Abstract
We determined the feasibility of using an anti-desmoglein (Dsg) monoclonal antibody, Px44, to deliver a biologically active protein to keratinocytes. Recombinantly produced Px44-green fluorescent protein (GFP) injected into mice and skin organ culture delivered GFP to the cell surface of keratinocytes. We replaced GFP with tumor necrosis factor -related apoptosis-inducing ligand (TRAIL) to produce Px44TRAIL. We chose TRAIL as a biologic model because it inhibits activated lymphocytes and causes apoptosis of hyperproliferative keratinocytes, features of various skin diseases. Px44TRAIL formed a trimer, the biologically active form of TRAIL. Standard assays of TRAIL activity showed that Px44TRAIL caused apoptosis of Jurkat cells and inhibited interferon-γ production by activated CD4+ T cells. Enzyme-linked immunoassay with Px44TRAIL showed delivery of TRAIL to Dsg. Immunofluorescence with Px44TRAIL incubated on skin sections and cultured keratinocytes or injected into mouse skin, human organ culture or human xenografts detected TRAIL on keratinocytes. Px44TRAIL caused apoptosis of hyperproliferative, but not differentiating, cultured keratinocytes through binding to Dsg3. Foldon, a small trimerization domain, cloned into Px44TRAIL maintained its stability and biological activity at 37° for at least 48 hr. These data suggest that such targeted therapy is feasible and may be useful for hyperproliferative and inflamed skin diseases.
INTRODUCTION
In this study we test the feasibility of targeting biologically active proteins to keratinocytes. Such a strategy might be useful in many scenarios depending on the agent delivered. For example, one could consider delivery of: agents that cause local immunosuppression for epidermal diseases modulated by activated lymphocytes (e.g. graft vs. host disease, lichen planus, discoid lupus erythematosus, vitiligo); inhibitors of cytokines that cause disease through actions on keratinocytes (e.g. in psoriasis); enzymes to activate or inactivate drugs; growth factor or growth factor inhibitors; and laser targets. Furthermore, agents that cause apoptosis of hyperproliferative keratinocytes or melanocytes in the epidermis might be useful in diseases such as psoriasis, actinic keratoses, skin and head and neck squamous cell carcinoma (HNSCC), and lentigo maligna.
Our hypothesis is that we can use non-pathogenic monoclonal anti-desmoglein (Dsg) single chain variable fragment antibodies (scFvs) that we have cloned from pemphigus patients (Payne et al., 2005) to deliver proteins to epidermis. These scFvs are ideal agents for targeting molecules to keratinocytes, which express Dsgs on their surface, for several reasons. They are small (approximately 25 kD) single-chain antibodies that contain only the antigen binding site and diffuse well through tissue. They do not contain the effector region of full length antibodies, therefore, will not elicit antibody-induced inflammation. They are human and, in themselves, would not elicit an immune reaction in most people. They are cloned and can be easily used to make and produce recombinant molecules.
We chose one of these non-pathogenic anti-Dsg scFvs to show proof of principle of our hypothesis. First we used it to deliver a marker protein, enhanced green fluorescent protein (GFP) to the surface of keratinocytes in normal epidermis. Once we showed that was feasible, we then used it to deliver biologically active tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). We chose TRAIL as a biologic model because it causes apoptosis of hyperproliferative keratinocytes and inhibits activated lymphocytes, features of many of the skin diseases discussed above.
The homotrimeric form of TRAIL has pro-apoptotic activity, while the dimer and monomer form are inactive (Trabzuni et al., 2000). TRAIL is known as a selective anti-tumor agent which induces apoptosis of various malignant hyperproliferative cells, including melanocytes (Thomas and Hersey, 1998; Zhang et al., 1999) and keratinocytes (Basile et al., 2001; Jansen et al., 2003; Kothny-Wilkes et al., 1998; Leverkus et al., 2000; Qin et al., 2001), usually without toxicity, or with much less toxicity, for normal cells, including keratinocytes (Ashkenazi et al., 1999; Basile et al., 2001; Jansen et al., 2003; Kothny-Wilkes et al., 1998; Leverkus et al., 2000; Qin et al., 2001). Thus, TRAIL may be a potential therapeutic agent with a selective inhibitory effect for skin diseases with hyperproliferative keratinocytes or melanocytes. Supporting this notion, in a mouse model of chemical-induced skin carcinogenesis, forced expression of TRAIL on keratinocytes (using a K14-TRAIL transgenic mouse) markedly inhibited pre-neoplastic lesions and tumor formation (Kedinger et al., 2011). Finally, in head and neck squamous cell carcinoma (HNSCC), which highly express Dsg 3 (Chen et al., 2007; Huang et al., 2010; Solassol et al., 2010), TRAIL death receptors are expressed, although in some cases they are down-modulated in metastases (Elrod et al., 2010; Yoldas et al., 2011). In addition, many HNSCC are associated with oncogenic papilloma viruses and keratinocytes expressing papillomavirus-16 E7 oncogene are susceptible to TRAIL-induced apoptosis (Basile et al., 2001). Thus targeted delivery of TRAIL to such neoplasms may be therapeutic.
Finally, TRAIL is also shown to be an immunosuppressive agent which inhibits inflammation in several autoimmune disease animal models including rheumatoid arthritis (Martinez-Lostao et al., 2010), autoimmune encephalomyelitis (Hirata et al., 2005) , and autoimmune thyroiditis (Wang et al., 2009).
Delivering TRAIL to normal epidermis could potentially establish a type of immunological privilege, as proposed for its natural expression in the eye and at the maternal-fetal interface (Niederkorn, 2006; Phillips et al., 1999). Such a function might be useful in various diseases thought to be possibly mediated by activated lymphocytes in epidermis, as discussed above.
In these studies we demonstrate that we can recombinantly produce TRAIL linked to anti-Dsg scFv, that the TRAIL is biologically active and stable, and that it can be delivered to Dsg on the cell surface of keratinocytes in the epidermis.
RESULTS
Design of a baculovirus modular vector to clone and produce an anti-Dsg scFv fusion protein to be used as an epidermal targeting vehicle
Previously, we isolated, by antibody phage display, monoclonal anti-Dsg scFv antibodies from pemphigus patients. Most of these antibodies, although they bound to Dsg and epidermis, were non-pathogenic. We used one of these non-pathogenic anti-Dsg antibodies, Px44, to test the feasibility of delivering a protein to the cell surface of keratinocytes in epidermis. Px44 injected intraperitoneally into mice localizes to multiple stratified squamous epithelial surfaces and persists there for at least 5 days (Supplemental Figure S1 online and unpublished).
To use scFv for protein delivery, we designed a baculovirus vector (Figure 1a) with two cassettes to produce a fusion protein: one 5’ with SfiI sites for a phage display-cloned scFv (which typically has SfiI sites at both ends); and one 3’ with a NotI site that can be used to clone cDNA, PCR-amplified with primers containing NotI sites, for essentially any protein (see example in Figure 1b). We chose a baculovirus vector (used in insect cells) to allow eukaryotic post-translational modification and folding to preserve physiologic function of the fused protein. In addition the vector encodes gp67, an extremely efficient signal peptide that allows secretion of the recombinant protein into the insect cell media for easy purification, and both a histidine x 6 (H6) tag (for purification of encoded fusion protein on a nickel or cobalt column) and a hemaggultinin epitope tag (HA) on the 3’ end.
Figure 1.
Recombinant production and immunofluorescent analysis of anti-Dsg scFv fused to GFP. (a) Modular baculovirus vector with cDNA encoding scFv in an SfiI restriction site and enhanced GFP (EGFP) in a NotI restriction site. cDNA encoding histidine x 6 (H6) and a hemaggultinin epitope tag (HA) are on the 3’ end. The polyhedron promoter is a strong promoter in insect cells. The gp67 signal peptide allows secretion of the translated fusion protein. (b) PCR strategy for amplifying cDNA for TRAIL (or any other protein) to insert in the NotI site of the vector. (c) The Px44GFP fusion protein as injected intradermally into human skin in organ culture, which was harvested 18 hr after injection and microscopically examined for GFP fluorescence. GFP is present on the cell surface of keratinocytes. (Marker 12 μm). (d) The Px44GFP fusion protein was injected in the tail vein of a mouse that was sacrificed 6 hr later. The ear (shown here enface) shows GFP fluorescence on the cell surface of keratinocytes. (Marker 10 μm).
To determine whether Px44 can deliver a fused protein to the epidermis, we used this vector to clone cDNA encoding enhanced green fluorescent protein (GFP) (27 kD) to the 3’ end of cDNA encoding Px44 (Figure 1a). The encoded fusion protein, called Px44GFP, was produced in insect cell media then purified by metal-affinity chromatography on a Talon (cobalt) column. Direct immunofluorescence showed GFP bound to the cell surface of epidermal keratinocytes after purified Px44GFP was injected intradermally into normal human skin organ culture or intravenously into an adult mouse (Figure 1c, d). These results demonstrate that Px44 can be used to deliver a protein to epidermal keratinocytes.
Biochemical and immunological characterization of Px44-mouse (m) TRAIL
To investigate the feasibility of targeted drug delivery of a biologically active protein by Px44, we substituted the extracellular domain of mouse TRAIL (mTRAIL) for GFP in the baculovirus vector (Figure 1a, b), then produced and purified the Px44mTRAIL by binding to an anti-HA agarose column and elution with excess HA peptide, because this gave consistently better purity than metal-affinity chromatography. Coomassie Blue staining of SDS-PAGE of the purified Px44mTRAIL showed a single major band that migrated slightly slower than the 53 kD standard and that stained with an anti-mTRAIL monoclonal antibody on immunoblot (Figure 2a). This apparent molecular weight was slightly larger than the predicted molecular weight based on the amino acid sequence (54 kD), perhaps due to N-glycosylation for which there are 1-2 predicted sites. Since TRAIL is biologically active as a homotrimer, while its monomer or dimer is inactive, we analyzed whether Px44-mTRAIL forms a homotrimer by size exclusion chromatography (Figure 2b). A major peak eluting at a position somewhat ahead of IgG (150 kDa) is consistent with a homotrimer of Px44TRAIL monomers, each with a mass of 54 kDa. (The peaks at smaller molecular weight indicate the MOPS buffer added to the sample prior to analysis (indicating the internal volume of the column) and the small peak eluting after the internal volume is HA peptide (used to purify the fusion protein) that was not completely removed by dialysis. These results show that the normal spontaneous homotrimer formation of TRAIL can occur when it is fused to the Px44 scFv, resulting in a trimer of the fusion protein.
Figure 2.
Biochemical and immunological characterization of Px44-mTRAIL. (a) Western blotting with anti-mTRAIL antibody (left panel), and Coomassie Blue staining (right panel) of SDS-PAGE of Px44-mTRAIL and commercial recombinant mouse TRAIL protein (rmTRAIL) show bands of expected approximate molecular weights. (b) Size exclusion chromatography of Px44-mTRAIL. The peak slightly larger than the 150 kD (IgG) marker indicates trimer formation of Px44-mTRAIL. (c) Dsg3 ELISA by using anti-HA antibody (left panel) and anti-mTRAIL antibody (right panel) shows binding of Px44-mTRAIL to Dsg3.
Next we determined whether the anti-Dsg scFv in the fusion protein retained its antigenic specificity. Dsg 3 ELISA developed with anti-HA and anti-mTRAIL antibody demonstrated that Px44-mTRAIL retained the antigenic specificity of the Px44 scFv antibody, and that Px44 binds the fused protein to the target antigen, Dsg3 (Figure 2c).
TRAIL in the Px44-mTRAIL fusion protein is biologically active
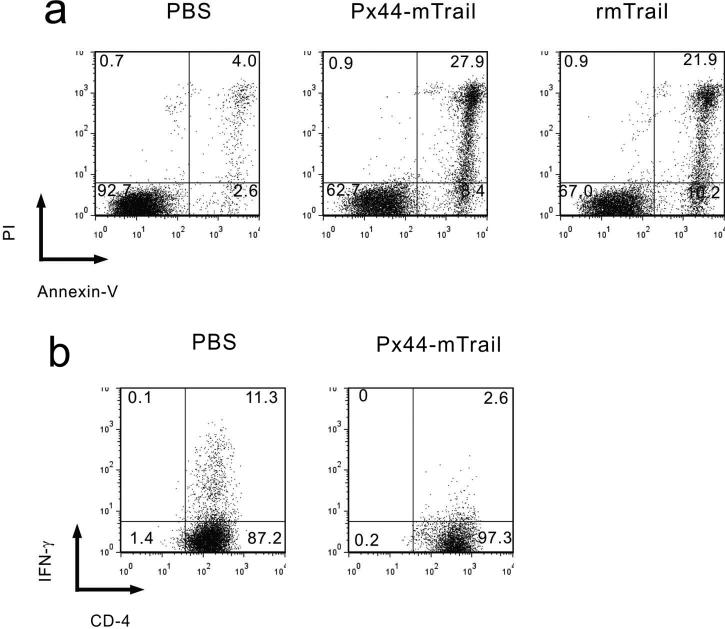
To examine the biological activity of the TRAIL in the fused mTRAIL protein, we used a standard assay of TRAIL activity, its ability to cause apoptosis of Jurkat cells, which are a hyperproliferative T-lymphocyte leukemia cell line (Figure 3a). Px44mTRAIL, recombinant mTRAIL (rmTRAIL, positive control) or PBS (negative control) was incubated for 16 hrs with Jurkat cells in suspension culture. Apoptotic cells were quantitated by flow cytometry after staining with anti-annexin-V for early apoptosis (dying cells) and propidium iodide (PI) for late apoptosis (dead cells). TRAIL in Px44mTRAIL needed anti-HA cross-linking to show activity consistently, probably because it helped aggregate the receptors (also see below). Px44mTRAIL (with anti-HA crosslinking) had similar activities to about twice the molar amount of recombinant TRAIL (without antibody crosslinking) with 36% and 32% dead and dying cells, respectively.
Figure 3.
Biological activity of mTRAIL fused to Px44 determined by flow cytometry. (a) Px44-mTRAIL (with anti-HA antibodies) induced apoptosis of Jurkat cells, as did rmTRAIL. (b) Px44-mTRAIL (with anti-HA antibodies) inhibited IFN-γ production of living activated mouse CD4+ T cells. (Dead cells were gated out).
TRAIL has also been reported to inhibit activated T cells by decreasing cytokine production (e.g. interferon-γ), but not necessarily by apoptosis (Lunemann et al., 2002). To test Px44mTRAIL for such activity, we showed that it decreased interferon-γ expression by living activated mouse CD4 + T cells (Figure 3b).
These results indicated that the fused TRAIL protein in Px44-mTRAIL maintained its biological activity both in apoptosis of hyperproliferative cells and in the inhibition of activated T-cells.
Px44-mTRAIL binds to keratinocytes and causes Dsg3-specific apoptosis of cultured proliferating, but not more differentiating, keratinocytes
TRAIL is known to trigger apoptosis in undifferentiated, hyperproliferative cultured keratinocytes, but not in differentiating keratinocytes (Jansen et al., 2003). To test whether the Px44mTRAIL fusion protein could cause antigen-specific apoptosis of proliferating keratinocytes by binding to Dsg, we first tested binding of Px44mTRAIL to cultured normal human epidermal keratinocytes. For a control, we produced AM3-13-mTRAIL, in which the irrelevant scFv antibody AM3-13 was linked to mTRAIL. To produce the vector encoding this fusion protein we replaced the cDNA encoding Px44 with that encoding AM3-13 in the SfiI site of the baculovirus vector described above (Figure 1a). As determined by immunofluorescence with antibodies to the HA-epitope tag, Px44-mTRAIL, but not AM3-13mTRAIL, bound on the cell surface of cultured keratinocytes (Figure 4a). The binding of Px44-mTRAIL on keratinocytes was also detected with an anti-mTRAIL antibody. Therefore, Px44 can deliver the fused mTRAIL protein to the specific target antigen on living keratinocytes. To demonstrate antigen-specific apoptosis of proliferating keratinocytes, we added Px44-mTRAIL (and AM3-13-mTRAIL with equal TRAIL specific activity, as a negative control) to undifferentiated human keratinocytes cultured in low calcium, and then washed the cells. We then determined apoptosis of keratinocytes by flow cytometry after another 16 hr of culture. We found that Px44-mTRAIL resulted in about 47% dead (propidium iodide [PI] positive) and dying (annexin-V positive, PI negative) cells compared to 18% with AM3-13-TRAIL (Figure 4b, left, upper). To confirm the antigen-specificity of the delivery of biologically active TRAIL to the keratinocytes, we showed that soluble recombinant Dsg3 blocked the apoptosis of keratinocytes induced by Px44-mTRAIL (Figure 4b, left, lower). These data demonstrate that the Px44-mTRAIL binding to Dsg3 enhances its ability to cause apoptosis of keratinocytes by binding it to the keratinocytes, allowing its effect after washout of the soluble molecule, whereas the short incubation without binding (either from AM3-13mTRAIL or Px44mTRAIL blocked from binding with soluble Dsg3) is much less effective.
Figure 4.
Target antigen-specific function of Px44-mTRAIL. (a) Px44-mTRAIL bound on the cell surface of normal human epidermal keratinocytes in low calcium (0.4mM) (left panel) whereas control fusion protein AM3-13-mTRAIL did not (middle panel). Binding of Px44-mTRAIL was detected by anti-HA antibody (left panel) and anti-mTRAIL antibody (right panel). (b) The normal human epidermal keratinocytes in low calcium (0.4mM) was treated with Px44-mTRAIL or AM3-13-mTRAIL. After 2 hours incubation, cells were washed then cultured for a further 16 hours, and, then, analyzed by flow cytometry for apoptosis. Increased cell death was seen in Px44-mTRAIL treated keratinocytes (upper left) compared to cells treated with AM3-13-mTRAIL. In the presence of recombinant Dsg3 during the 2 hours incubation with fusion proteins, the effect of Px44-mTRAIL-HA treatment was inhibited (lower left). In high calcium (1.2mM), keratinocytes were resistant to Px44-mTRAIL induced apoptosis.
Finally, we examined the sensitivity of differentiating keratinocytes cultured in high calcium (1.2mM) for 48 hrs to Px44-mTRAIL-induced apoptosis (Figure 4b, right). Although dead or dying cells (24%) due to differentiation were observed, as expected, in differentiating keratinocytes without any active reagent added, adding Px44-mTRAIL hardly increased their number (27%). Thus, unlike more proliferative cells cultured in lower calcium medium, the differentiating, less proliferative, keratinocytes were resistant to Px44-mTRAIL-induced apoptosis.
Taken together these results indicate that Px44-mTRAIL binds to keratinocytes and causes apoptosis of cultured hyperproliferative, but not more differentiating, keratinocytes.
Cloning and characterization of Px44 fused to human (h) TRAIL and stabilization of trimer formation and biological activity with foldon
Ultimately for human targeted therapy with TRAIL we would prefer human TRAIL (hTRAIL) fused to Px44. In addition, hTRAIL is thought to be more potent than mTRAIL (Berg et al., 2007). Finally, enforced trimerization with a potent trimerization domain is thought to preserve stability and activity of TRAIL. Therefore, we used the baculovirus cloning vector (Figure 1a, b) to substitute PCR-amplified cDNA encoding hTRAIL for the cDNA encoding mTRAIL to produce a vector encoding Px44hTRAIL. We also added a small potent trimerization domain called foldon (Meier et al., 2004) encoded within the PCR primers to produce Px44-foldon-hTRAIL. We tested the fusion protein produced by these constructs for biological activity, stability at 37°, and ability to diffuse from the dermis to bind the cell surface of keratinocytes in intact epidermis.
Incubation of Jurkat cells for 16 hr with Px44hTRAIL at 1 μg/ml (without pre-incubation at 37°) caused approximately 53% dead and dying cells (Fig. 5a “0h” column, bottom). Px44-foldon-hTRAIL was more potent under these conditions causing approximately 79% dead and dying cells (Figure 5a “0h”, top). Furthermore, Px44hTRAIL lost activity during pre-incubation at 37° before incubation with Jurkat cells. After 16 hr pre-incubation Px44hTRAIL caused approximately 40% dead and dying cells (down from 53%) whereas preincubation of Px44-foldon-hTRAIL at 37° did not cause loss of activity; it maintained its ability to cause 80% dead or dying cells. Even after preincubation for 48 hr at 37°, Px44-foldon-hTRAIL maintained its ability to cause apoptosis of approximately 80% of Jurkat cells (Figure 5b top row), while Px44hTRAIL gradually further lost that ability. However, using anti-HA to externally cross link Px44hTRAIL fully restored its apoptotic ability to over 80%, (Figure 5b bottom row). This data suggests that Px44hTRAIL at 37° gradually loses its trimerization and, with that, its ability to cross-link its receptor, but the addition of anti-HA allows external cross-linking and activity restoration. The greater stability of Px44-foldon-hTRAIL is confirmed by size exclusion chromatography (Figure 5c) in which Px44-foldon-hTRAIL maintains trimer formation even for 48 hr at 37° but the trimer of Px44-hTRAIL is gradually lost.
Figure 5.
Enforced trimerization by foldon enhanced and stabilized the biological activity of Px44-hTRAIL. (a and b) Flow cytometric analysis of the apoptosis-inducing activity on Jurkat cells of Px44-Foldon-hTRAIL and Px44-hTRAIL after incubation at 37°C (before incubation with the cells). (a) Px44-hTRAIL's ability to induce apoptosis decreased over time incubated at 37° (lower panel) compared to Px44-foldon-hTRAIL (upper panel). (b) Px44-Foldon-hTRAIL kept its activity even after 48 hr at 37° (upper panels). The reduction of activity of Px44-hTRAIL was restored by anti-HA cross-linking (lower panels). (c) Size exclusion chromatography of Px44-Foldon-hTRAIL and Px44-hTRAIL before and after 37°C incubation for 48 hours. Px44-hTRAIL before 37°C incubation, and Px44-foldon-hTRAIL formed homotrimers (arrowhead). A lower molecular weight peak developed in Px44-hTRAIL after the 48 hours incubation at 37°C (arrow), indicating instability of the homotrimer with dissociation into smaller units.
Px44-mTRAIL binds to the cell surface of keratinocytes in normal epidermis
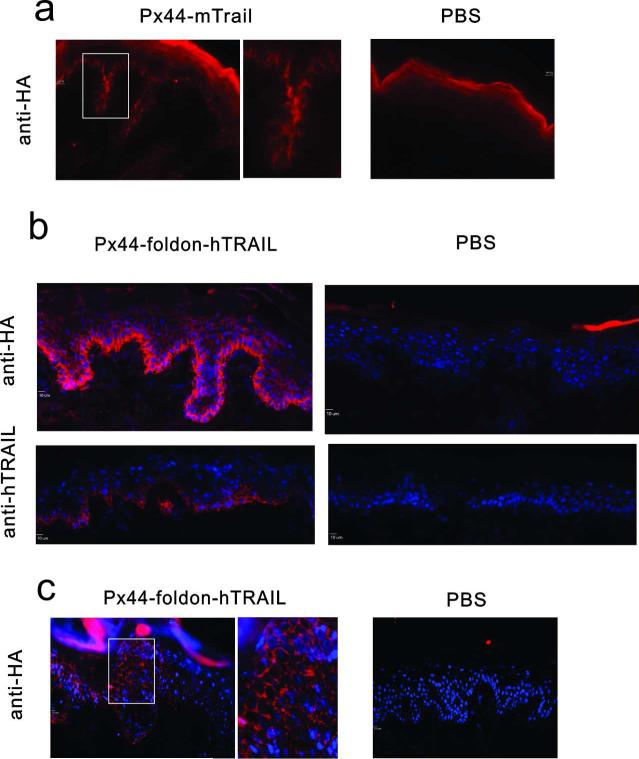
Because biologically active Px44TRAIL is a trimer and we ultimately want to use it to deliver TRAIL to the epidermis, we wanted to show that Px44TRAIL could diffuse from dermis to epidermis and bind the keratinocyte cell surface. First, we injected Px44-mTRAIL intradermally into neonatal mouse back skin. At 2 hours after subcutaneous injection Px44-mTRAIL bound on the epidermal cell surface of basal and hair follicle keratinocytes as detected by immunofluorescence with anti-HA (Figure 6a).
Figure 6.
Px44-mTRAIL and Px44-foldon-hTRAIL diffuse across the basement membrane of mouse and human epidermis to bind to the cell surface of keratinocytes. (a) Px44-mTRAIL injected into neonatal mice binds to the cell surface of hair follicle and basal keratinocytes as detected by anti-HA immunofluorescence. Area in white box is shown closer up to the right of its image. (Marker, 10 μ). (b) Px44-foldon-hTRAIL injected intradermally into human skin in organ culture binds the cell surface of keratinocytes as detected by immunofluorescence with anti-HA and anti-hTRAIL. (Marker, 10 μm). (c) Px44-foldon-hTRAIL injected intradermally into human skin xenografts on mice binds to the cell surface of keratinocytes as detected by immunofluorescence with anti-HA antibodies. Area in white box is shown closer up to the right of its image. (Marker, 10 μm)
To examine the binding of Px44-foldon-hTRAIL, we injected it intradermally into human skin organ culture. At 2 hrs after injection, Px44-foldon-hTRAIL bound on the keratinocyte cell surface, as detected by anti-HA antibodies and anti-hTRAIL antibodies (Figure 6b). Px44-foldon-hTRAIL also bound on the keratinocytes of human skin xenografts on immunodeficient mice 1 hr after intradermal injection (Figure 6c). Finally, we injected Px44-foldon-hTRAIL into mouse back skin once then again in 16 hr. 30 min after the last injection harvested skin was positive by anti-HA immunofluorescence but negative by TUNEL staining (data not shown). These results demonstrate that Px44-foldon-hTRAIL can cross the basement membrane of normal human skin to be delivered to the surface of keratinocytes but does not cause apoptosis in normal epidermis.
DISCUSSION
In this study we show that it is feasible to molecularly clone and produce an anti-Dsg3 scFv linked as a fusion protein with an indicator protein, GFP, or a biologically active protein, TRAIL, and that the scFv targets the fused protein to the keratinocyte cell surface in culture or in tissue.
TRAIL and agonist antibodies against its cell death receptors have been, or are, in clinical trials for various cancers (Abdulghani and El-Deiry, 2010). However, one major problem is that the therapy is, in general, not targeted and there may be some toxicity to normal organs, usually liver, and especially at higher doses or with more active TRAIL preparations. Some preclinical studies have shown the feasibility of targeted therapy of TRAIL with scFv antibodies to cancer-specific antigens or antigens overexpressed in the neoplastic cells (Bremer et al., 2004; Bremer et al., 2005; de Bruyn et al., 2010; Schneider et al., 2010). In these studies the targeted TRAIL showed greater activity in causing apoptosis of tumor cells than untargeted TRAIL and also showed a “bystander effect”, i.e. it kills neighboring cells that don't have the targeted antigen. It is thought that “attaching” TRAIL to a cell surface also increases its specific activity. The unique aspect of our approach is to use a human scFv to target normal tissue (epidermis) to prevent inflammation or to target developing hyperproliferative lesions in that tissue (e.g. actinic keratoses, early HNSCC, psoriasis, lentigo maligna) lesions. Adding credence to the idea that cell surface TRAIL might contribute to tumor surveillance, a recent study showed that imiquimod and interferon alpha induce TRAIL expression on plasmacytoid dendritic cells which then developed the ability to cause apoptosis of melanoma cells (Kalb et al., 2012). It was proposed that it is partially through this mechanism that imiquimod can be therapeutically active against actinic keratoses and skin cancer.
Although detailed analysis of the effects of Px44-TRAIL in in vivo disease models was beyond the scope of this report, we did test its activity in a K14-activated Fyn mouse model of actinic keratoses and early squamous cell carcinoma (Zhao et al., 2009). In this model we could show in two experiments that Px44-mTRAIL and Px44-foldon-hTRAIL caused apoptosis in such neoplasms (Supplemental Figure S2 online), confirming the ideas above.
Another unique aspect of our study is that the modular vector we developed can fuse essentially any protein to an anti-Dsg scFv to deliver it to epidermis. Using TRAIL or other proteins that inhibit activated lymphocytes would have the potential to suppress certain immunological reactions that cause epidermal disease..
There are potential problems with targeted therapy of TRAIL to epidermis that will have to be addressed in future studies. For example, even though the anti-Dsg3 scFv and TRAIL used here are human, there could be an immune response against the linker or foldon. In the case of foldon, other less antigenic trimerization domains such as leucine or isoleucine zippers could be substituted (Abdulghani and El-Deiry, 2010). Another issue is that although soluble TRAIL usually does not affect normal epidermis, targeted TRAIL might. However our initial studies in mouse epidermis suggest, at least under certain conditions, it does not. In addition, K14-TRAIL transgenic mice that overexpress TRAIL in the epidermis do not show toxicity of normal epidermis (Kedinger et al., 2011). However, with each particular TRAIL delivery method, more extensive studies would need to address this issue. Finally, how fast the targeted TRAIL turns over in the skin (either through keratinocyte shedding or cellular internalization) will affect its therapeutic efficacy in different situations. In treating tumors, TRAIL, although beneficial in killing proliferating cells, might be detrimental if it downregulates the host immune response. But these issues have to be determined empirically; for example it may only require short contact with hyperproliferative cells to eliminate that pool, however, it may require more sustained contact to affect activated lymphocytes if they are continually renewed.
In any case, we show here that targeting proteins to the epidermis with an anti-Dsg3 scFv, derived from a human PV patient, is practical. This work establishes the practicality and basis for testing this fusion protein as well as others targeted to keratinocytes in various immunological skin diseases and precancerous and cancerous lesions.
MATERIALS AND METHODS
(Supplemental Materials and Methods have further details)
Design of baculovirus vector for producing fusion proteins
We modified the cloning vector pAcGP67 Baculovirus Transfer Vector (BD Bioscience, San Jose, CA) to express and secrete scFv fusion proteins in insect cells (Figure 1a).
Production and purification of recombinant proteins
Chimeric protein in the culture supernatant of a baculovirus expression system in insect cells was bound to anti-HA agaraose beads then eluted with excess HA peptide.
ELISA
A Dsg3 ELISA kit (MBL, Woburn, MA) was used.
Cell culture
Jurkat cells were cultured in RPMI 1640 in 96 well plates.
Normal human epidermal keratinocytes (NHEK) obtained from neonatal foreskins were cultured in Defined K-SFM (Life Technologies, Grand Island, NY)..
Mouse CD4+ T cells were enriched from splenocytes of C57BL/6 mice by positive selection using the MACS cell separation system (Miltenyi Biotec, Berisch Gladbach, Germany). For Th1 skewed culture conditions, cells were cultured with anti-CD3, anti-CD28, anti-IL-4, and IL-12.
Flow cytometry
Analysis of apoptosis of Jurkat cells and NHEK used the FITC-Annexin-V Apoptosis Detection Kit I (BD Bioscience). For analysis of NHEK, both floating and adherent cells were harvested. For intracellular cytokine staining, mouse CD4+ T cells were treated with PMA, ionomycin, and brefeldin A (Sigma), and stained with FITC-anti mouse CD4 (Biolegend) and PE-anti mouse interferon (IFN)-γ (Biolegend, San Diego, CA).
Immunohistochemistry
Immunofluorescence was performed as previously described (Payne et al., 2005).
Immunoblot
General procedures were as previously described (Ishii et al., 2008).
Human skin organ culture injection
5 mm square pieces of normal skin were injected intradermally with 50 μl of protein (1mg/ml), then, after 2-18 hours culture, harvested for immunofluorescence.
Protein injection into the mice
Chimeric proteins were injected intravenously or intradermally and binding to epidermis detected by immunofluorescence.
Size exclusion chromatography
Samples of 100 μl were analyzed on a Superose 12 HPLC column (Bio-Rad, Hercules, CA).
Animals and human subjects
Approvals for animal protocols and for human subjects were obtained from appropriate review boards at the University of Pennsylvania. Only discarded, de-identified human material was used.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Brendan Hilliard and Mark Tykocinski for providing cDNA encoding mouse TRAIL (originally through Dr. Hideo Yagita) and human TRAIL; and Aimee Payne, Christoph Hammers, and Tomoaki Yokoyama for their comments. This paper was supported by grants to JRS: R01 AR052672, AR052672S1, R03 DA032475, and P30 AR057217 (Skin Disease Research Center) from the National Institutes of Health.
Abbreviations
- Dsg
Desmoglein
- GFP
Green fluorescent protein
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- HNSCC
head and neck squamous cell carcinoma
- scFvs
single chain variable fragment antibodies
- HA
hemaggultinin
Footnotes
CONFLICT OF INTEREST
Authors JS and DS have a U.S. patent for this technology
References
- Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010;14:1091–108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Zacny V, Munger K. The cytokines tumor necrosis factor-alpha (TNF-alpha) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J Biol Chem. 2001;276:22522–8. doi: 10.1074/jbc.M010505200. [DOI] [PubMed] [Google Scholar]
- Berg D, Lehne M, Muller N, Siegmund D, Munkel S, Sebald W, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 2007;14:2021–34. doi: 10.1038/sj.cdd.4402213. [DOI] [PubMed] [Google Scholar]
- Bremer E, Samplonius D, Kroesen BJ, van GL, de LL, Helfrich W. Exceptionally potent anti-tumor bystander activity of an scFv:sTRAIL fusion protein with specificity for EGP2 toward target antigen-negative tumor cells. Neoplasia. 2004;6:636–45. doi: 10.1593/neo.04229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E, Samplonius DF, Peipp M, van GL, Kroesen BJ, Fey GH, et al. Target cell-restricted apoptosis induction of acute leukemic T cells by a recombinant tumor necrosis factor-related apoptosis-inducing ligand fusion protein with specificity for human CD7. Cancer Res. 2005;65:3380–8. doi: 10.1158/0008-5472.CAN-04-2756. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, et al. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. 2007;26:467–76. doi: 10.1038/sj.onc.1209802. [DOI] [PubMed] [Google Scholar]
- de Bruyn M, Rybczynska AA, Wei Y, Schwenkert M, Fey GH, Dierckx RA, et al. Melanoma-associated Chondroitin Sulfate Proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo. Mol Cancer. 2010;9:301. doi: 10.1186/1476-4598-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod HA, Fan S, Muller S, Chen GZ, Pan L, Tighiouart M, et al. Analysis of death receptor 5 and caspase-8 expression in primary and metastatic head and neck squamous cell carcinoma and their prognostic impact. PLoS One. 2010;5:e12178. doi: 10.1371/journal.pone.0012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174:1888–97. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lee TJ, Chang PH, Lee YS, Chuang CC, Jhang YJ, et al. Desmoglein 3 is overexpressed in inverted papilloma and squamous cell carcinoma of sinonasal cavity. Laryngoscope. 2010;120:26–9. doi: 10.1002/lary.20151. [DOI] [PubMed] [Google Scholar]
- Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–48. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen BJ, van Ruissen F, Cerneus S, Cloin W, Bergers M, van Erp PE, et al. Tumor necrosis factor related apoptosis inducing ligand triggers apoptosis in dividing but not in differentiating human epidermal keratinocytes. J Invest Dermatol. 2003;121:1433–9. doi: 10.1046/j.1523-1747.2003.12636.x. [DOI] [PubMed] [Google Scholar]
- Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. J Immunol. 2012;188:1583–91. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- Kedinger V, Muller S, Gronemeyer H. Targeted expression of tumor necrosis factor-related apoptosis-inducing ligand TRAIL in skin protects mice against chemical carcinogenesis. Mol Cancer. 2011;10:34. doi: 10.1186/1476-4598-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothny-Wilkes G, Kulms D, Poppelmann B, Luger TA, Kubin M, Schwarz T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 1998;273:29247–53. doi: 10.1074/jbc.273.44.29247. [DOI] [PubMed] [Google Scholar]
- Leverkus M, Neumann M, Mengling T, Rauch CT, Brocker EB, Krammer PH, et al. Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000;60:553–9. [PubMed] [Google Scholar]
- Lunemann JD, Waiczies S, Ehrlich S, Wendling U, Seeger B, Kamradt T, et al. Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto)antigen-specific T cells. J Immunol. 2002;168:4881–8. doi: 10.4049/jimmunol.168.10.4881. [DOI] [PubMed] [Google Scholar]
- Martinez-Lostao L, Garcia-Alvarez F, Basanez G, Alegre-Aguaron E, Desportes P, Larrad L, et al. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010;62:2272–82. doi: 10.1002/art.27501. [DOI] [PubMed] [Google Scholar]
- Meier S, Guthe S, Kiefhaber T, Grzesiek S. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable beta-hairpin: atomic details of trimer dissociation and local beta-hairpin stability from residual dipolar couplings. J Mol Biol. 2004;344:1051–69. doi: 10.1016/j.jmb.2004.09.079. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–9. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TA, Ni J, Pan G, Ruben SM, Wei YF, Pace JL, et al. TRAIL (Apo-2L) and TRAIL receptors in human placentas: implications for immune privilege. J Immunol. 1999;162:6053–9. [PubMed] [Google Scholar]
- Qin J, Chaturvedi V, Bonish B, Nickoloff BJ. Avoiding premature apoptosis of normal epidermal cells. Nat Med. 2001;7:385–6. doi: 10.1038/86401. [DOI] [PubMed] [Google Scholar]
- Schneider B, Munkel S, Krippner-Heidenreich A, Grunwald I, Wels WS, Wajant H, et al. Potent antitumoral activity of TRAIL through generation of tumor-targeted single-chain fusion proteins. Cell Death Dis. 2010;1:e68. doi: 10.1038/cddis.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solassol J, Burcia V, Costes V, Lacombe J, Mange A, Barbotte E, et al. Pemphigus vulgaris antigen mRNA quantification for the staging of sentinel lymph nodes in head and neck cancer. Br J Cancer. 2010;102:181–7. doi: 10.1038/sj.bjc.6605470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–200. [PubMed] [Google Scholar]
- Trabzuni D, Famulski KS, Ahmad M. Functional analysis of tumour necrosis factor-alpha-related apoptosis-inducing ligand (TRAIL): cysteine-230 plays a critical role in the homotrimerization and biological activity of this novel tumoricidal cytokine. Biochem J. 2000;350(Pt 2):505–10. [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Chen GH, Fan Y, Van Antwerp M, Baker JR., Jr. Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells. Endocrinology. 2009;150:2000–7. doi: 10.1210/en.2008-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoldas B, Ozer C, Ozen O, Canpolat T, Dogan I, Griffith TS, et al. Clinical significance of TRAIL and TRAIL receptors in patients with head and neck cancer. Head Neck. 2011;33:1278–84. doi: 10.1002/hed.21598. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–53. [PubMed] [Google Scholar]
- Zhao L, Li W, Marshall C, Griffin T, Hanson M, Hick R, et al. Srcasm inhibits Fyn-induced cutaneous carcinogenesis with modulation of Notch1 and p53. Cancer Res. 2009;69:9439–47. doi: 10.1158/0008-5472.CAN-09-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.