Abstract
This study was performed to demonstrate the presence of anti-Plasmodium falciparum antibodies in a population living in Irian Jaya, Indonesia that cross-react with human T-lymphotropic virus type I (HTLV-I) proteins. Serum samples from 63 volunteers living in Oksibil, a secluded highland valley in Irian Jaya, were tested for anti-P. falciparum antibodies by an immunofluorescence assay and for anti-HTLV-I antibodies by an enzyme immunoassay (EIA). All samples were positive for anti-P. falciparum antibodies at titers of > or = 1:256. Twenty-four samples were reactive by EIA for HTLV-I, and of these, 23 were tested by western blotting (immunoblotting). Five of the 23 samples were classified as western blot positive and 18 were classified as western blot indeterminate. In competitive blocking assays with malaria proteins, western blot immunoreactivity to all HTLV-I Gag proteins was either reduced or eliminated. Significant reductions in the HTLV-I EIA optical density values of the Oksibil sera occurred when the sera were competitively blocked with the malaria antigens. The optical density values of HTLV-I-positive control sera showed no significant change. Competitive blocking with HTLV-I antigens produced reductions in the optical density values of both the Oksibil sera and the HTLV-I-positive control sera. These data suggest that in this population, anti-P. falciparum antibodies are cross-reactive with HTLV-I proteins in the western blot and EIA tests.
Full text
PDF

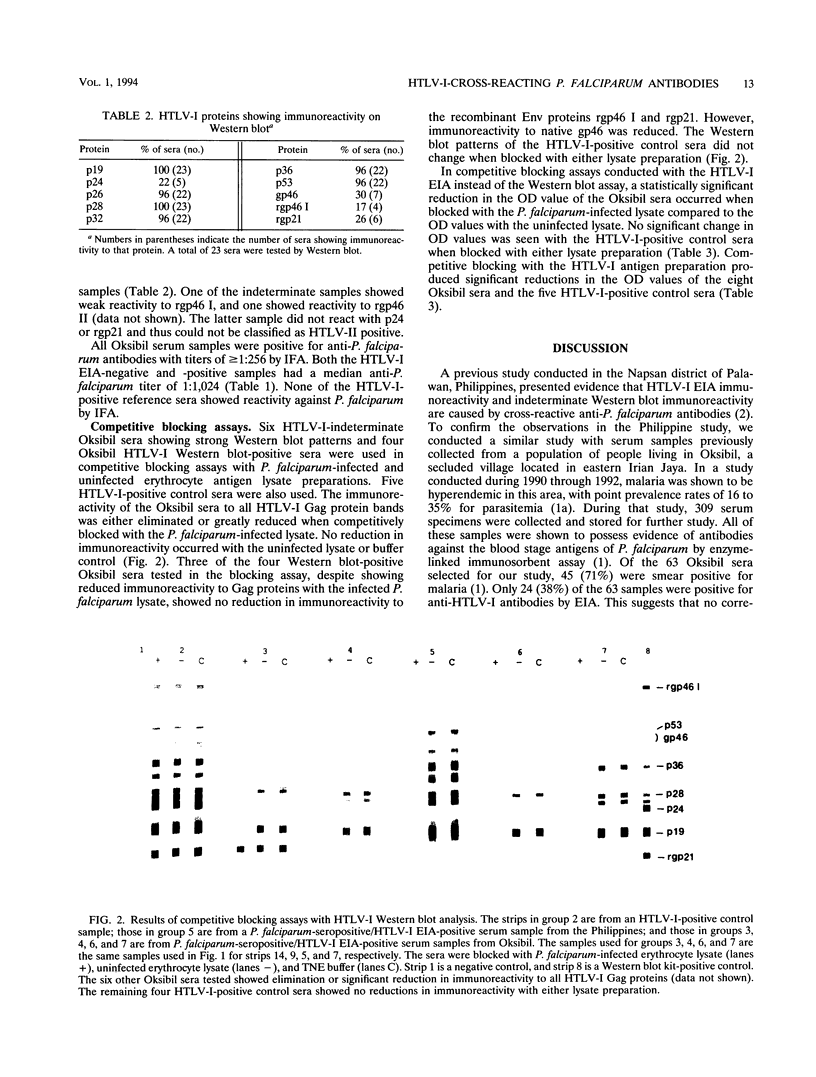
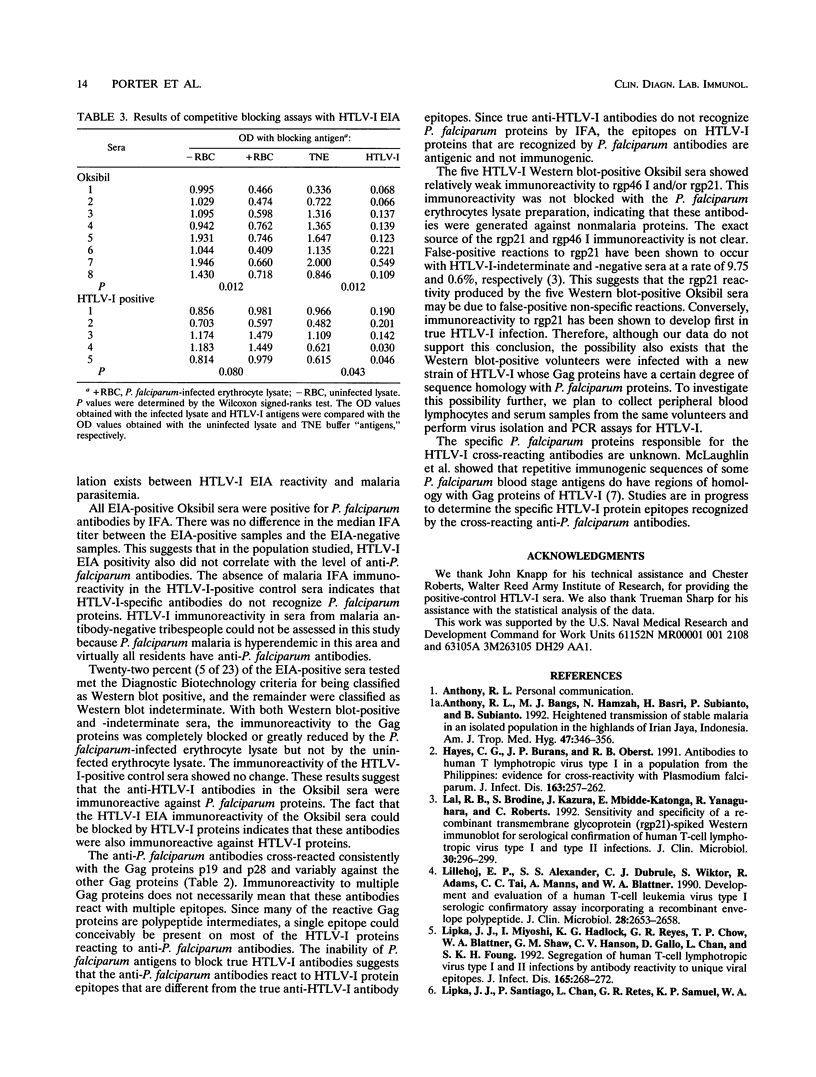

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. L., Bangs M. J., Hamzah N., Basri H., Purnomo, Subianto B. Heightened transmission of stable malaria in an isolated population in the highlands of Irian Jaya, Indonesia. Am J Trop Med Hyg. 1992 Sep;47(3):346–356. doi: 10.4269/ajtmh.1992.47.346. [DOI] [PubMed] [Google Scholar]
- Hayes C. G., Burans J. P., Oberst R. B. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis. 1991 Feb;163(2):257–262. doi: 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Brodine S., Kazura J., Mbidde-Katonga E., Yanagihara R., Roberts C. Sensitivity and specificity of a recombinant transmembrane glycoprotein (rgp21)-spiked western immunoblot for serological confirmation of human T-cell lymphotropic virus type I and type II infections. J Clin Microbiol. 1992 Feb;30(2):296–299. doi: 10.1128/jcm.30.2.296-299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. P., Alexander S. S., Dubrule C. J., Wiktor S., Adams R., Tai C. C., Manns A., Blattner W. A. Development and evaluation of a human T-cell leukemia virus type I serologic confirmatory assay incorporating a recombinant envelope polypeptide. J Clin Microbiol. 1990 Dec;28(12):2653–2658. doi: 10.1128/jcm.28.12.2653-2658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J. J., Miyoshi I., Hadlock K. G., Reyes G. R., Chow T. P., Blattner W. A., Shaw G. M., Hanson C. V., Gallo D., Chan L. Segregation of human T cell lymphotropic virus type I and II infections by antibody reactivity to unique viral epitopes. J Infect Dis. 1992 Feb;165(2):268–272. doi: 10.1093/infdis/165.2.268. [DOI] [PubMed] [Google Scholar]
- Lipka J. J., Santiago P., Chan L., Reyes G. R., Samuel K. P., Blattner W. A., Shaw G. M., Hanson C. V., Sninsky J. J., Foung S. K. Modified Western blot assay for confirmation and differentiation of human T cell lymphotropic virus types I and II. J Infect Dis. 1991 Aug;164(2):400–403. doi: 10.1093/infdis/164.2.400. [DOI] [PubMed] [Google Scholar]
- McLaughlin G. L., Benedik M. J., Campbell G. H. Repeated immunogenic amino acid sequences of Plasmodium species share sequence homologies with proteins from humans and human viruses. Am J Trop Med Hyg. 1987 Sep;37(2):258–262. doi: 10.4269/ajtmh.1987.37.258. [DOI] [PubMed] [Google Scholar]
- Sulzer A. J., Wilson M., Hall E. C. Indirect fluorescent-antibody tests for parasitic diseases. V. An evaluation of a thick-smear antigen in the IFA test for malaria antibodies. Am J Trop Med Hyg. 1969 Mar;18(2):199–205. [PubMed] [Google Scholar]
- Weber J. N., Banatvala N., Clayden S., McAdam K. P., Palmer S., Moulsdale H., Tosswill J., Dilger P., Thorpe R., Amann S. HTLV-1 infection in Papua New Guinea: evidence for serologic false positivity. J Infect Dis. 1989 Jun;159(6):1025–1028. doi: 10.1093/infdis/159.6.1025. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., Ajdukiewicz A. B., Garruto R. M., Sharlow E. R., Wu X. Y., Alemaena O., Sale H., Alexander S. S., Gajudusek D. C. Human T-lymphotropic virus type I infection in the Solomon Islands. Am J Trop Med Hyg. 1991 Feb;44(2):122–130. doi: 10.4269/ajtmh.1991.44.122. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., Jenkins C. L., Alexander S. S., Mora C. A., Garruto R. M. Human T lymphotropic virus type I infection in Papua New Guinea: high prevalence among the Hagahai confirmed by western analysis. J Infect Dis. 1990 Sep;162(3):649–654. doi: 10.1093/infdis/162.3.649. [DOI] [PubMed] [Google Scholar]