Abstract
Tuberculosis (TB) is mainly a disease of the lungs, but Mycobacterium tuberculosis (Mtb) can establish infection in virtually any organ in the body. Rising rates of extrapulmonary (EP) TB have been largely associated with the HIV epidemic, as patients co-infected with HIV show a four-fold higher risk of EPTB. Spinal TB (Pott's Disease), one of the most debilitating extrapulmonary forms of disease, is difficult to diagnose and can cause deformity and/or neurological deficits. This study examined the histopathology and distribution of immune cells within spinal TB lesions and the impact of HIV on pathogenesis. The overall structure of the spinal granulomas resembled that seen in lung lesions from patients with pulmonary TB. Evidence of efficient macrophage activation and differentiation were detectable within organized structures in the spinal tissue, irrespective of HIV status. Interestingly, the granulomatous architecture and macroscopic features were similar in all samples examined, despite a reversal in the ratio of infiltrating CD4 to CD8 T cells in the lesions from HIV-infected patients. This study provides a foundation to understand the mechanism of tissue destruction and disease progression in Spinal TB, enabling the future development of novel therapeutic strategies and diagnostic approaches for this devastating disease.
Keywords: Spinal Tuberculosis, Pott's Disease, Immunopathogenesis, HIV/AIDS, Granuloma
INTRODUCTION
Tuberculosis (TB) is a resurgent and significant global health emergency. In addition to roughly 1.4 million deaths due to TB in 20111_ENREF_1, an estimated 1.8 billion people are latently infected with the causative agent Mycobacterium tuberculosis (Mtb). While pulmonary TB is the most common form, the disease can affect virtually all organs in the body. In recent years, the rates of extrapulmonary TB (EPTB) have been increasing, largely in conjunction with the rising prevalence of HIV infection2, 3. Whereas 10–20% of HIV-uninfected cases develop EPTB, 40–80% of co-infected individuals may develop these disease manifestations4, 5. This is particularly relevant in endemic areas with high disease burdens, such as South Africa, where 15% of all new TB cases were EP and 65% of the 323,440 TB cases tested in 2011 were HIV positive1.
Spinal TB (Pott's Disease) is one of the most debilitating and destructive extrapulmonary manifestations, accounting for 1–5% of TB cases worldwide6. TB of the spine is characterized by destruction of the vertebral bodies and discs and the formation of abscesses, which may impinge on the spinal cord, ultimately resulting in collapse of the spinal column and risk of paralysis. Because spinal TB is paucibacillary and tissue biopsies are not readily available, diagnosis relies on nonspecific clinical presentations, rather than conventional microbiologic tests7. Consequently, diagnosis and treatment of patients with spinal TB is frequently delayed8. Treatment includes anti-TB chemotherapy for 6 to 9 months, and surgical intervention is recommended in severe cases to manage neurological deficits and/or deformity6. Surgery involves debridement (removal of the abscess and granulomatous tissue), followed by spinal reconstruction9, 10. Previously published studies of spinal TB have focused on clinical descriptions and surgical interventions to treat severe cases9, 11–15, but our understanding of the local pathology is limited.
Most reported studies of TB immunity and pathogenesis have been based on analysis of peripheral blood leukocytes, which are not fully representative of the local site of infection16. Recently, data from studies using resected lung tissue from pulmonary TB patients, combined with work from animal models, have begun to elucidate the complex host response to Mtb. Following inhalation, the bacilli are phagocytosed by macrophages, which produce cytokines and chemokines to recruit peripheral blood leukocytes, including granulocytes, monocytes, T lymphocytes and B lymphocytes. Upon activation, these cells drive antimicrobial activity by the infected phagocytes, leading to bacillary control. The hallmark of TB is the granuloma, an organized aggregate of leukocytes that forms in response to chronic Mtb-driven macrophage stimulation17–21. The unique microenvironment of the granuloma includes activated macrophages that differentiate into epithelioid cells that can fuse to become multinucleated giant cells or differentiate into foam cells, characterized by lipid droplet accumulation22, 23. Pulmonary granulomas can differentially mature into lesions with distinct histological and immunological characteristics within the same lung24. Granulomas can be non-necrotizing, marked by high levels of mononuclear cellular infiltration (cellular), or may contain a necrotic center, which can liquefy and drain, giving rise to cavitary disease. Pulmonary cavities favor unrestricted bacillary growth and facilitate aerosol spread of infection. In contrast to the lungs, the physiology of the spine is non-permissive to cavity formation. Thus, as granulomas mature and enlarge, central necrotic tissue can liquefy, but has nowhere to drain and instead forms characteristic paraspinal, psoas and epidural abscesses. The underlying mechanisms that drive the granulomatous process, especially at EP sites, remain poorly defined18. In particular, whether the cellular composition and histological features of the granuloma are similar or not in different tissues is not well studied. Moreover, the impact of the Mtb-driven immune response on tissue-specific cell populations, such as those involved in bone and cartilage homeostasis in the spine, is not clear. In the absence of animal models of EPTB, human specimens are needed to address these questions.
The devastating impact of HIV co-infection on TB is well documented: TB is an AIDS-defining illness in many parts of the world and one of the leading causes of death in individuals with HIV infection25. HIV depletes the activated CD4+ T cell pool26 and reportedly may target Mtb-specific cells, which are a key component of protective immunity against TB27. The clinical manifestations of TB often vary between HIV-positive and - negative individuals, likely reflecting differences in the cellular and molecular interactions elicited during co-infection5. Pulmonary TB patients with HIV are less likely to have cavitary disease and roughly 20% will have normal chest x-rays, implying alterations in tissue damage and remodelling pathways25, 28. Overall, HIV co-infection is thought to negatively impact granuloma formation and TB-specific immunity, leading to decreased killing of Mtb-infected cells and more extensive disease progression26, 29_ENREF_28. However, previous studies aimed at understanding the underlying mechanisms of the clinical consequences of co-infection have primarily focused on patients with pulmonary TB. Thus, our understanding of the impact of HIV on the granulomatous process and pathogenesis in patients with EPTB is incomplete.
To begin unravelling the Mtb-induced host response within the spine, we performed an immunohistological characterization of spinal TB granulomatous tissue isolated from HIV-negative and -positive patients treated at a central referral hospital in KwaZulu-Natal, South Africa. Our goal was to describe the cellular recruitment to, and subsequent organization of, the granuloma, as well as the extent of tissue damage and pathology, in spinal TB. We also characterized the influence of HIV infection on the granulomatous process and tissue pathology in patients with spinal TB.
METHODS
Study cohort
Participants were recruited from 2002 – 2003 from the Spinal Unit of King George V Hospital (Durban, South Africa), a specialized referral unit at this public district hospital, which handles spinal pathology, including infection. Management of patients followed the South African Department of Health (SA-DOH) guidelines30. Patients who had been on anti-TB treatment for 5–24 weeks, with progressive spinal pathology requiring surgical intervention, were recruited into the study15. Patients with active pulmonary TB or concomitant immunosuppressive disorders, such as diabetes, chronic steroid use or congenital disorders were excluded. None of the HIV-positive patients were on anti-retroviral therapy (ART), as it was unavailable in the public sector at the time of this study. Magnetic resonance imaging (MRI) scans were taken for diagnostic purposes and to direct surgical removal of the diseased tissue and bone. Whole blood (EDTA anti-coagulant) was collected at surgery for haematology. Quantification of T cells (CD4 and CD8 counts) was done using TetraOne technology (Beckman Coulter). The HIV-1 status of patients was determined by ELISA (Organon Teknika Vironostika and Murex Wellcozyme HIV 1+2 GAC assays) and viral loads of sero-positive patients were quantified (NucliSens™ QT kit; Organon Teknika) from plasma (100μl) and tissue (10–12mg). The input mass of tissue was standardized to correspond to an input volume of 1ml of plasma as per the manufacturer's instructions, thus allowing a direct comparison of plasma versus tissue HIV viral loads. The Biomedical Research Ethics Committee at the Nelson R. Mandela School of Medicine at the University of KwaZulu-Natal approved sample collection and immunohistology studies (H112/02). Subsequently, approval from Institutional Review Board (IRB) at the University of Medicine and Dentistry of New Jersey (UMDNJ) was granted for immunohistochemistry on the tissue sections. All participants provided written, informed consent.
Histology & Immunohistochemistry
Tissue biopsies were fixed in formalin (41% formaldehyde + 0.9% NaCl, 1:8v/v) within 1 hour of collection, processed and embedded in paraffin wax using conventional protocols (FFPE blocks). The FFPE tissue blocks were sectioned (5mm thickness) serially. The first section was stained with Haematoxylin and Eosin (H&E) according to conventional protocols. Of the 60 patient specimens collected, 13 HIV-uninfected and 9 HIV-infected cases were chosen for further detailed microscopic evaluation, based on the initial assessment of their histopathology. The 22 selected specimens displayed intact granulomas, which enabled orientation of the lesions. Characteristics of this subset of patients are displayed in Table 1. T cells were immunolocalized using anti-human CD3 (Clone BBK-2), CD4 (Clone BC/IF6) and CD8 (Clone BC/1A5) antibodies (Biocare Medical, CA, USA). The lysosomal marker, CD68 (Clone KP1) localized macrophages, monocytes and cells of the macrophage/monocyte lineage. Immunohistochemistry was performed using standard protocols. Sections were deparaffinized in xylene, hydrated in graded ethanols and antigen sites were unblocked in an Antigen Decloaking chamber in either Borg (for CD3, CD4 and CD8 antibodies) or Diva (for the CD68 antibody) decloaking buffers (Biocare Medical, CA, USA). The Sniper Blocker (Biocare Medical, CA, USA) solution was used to block endogenous peroxidase activity, and non-specific staining was minimized by incubation with 10% (w/v) milk protein. Sections were incubated in each of the 4 ready-to-use primary antibodies for 30 minutes as per the manufacturer's recommendations. The Mach4 Mouse Probe (Biocare Medical, CA, USA) was used as a linker, followed by visualisation with the DAB chromogen (Biocare Medical, CA, USA) and nuclear visualisation using Mayer's Haematoxylin (Biocare Medical, CA, USA).
Table 1.
Characteristics of Patient Cohort
| Parametera | HIV-negative (n=13) | HIV-positive (n=9) | Significanceb |
|---|---|---|---|
| Age in years (min - max) | 44.4 ± 18.7 (7 – 71) | 26.4 ± 10.2 (3 – 39) | p < 0.01 |
| No. Pediatric | 1 | 1 | |
| No. Adults | 12 | 8 | |
|
| |||
| Sex: No. Male (%) | 3 (23.1) | 2 (22.2) | n.s. |
|
| |||
| Mean CD4 Count (cells/ml) | |||
| Absolute No.(min - max) | 790.6 ± 417.7 (281 – 1370) | 544.6 ± 315.4 (100.1 – 885.4) | p < 0.01 |
| Percentage (min - max) | 44.9 ± 13.2 (22 – 64) | 27.3 ± 10.4 (10 – 43) | P < 0.01 |
|
| |||
| Mean CD8 Count (cells/ml) | |||
| Absolute No. (min - max) | 795.0 ± 574.5 (194 – 1711.6) | 1148.9 ± 549.1 (355 – 1990) | p < 0.01 |
| Percentage (min - max) | 43 ± 12.9 (20 – 65) | 62 ± 11.3 (50 – 84) | p < 0.01 |
|
| |||
| Mean viral load (log copies/ml) | |||
| Plasma (min - max) | --- | 4.57 ± 1.04 (2.92 – 5.80) | p<0.05 |
| Tissue (min - max) | 5.80 ± 1.87 (3.00 – 8.10) | ||
Absolute values given as mean ± standard deviation.
Significance determined by two-tailed Student's t-test.
Image analysis
Images of whole sections were acquired with the PathScan Enabler IV (Meyer Instruments, Houston, TX). The stained sections were analyzed using a Nikon DXM 1200C microscope and photographed at ×10, ×20, ×40 or ×100 magnification, using NIS-Elements F3.0 software (Nikon Instruments Inc., Melville, NY). The number of positively-stained cells in a selected region of interest (ROI) (at the same magnification) was quantified manually by the same investigator (SD). The nuclei of stained and unstained cells were counted to determine the absolute number and percent positive cells per ROI. Statistical significance was determined by a two-tailed t-test, using GraphPad Prism (GraphPad, La Jolla, CA).
RESULTS
The Patient Population
The purpose of this study was to characterize the cellular immune response to Mtb infection of the spine and explore the impact of HIV co-infection on this response. Accordingly, diseased tissue removed from patients who underwent surgery to treat their spinal TB pathology, was analyzed by histology and immunohistology. Tissue from 13 HIV-negative and 9 HIV-positive patients was studied (Table 1). HIV-negative patients were on average older than those who were co-infected. Both groups included 1 pediatric case and a similar percentage of males and females. As expected, significant differences in circulating CD4+ and CD8+ T cell numbers were noted between the HIV-negative and -positive TB patients. A wide range of viral loads was detected in the blood and tissues of all HIV infected patients, with significantly (p<0.05) greater viral titers found within the spinal tissue.
The Granulomatous Response in the Spine of Patients with Pott's Disease
Representative MRI scans revealed the location of the diseased tissue and showed how the abscess impinged on the spinal cord (Figure 1A and B). Multiple tissue fragments, excised during surgery from regions close to the vertebrae and proximate to the dura of the spinal cord, were obtained from each patient. Histologic examination of low power images of H&E stained tissue sections were used to orient each lesion. These revealed diverse features of spinal pathology, including mononuclear cellular infiltration associated with fragments of bone and cartilage, as well as dura and nervous tissue; extensive tissue necrosis and fibrosis were evident (Figure 1C and D).
Figure 1. Orientation of spinal abscess tissue.
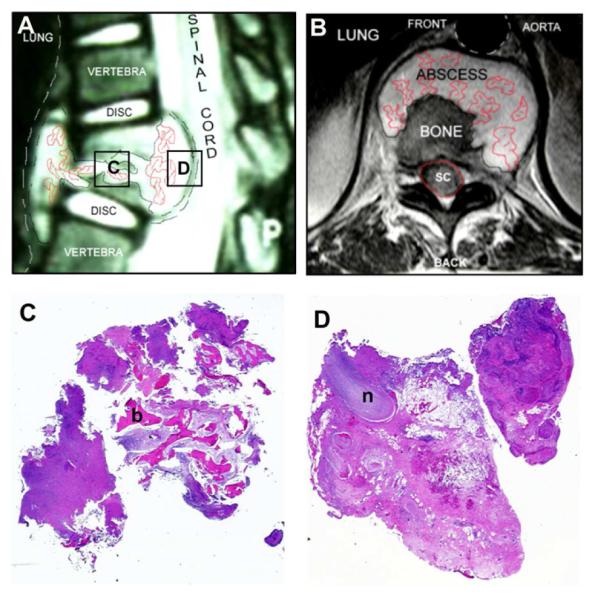
A) Mid-sagittal MRI sequence illustrating the abscess surrounding the vertebrae, with destruction of the involved discs and impact on the spinal cord. Boxes indicate regions subsequently analyzed by histology. B) A coronal MRI image of the same abscess. C & D) Low power micrographs (×4 magnification) of excised spinal tissue: C) highlights the involvement of bone (b); D) depicts the abscess wall in relation to nervous tissue (n).
Representative cross-sections of H&E stained, diseased tissue from an HIV-negative (Figure 2, top panels) and - positive patient (Figure 2, bottom panels) are shown, demonstrating heterogeneous architecture within the cellular granulomatous area. Regions of dense mononuclear cellular accumulation were evident, including macrophage-rich zones, surrounded by peripheral lymphocyte-rich cuffs (Figure 2A and D). Necrotic areas, devoid of intact cellular structures, were seen in the centers of the macrophage-rich zones. Macrophages were specifically identified by CD68 immunostaining (Figure 2B and E). Epithelioid cell aggregates and multinucleated giant cells were detectable in tissue samples from both HIV-negative and -positive patients. To localize T lymphocytes in the diseased tissue, immunostaining for CD3 was performed (Figure 2C and F). Dense zones of CD3+ T cells were observed surrounding the macrophage-rich areas. In addition, individual T-cells were seen scattered amongst the macrophages. Overall, evidence of localized, well-organized granulomas was noted, irrespective of HIV status.
Figure 2. Cellular architecture of spinal TB granulomas.
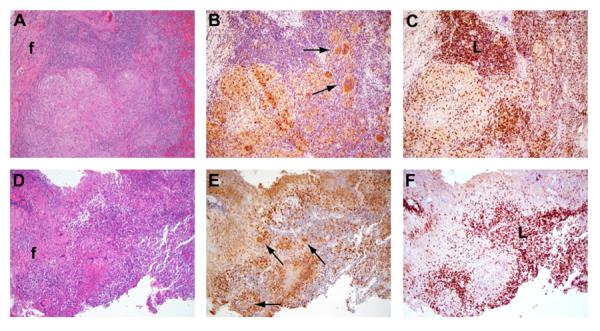
Images show areas of macrophage rich-regions, surrounded by lymphoctes in HIV-negative (A–C) and HIV-positive specimens (D–F). A & D) H&E staining. CD68 (B & E) and CD3 (C & F) immunostaining, in which positively stained cells are brown. Fibrotic areas (f), lymphoid-rich regions (L), and multinucleated giant cells (arrows) are evident (×10 magnification).
T cell Subsets and Mononuclear Phagocyte Distribution
Immunostaining for CD3, CD4 and CD8 expression by cells in the granulomatous tissue facilitated the evaluation of the distribution and organization of T lymphocytes. Both CD4+ and CD8+ T cells were detectable in the HIV-negative (Figure 3, top panels) and HIV-positive (Figure 3, bottom panels) tissue sections. Enumeration of the total number of infiltrating CD3+ T cells revealed no statistically significant differences between the two groups (Figure 4A). On the other hand, whereas CD4+ T cells were enriched in the granulomatous tissue from HIV-negative patients, T cells expressing the CD8 co-receptor were more abundant in the specimens from HIV-positive patients (compare Figure 3B and C to 3E and F; Figure 4A). Thus, the characteristic reversal in the CD4 to CD8 ratio observed in the blood of HIV-positive patients was also reflected within the infected tissue (Figure 4B). Taken together, we conclude that T lymphocytes were efficiently recruited and retained in the infected spinal tissue, irrespective of HIV status, with CD8+ T cells apparently compensating for the relative paucity of systemic CD4+ T cells in HIV-infected individuals (Table 1).
Figure 3. Distribution of T lymphocytes within the spinal TB granuloma.
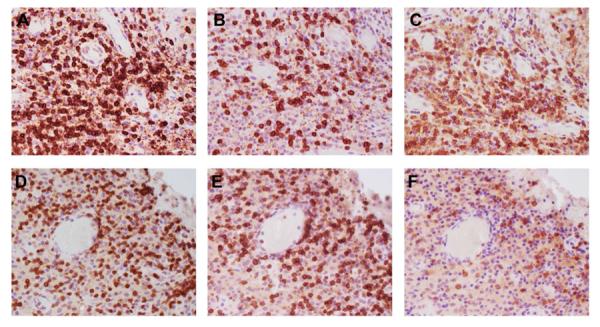
CD3 (A & D), CD8 (B & E) and CD4 (C & F) immunostaining depicts the distribution of T cells. Samples from HIV-negative (A–C) and HIV-positive (D–F) patients (×40 magnification).
Figure 4. Quantification of the T cells in blood and tissue.
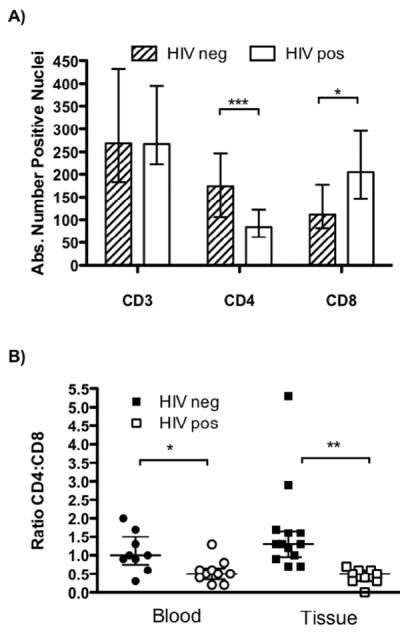
A) The absolute number of cells that positively stain for CD3, CD8 and CD4 expression in granulomatous tissue from one region of interest (×40 magnification) per HIV-negative and -positive patient. Results are the median and the error bars indicate the IQR. B) The ratio of CD4:CD8 T cells in the peripheral blood (circles) and in the infected tissue (squares) of the patient cohort. Closed symbols: HIV-negative samples and open symbols: HIV-positive samples. * p < 0.05, ** p < 0.01, *** p < 0.005 by two-tailed t-test.
Immunostaining for CD68 expression revealed distinct clusters of positive staining macrophages in several tissue microenvironments. CD68+ epithelioid cells were seen in clearly demarcated aggregates in the cellular granulomatous tissue of both HIV-negative and -positive patients (Figure 2B and E). In addition, multinucleated giant cells, discernible by their distinct morphology, were detectable in 10 of 13 (76.9%) HIV-negative and 5 of 9 (55.6%) HIV-positive patients (Figure 5A and B). Enumeration of the total numbers of giant cells, per ×10 microscopic field, as well as the average number of nuclei per giant cell revealed similar numbers for both patient groups (Table 2). We thus conclude that the general macrophage response to Mtb infection of the spine, as indicated by the differentiation of the cells into characteristic epithelioid and multinucleated giant cells, proceeded efficiently in these patients independently of HIV co-infection.
Figure 5. Evidence of macrophage differentiation and bone-remodeling.
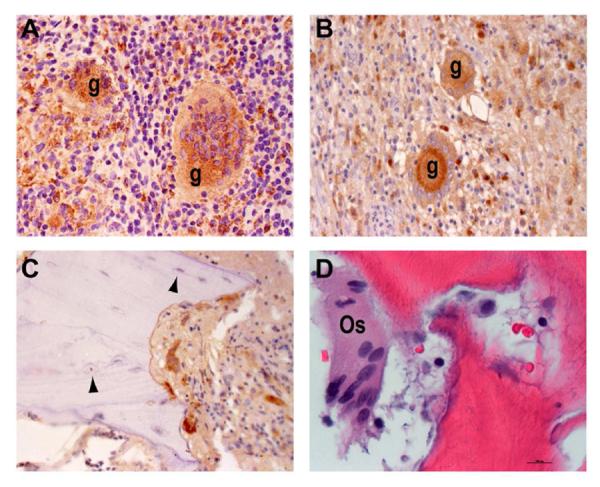
CD68 immunostaining from an HIV-negative (A) and two HIV-positive (B, C) specimens. CD68+ individual macrophages (arrows) and multinucleated giant cells (g), as well as CD68− osteocytes (arrowheads) are indicated (×40 magnification). D) H&E staining depicts a multinucleated osteoclast (Os) in close proximity to damaged bone from an HIV-negative patient (×80 magnification).
Table 2.
Quantification of Multinucleated Giant Cells
| Parameter | HIV negativea (n=10) | HIV positive (n=5) | Total (n=15) |
|---|---|---|---|
| Total number of Giant cells in ROI (min – max) | 4.8 ± 4.6 (1 – 12) | 5.4 ± 4.3 (3 – 13) | 5.0 ± 4.3 (1 – 13) |
|
| |||
| Mean number of nuclei / giant cell in ROI (min – max) | 9.3 ± 4.9 (4 – 18) | 10.9 ± 3.8 (5 – 15) | 9.8 ± 4.5 (4 – 18) |
Giant cells were not detected in the tissue obtained from 3/13 of the HIV-negative and 4/9 of the HIV-positive patients.
Histologic Evidence of Bone Remodeling and Tissue Damage
Osteoclasts and osteoblasts mediate bone remodeling. Osteoblasts are mesenchymal-lineage cells responsible for the generation of new bone. Osteocytes, mature osteoblasts embedded within the bone matrix that are CD68 negative, were evident in the sampled tissue (Figure 5C, arrowheads). Osteoclasts are multinucleated bone marrow-derived cells of similar lineage as monocytes. These CD68+ cells were detected in close proximity to bone fragments (Figure 5C and D). Evidence of active bone resorption and osteogenesis, indicative of the robust, chronic inflammation and resultant damage elicited in response to Mtb infection of the spine, was seen in specimens from both HIV-infected and -uninfected TB patients.
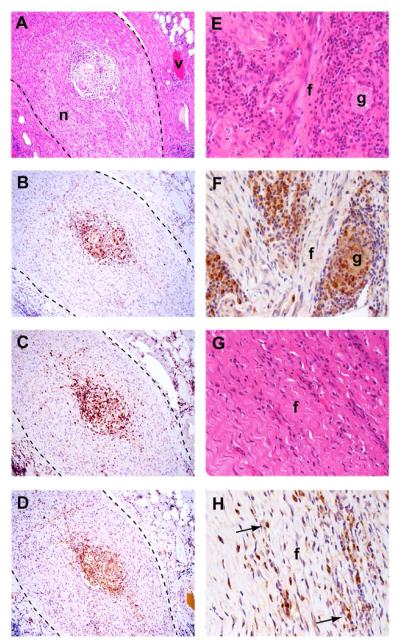
Neurological complications are one of the most severe consequences of spinal TB infection and are an important indication for surgical intervention. Careful examination of excised tissue demonstrated the presence of granulomas within nerve tissue (Figure 6, left panels). This feature was confined to tissue specimens localized proximate to the dura of the spinal cord, which includes a large network of nerve bundles. Immunohistology confirmed the presence of CD68+ macrophages and multinucleated giant cells (Figure 6B), as well as CD4+ and CD4− CD3+ T cells (Figure 6C and D) within the granulomatous region. Fibrosis is a protective tissue remodeling response elicited to repair damaged tissue and is a characteristic feature of the TB-induced granuloma. We detected regions of extensive fibrosis in both HIV-negative (Figure 6E and F) and HIV-positive specimens (Figure 6G and H).
Figure 6. Pathological features of Spinal TB infection.
A – D) Granuloma formation within a nerve bundle (n), surrounded by connective tissue, from an HIV-negative patient. Blood vessel (v). A) H&E staining. Immunostaining for CD68 (B), CD3 (C) and CD4 (D). (×10 magnification). E – H) Evidence of fibrosis (f) in an HIV-negative (E, F) and an HIV-positive (G, H) specimen. E, G) H&E staining. F, H) CD68 immunostaining, marking the presence of multinucleated giant cells (g) and scattered, individual macrophages (arrows) (×40 magnification).
As the granulomas enlarge, areas of central necrosis expand, ultimately resulting in abscess formation. A representative granuloma with a central necrotic zone (Figure 7, highlighted) and the presence of residual cellular debris and polymorphonuclear neutrophils (Figure 7B, arrows) is shown. Immunolocalization of CD3+ T cells demonstrated their distribution within and surrounding the developing abscess (Figure 7C). This entire structure was surrounded by a fibrotic layer with a much lower density of cells. A fully developed abscess, from which the pus has been drained by surgical excision, demonstrated a clear necrotic region surrounded by granulomatous tissue (Figure 7D). No macrophages or T cells were seen within the necrotic zone or on the luminal surface of the necrotic layer of the abscess (data not shown). This is in contrast to pulmonary TB granulomas, in which CD68+ macrophages were clearly noted on the luminal surface of the cavity24.
Figure 7. Abscess formation.
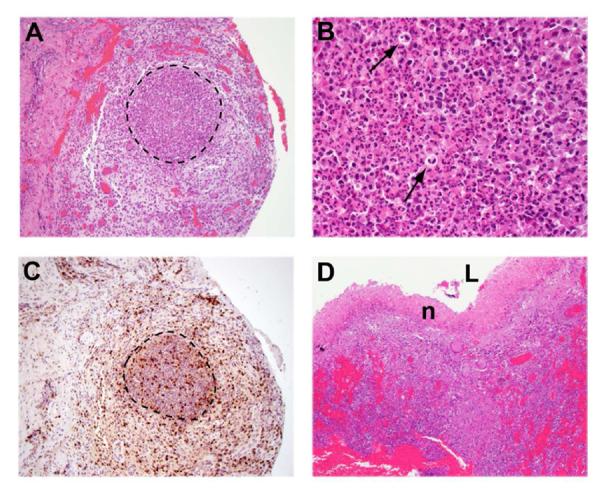
H&E staining shows the early formation of an abscess under low (A: ×10) and high magnification (B: ×80), including the presence neutrophils (arrows). Immunostaining for CD3 (C) depicts T lymphocyte infiltration within and around the abscess. D) H&E staining of a separate specimen in which the abscess has drained and the lumen (L) is surrounded by necrotic tissue (n) (×10 magnification).
DISCUSSION
We have described the tissue architecture and organization of infiltrating immune cells in granulomatous lesions from spinal TB patients. Our IHC analysis revealed the differentiation of epithelioid cells and multinucleated giant cells, as well as evidence of active tissue remodeling, including areas of fibrosis and bone resorption. Interestingly, despite statistically significantly lower CD4+ T cell counts and relatively high viral titers in the HIV/TB co-infected patients, the granulomatous tissue displayed similar features, irrespective of HIV status. Additionally, our analysis of the immune cell composition of spinal TB granulomas from these patients revealed that CD8+ T cells numerically compensated for HIV-dependent reductions in the CD4+ T cell compartment.
The granuloma is the characteristic manifestation of chronic macrophage activation and maturation in response to Mtb infection17–19, 21. Whereas resting macrophages permit the replication of Mtb, activated macrophages can suppress the growth or directly kill intracellular Mtb. The unique inflammatory and cytokine milieu in the TB granuloma triggers macrophages to adopt several distinct morphological phenotypes, including epithelioid cells, foamy cells and multinucleated giant cells. We detected giant cells in the spinal lesions, as well as extensive areas of epithelioid cells. Furthermore, we found that the giant cell differentiation program was not altered by HIV co-infection, evidenced by similar numbers of giant cells (containing similar numbers of nuclei) in specimens from both patient groups. The functional significance of this unique cell type is not well understood; giant cells reportedly form in direct response to Mtb-derived components31 and lose their phagocytic ability32. Giant cells within lesions in TB lymphadenitis are reportedly not very pro-inflammatory, expressing little TNFα and IFNγ and high levels of IL-10 and TGFβ33. Collectively, the published data support a hypothesis in which giant cells may be associated with a favorable environment for bacillary growth. Further studies to determine the functional characteristics of giant cells and epithelioid cells within the paucibacillary granulomas of the spine may provide novel information on host-Mtb interactions, shedding light on the differences between protective immunity and progressive, necrotic, pathological events.
Chronic inflammation underlies much of the pathogenesis in pulmonary TB, as exemplified by the excessive fibrotic response and cell death, which can lead to compromised organ function even after successful antibiotic treatment34, 35. Pathological fibrosis is reportedly one of the determinants of genetic susceptibility in a murine model of pulmonary TB36. In the spinal TB lesions from both HIV-infected and -uninfected patients, we found extensive areas of fibrosis. Also of note, we observed evidence of active bone resorption, including CD68+ multinucleated osteoclasts in close association with bone fragments, demonstrating that Mtb infection of the spine triggers alterations in bone homeostasis. Consistent with this observation, elevated deoxypyridinoline, a breakdown product of collagen type I, the main constituent of bone, has been detected in urine from spinal TB patients, but not in pulmonary TB patients or healthy controls37. In general, the extent of pathology was similar in the HIV-positive and -negative specimens from our two patient cohorts. A recent study of MRI scans of spinal TB patients reported a greater degree of vertebral collapse/kyphosis in HIV-negative cases compared to HIV co-infected cases, while the total number of affected vertebrae did not differ between the cohorts38. This observation suggests that HIV-dependent impairments in the immune response can lead to decreased tissue pathology. Additionally, a previous analysis of granulomas from pleural TB patients revealed an increased proportion of necrotic lesions in HIV-positive patients, which correlated with elevated expression of TNFα mRNA39. Tissue-specific differences, as well as the extent of immune compromise, may account for variations in pathogenesis, and highlight the importance of studying the local disease site to understand the complex interplay between the host, Mtb and HIV.
T cells play a critical role in the control of mycobacterial infections. In the spinal lesions from both patient groups, we detected distinct zones of T cell accumulation with equal numbers of infiltrating CD3+ T cells, irrespective of HIV status. This finding differs somewhat from prior reports describing pulmonary or pericardial lesions, in which higher proportions of CD3+ T cells were found in HIV-uninfected patients compared to co-infected TB patients40, 41. Consistent with these studies, we observed a switch in the ratio of CD4+ to CD8+ T cells based on HIV status. Prior understanding of the Mtb-specific T cell response has assumed that CD4+ T cells are the predominant cell type responsible for maintaining macrophage activation. However, we found that CD8+ T cells numerically replace CD4+ T cells in the spinal lesions of the HIV positive patients. While the lack of gross impairment or abnormalities that we observed in the granuloma architecture in specimens from co-infected patients suggests the possibility that CD8+ T cells may directly contribute to sustaining the granulomatous response, further studies are required to determine the functional significance of this observation. The functional quality and magnitude of the CD4+ and CD8+ T cell responses have been reported to vary within individual granulomas42–44 and also differ depending on the infected tissue42, 45–47. In studies of BCG-vaccinated infants, we have previously shown that CD8+ T cells contribute to IFNγ production48. Additionally, CD4+ T cell-specific IFNγ production was dispensable for Mtb control in mice49, while the expression of IFNγ and iNOS activity was unimpaired in CD4+ T cell-depleted mice50. Taken together, these findings support our data that significant levels of macrophage activation can be achieved in the absence of an intact CD4+ T cell compartment.
Unlike pulmonary granulomas, the lesions in Pott's disease are paucibacillary. In a previous study of cavitary lesions from patients with pulmonary TB, we showed that bacilli were most often localized to the cavity surface, in close proximity to open airways, within macrophages permissive to Mtb growth (T cells are absent from this region)24. In contrast, the luminal surface of the spinal abscess were acellular. Thus, the lack of macrophages may partly explain the absence of bacillary growth at this site. The low oxygen levels in spinal tissue, as compared to the lungs, is also unfavorable to Mtb, which does not grow in anoxic conditions51, 52_ENREF_50. Additionally, it has recently been hypothesized that epithelial cell-derived MMP9 (matrix metallo-proteinase 9) expression, not only facilitates granuloma formation, but also promotes Mtb replication17, 53. The absence of this epithelial cell-driven pathway in the spine may be another reason that spinal TB is paucibacillary. The extremely destructive nature of spinal TB indicates that high Mtb burdens are not necessary to drive tissue damage. However, the pathology in spinal TB is not self-limiting, and patients must be treated with antibiotics to stop tissue destruction54. The extensive pathology associated with spinal TB is likely a reflection of chronic inflammation triggered by relatively low numbers of Mtb, which may then be perpetuated by persistent bacterial-derived components.
Our current analysis was confined to the host response to infection, but both Mtb and HIV adapt to and influence the pathological process. We found the viral loads in the HIV-infected group to be significantly higher in the spinal tissue compared with plasma. This may reflect heightened immune stimulation at the site of infection promoted by the anti-TB immune response. Evidence from in vitro models has shown that Mtb can drive HIV replication in macrophages55. Furthermore, TNF-α, a key component of anti-mycobacterial immunity, is a strong transactivator of the HIV LTR region, promoting increased rates of viral replication55, 56. Increased viral replication promotes divergent HIV evolution, increasing viral diversity and enabling the virus to better evade the immune system. Evidence of such viral compartmentalization has been detected in HIV patients with pleural TB57 and pulmonary TB58.
Spinal TB is a chronic, insidious disease responsible for significant morbidity and mortality. Diagnostic limitations are responsible for significant delays in the initiation of treatment, leading to exacerbation of pathology and increased risk of permanent deformity and neurological impairment. This study provides a foundation for understanding the unique disease progression of Mtb infection within the spine and also sheds light on the influence of HIV on the immunopathogenesis. To more effectively and rapidly diagnose spinal TB and develop novel treatment approaches, further investigations of the pathogenesis in human tissue specimens are warranted.
Acknowledgments
FUNDING SOURCES Funded through a K-RITH/Howard Hughes Medical Institute Traveling Scholar's Award to GK and a Fogarty AIDS International Training and Research Program (AITRP) Award to SD. No study sponsor played a role in the study design, data collection and interpretation or preparation of the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST All authors declare no conflicts of interest.
REFERENCES
- 1.WHO . Global Tuberculosis Report 2012. WHO; Geneva, Switzerland: 2012. [Google Scholar]
- 2.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax. 2009;64:1090–1095. doi: 10.1136/thx.2009.118133. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Graviss EA. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003;7:1178–1185. [PubMed] [Google Scholar]
- 4.Naidoo K, Padayatchi N, Abdool Karim Q. HIV-Associated Tuberculosis. Clin Dev Immunol. 2011;2011 doi: 10.1155/2011/585919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterling TR, Pham PA, Chaisson RE. HIV infection-related tuberculosis: clinical manifestations and treatment. Clin Infect Dis. 2010;50(Suppl 3):S223–230. doi: 10.1086/651495. [DOI] [PubMed] [Google Scholar]
- 6.Trecarichi E, Di Meco E, Mazzotta V, Fantoni M. Tuberculous spondylodiscitis: epidemiology, clinical features, treatment, and outcome. European review for medical and pharmacological sciences. 2012;16(Suppl 2):58–72. [PubMed] [Google Scholar]
- 7.Delogu G, Zumbo A, Fadda G. Microbiological and immunological diagnosis of tuberculous spondylodiscitis. European review for medical and pharmacological sciences. 2012;16(Suppl 2):73–78. [PubMed] [Google Scholar]
- 8.Kamara E, Mehta S, Brust JC, Jain AK. Effect of delayed diagnosis on severity of Pott's disease. Int Orthop. 2012;36:245–254. doi: 10.1007/s00264-011-1432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govender S, Kumar KP. Cortical allografts in spinal tuberculosis. Int Orthop. 2003;27:244–248. doi: 10.1007/s00264-003-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Kumar J. Tuberculosis of spine: neurological deficit. Eur Spine J. 2012 doi: 10.1007/s00586-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanty SP, Bhat S, Nair NS. An analysis of clinicoradiological and histopathological correlation in tuberculosis of spine. J Indian Med Assoc. 2011;109:161–165. [PubMed] [Google Scholar]
- 12.Ferrer MF, Torres LG, Ramirez OA, Zarzuelo MR, Del Prado Gonzalez N. Tuberculosis of the spine. A systematic review of case series. Int Orthop. 2011 doi: 10.1007/s00264-011-1414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govender S, Parbhoo AH, Kumar KP, Annamalai K. Anterior spinal decompression in HIV-positive patients with tuberculosis. A prospective study. J Bone Joint Surg Br. 2001;83:864–867. doi: 10.1302/0301-620x.83b6.11995. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318065b7c3. [DOI] [PubMed] [Google Scholar]
- 15.Danaviah S, Govender S, Cassol S. Histopathology and genotyping in infectious spondylitis of HIV− and HIV+ patients. Clin Orthop Relat Res. 2007;460:50–55. doi: 10.1097/BLO.0b013e31806a9147. [DOI] [PubMed] [Google Scholar]
- 16.Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis. 2012;205(Suppl 2):S316–324. doi: 10.1093/infdis/jis043. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 18.Reece ST, Kaufmann SH. Floating between the poles of pathology and protection: can we pin down the granuloma in tuberculosis? Curr Opin Microbiol. 2012;15:63–70. doi: 10.1016/j.mib.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 20.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–268. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, Kaplan G, Russell DG. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2:258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, Barry CE, 3rd, Bekker LG. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwan C, Ernst J. HIV and tuberculosis: a deadly human syndemic. Clinical microbiology reviews. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7:268–275. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- 27.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave E, Casazza J, Ambrozak D, Louder M, Ampofo W, Pollakis G, Hill B, Sanga E, Saathoff E, Maboko L, Roederer M, Paxton W, Hoelscher M, Koup R. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg SD, Frager D, Suster B, Walker S, Stavropoulos C, Rothpearl A. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;193:115–119. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- 29.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SA-DOH . In: The National Infection, Prevention and Control Policy for TB, MDRTB and XDRTB. Health DO, editor. Pretoria: 2007. [Google Scholar]
- 31.Puissegur M-P, Lay G, Gilleron M, Botella L, Nigou Jrm, Marrakchi H, Mari B, Duteyrat J-L, Guerardel Y, Kremer L, Barbry P, Puzo G, Altare Fdr. Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent, ADAM9- and beta1 integrin-mediated pathway. Journal of immunology (Baltimore, Md : 1950) 2007;178:3161–3169. doi: 10.4049/jimmunol.178.5.3161. [DOI] [PubMed] [Google Scholar]
- 32.Lay G, Poquet Y, Salek-Peyron P, Puissegur MP, Botanch C, Bon H, Levillain F, Duteyrat JL, Emile JF, Altare F. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol. 2007;211:76–85. doi: 10.1002/path.2092. [DOI] [PubMed] [Google Scholar]
- 33.Mustafa T, Mogga SJ, Mfinanga SG, Morkve O, Sviland L. Immunohistochemical analysis of cytokines and apoptosis in tuberculous lymphadenitis. Immunology. 2006;117:454–462. doi: 10.1111/j.1365-2567.2005.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 36.Marquis JF, Nantel A, LaCourse R, Ryan L, North RJ, Gros P. Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in mice. Infect Immun. 2008;76:78–88. doi: 10.1128/IAI.00369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Wang Z, Li H, Yuan H. Diagnostic performance of the urinary deoxypyridinoline in spinal tuberculosis. Orthopedics. 2012;35:e922–926. doi: 10.3928/01477447-20120525-36. [DOI] [PubMed] [Google Scholar]
- 38.Anley CM, Brandt AD, Dunn R. Magnetic resonance imaging findings in spinal tuberculosis: Comparison of HIV positive and negative patients. Indian J Orthop. 2012;46:186–190. doi: 10.4103/0019-5413.93688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezuidenhout J, Roberts T, Muller L, van Helden P, Walzl G. Pleural tuberculosis in patients with early HIV infection is associated with increased TNF-alpha expression and necrosis in granulomas. PLoS One. 2009;4:e4228. doi: 10.1371/journal.pone.0004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law KF, Jagirdar J, Weiden MD, Bodkin M, Rom WN. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153:1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 41.Matthews K, Ntsekhe M, Syed F, Scriba T, Russell J, Tibazarwa K, Deffur A, Hanekom W, Mayosi BM, Wilkinson RJ, Wilkinson KA. HIV-1 infection alters CD4+ memory T-cell phenotype at the site of disease in extrapulmonary tuberculosis. Eur J Immunol. 2012;42:147–157. doi: 10.1002/eji.201141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, Aseffa A, Derbew M, Svensson M, Andersson J, Brighenti SG. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol. 2009;174:2211–2224. doi: 10.2353/ajpath.2009.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenhalls G, Stevens L, Bezuidenhout J, Amphlett GE, Duncan K, Bardin P, Lukey PT. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology. 2002;105:325–335. doi: 10.1046/j.1365-2567.2002.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenhalls G, Squires GR, Stevens-Muller L, Bezuidenhout J, Amphlett G, Duncan K, Lukey PT. Associations between toll-like receptors and interleukin-4 in the lungs of patients with tuberculosis. Am J Respir Cell Mol Biol. 2003;29:28–38. doi: 10.1165/rcmb.2002-0163OC. [DOI] [PubMed] [Google Scholar]
- 45.Mitra DK, Sharma SK, Dinda AK, Bindra MS, Madan B, Ghosh B. Polarized helper T cells in tubercular pleural effusion: phenotypic identity and selective recruitment. Eur J Immunol. 2005;35:2367–2375. doi: 10.1002/eji.200525977. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, Liu H, Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 47.Caramori G, Lasagna L, Casalini AG, Adcock IM, Casolari P, Contoli M, Tafuro F, Padovani A, Chung KF, Barnes PJ, Papi A, Rindi G, Bertorelli G. Immune response to Mycobacterium tuberculosis infection in the parietal pleura of patients with tuberculous pleurisy. PLoS One. 2011;6:e22637. doi: 10.1371/journal.pone.0022637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray R, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, Hawkridge A, Hussey G, Maecker H, Kaplan G, Hanekom W. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. Journal of immunology (Baltimore, Md : 1950) 2006;177:5647–5651. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 49.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scanga C, Mohan V, Yu K, Joseph H, Tanaka K, Chan J, Flynn J. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rustad TR, Sherrid AM, Minch KJ, Sherman DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol. 2009;11:1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 53.Volkman H, Pozos T, Zheng J, Davis J, Rawls J, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science (New York, NY) 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 55.Ranjbar S, Boshoff H, Mulder A, Siddiqi N, Rubin E, Goldfeld A. HIV-1 replication is differentially regulated by distinct clinical strains of Mycobacterium tuberculosis. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falvo J, Ranjbar S, Jasenosky L, Goldfeld A. Arc of a vicious circle: pathways activated by Mycobacterium tuberculosis that target the HIV-1 long terminal repeat. Am J Respir Cell Mol Biol. 2011;45:1116–1124. doi: 10.1165/rcmb.2011-0186TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins KR, Quinones-Mateu ME, Wu M, Luzze H, Johnson JL, Hirsch C, Toossi Z, Arts EJ. Human immunodeficiency virus type 1 (HIV-1) quasispecies at the sites of Mycobacterium tuberculosis infection contribute to systemic HIV-1 heterogeneity. Journal of virology. 2002;76:1697–1706. doi: 10.1128/JVI.76.4.1697-1706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins KR, Mayanja-Kizza H, Sullivan BA, Quinones-Mateu ME, Toossi Z, Arts EJ. Greater diversity of HIV-1 quasispecies in HIV-infected individuals with active tuberculosis. J Acquir Immune Defic Syndr. 2000;24:408–417. doi: 10.1097/00126334-200008150-00002. [DOI] [PubMed] [Google Scholar]