CD4 Negative cells that were CD11a High.\
Because chronic lymphocytic leukemia/low grade B cell lymphoma patients may have circulating malignant B cells for several months, these patients were omitted from B cell analyses (n=6).
Day 7 immune cell subset counts could not be determined using the above methods, as the white blood cell (WBC) count was typically very low (≤0.1/nl) which precluded accurate determination of MNC and neutrophil counts by our clinical hematology lab. For enumeration of MNC subsets, it was arbitrarily assumed that WBC count (determined by clinical hematology lab) = MNC count. Each absolute MNC subset count was calculated as WBC count multiplied by the subset percentage (among MNCs) divided by 100. For neutrophils, it was arbitrarily assumed that absolute neutrophil count = WBC count. Thus, this method does not accurately measure immune cell subset counts on day 7. It only provides a conservative (probably higher than true) estimate of the counts to show that the counts on day 7 were extremely low.
Immunoglobulins
As a rough surrogate of total body plasma cell count and function, IgM, IgG and IgA serum levels were measured by a clinical laboratory (using automated immunoturbidimetry analyzer, Roche Diagnostics Integra 800). Patients who received IgG supplementation within 2 months before a time point were excluded from analysis for that time point.
For CMV serostatus determination (presence or absence of CMV-specific IgG), a microplate enzyme immunoassay (Siemens Enzygnost, Marburg, Germany) was used.
ATG Levels
Serum levels of rabbit IgG capable of binding to human lymphocytes were determined using a flow cytometry-based assay as described (14).
Statistics
Mann-Whitney rank sum test was used to compare subset counts between subject groups. Spearman rank correlation test was used to determine associations between subset counts and ordinal variables like ATG levels. For most analyses, p<0.05 (2-tailed) was considered significant. When analyzing factors associated with immune cell subset counts, spurious associations could be found due to multiple comparisons. To minimize the chance of spurious associations, p<0.005 was considered significant, unless the associations appeared significant for two adjacent time points (p<0.05 for both time points), because in such situations the associations were less likely spurious.
Results
Immune reconstitution after ATG-conditioned transplantation
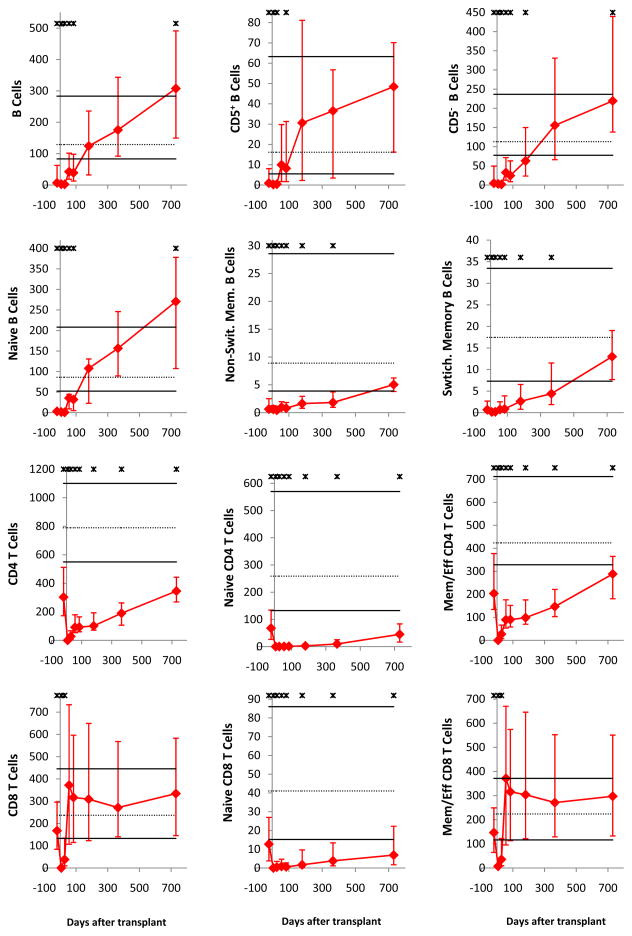
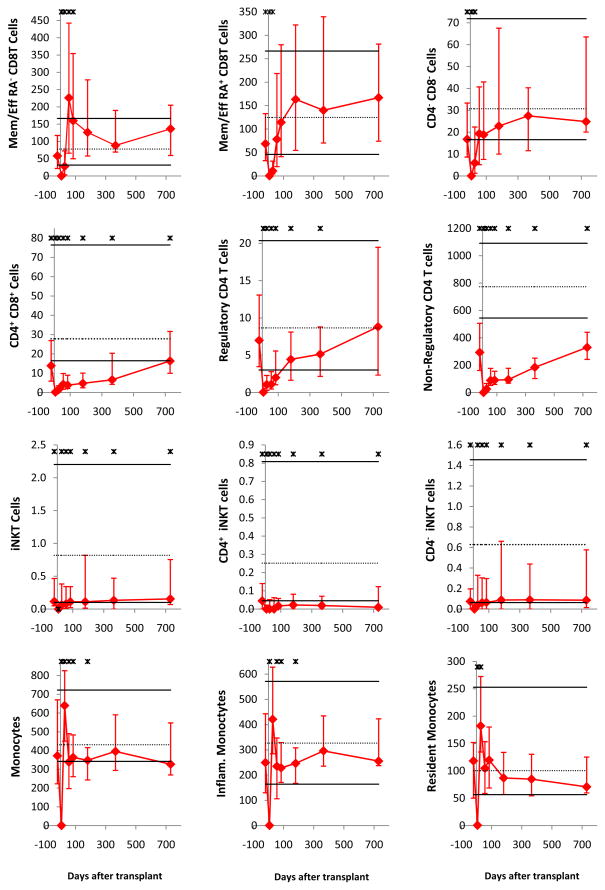
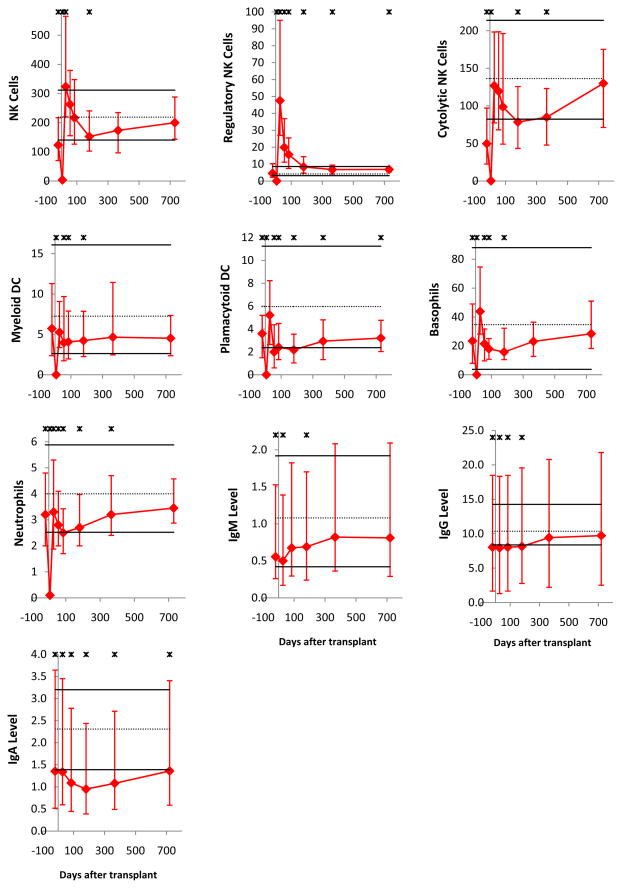
All immune cell subsets were virtually undetectable on day 7. Subsequently, as published for non-ATG-conditioned transplants (25–29, 31–33), innate immune cells recovered faster than adaptive immune cells (Figure 1). Early normalization (counts on day 28 not significantly lower than in healthy controls) was noted for NK cells, monocytes, basophils, myeloid dendritic cells (MDCs), and plasmacytoid dendritic cells (PDCs); for neutrophils, the median day 28 count was statistically significantly lower than in healthy controls, nevertheless within the normal range (10th–90th percentile of healthy controls). Late normalization (by day 180) occurred for B cells, CD8 T cells, and CD4−CD8− T cells. No normalization by day 730 was noted for CD4 T cells, CD4+CD8+ T cells and iNKT cells.
Figure 1. Median immune cell subset counts in recipients of ATG-conditioned blood stem cell transplantation.
The time points displayed are before transplantation (pre-conditioning) and day 7, 28, 56, 84, 180, 365 and 730 after transplantation (for Ig levels, days 7 and 56 were unavailable). Error bars indicate the 25th to 75th percentiles. Stars indicate a significant difference (P < .05) from graft donors. Normal values (derived from graft donors) are shown as horizontal lines (solid black lines for the 10th and 90th percentiles and dotted line for the median). Days after transplantation are shown on all x-axes. On all y-axes, values are cells per microliter blood, except for neutrophils (per nanoliter blood) and IgM, IgG, and IgA (g/L serum).
Substantial differences in the tempo of recovery were noted for subpopulations of the above subsets. Specifically, naïve B cells recovered faster than memory B cells, and CD5+ B cells recovered faster than CD5− B cells. Memory/effector CD4 and memory/effector CD8 T cells recovered faster than naïve cells, and CD25high (“regulatory”) CD4 T cells recovered faster than CD25low/neg (“non-regulatory”) CD4 T cells. CD4−iNKT cells recovered faster than CD4+ iNKT cells. CD56highCD16neg (“regulatory”) NK cells recovered much faster than CD56intCD16+ (“cytolytic”) NK cells. Of note, the CD56highCD16neg NK cells were significantly higher than normal at all time points from day 28 to day 730.
Immunoglobulin levels were mildly subnormal (patient median significantly lower than donor median) early post-transplant. IgM normalized virtually by day 84, IgG normalized by day 365 and IgA has not normalized by day 730.
Late nadir (on day 56–365) of innate immune cells was noted: On at least two of day 56, 84, 180 and 365 time points, counts of neutrophils, monocytes, basophils, MDCs, PDCs and cytolytic NK cells were significantly lower than in healthy controls.
Factors influencing immune reconstitution after ATG-conditioned transplantation
Next we evaluated whether factors associated with immune reconstitution after non-ATG-conditioned transplantation are also associated with immune reconstitution after ATG-conditioned transplantation, and whether ATG levels are associated with the reconstitution (Table 3). The associations were evaluated for day 28, 56, 84, 180 and 365 and not for day 730 as only a limited number of patients were studied on day 730. If pretransplant factors were significantly associated with posttransplant immune cell count, we assumed a cause and effect relationship. We also evaluated posttransplant factors (GVHD and ATG levels) for which a direct effect on post-transplant cell counts (cause and effect relationship) may be less clear.
Table 3.
Factors associated with immune cell subset counts*
| Factor | Increased Immune subset | Day 28 | Day 56 | Day 84 | Day 180 | Day 365 | Decreased Immune subset | Day 28 | Day 56 | Day 84 | Day 180 | Day 365 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↑ CD34 Cells in Graft | ||||||||||||
| IgA levels | ||||||||||||
| ↑ Subset in Graft** | ||||||||||||
| Memory B cells, switched | ||||||||||||
| Naive CD4 T Cells | ||||||||||||
| Naïve CD8 T cells | ||||||||||||
| CD4-CD8- T cells | ||||||||||||
| Regulatory CD4 T cells | ||||||||||||
| Invariant NKT cells | ||||||||||||
| CD4- invariant NKT cells | ||||||||||||
| Myeloid dendritic cells | ||||||||||||
| ↑ Age of Recipient | ||||||||||||
| Naïve CD4 T cells | ||||||||||||
| Naïve CD8 T cells | ||||||||||||
| CD4-CD8- T cells | ||||||||||||
| Recipient CMV Seropositive | ||||||||||||
| Memory/effector CD4 T cells | ||||||||||||
| CD8 T cells | ||||||||||||
| Memory/effector CD8 T cells | ||||||||||||
| CD4+CD8+ T cells | ||||||||||||
| Significant GVHD | ||||||||||||
| Naïve CD8 T cells | Naïve B cells | |||||||||||
| CD4- invariant NKT cells | CD5+ B cells | |||||||||||
| CD5− B cells | ||||||||||||
| Plasmacytoid dendritic cells | ||||||||||||
| IgM | ||||||||||||
| IgG | ||||||||||||
| IgA | ||||||||||||
| Matched Sib Donor (compared to other donor) | ||||||||||||
| Naïve CD4 T cells | ||||||||||||
| Higher ATG level on Day 7 | ||||||||||||
| NK cells | CD4 T cells | |||||||||||
| Cytolytic NK cells | Naïve CD4 T cells | |||||||||||
| Memory/effector CD4 T cells | ||||||||||||
| CD8 T cells | ||||||||||||
| Naïve CD8 T cells | ||||||||||||
| Memory/effector CD8 T cells | ||||||||||||
| CD4-CD8- T cells | ||||||||||||
| CD4+CD8+ T cells | ||||||||||||
| Regulatory T cells | ||||||||||||
| Invariant NKT cells | ||||||||||||
| CD4+ invariant NKT cells | ||||||||||||
| CD4- invariant NKT cells | ||||||||||||
| Higher ATG level on Day 28 | ||||||||||||
| B cells | B cells | |||||||||||
| CD5+ B cells | CD5+ B cells | |||||||||||
| CD5- B cells | CD5- B cells | |||||||||||
| Naïve B cells | Naïve B cells | |||||||||||
| CD4 T cells | ||||||||||||
| Naïve CD4 T cells | ||||||||||||
| Memory/effector CD4 T cells | ||||||||||||
| CD8 T cells | ||||||||||||
| Naïve CD8 T cells | ||||||||||||
| Memory/effector CD8 T cells | ||||||||||||
| CD4-CD8− T cells | ||||||||||||
| CD4+CD8+ T cells | ||||||||||||
| Regulatory T cells | ||||||||||||
| Invariant NKT cells | ||||||||||||
| CD4+ invariant NKT cells | ||||||||||||
| CD4- invariant NKT cells | ||||||||||||
Dark grey denotes “definitely associated” (p<0.005 for one time point, or p value between 0.005 and 0.05 for two or more consecutive time points). Light grey denotes “possibly associated” (p value between 0.005 and 0.05 for one time point). More comprehensive information can be found in Supplementary Table 3.
Correlation between immune cell subset count in the graft (per kg recipient body weight) and the posttransplant blood count of the same subset.
CD34 cell graft content
There was no significant correlation between CD34 cell graft content and posttransplant counts of any of the subsets on day 28, 56, 84, 180 or 365. Unexpectedly, there was a significant correlation between CD34 cell graft content and IgA levels on days 84, 180 and 365.
Immune cell graft content
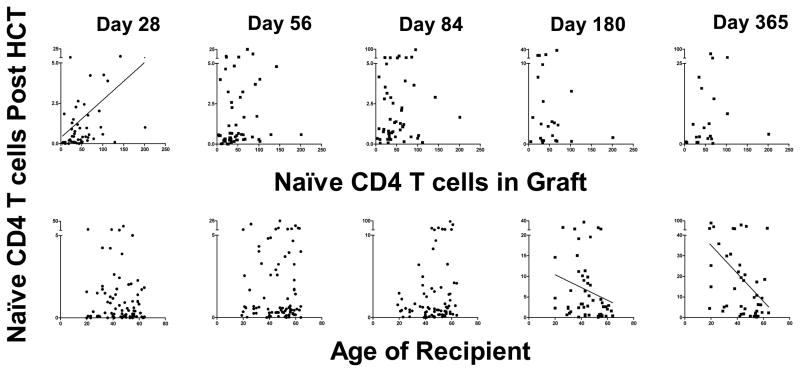
There were significant correlations between the number of immune cells in the graft and the count of the same immune cells posttransplant for multiple subsets, specifically, IgD/M→IgG/A/E switched memory B cells, naïve CD4 T cells, total and naïve CD8 T cells), CD4−CD8− T cells, regulatory CD4 T cells, total and CD4− iNKT cells, and MDCs (See Figure 2 for naïve CD 4 cells and Supplementary Figure 2 for naive CD8 T cells). This suggests that not all of these cells were killed by ATG and that their de novo production was limited early posttransplant.
Figure 2. Effects of naïve CD4 T cell graft content (top) and of recipient age (bottom) on naïve CD4 T cell counts on day 28, 56, 84, 180 and 365 post transplant.
Correlation trend line is shown in case of p<0.05. On x-axes, the naïve CD4 T cell number in graft is expressed as ×106/kg recipient body weight, and the recipient age at the time of transplantation is expressed as years. On all y-axes, naïve CD4 T cell counts are per microliter blood.
Age
Higher recipient age resulted in decreased counts of naïve CD4 T cells, naïve CD8 T cells and CD4−CD8− T cells on days 180 and 365. This suggests that thymopoiesis was unable to increase the pool of these cells in older patients in the second half of the first year (Figure 2 & Supplementary Figure 2).
CMV serostatus
Recipient CMV seropositivity pretransplant was associated with high counts of memory/effector CD4 T cells on days 84 and 180, total and memory/effector CD8 T cells on days 28 through 365 and CD4+CD8+ T cells on days 84 through 365 (Figure 3), suggesting that substantial fractions of these cells were CMV specific. The donor’s CMV serostatus did not affect the counts of these subsets in either seropositive or seronegative recipients (data not shown).
Figure 3. Effect of recipient CMV serostatus (pretransplant) on memory/effector CD8 T cell counts.
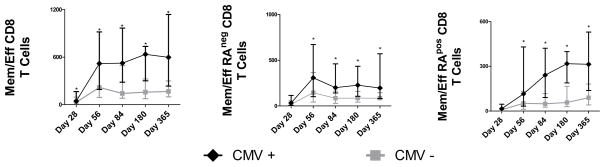
Shown are total memory/effector CD8 T cells, CD45RAnegative (memory-like) memory/effector CD8 T cells, and CD45RApositive (terminally differentiated) memory/effector CD8 T cells. Asterisks (*) denote p<0.05 (Man-Whitney rank sum test). On all y-axes, displayed are cell counts per microliter blood.
Donor match
The only significant association was that of higher naïve CD4 T cells on days 56 and 84 in patients receiving grafts from HLA-matched sibs compared to other donors.
GVHD
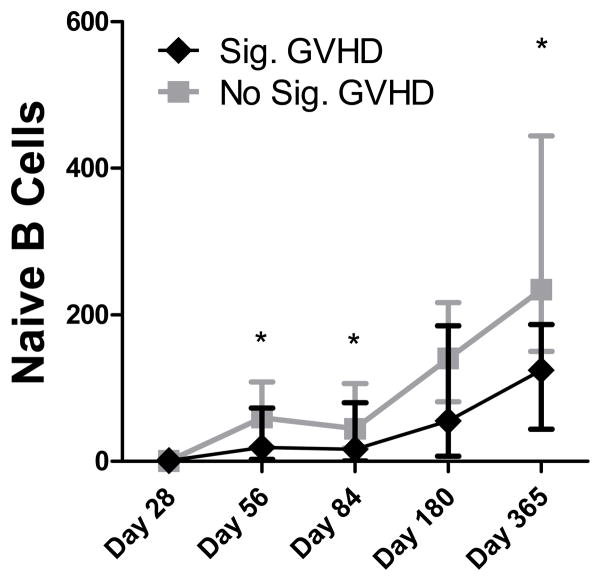
Subset counts were compared between patients who did and did not develop significant GVHD (grade 2–4 aGVHD or cGVHD treated with systemic immunosuppressive therapy at any time during follow up). Naïve CD8 T cells and CD4− iNKT cells were significantly higher and PDCs significantly lower in GVHD patients on day 28, before grade 2–4 aGVHD developed in most patients who developed it (median onset was on day 31). Thus, it was impossible to determine whether the high/low counts of these subsets on day 28 influenced the development of GVHD or the GVHD (possibly subclinical) influenced the subset counts. Negative influence of GVHD was assumed to exist if a subset count (or Ig level) was not significantly different between patients with or without GVHD on day 28 but was significantly lower in patients with GVHD at a later time point. This was the case for total, CD5+, CD5− and naive B cells (Figure 4) and IgM, IgG and IgA levels. Thus, reconstitution of humoral immunity was hampered by GVHD and/or its treatment.
Figure 4. Effect of GVHD on naive B cell counts.
ATG-conditioned and non- ATG-conditioned patients were divided into those with vs. without significant GVHD (grade 2–4 aGVHD or cGVHD requiring systemic immunosuppressive therapy at any time during follow up). Asterisk (*) denotes p<0.05 per the Mann-Whitney rank sum test. On y-axis, displayed are naïve B cell counts per microliter blood.
ATG levels
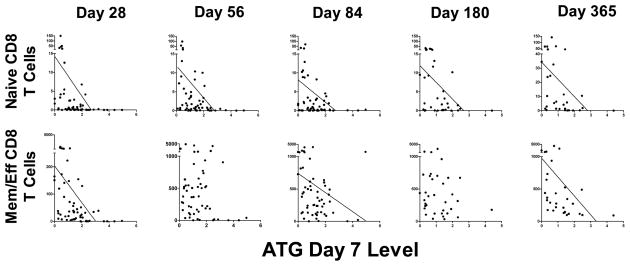
In an attempt to study the effect rather than the cause of the ATG levels on immune cell subset counts, we studied sera from the earliest time points available to us, ie, days 7 and 28. Despite the high content of antibodies against multiple immune cell subsets in ATG, we only found definite association (determined by Spearman rank correlation) between higher ATG levels and lower subset counts for T cell subsets (Table 3 and Figure 5). Remarkably, for most of the T cell subsets the association was detectable not only in the first 3 months but also as late as day 180 or 365, after clearance of ATG (14, 34, 35). This suggests a profound influence on T cell counts long-term. The only T cell subsets for which day 180 or 365 counts were not significantly lower in patients with high day 7 or 28 ATG levels were regulatory T cells and CD4+ iNKT cells, the former perhaps because ATG can stimulate regulatory T cell expansion (36). IgG and IgA levels were lower on day 28 in patients with high day 7/28 ATG levels. This presumed negative ATG effect on IgG/IgA plasma cells appeared to be only transient, as there was no association between day 7/28 ATG levels and IgG/IgA levels at later time points.
Figure 5. Effect of day 7 ATG serum levels on day 28, 56, 84, 180 and 365 counts of naïve (top) and memory/effector (bottom) CD8 T cells.
Trend line is shown in cases of p<0.05 as determined by Spearman rank correlation, demonstrating that higher day 7 ATG levels were associated with lower cell counts. On all x-axes, displayed are ATG levels on day 7 as mg/L serum. On all y-axes, displayed are cell counts per microliter blood.
Of note, in patients with high day 7/28 ATG levels, total and memory/effector CD8 T cells were lower on day 28 but not on day 56. Given that the memory/effector CD8 T cell count increased between day 28 and 56 median 10-fold, this suggests that ATG may have killed or hampered expansion of CD8 T cells in the first month, but paradoxically allowed expansion in the second month. Similarly, for B cells, day 28 ATG levels tended to be associated with low counts of total, CD5+, CD5− and naïve B cells on day 28 but with high counts on day 56. Given that B cell count increased between day 28 and 56 median 19-fold, this suggests that a fraction of antibodies contained in ATG may have killed or hampered generation/expansion of B cells in the first month, but paradoxically improved generation/expansion of B cells in the second month. For NK cells, it was noted that there was a trend towards a paradoxical increase of total and cytolytic NK cells by day 28 in patients with high day 7 ATG levels, suggesting that reconstitution of NK cells (especially cytolytic NK cells) was not hampered but was possibly improved by ATG.
Immune reconstitution after ATG-conditioned vs. non-ATG-conditioned transplantation
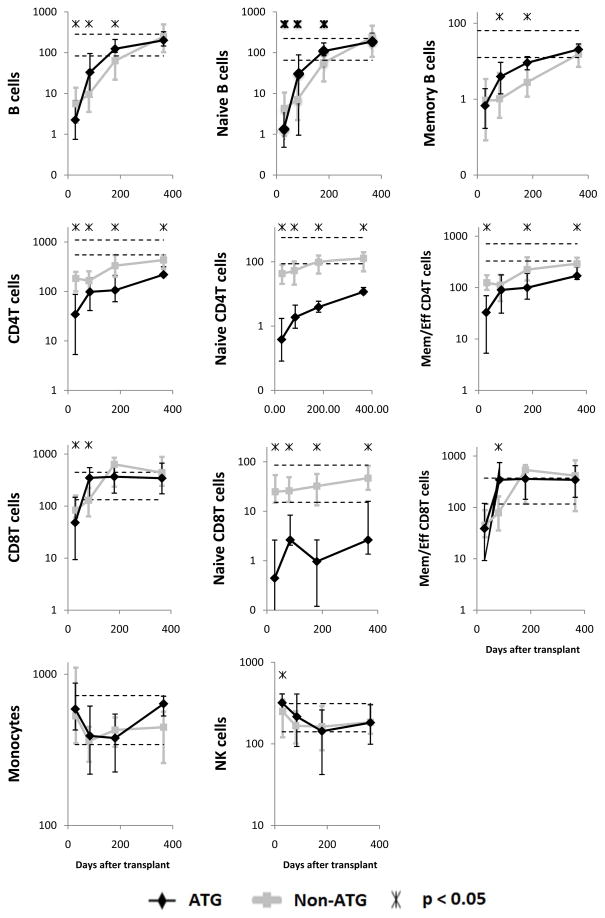
To evaluate further the effect of ATG on immune reconstitution, we compared the subset counts between matched sib graft recipients who received ATG-based and non-ATG based conditioning (Table 1). This was only possible for total/naïve/memory B, CD4 T and CD8 T cells, total NK cells and total monocytes, which were measured in a comparable way in both cohorts.
The comparisons (Figure 6) confirmed the findings derived from the above analyses of associations of subset counts with ATG levels in ATG-conditioned patients. Specifically, counts of total, naïve and memory B cells, total, naïve and memory/effector CD4 T cells and total, naïve and memory/effector CD8 T cells were lower after ATG-based than after non-ATG based conditioning in the first month post transplant (significant for all subsets except for memory B cells and memory/effector CD8 T cells). Thereafter, counts of both naïve and memory CD4 T cells and naïve CD8 T cells remained lower in ATG-conditioned patients for at least 1 year (significant for all 3 subsets at all 3 later time points (3, 6, 12 months) except only a trend for memory/effector CD8 T cells at 3 months). Conversely, in at least one later time point, counts of memory/effector CD8 T cells and both naïve and memory B cells were significantly higher in ATG conditioned patients. NK cell counts were significantly higher in ATG-conditioned patients at 1 month posttransplant.
Figure 6. Median immune cell subset counts in recipients of blood stem cells from HLA-matched siblings conditioned with ATG (black diamonds) vs. without ATG (gray squares).
The time points displayed are 1, 3, 6 and 12 months after transplantation. Error bars indicate the 25th to 75th percentiles. Stars indicate a significant difference (P < .05) between ATG-conditioned and non-ATG-conditioned patients. Normal values are shown as horizontal dashed lines (10th and 90th percentiles). Days after transplantation are shown on all x-axes. On all y-axes, values are cells per microliter blood.
To address whether the improved reconstitution of B cells and memory/effector CD8 T cells was due to the lower incidence of GVHD after ATG conditioning or due to ATG itself, we compared the counts in ATG-conditioned vs. non-ATG-conditioned patients separately for patients with and without significant GVHD. The results showed an effect of ATG itself (Supplementary Figure 3).
Discussion
Based on the above data, we can summarize immune reconstitution after ATG-conditioned HCT by time to recovery, as follows:
Early recovery occurs for monocytes, NK cells (in particular, regulatory NK cells), basophils, neutrophils, MDCs and PDCs.
Intermediate recovery occurs for B cells (faster for naïve than memory B cells, and for CD5+ than CD5− B cells), CD8 T cells (faster for memory/effector than naïve CD8 T cells), and CD4−CD8− T cells.
Very late recovery occurs for CD4 T cells (faster for memory/effector than naïve CD4 T cells), CD4+CD8+ T cells and iNKT cells (faster for CD4− than CD4+ iNKT cells).
Overall, this is consistent with reported literature on non-ATG-conditioned HCT (26, 37–39), except for the markedly slow recovery of CD4 T cells and iNKT cells.
The most important finding of our study is that of the differential impact of ATG on reconstitution of immune subsets. Four patterns of the ATG impact were noted based on the comparison of subset counts between ATG-conditioned vs. non-ATG-conditioned patients and/or the analysis of correlation between ATG levels and subset counts:
Both short and long term subset deficiency, applicable to naïve and memory/effector CD4 T cells, and naïve CD8 T cells.
Short-term subset deficiency followed by fast recovery (faster than in non-ATG-conditioned patients), applicable to naïve and memory B cells, and memory/effector CD8 T cells.
Short-term subset excess, applicable to NK cells, and
No or only minor impact, applicable to monocytes, dendritic cells, and neutrophils.
The long-lasting deficiency of naïve T cells (both CD4 and CD8) suggests that ATG killed or inhibited expansion of a substantial fraction of naïve T cells or stimulated their differentiation to memory/effector T cells. These effects of ATG have been documented in vitro (40) and are supported by limited clinical studies (22–24). Late posttransplant, de novo production of T cells (thymopoiesis) regenerated naïve T cell pool very slowly, particularly in older transplant recipients. This can be attributed to the fact that we studied adult patients, whose thymi are less likely to undergo posttransplant hypertrophy than in pediatric patients (28, 41, 42). Regarding memory/effector CD4 T cells, their prolonged deficiency may be due to their limited capacity to undergo peripheral expansion, the mechanism of which is unknown (30, 43).
The reason for the more rapid recovery of B cells and memory/effector CD8 T cells during or after the second and third months following ATG-based conditioning remains unexplained. We hypothesize that in the case of B cells this could be due in part to the preventive effect of ATG on GVHD, as naïve B cell counts in our ATG-conditioned patients were lower on day 56 and 84 in those with GVHD (Table 3). This observation is consistent with the known negative impact of GVHD and/or its treatment on B-lymphopoiesis (44–47). There also appears to be a GVHD-independent effect of ATG (Supplementary Figure 3) which we hypothesize is related to the known positive effect of ATG on Th2 cells (48). For memory/effector CD8 T cells, the reason is not clear. The low incidence of GVHD in ATG-conditioned patients cannot be the cause of the fast regeneration of memory/effector CD8 T cells between 1 and 3 months, as there was no association between memory/effector CD8 T cell count and GVHD (Table 3) and in bivariate analysis it was ATG conditioning and not GVHD that was associated with high memory/effector CD8 T cell counts at 3 months (Supplementary Figure 3). Moreover, GVHD has been associated with high (not low) memory/effector (or total) CD8 T cell counts (49). Perhaps, ATG at low concentration (<0.05 mg/L serum, typical of our patients past 1 month post transplant) (14) may stimulate the expansion of memory/effector CD8 T cells. This is a testable hypothesis.
Why were NK cell counts higher at 1 month after ATG- than non-ATG-conditioned transplantation? One possibility is a stimulatory effect of ATG on NK-lymphopoiesis/expansion; this is purely speculative. It is also possible that the high NK cell count was compensatory to the profound T-lymphopenia. In other settings of T-lymphopenia (not induced by ATG), high NK cell counts have been described (26, 37, 50, 51), perhaps as a reaction to viral reactivation (51).
Plasma cell-specific antibodies capable of inducing apoptosis of malignant plasma cells in vitro are contained in ATG (15, 52). In spite of that, our study did not detect any significant decline of IgM or IgG and only a minor decline of IgA levels (p=0.022 for pre-transplant to day 28 by Wilcoxon signed rank test) (Figure 1). As the half life of IgG is approximately 23 days and IgM and IgA only 3–5 days (53), we expected to see a substantial decline in IgG and particularly IgM and IgA levels in the first 3 months, given the near-zero B cell counts on day 7 and 28 and low B cell counts on day 56 and 84. Paradoxically, at 3 and 12 months, IgG levels were higher in ATG-conditioned than non-ATG-conditioned patients (8.15 g/L vs. 5.45 g/L at 3 mo, p=0.004, and 9.73 g/L vs. 6.67 g/L at 12 mo, p=0.236). The above facts suggest that long-lived recipient plasma cells were relatively resistant not only to chemo/radiotherapy (54, 55), but also to ATG, and that ATG did not impair, but may have improved, the differentiation of donor-type B cells to plasma cells. Nevertheless a minor, short-term, negative effect of ATG on recipient plasma cells may have occurred as day 28 IgG and IgA levels were lower in patients with high day 7/28 ATG levels (Table 3).
Factors influencing immune reconstitution after ATG-conditioned HCT were, apart from ATG levels, similar to factors influencing immune reconstitution after non-ATG-conditioned HCT (graft contents of immune cells, age of recipient, CMV serostatus of recipient, GVHD). This suggests that the impact of ATG at the doses given, though statistically significant, is not overwhelming as it still allows the other factors to be relevant. Specifically, the correlation between graft content and early posttransplant naïve T and memory B cell counts suggests that ATG did not completely destroy naïve T and memory B cells transferred with the graft. The inverse correlation between age and naïve T cell counts suggests that ATG, though containing antibodies against antigens expressed on thymocytes and thymic stromal cells, allowed for thymopoiesis, at least in younger patients. This may be due to existence of a putative blood-thymus barrier that makes the thymus inaccessible to ATG (56), or due to the fact that by the time thymopoiesis became operational (beyond 3 months post-HCT) (57, 58), ATG has been cleared. The association between high memory/effector T cell (both CD4 and CD8) counts and CMV seropositivity suggests that ATG did not abolish the ability of CMV-specific T cells to expand.
The late nadir of innate immune cell counts is a surprising finding not previously reported. This appears to be a common phenomenon of the innate immune cells, specifically neutrophils, monocytes, basophils, MDCs, PDCs and cytolytic NK cells. We hypothesize that this phenomenon is not unique for ATG-conditioned HCT, as a trend toward late low monocyte and NK cell count was also observed in the non-ATG-conditioned patients (Figure 6). Also, there was no significant difference between the number of patients with severe late neutropenia (at least one episode of neutrophil count <0.5× 109/l on day 30–365) – it occurred in 3/34 (9%) ATG-conditioned and 3/46 (7%) non-ATG-conditioned matched sib graft recipients. This late decline in innate immune cells may suggest a dynamic change in the bone marrow microenvironment particularly in the cytokine production and consumption akin to neutrophil recovery after rituximab therapy (59). Other factors that may also contribute to the late nadir include infection, graft-versus-host disease or myelosuppressive drugs like cotrimoxazole or acyclovir.
This study is the first to describe iNKT reconstitution post marrow/mobilized blood stem cell transplantation. The iNKT cells should theoretically be useful post-transplant as they have both anti-GVHD (60) and anti-tumor effects (61). Unfortunately, per our study, iNKT cell recovery is extremely slow. In addition, as there was a significant correlation between iNKT cell graft content and posttransplant iNKT counts not only early (day 28, 56 and 84) but also late (day 180 and 365), iNKT cells may be produced de novo after HCT only minimally, if at all.
Limitations of our study include: 1. Studying immune cells in blood. Although blood is the easiest source to measure immune reconstitution, it is important to note that an all-inclusive understanding would require analysis of immune cells also in lymph nodes, spleen and other organs. 2. The difference between the ATG and non-ATG-conditioned cohorts in the incidence of GVHD, immune cell subset graft contents and median days of blood draw (Table 1). Fortunately, the different incidence of GVHD did not preclude our finding that ATG improves recovery of B cells and memory/effector CD8 T cells after 1 month post transplant (Figure S3). The different immune cell numbers in the grafts of ATG-conditioned vs. non-ATG-conditioned patients could have theoretically contributed to the observed differences in posttransplant counts of the subsets for which correlation was found between graft content and posttransplant count, i.e., naïve T cells and memory B cells. However, for naïve CD4 T cells, the graft content was 1.3-fold higher whereas the day 28 cell count was 112.4-fold higher in Seattle than in Calgary, suggesting that the lower posttransplant T cell count in Calgary was due to the administration of ATG rather than lower naïve CD4 T cell graft content. Likewise, for naïve CD8 T cells, the graft content was 4-fold higher whereas the day 28 cell count was 56-fold higher in Seattle than Calgary, suggesting ATG as the primary factor. For memory B cell counts, the 2-fold higher memory B cell graft content in Seattle vs. Calgary likely did not cause the higher memory B cell counts on day 84 and 180 in Calgary, as the opposite would be expected (higher memory B cell counts in Seattle patients).
Regarding the differences in the median days of blood draw, even though they were statistically significant, they were probably not biologically/clinically significant (differences of only 3.5 days for the 1 month time point, 6 days for 3 month time point, 4 days for the 6 month time point and 8.5 days for the 12 month time point). Moreover, for NK cells, this should not change the conclusion of paradoxically improved recovery in ATG than non-ATG-conditioned patients, because at 1 month, when the NK cell count was significantly higher in the ATG-conditioned patients, the blood was drawn median 3.5 days earlier (not later) in the ATG-conditioned patients. 3. Differences between enumeration of cell subsets in Seattle and Calgary, e.g., using density-gradient separated fresh MNCs in Seattle vs. total fresh WBCs in Calgary for flow cytometry, and more current definitions of cell subsets for the Calgary patient cohort compared to the historical cohort in Seattle. This problem was mitigated by the fact that the same investigator (J.S.) supervised the analysis in both Seattle and Calgary and personally reviewed all flow cytometry dot plots for all patients at all time points, and by using near-identical subset definitions for the comparison of Seattle and Calgary matched sib transplant recipients.
In summary, this is the first comprehensive study to show the impact of ATG on immune reconstitution. ATG appears to have different impact on different subsets, on one hand producing long-term deficiency of CD4 T cells, naïve CD8 T cell and iNKT cells, and on the other hand improving reconstitution of NK cells, B cells and memory/effector CD8 T cells. Clinical relevance of these findings like influence on viral, bacterial and fungal infections and relapse should be explored.
Supplementary Material
Acknowledgments
Grant support: Alberta Heritage Foundation for Medical Research, Canada Research Chair Program, Alberta Cancer Foundation, and U.S. National Institutes of Health (CA18029).
The authors would like to thank the patients for participating in research that could not benefit them but only future patients. This study could not happen without the dedication of Polly Louie, Lynne Fisk, Judy Wu, Glennis Doiron, Vandana Singh, Monja Metcalf, as well as the staff of the Alberta Blood and Marrow Transplant Program, including inpatient and outpatient nurses and physicians, including Drs. Ahsan Chaudhry, Nancy Zacarias, Ping Yue, Nizar Bahlis, Chris Brown, Andrew Daly, Peter Duggan, Michelle Geddes, Lynn Savoie, Douglas Stewart, Mona Shafey, Loree Larratt, and Robert Turner.
Abbreviations
- ATG
Antithymocyte globulin
- GVHD
Graft-vs-host disease
- aGVHD
Acute graft-vs-host disease
- cGVHD
Chronic graft-vs-host disease
- HCT
Hematopoietic stem cell transplantation
- TBI
Total body irradiation
- PBS
Phosphate buffered saline
- MNC
Mononuclear cells
- MDC
Myeloid dendritic cells
- PDC
Plasmacytoid dendritic cells
Footnotes
Disclosure of Interest: J.S. has received a grant from Genzyme, Otherwise the authors declare no competing financial interests.
This work was presented in abstract form at American Society of Hematology meeting, San Diego, December 2011.
References
- 1.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012 doi: 10.1097/MOH.0b013e328353bc7d. Epub 2012/04/21. [DOI] [PubMed] [Google Scholar]
- 2.Mackall C, Fry T, Gress R, Peggs K, Storek J, Toubert A. Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone marrow transplantation. 2009;44(8):457–62. doi: 10.1038/bmt.2009.255. Epub 2009/10/29. [DOI] [PubMed] [Google Scholar]
- 3.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8. doi: 10.1182/blood-2009-12-234096. Epub 2010/03/11. [DOI] [PubMed] [Google Scholar]
- 4.Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(9):919–27. doi: 10.1016/j.bbmt.2006.05.008. Epub 2006/08/22. [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Petersen M, Sy S, Ugarte-Torres A, Williamson TS, Eliasziw M, Mansoor A, et al. High Epstein-Barr virus-specific T-cell counts are associated with near-zero likelihood of acute myeloid leukemia relapse after hematopoietic cell transplantation. Leukemia. 2012;26(2):359–62. doi: 10.1038/leu.2011.195. Epub 2011/07/30. [DOI] [PubMed] [Google Scholar]
- 6.Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N, et al. Impaired recovery of Epstein-Barr virus (EBV)--specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101(11):4290–7. doi: 10.1182/blood-2002-10-3001. Epub 2003/02/11. [DOI] [PubMed] [Google Scholar]
- 7.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186. Epub 2009/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. Epub 1997/03/27. [DOI] [PubMed] [Google Scholar]
- 9.Lowsky R, Lipton J, Fyles G, Minden M, Meharchand J, Tejpar I, et al. Secondary malignancies after bone marrow transplantation in adults. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12(10):2187–92. doi: 10.1200/JCO.1994.12.10.2187. Epub 1994/10/01. [DOI] [PubMed] [Google Scholar]
- 10.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. Epub 2009/08/22. [DOI] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–5. doi: 10.1016/j.bbmt.2005.12.034. Epub 2006/04/26. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, et al. Preemptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 2010;45(2):385–91. doi: 10.1038/bmt.2009.151. Epub 2009/07/09. [DOI] [PubMed] [Google Scholar]
- 13.Remberger M, Sundberg B. Low serum levels of total rabbit-IgG is associated with acute graft-versus-host disease after unrelated donor hematopoietic stem cell transplantation: results from a prospective study. Biol Blood Marrow Transplant. 2009;15(8):996–9. doi: 10.1016/j.bbmt.2009.04.013. Epub 2009/07/11. [DOI] [PubMed] [Google Scholar]
- 14.Podgorny PJ, Ugarte-Torres A, Liu Y, Williamson TS, Russell JA, Storek J. High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant. 2010;16(7):915–26. doi: 10.1016/j.bbmt.2010.02.027. Epub 2010/03/17. [DOI] [PubMed] [Google Scholar]
- 15.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–94. doi: 10.1038/sj.leu.2404683. Epub 2007/04/06. [DOI] [PubMed] [Google Scholar]
- 16.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79(11):1507–15. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 17.Bonnefoy-Berard N, Flacher M, Revillard JP. Antiproliferative effect of antilymphocyte globulins on B cells and B-cell lines. Blood. 1992;79(8):2164–70. Epub 1992/04/15. [PubMed] [Google Scholar]
- 18.Bonnefoy-Berard N, Revillard JP. Mechanisms of immunosuppression induced by antithymocyte globulins and OKT3. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1996;15(5):435–42. [PubMed] [Google Scholar]
- 19.LaCorcia G, Swistak M, Lawendowski C, Duan S, Weeden T, Nahill S, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009;87(7):966–74. doi: 10.1097/TP.0b013e31819c84b8. Epub 2009/04/09. [DOI] [PubMed] [Google Scholar]
- 20.Eiermann TH, Lambrecht P, Zander AR. Monitoring anti-thymocyte globulin (ATG) in bone marrow recipients. Bone Marrow Transplant. 1999;23(8):779–81. doi: 10.1038/sj.bmt.1701645. Epub 1999/05/07. [DOI] [PubMed] [Google Scholar]
- 21.Haidinger M, Geyeregger R, Poglitsch M, Weichhart T, Zeyda M, Vodenik B, et al. Antithymocyte globulin impairs T-cell/antigen-presenting cell interaction: disruption of immunological synapse and conjugate formation. Transplantation. 2007;84(1):117–21. doi: 10.1097/01.tp.0000266677.45428.80. Epub 2007/07/14. [DOI] [PubMed] [Google Scholar]
- 22.Nakai K, Mineishi S, Kami M, Saito T, Hori A, Kojima R, et al. Antithymocyte globulin affects the occurrence of acute and chronic graft-versus-host disease after a reduced-intensity conditioning regimen by modulating mixed chimerism induction and immune reconstitution. Transplantation. 2003;75(12):2135–43. doi: 10.1097/01.TP.0000066453.32263.F7. Epub 2003/06/28. [DOI] [PubMed] [Google Scholar]
- 23.Fehse N, Fehse B, Kroger N, Zabelina T, Freiberger P, Kruger W, et al. Influence of anti-thymocyte globulin as part of the conditioning regimen on immune reconstitution following matched related bone marrow transplantation. Journal of hematotherapy & stem cell research. 2003;12(2):237–42. doi: 10.1089/152581603321628377. Epub 2003/06/14. [DOI] [PubMed] [Google Scholar]
- 24.Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 1997;3(2):65–75. Epub 1997/06/01. [PubMed] [Google Scholar]
- 25.Behringer D, Bertz H, Schmoor C, Berger C, Dwenger A, Finke J. Quantitative lymphocyte subset reconstitution after allogeneic hematopoietic transplantation from matched related donors with CD34+ selected PBPC grafts unselected PBPC grafts or BM grafts. Bone Marrow Transplant. 1999;24(3):295–302. doi: 10.1038/sj.bmt.1701889. Epub 1999/08/24. [DOI] [PubMed] [Google Scholar]
- 26.Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380–9. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 27.Fallen PR, McGreavey L, Madrigal JA, Potter M, Ethell M, Prentice HG, et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32(10):1001–14. doi: 10.1038/sj.bmt.1704235. Epub 2003/11/05. [DOI] [PubMed] [Google Scholar]
- 28.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. The Journal of clinical investigation. 2005;115(4):930–9. doi: 10.1172/JCI22492. Epub 2005/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91(9):3481–6. Epub 1998/05/23. [PubMed] [Google Scholar]
- 30.Ugarte-Torres A, Hoegh-Petersen M, Liu Y, Zhou F, Williamson TS, Quinlan D, et al. Donor Serostatus Impacts Cytomegalovirus-Specific Immunity, Cytomegaloviral Disease Incidence and Survival in Seropositive Hematopoietic Cell Transplant Recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. doi: 10.1016/j.bbmt.2010.07.020. Epub 2010/08/07. [DOI] [PubMed] [Google Scholar]
- 31.Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–7. doi: 10.1182/blood-2002-11-3472. Epub 2003/07/05. [DOI] [PubMed] [Google Scholar]
- 32.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–80. Epub 1999/01/13. [PubMed] [Google Scholar]
- 33.Noel DR, Witherspoon RP, Storb R, Atkinson K, Doney K, Mickelson EM, et al. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978;51(6):1087–105. Epub 1978/06/01. [PubMed] [Google Scholar]
- 34.Kakhniashvili I, Filicko J, Kraft WK, Flomenberg N. Heterogeneous clearance of antithymocyte globulin after CD34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):609–18. doi: 10.1016/j.bbmt.2005.05.001. Epub 2005/07/26. [DOI] [PubMed] [Google Scholar]
- 35.Bunn D, Lea CK, Bevan DJ, Higgins RM, Hendry BM. The pharmacokinetics of anti-thymocyte globulin (ATG) following intravenous infusion in man. Clinical nephrology. 1996;45(1):29–32. Epub 1996/01/01. [PubMed] [Google Scholar]
- 36.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. Journal of the American Society of Nephrology : JASN. 2006;17(10):2844–53. doi: 10.1681/ASN.2006050422. Epub 2006/08/18. [DOI] [PubMed] [Google Scholar]
- 37.Federmann B, Hagele M, Pfeiffer M, Wirths S, Schumm M, Faul C, et al. Immune reconstitution after haploidentical hematopoietic cell transplantation: impact of reduced intensity conditioning and CD3/CD19 depleted grafts. Leukemia. 2011;25(1):121–9. doi: 10.1038/leu.2010.235. Epub 2010/10/15. [DOI] [PubMed] [Google Scholar]
- 38.Martinez C, Urbano-Ispizua A, Rozman C, Marin P, Rovira M, Sierra J, et al. Immune reconstitution following allogeneic peripheral blood progenitor cell transplantation: comparison of recipients of positive CD34+ selected grafts with recipients of unmanipulated grafts. Experimental hematology. 1999;27(3):561–8. doi: 10.1016/s0301-472x(98)00029-0. Epub 1999/03/25. [DOI] [PubMed] [Google Scholar]
- 39.Theilgaard-Monch K, Raaschou-Jensen K, Palm H, Schjodt K, Heilmann C, Vindelov L, et al. Flow cytometric assessment of lymphocyte subsets, lymphoid progenitors, and hematopoietic stem cells in allogeneic stem cell grafts. Bone Marrow Transplant. 2001;28(11):1073–82. doi: 10.1038/sj.bmt.1703270. Epub 2002/01/10. [DOI] [PubMed] [Google Scholar]
- 40.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(3):465–74. doi: 10.1111/j.1600-6143.2005.00759.x. Epub 2005/02/15. [DOI] [PubMed] [Google Scholar]
- 41.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunological reviews. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. Epub 2005/05/11. [DOI] [PubMed] [Google Scholar]
- 42.Castermans E, Hannon M, Dutrieux J, Humblet-Baron S, Seidel L, Cheynier R, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96(2):298–306. doi: 10.3324/haematol.2010.029702. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouse TARRR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HWJ. Immune response to viruses. Clinical Immunology. 2001:10–28. [Google Scholar]
- 44.Storek J, Wells D, Dawson MA, Storer B, Maloney DG. Factors influencing B lymphopoiesis after allogeneic hematopoietic cell transplantation. Blood. 2001;98(2):489–91. doi: 10.1182/blood.v98.2.489. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Garcia J, Serrano J, Gomez P, Martinez F, Martin C, Roman-Gomez J, et al. The impact of acute and chronic graft-versus-host disease on normal and malignant B-lymphoid precursors after allogeneic stem cell transplantation for B-lineage acute lymphoblastic leukemia. Haematologica. 2006;91(3):340–7. Epub 2006/03/15. [PubMed] [Google Scholar]
- 46.Storek J, Witherspoon RP, Webb D, Storb R. Lack of B cells precursors in marrow transplant recipients with chronic graft-versus-host disease. Am J Hematol. 1996;52(2):82–9. doi: 10.1002/(SICI)1096-8652(199606)52:2<82::AID-AJH3>3.0.CO;2-1. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber KL, Forman J. The effect of chronic graft-versus-host disease on B cell development. Transplantation. 1993;55(3):597–604. doi: 10.1097/00007890-199303000-00025. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Parker M, Xia C, Peng R, Wasserfall C, Clarke T, et al. Rabbit polyclonal mouse antithymocyte globulin administration alters dendritic cell profile and function in NOD mice to suppress diabetogenic responses. J Immunol. 2009;182(8):4608–15. doi: 10.4049/jimmunol.0713269. Epub 2009/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16(3):413–25. Epub 1995/09/01. [PubMed] [Google Scholar]
- 50.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172(2):864–70. doi: 10.4049/jimmunol.172.2.864. Epub 2004/01/07. [DOI] [PubMed] [Google Scholar]
- 51.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. The Journal of experimental medicine. 2011;208(2):357–68. doi: 10.1084/jem.20100479. Epub 2011/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timm MM, Kimlinger TK, Haug JL, Kline MP, Greipp PR, Rajkumar SV, et al. Thymoglobulin targets multiple plasma cell antigens and has in vitro and in vivo activity in multiple myeloma. Leukemia. 2006;20(10):1863–9. doi: 10.1038/sj.leu.2404359. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder HWJTR. Clinical Immunology. London: Mosby; 2001. p. 9. [Google Scholar]
- 54.Miller JJ, Cole LJ. The radiation resistance of long-lived lymphocytes and plasma cells in mouse and rat lymph nodes. J Immunol. 1967;98(5):982–90. Epub 1967/05/01. [PubMed] [Google Scholar]
- 55.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 56.Ruzek MC, Neff KS, Luong M, Smith KA, Culm-Merdek K, Richards SM, et al. In vivo characterization of rabbit anti-mouse thymocyte globulin: a surrogate for rabbit anti-human thymocyte globulin. Transplantation. 2009;88(2):170–9. doi: 10.1097/TP.0b013e3181abc061. Epub 2009/07/23. [DOI] [PubMed] [Google Scholar]
- 57.Heitger A, Neu N, Kern H, Panzer-Grumayer ER, Greinix H, Nachbaur D, et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90(2):850–7. Epub 1997/07/15. [PubMed] [Google Scholar]
- 58.Krenger W, Blazar BR, Hollander GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011;117(25):6768–76. doi: 10.1182/blood-2011-02-334623. Epub 2011/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemieux B, Tartas S, Traulle C, Espinouse D, Thieblemont C, Bouafia F, et al. Rituximab-related late-onset neutropenia after autologous stem cell transplantation for aggressive non-Hodgkin’s lymphoma. Bone marrow transplantation. 2004;33(9):921–3. doi: 10.1038/sj.bmt.1704467. [DOI] [PubMed] [Google Scholar]
- 60.Kohrt HE, Pillai AB, Lowsky R, Strober S. NKT cells, Treg, and their interactions in bone marrow transplantation. European journal of immunology. 2010;40(7):1862–9. doi: 10.1002/eji.201040394. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Exley MA, Lynch L, Varghese B, Nowak M, Alatrakchi N, Balk SP. Developing understanding of the roles of CD1d-restricted T cell subsets in cancer: reversing tumor-induced defects. Clin Immunol. 2011;140(2):184–95. doi: 10.1016/j.clim.2011.04.017. Epub 2011/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.