SUMMARY
Active tuberculosis (TB) among HIV-infected patients, even when successfully treated, may be associated with excess mortality. We conducted a prospective cohort study nested in a randomized TB vaccine trial to compare mortality between HIV-infected patients diagnosed and treated for TB (TB, n=77) and HIV-infected patients within the same CD4 range, who were not diagnosed with or treated for active TB (non-TB, n=308) in the period 2001–2008. Only twenty four subjects (6%) were on antiretroviral therapy at the beginning of this study. After accounting for covariate effects including use of antiretroviral therapy, isoniazid preventive therapy, and receipt of vaccine, we found a four-fold increase in mortality in TB patients compared with non-TB patients (adjusted Hazard Ratio 4.61; 95% Confidence Interval (CI): 1.63, 13.05). These findings suggest that treatment for TB alone is not sufficient to avert the excess mortality associated with HIV-related TB and that prevention of TB may provide a mortality benefit.
Keywords: TB prevention, survival, CD4 counts
INTRODUCTION
Tuberculosis (TB) is the leading cause of death among human immunodeficiency virus (HIV)-infected patients in Africa.1 The impact of TB is more evident in areas with a heavy burden of HIV-TB co-disease. In Tanzania for instance, an estimated 2.7% (28,116/1.05 million) of all HIV-infected persons in 2007 also had active TB, of whom, 71% died.2 The advent of combination antiretroviral therapy (cART) in the mid-1990s3 transformed the treatment for HIV worldwide. As a result, AIDS-associated morbidity dropped dramatically in many high-burden countries, followed by mortality decline a decade later.4 In spite of this success, the prevalence of HIV-associated TB remains high in most sub-Saharan African countries.2
Several studies have examined the effect of HIV-associated TB on mortality.5–10 Most of these studies have found increased mortality, but it is not clear if this mortality is the result of death due to TB disease, increased progression to AIDS, or both. We therefore performed a prospective cohort analysis to examine whether HIV-infected patients who completed TB treatment had a higher mortality than similar HIV-infected patients who did not develop active TB, using a cohort of adults enrolled in a randomized trial of a TB vaccine in Tanzania. This study was conducted before recent trials demonstrated the mortality benefit of early cART intake in HIV-associated TB.11,12,13
METHODS
Study population
The DarDar randomized vaccine trial was a collaborative work between Dartmouth Medical School (New Hampshire, USA), Muhimbili University of Health and Allied Sciences (MUHAS) (Dar es Salaam, Tanzania), and Boston University School of Public Health (BUSPH) (Massachusetts, USA). The aim of the trial was to examine the efficacy of an inactivated whole cell mycobacterial vaccine for HIV-infected adults. The trial was conducted at the Infectious Diseases Centre (IDC) in Dar es Salaam, Tanzania between September 2001 and January 2008.14
Subjects in the DarDar trial were recruited from voluntary counseling and testing (VCT) sites, and non-governmental organizations (NGOs). A study nurse was designated to visit VCT sites each weekday to assist in recruitment process. Study visits for the trial vaccine were done at baseline, then after 2 months, 4 months, 6 months, 12 months, and then every 3 months. Eligibility criteria were age 18 or above, a Bacillus Calmette-Gurin (BCG) scar, CD4 counts ≥200/mm3, two positive tests for HIV enzyme-linked immunosorbant assay (ELISA), no active TB, and a signed informed consent for HIV testing and participation in the study. In addition, for the present analysis, we excluded two observations with unusual recorded CD4 counts measurements (>3,000 cells/mm3) and 166 patients with history of treatment for active TB prior to inclusion in the vaccine trial, as the interest of this study was on the effect of treated TB that occurred during follow-up, when administration of TB drugs during follow-up could be closely monitored by our study team.
Pregnant women were not immediately eligible for the vaccine trial but could defer participation until after giving birth. The study was approved by Dartmouth College, Boston University, and MUHAS Institutional Review Boards.
Assessment for TB
All study subjects were HIV-infected. Baseline HIV tests were performed in the Department of Medicine Research Laboratory at MUHAS using ELISA (Beringer, Wellcozyme and Veronstika).
Patients were questioned about symptoms of TB at all vaccine and clinic visits. Those suspected to have active TB were evaluated on a case-by-case basis by project clinicians using routine clinical, radiographic, and microbiologic diagnostic tools.14 In certain situations, evaluation also included a 10-day course of antibiotics for common bacterial pneumonia. Based on these assessments, suspected TB patients were referred by project clinicians to the National Program for TB and Leprosy (NTLP) for the final decision on whether or not to treat for TB.
Evaluation for active TB was conducted to all subjects by study staff and included a physical examination, chest radiograph, three expectorated sputum samples for AFB stain and culture (one spot sample and two first morning samples; standard techniques including culture on Lowenstein Jensen agar), and an automated mycobacterial blood culture (a single 5-ml sample until March 2004, then two 5-ml samples; MBBacT; bioMérieux, Durham, North Carolina, USA). All potential TB cases were reviewed by a blinded panel of three consultants who independently classified cases as definite, probable, possible, or unlikely based on rigorous study definitions.14 The current analysis was based on definite and probable cases.
Treatment for active TB was based on the NTLP guidelines, independent of vaccine status. Standard treatment at the inception of the study was two months of isoniazid, rifampicin, pyrazinamide and ethambutol followed by six months of isoniazid and ethambutol (eight months total). Standard treatment after the change in NTLP guidelines on February 1, 2006 (which excluded ethambutol) was two months of isoniazid, rifampicin and pyrazinamide followed by four months of isoniazid and rifampicin (six months total). Both regimens were given by DOT. Drug sensitivity tests were performed at the MUHAS laboratory to ensure that patients were offered appropriate treatment. All patients on treatment were instructed to bring their TB treatment record into each study visit.
At each examination, patients were evaluated for referral to an appropriate HIV Care and Treatment Center (CTC), and were informed if they met criteria for initiation of ART as outlined in the Tanzania National AIDS Control Programme (NACP) guidelines.15 All subjects with a positive tuberculin skin test and no prior history of active TB were offered a six-month course of IPT. Co-trimozaxole was offered to subjects with CD4 counts < 200.
We defined a ‘TB’ subject as someone diagnosed for active definite or probable TB for the first time during follow-up and completed TB treatment. Conversely, we defined a ‘non-TB’ subject as someone who did not develop active TB during the study.
Matching and controlling for confounding
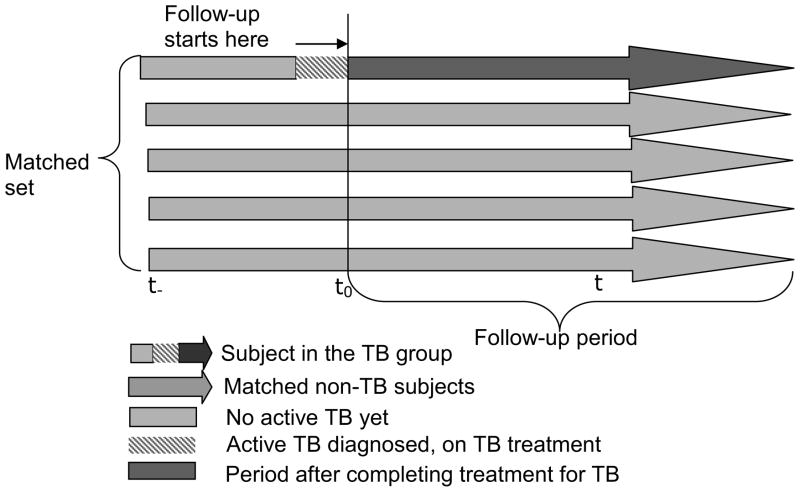
To exclude deaths directly attributable to TB disease, we excluded subjects who were still receiving TB therapy. We matched non-TB subjects to TB subjects beginning at the completion of TB treatment for the TB subjects and that time of a similar CD4 counts for the non-TB subjects (CD4 counts were matched to within 50 cells/mm3). Thus, follow-up in both groups was initiated at the matching time (t0, Figure 1). We matched on CD4 counts at the matching time but not with other potential confounders to avoid overmatching. These other potential confounders were controlled for in the regression model: ART status at the time of matching, co-trimoxazole status at the time of matching, trial vaccine status, age, DarDar baseline tuberculin skin test (TST) status, and gender. We matched every TB subject with four non-TB subjects. When CD4 counts were not available at the exact time of interest, we linearly interpolated counts assuming a constant decline between recorded observations.
Figure 1.
Diagrammatic representation of the matching process. Each arrow represents one subject.
Outcome assessment
The outcome was death (i.e. all-cause mortality). Information regarding the date of death was obtained from medical records, relatives, or confidantes. Putative causes of deaths were obtained by interviewing relatives of the deceased regarding symptoms, medical evaluations, and medications prescribed in the period immediate before death. Neither autopsies nor reliable information on the causes of deaths were available for most patients. We censored two patients who died from a motor accident. Subjects were followed from the start of follow-up to death, withdrawal/loss to follow-up or study termination (i.e. 31st January 2008), whichever occurred first.
Statistical analysis
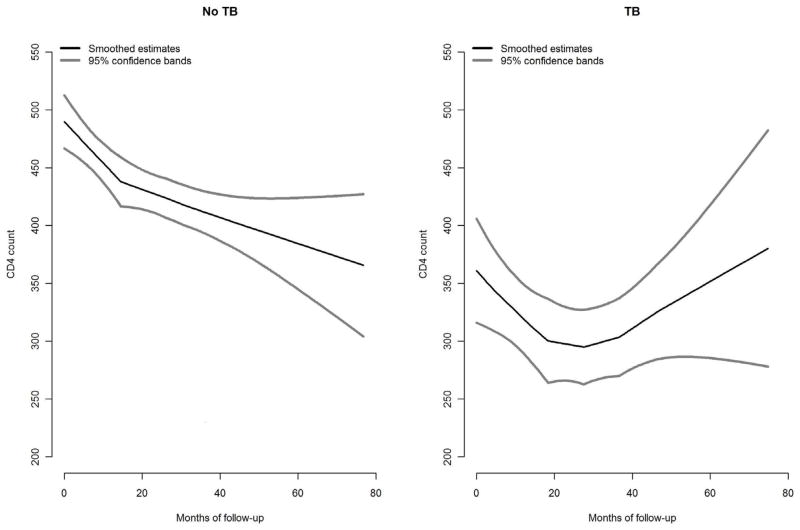
We performed descriptive analyses to explore distribution characteristics of subjects in the matched sample. Kaplan Meier curves were plotted to compare the death rates between the TB group and the non-TB group. Although CD4 counts were measured on multiple occasions, there were very few measurements taken after matching to allow us to perform accurate assessment of CD4 change over time in the two groups. However, we explored the overall trajectory of CD4 counts across groups (by including both pre-match and post-match CD4 counts) using non-parametric locally weighted scatter plot smoothing curves (figure 2). A Cox proportional hazards model was used to analyze the effect of TB on mortality, accounting for potential confounders. Although information on ART and co-trimoxazole was collected at matching and during follow-up, there were not sufficient follow-up data points on these variables to accurately fit models for time-dependent confounding (i.e. structural nested models or marginal structural models). Thus, to overcome the problem, confounding by these variables was accounted for by regression adjustment at matching, and their effects as competing risks during follow-up was accounted for in the primary analysis by censoring subjects immediately after initiation of ART or co-trimoxazole treatment. Statistical analyses were performed to ensure that the proportional hazards assumptions for the validity of the Cox model were not violated. Analysis was performed using SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina) and S-Plus version 8.0.4 (TIBCO Software Inc., Palo Alto, California).
Figure 2.
Left panel shows mean CD4 count among non-TB patients. Right panel shows mean CD4 count among TB patients. The curves cover the entire duration of the DarDar trial, pre- and post- matching periods. This study covers only the post- matching period.
RESULTS
Study population
The DarDar trial enrolled 2013 subjects. One hundred and sixty nine subjects had a history of treatment for TB before being enrolled in the trial and were excluded from the present analysis. Also excluded were seven patients who had no follow-up and 27 patients with unconfirmed TB (“possible” or “unlikely”) who completed TB treatment. We therefore had 1810 subjects before matching. There were 135 subjects with definite or probable active TB. Of these, we excluded seven subjects with TB but no evidence of ever receiving TB treatment following diagnosis, 51 subjects who did not complete TB treatment, and one subject with missing TST results. We thus had 77 subjects in the TB group. Of these, thirty (39%) met the definition for ‘definite TB’ and 47 (61%) met the definition for ‘probable TB’. We selected 308 CD4-matched subjects who never developed active TB during follow-up as a comparison group. We therefore had a total of 385 subjects for analysis, contributing 9531 person-months of follow-up.
The subjects were predominantly female (75%), single (63%), with median age 33 years, and had never been on ART medication (92%). The median follow-up was two years. There were 32 deaths, 11 in the TB group and 21 in the non-TB group (Tables 1 and 2). A previous study done on DarDar participants16 identified two eligible patients with multidrug-resistance TB. None of these patients were part of this substudy. Twenty nine of 56 TB patients (52%) who did not complete treatment died during the duration of the DarDar trial.
Table 1.
Distribution characteristics of patients
| TB group (N=77) | Non-TB group (N=308) | P value for the absolute difference in IP | |||
|---|---|---|---|---|---|
|
| |||||
| Variable | Total | No. Deaths (IP in %)1 | Total | No. Deaths (IP in %) | |
| Overall | 77 | 11 (14.3) | 308 | 21 (6.95) | 0.071 |
| Age | |||||
| 18–<30 | 27 | 5 (18.52) | 113 | 4 (3.54) | 0.026 |
| 30–<40 | 34 | 3 (8.82) | 116 | 9 (7.76) | 0.826 |
| 40–<50 | 13 | 3 (23.08) | 63 | 7 (11.11) | 0.359 |
| 50–70 | 3 | 0 (0.00) | 10 | 1 (10.00) | 0.786 |
| Gender | |||||
| Females | 59 | 8 (13.56) | 228 | 12 (5.26) | 0.060 |
| Males | 18 | 3 (16.67) | 74 | 9 (12.16) | 0.652 |
| Marital status | |||||
| Married or cohabiting | 28 | 4 (14.29) | 110 | 9 (8.18) | 0.396 |
| Single | 49 | 7 (14.29) | 192 | 12 (6.25) | 0.116 |
| Source of referral | |||||
| Any Muhimbili clinic | 7 | 0 (0.00) | 26 | 2 (7.69) | 0.635 |
| Infectious Diseases Centre | 5 | 1 (20.00) | 18 | 0 (0.00) | 0.250 |
| NGO VCT | 39 | 7 (17.95) | 157 | 10 (6.37) | 0.061 |
| Self referred | 12 | 1 (8.33) | 38 | 4 (10.53) | 0.907 |
| Other | 14 | 2 (16.67) | 63 | 5 (7.94) | 0.523 |
| CD4 count at or around the matching time | |||||
| <200 | 49 | 7 (14.29) | 191 | 11 (5.76) | 0.090 |
| 200–<250 | 6 | 2 (33.33) | 23 | 4 (17.39) | 0.537 |
| 250–<300 | 7 | 0 (0.00) | 28 | 4 (14.29) | 0.437 |
| 300–<350 | 6 | 0 (0.00) | 24 | 1 (4.17) | 0.806 |
| ≥350–2000 | 9 | 2 (22.22) | 36 | 1 (2.78) | 0.137 |
| On ART at matching | |||||
| On medication | 4 | 0 (0.00) | 20 | 2 (10.00) | 0.711 |
| Not on medication | 73 | 11 (15.07) | 282 | 19 (6.74) | 0.054 |
| On ART at some point during follow-up | |||||
| On medication | 6 | 0 (0.00) | 20 | 2 (10.00) | 0.611 |
| Not on medication | 71 | 11 (15.49) | 282 | 19 (6.74) | 0.047 |
| Vaccine arm | |||||
| M. vaccae | 28 | 7 (25.00) | 146 | 9 (6.16) | 0.016 |
| Placebo | 49 | 4 (8.16) | 156 | 12 (7.69) | 0.893 |
| Isoniazid preventive therapy (at DarDar baseline) | |||||
| Not eligible for therapy | 50 | 6 (12.00) | 214 | 15 (7.01) | 0.303 |
| Eligible, not adhered | 7 | 4 (57.14) | 10 | 2 (20.00) | 0.335 |
| Eligible, adhered | 20 | 1 (5.00) | 78 | 4 (5.13) | 0.957 |
| On co-trimoxazole at matching | |||||
| On medication | 38 | 6 (15.79) | 78 | 5 (6.41) | 0.173 |
| Not on medication | 39 | 5 (12.82) | 224 | 16 (7.14) | 0.296 |
| On co-trimoxazole at some point during follow-up | |||||
| On medication | 68 | 10 (14.71) | 135 | 8 (5.93) | 0.073 |
| Not on medication | 9 | 1 (11.11) | 167 | 13 (7.78) | 0.705 |
IP stands for incidence proportion
Table 2.
Distribution characteristics of patients by death and person-time
| TB group (N=77) | Non-TB group (N=308) | P value for the absolute difference in death rate | |||
|---|---|---|---|---|---|
|
| |||||
| Variable | Total person-months | No. Deaths | Total person-months | No. Deaths | |
| Overall | 1333.27 | 11 | 7801.57 | 21 | 0.002 |
| Age | |||||
| 18–<30 | 508.70 | 5 | 2884.00 | 4 | 0.001 |
| 30–<40 | 564.53 | 3 | 3116.77 | 9 | 0.353 |
| 40–<50 | 178.07 | 3 | 1561.43 | 7 | 0.039 |
| 50–70 | 81.97 | 0 | 239.37 | 1 | 0.558 |
| Gender | |||||
| Females | 962.23 | 8 | 5960.53 | 12 | 0.001 |
| Males | 371.03 | 3 | 1841.03 | 9 | 0.446 |
| Marital status | |||||
| Married or cohabiting | 493.00 | 4 | 4983.83 | 9 | 0.006 |
| Single | 840.27 | 7 | 2817.73 | 12 | 0.151 |
| Source of referral | |||||
| Any Muhimbili clinic | 136.20 | 0 | 646.80 | 2 | 0.516 |
| Infectious Diseases Centre | 97.57 | 1 | 544.40 | 0 | - |
| NGO VCT | 693.33 | 7 | 4232.37 | 10 | 0.001 |
| Self referred | 178.20 | 1 | 726.73 | 4 | 0.986 |
| Other | 229.97 | 2 | 1651.26 | 5 | 0.187 |
| CD4 count at or around the matching time | |||||
| <200 | 790.13 | 7 | 4896.53 | 11 | 0.002 |
| 200–<250 | 108.43 | 2 | 680.77 | 4 | 0.163 |
| 250–<300 | 178.13 | 0 | 777.83 | 4 | 0.339 |
| 300–<350 | 92.77 | 0 | 497.70 | 1 | 0.666 |
| ≥350–2000 | 163.80 | 2 | 948.73 | 1 | 0.010 |
| On ART at matching | |||||
| On medication | 104.10 | 0 | 428.47 | 2 | 0.486 |
| Not on medication | 1229.17 | 11 | 7373.10 | 19 | <0.001 |
| On ART at some point during follow-up | |||||
| On medication | 157.03 | 0 | 428.47 | 2 | 0.392 |
| Not on medication | 1176.23 | 11 | 7373.10 | 19 | <0.001 |
| Vaccine arm | |||||
| M. vaccae | 450.97 | 7 | 3824.87 | 9 | <0.001 |
| Placebo | 882.30 | 4 | 3966.7 | 12 | 0.481 |
| Isoniazid preventive therapy (at DarDar baseline) | |||||
| Not eligible for therapy | 845.67 | 6 | 5431.27 | 15 | 0.043 |
| Eligible, not adhered | 116.37 | 4 | 254.63 | 2 | 0.062 |
| Eligible, adhered | 371.23 | 1 | 2115.67 | 4 | 0.003 |
| On co-trimoxazole during matching | |||||
| On medication | 506.80 | 6 | 1550.70 | 5 | 0.021 |
| Not on medication | 826.47 | 5 | 6250.87 | 16 | 0.083 |
| On co-trimoxazole at some point during follow-up | |||||
| On medication | 1227.37 | 10 | 3562.83 | 8 | 0.004 |
| Not on medication | 105.90 | 1 | 4238.73 | 13 | 0.254 |
Outcomes
Kaplan-Meier curves showed a higher mortality for patients in the TB group than patients in the non-TB group (Figure 3). Crude death rate was eight per 1000 person-months in the TB group compared with three per 1000 person-months in the non-TB group (log rank P value= 0.01). The adjusted Cox proportional hazards model indicated a four-fold increased mortality in the TB group than the non-TB group (Hazard Ratio= 4.61; 95% Confidence Interval (CI): 1.63, 13.05) (Table 3). The median time to TB for cases was 22 months (interquantile range (IQR): 13–36 months), the median time to completion of TB treatment for cases was 30 months (IQR: 21–43 months), and the median time to t0 for non-TB cases was 23 months (IQR: 14–36 months). Throughout follow-up there was no TB patient who had TB recurrence after successfully completion of therapy.
Figure 3.
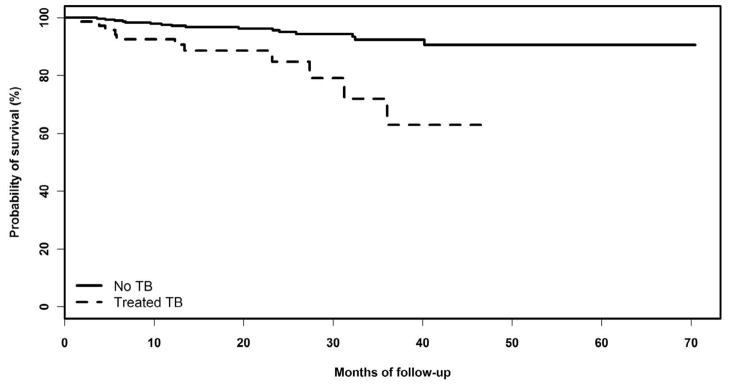
Kaplan-Meier curves comparing the probability of survival between the TB group (dashed line) and the non-TB group (solid line).
Table 3.
Comparison of mortality between the TB and the non-TB group
| Hazard ratio [95% CI] | ||
|---|---|---|
|
| ||
| Crude | Adjusted1 | |
| TB group (n=77) | 4.42 [1.72,11.33] | 4.61 [1.63,13.05] |
|
| ||
| Non-TB group (n=308) | 1.00 | 1.00 |
Adjusted for age, sex, vaccine status, and co-trimoxazole
CD4 count declines over time
When analyzed from the beginning of the vaccine study follow-up, it can be seen that TB patients had a lower initial CD4 counts than non-TB patients (Figure 2). In the early part of follow-up, CD4 counts decreased in both groups. However, as can be seen in figure 2, the average counts for TB patients rebounded after about 40 months of being in the study, while there was no rebound seen in the non-TB group. These results were essentially unchanged when persons receiving ART were excluded, or when persons who died were excluded (data not shown).
DISCUSSION
Our findings suggest a strong association between successfully treated TB disease and increased mortality in HIV-infected adults in Tanzania. Compared with patients with a similar severity of HIV disease who did not develop active TB, patients who developed and completed treatment for active TB had a greater than four-fold increase in mortality.
In most other studies that have observed such an increase, patients with TB also had a more rapid progression to new onset AIDS-associated opportunistic infections, suggesting that the mechanism for this increased mortality was an enhanced immune deficit. However, only two of the studies looked directly at decreases in CD4 counts or increases in HIV viral load. Whalen et al. found that patients with TB had a greater rise in viral load after TB than did control patients, but this difference was not statistically significant (p=0.17),8 while Leroy et al found that a higher proportion of patients with TB progressed to CD4<50 than did control patients, but this also was not statistically significant (p=0.09).10 We were also unable to document a significantly more rapid decline of CD4 cell counts after TB. This suggests that other factors may be contributing to the increased mortality in this population. One possibility might be M. tuberculosis–induced impairment of the lung function; a recent report showed that TB, even when successfully treated, leaves patients with substantial pulmonary impairment.17
While we cannot draw firm conclusions from our study, the increase in CD4 counts over time among the TB patients suggests that a more rapid decrease in immune competence may not be the cause of increased mortality in our patients who were treated for TB. This is consistent with a recent report from Uganda, where TB patients had decreased cellular markers of immune activation as TB treatment progressed (in the absence of ART), despite the absence of an effect on the CD4 counts or HIV loads of the patients.18
In our study we observed increases in CD4 counts in TB patients after TB treatment. One possible explanation would be systematic ART initiation following TB treatment. However, only 6 of our TB patients were known to have initiated ART during follow-up, and when these 6 were excluded from the CD4 count analysis, the effect persisted. Similarly, exclusion of subjects with TB who died during the study did not markedly change the curve, indication that death of those with low CD4 counts did not explain the rise in the group mean CD4 count. Thus, we conclude that this rise in CD4 counts can be attributed to the effect of TB treatment on CD4 counts, as reported previously.19
Our study had three important limitations. First, about a quarter of patients had their CD4 counts interpolated at the time of matching (all in the non-TB group), thus residual confounding on mortality results can not be ruled out. Second, due to the small number of subjects on ART, we could not evaluate the combined effect of TB treatment and ART on mortality prevention. However, of the 26 patients on ART in our study cohort (Table 1), only two died by the end of follow-up, suggesting that ART could have prevented deaths in some of these patients. All patients meeting WHO guidelines for ART initiation were referred repeatedly to CTC designated clinics, but the uptake was low at the time of the trial. Finally, there could have been residual confounding due to unmeasured factors such as lifestyle variables, HIV subtypes, and comorbidities that could have affected mortality in ways that we could no assess.
The main strengths of this study included a close monitoring on administration of TB treatment which reduced misclassification of treatment effect and the availability of CD4 counts at multiple visits over time, which allowed us to asses the overall trends in CD4 counts over time.
In summary, we show that active TB, even when adequately treated, is strongly associated with increased mortality among HIV-infected patients treated for TB in the absence of antiretroviral therapy. This study confirms findings from previous studies that active TB is associated with increased mortality among HIV-infected patients. Therefore, prevention of TB disease should be a high priority among HIV-infected populations in areas where co-infection is common. Results from this study underscore the need for more widespread and effective implementation of Isoniazid preventive therapy, as recommended by WHO in the “3 I’s” Initiative.20,21 This should go parallel with integration of TB and HIV treatment for prevention of TB and mortality.11,12,13
Acknowledgments
This study received a financial support from the Fogarty International Centre of the National Institutes of Health (grant number D43-TW006808 and grant number AI45407). We thank all the DarDar staff and participants for their support.
Footnotes
Competing interests
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Conrad Kabali, Email: ckabali@bu.edu.
Lillian Mtei, Email: lndefomiro@yahoo.com.
Daniel R. Brooks, Email: danbrook@bu.edu.
Richard Waddell, Email: Richard.d.waddell@dartmouth.edu.
Muhammad Bakari, Email: drbakari@yahoo.com.
Mecky Matee, Email: mmateemi@yahoo.com.
Robert D. Arbeit, Email: Robert.arbeit@gmail.com.
Kisali Pallangyo, Email: kpallangyo@gmail.com.
C. Fordham von Reyn, Email: fvonreyn@yahoo.com.
C. Robert Horsburgh, Email: rhorsbu@bu.edu.
References
- 1.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–937. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO Report. 2009. Global tuberculosis control -epidemiology, strategy, financing. WHO/HTM/TB/2009.411. [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. New Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. 2008 Report on the Global AIDS epidemic. UNAIDS/08.27E/JC1511E. [Google Scholar]
- 5.van der Sande MA, Schim van der Loeff MF, Bennett RC, Dowling M, Aveika AA, Togun TO, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS. 2004;18:1933–1941. doi: 10.1097/00002030-200409240-00009. [DOI] [PubMed] [Google Scholar]
- 6.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5(3):225–232. [PubMed] [Google Scholar]
- 7.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151(1):129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 8.Whalen CC, Nsubuga P, Okwera A, Johnson JL, HOM DL, Michael NL, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–28. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munsiff SS, Alpert PL, Gourevitch MN, Chang CJ, Klein RS. A prospective study of tuberculosis and HIV disease progression. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:361–366. doi: 10.1097/00042560-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Leroy V, Salmi LR, Dupon M, Sentilhes A, Jeannette Texier-Maugein, Laurence D, et al. Progression of human immunodeficiency virus infection in patients with tuberculosis disease. A cohort study in Bordeaux, France, 1988–1994. The Groupe d’Epidémiologie Clinique du Sida en Aquitaine (GECSA) Am J Epidemiol. 1997;145:293–300. doi: 10.1093/oxfordjournals.aje.a009105. [DOI] [PubMed] [Google Scholar]
- 11.Abdool Karim SS, Naidoo K, Padayatchi N, Baxter C, Gray A, Gengiah T, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc F, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette–Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24:675–685. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National AIDS Control Programme. National guidelines for the management of HIV and AIDS. 3. NACP; 2008. [Google Scholar]
- 16.Adams LV, Kreiswirth BN, Arbeit RD, Soini H, Mtei L, Matee M, et al. Molecular epidemiology of HIV-associated Tuberculosis in Dar es Salaam, Tanzania: Strain predominance, clustering, and polyclonal diesease. J Clin Microbiol. 2012;50(8):2645. doi: 10.1128/JCM.00624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasipanodya JG, Miller TL, Vecino M, Munguio G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131:1817–24. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 18.Mahan CS, Walusimbi M, Johnson DF, Lancioni C, Charlebois E, Baseke J, et al. Tuberculosis treatment in HIV infected Ugandans with CD4 counts >350 cells/mm3 reduces immune activation with no effect on HIV load or CD4 count. PLoS One. 2010;5:e9138. doi: 10.1371/journal.pone.0009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DJ, Sim JG, Sole GJ, Rymer L, Shalekoff S, van Niekerk AB, et al. CD4+ lymphocyte count in African patients co-infected with HIV and tuberculosis. J Acquir Immune Defic Synd. 1995;8:386–91. [PubMed] [Google Scholar]
- 20.Glaziou P, Floyd K, Korenromp EL, Sismanidis C, Bierrenbach A, Williams BG, et al. Lives saved by tuberculosis control and prospects for achieving the 2015 global target for reducing tuberculosis mortality. Bull World Health Organ. 2011;89:573–582. doi: 10.2471/BLT.11.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Three I’s Meeting: intensified case finding (ICF), isoniazid preventive therapy,(IPT) and TB infection control (IC) for people living with HIV. In Report of a joint World Health Organization HIV/AIDS and TB department meeting; 2008. [Google Scholar]