Abstract
Background
Reduced nicotine content (RNC) cigarettes have led to smoking fewer cigarettes, withdrawal relief, and facilitation of cessation. The aim of this study is to examine the effects RNC cigarettes with and without nicotine patch and patch alone on smoking behavior, toxicant exposure, withdrawal discomfort and as an exploratory analysis, on long-term abstinence.
Methods
This study involved a randomized, parallel arm design and six weeks of: 1) 0.05-0.09 mg nicotine yield cigarettes (N=79); 2) 21 mg nicotine patch (N=80) or 3) 0.05-0.09 nicotine yield cigarettes with 21 mg nicotine patch (N=76); all groups received six weeks of additional behavioral treatment with follow-ups up to six months.
Results
Combination approach led to lower rates of smoking assigned cigarettes and hence lower CO levels than RNC cigarettes alone. Additionally, the combination approach was associated with less withdrawal severity when switching from usual brand to assigned product, and less smoking of usual brand cigarettes during treatment, but not after treatment compared to the other approaches.
Conclusion
Combining very low nicotine content cigarettes with nicotine patch may improve the acute effects resulting from switching to either of these products alone.
Impact
These findings may have implications for smoking cessation treatment or a policy measure to reduce nicotine content in cigarettes.
Keywords: reduced nicotine, cigarettes-nicotine, patch-tobacco, addiction-cigarette, consumption-biomarkers
INTRODUCTION
The use of reduced nicotine content (RNC) cigarettes has been considered as a possible cessation tool and as a national policy measure (1-3). Unlike “light” cigarettes, the nicotine content in the RNC cigarette itself is substantially lower than conventional cigarettes. Reducing nicotine in cigarettes to levels that are non-addictive would potentially lead to cessation from smoking (because cigarettes are no longer reinforcing) and if implemented as a policy, has the potential to have significant public health benefit.
To date, the scientific literature shows that switching to RNC cigarettes leads to a reduction in cigarette intake with minimal compensatory smoking behavior, no greater exposure to toxicants than their usual brand cigarettes, decrease in dependence and facilitation of abstinence among smokers not interested (4, 5) and interested in quitting smoking (3, 6). As an example of the effects of RNC cigarettes in facilitating cessation, smokers interested in quitting who were assigned to the 0.05 mg nicotine yield cigarettes achieved a biochemically verified 7-day point prevalence smoking cessation rate of 35.9% as compared to 13.5% and 20.0% among those smokers assigned to a higher nicotine yield cigarettes (0.3 mg nicotine yield) or to nicotine lozenge at 6 weeks post-treatment, respectively. Another large study found that smokers calling a quit line and assigned to RNC cigarettes plus usual care, which involved use of nicotine replacement therapy (NRT), were observed to have significantly higher abstinence rates than those who were provided usual care alone (3). To date, no study has examined the effects of combining RNC cigarettes plus nicotine patch on smoking behavior, resultant toxicant exposure, withdrawal symptoms and craving, dependence scores and on abstinence rates compared with medicinal nicotine product alone and with RNC cigarettes alone. To address this gap, we conducted a study in which smokers were randomized to 6 weeks of 0.05-0.09 mg nicotine yield cigarettes, 21 mg nicotine patch, or a combination of both. We hypothesized that smoking behavior, toxicant exposure, withdrawal and craving upon switching to the assigned product would be less for the combined intervention condition compared to the products alone. These hypotheses are based on the assumption that the RNC cigarettes would decrease craving associated with the sensory aspects of smoking and reduce the reinforcing value of cigarettes, while the patch would provide nicotine (not associated with the act of smoking) for nicotine-related craving and withdrawal relief. Therefore, the combination therapy would lead to better treatment response than either product alone. In an exploratory analysis, we also hypothesized that abstinence rates would be highest with combined product treatment condition compared to the single product conditions.
Our goal was to examine the feasibility of using these cigarettes as a method to significantly reduce smoking behavior and the effects of adding the nicotine patch in augmenting beneficial effects from RNC cigarettes. If this combination approach proved more effective than the products alone, then RNC cigarettes can be considered an adjunct to existing nicotine replacement therapies. Additionally, in the event of a national policy to reduce nicotine in all cigarettes, the results would suggest the importance of making nicotine replacement therapies easily accessible to smokers.
MATERIAL AND METHODS
Subjects
Smokers between the ages of 18 and 70 interested in quitting smoking were recruited via advertisement from the Twin Cities and Duluth, Minnesota. In the advertisements, the study was described as testing a nicotine free cigarette or new tobacco product as a way to become smoke free. To be eligible, smokers had to a) have smoked 10 to 40 cigarettes daily for the past year (the range was instituted to reduce heterogeneity); b) be in good physical and psychiatric health; and c) have no contraindications for medicinal nicotine use. Smokers using other tobacco or nicotine products and smokers who were pregnant or nursing were excluded. The study was approved by our institutional review board and in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services.
Study Design
After a telephone screening to determine preliminary eligibility, an orientation session was held at which the study was further explained, written informed consent was obtained and a more thorough screening for eligibility was performed.
After a two week period during which baseline measurements were collected while subjects smoked their usual brand ad libitum, subjects were assigned to one of three conditions: a) 0.05-0.09 mg nicotine yield cigarettes, that is very low nicotine content (VLNC) cigarettes, b) 21 mg nicotine patch (NP), or c) combination of both. Subjects were initially assigned Quest 3 cigarettes (manufactured by Vector), a commercially available VLNC cigarette of ≤ 0.05 mg machine-determined nicotine yield, 0.7 to 0.9 mg nicotine and about 8 to 11 mg tar on per cigarette basis, and with reduced levels of tobacco-specific nitrosamines compared to conventional cigarettes (NNK=0.05 and NNN=0.83 ug/g tobacco wet weight for Quest 3 vs. NNK=0.68 and NNN=2.8 ug/g tobacco wet weight for Marlboro “Light” (7). These cigarettes no longer were available after randomizing 27% of our subjects, so we switched to Xodus, which contained 0.09 mg machine-determined nicotine yield, 1.2 mg nicotine, about 10 mg tar and similarly low carcinogens (e.g., NNK=0.05 ug/g and NNN=0.72 ug/g tobacco wet weight, unpublished data). The average nicotine yield per each cigarette puff was about from 0.005 to 0.008 and 0.010 to 0.011 mg, for the 0.05 and 0.09 mg nicotine yield cigarettes, respectively. Subjects were instructed to use only assigned products for six weeks, after which time they were to discontinue all product use. Subjects were seen weekly during the 6-week product assignment period and an additional 6 weeks at weeks 7, 8, 10 and 12 for continued behavioral treatment.
At each visit, subjects assigned to either cigarette condition were provided a supply equivalent to 150% of their baseline smoking rate (to allow for compensatory smoking) and were told to smoke these VLNC cigarettes ad libitum, that is, as they would smoke their usual cigarettes. Subjects assigned to receive nicotine patch were informed to replace the old patch with the new patch each morning. Subjects maintained a daily smoking diary where they recorded any cigarettes smoked (either those assigned to them or their usual brand). They were not penalized for smoking unassigned cigarettes, but told that although we do not encourage them to smoke cigarettes other than those assigned, it is crucial to the study that they accurately report all cigarette use.
Brief standardized counseling was provided at each visit during the intervention phase of the study. During the first six weeks, subjects assigned to the cigarette conditions were counseled to consider the use of these products as a step towards quitting and discussed behavioral strategies to resist smoking other (non-VLNC) cigarettes. Subjects assigned to the NP only condition were provided treatment tools recommended by the US Clinical Practice Guideline (8). During the second six week intervention phase, all subjects received counseling similar to that received by the subjects assigned to the nicotine patch condition. All three treatment groups received similar amounts of behavioral support.
Follow-up visits occurred at 16, 24 and 36 weeks. Subjects who completed the study were paid up to $330.
Outcome Measures
Biomarkers of tobacco exposure measures included a) urinary total nicotine equivalents (TNE) which is the sum of nicotine, cotinine, and 3’-hydroxycotinine and their glucuronides, altogether accounting for 73 – 96% of the nicotine dose (9, 10); b) urinary total cotinine, a metabolite of nicotine; c) alveolar carbon monoxide (CO) measured using the Bedfont Micro Smokerlyzer® (Bedfont Scientific Limited, Kent, UK); and d) urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL), metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; 11). All measures were assessed at baseline. Additionally, carbon monoxide was assessed at each clinic visit, cotinine at weeks 2, 6 and 12 of intervention and at follow-up visits, and biomarkers for other exposures at week 6 of intervention.
Subjective measures included: a) a Tobacco Use Questionnaire asking about current tobacco use status (cigarettes and other tobacco products); b) a daily diary detailing the number of assigned products used and usual cigarettes smoked; c) the Minnesota Nicotine Withdrawal Scale, a widely used scale assessing withdrawal from cigarettes (12-14), nicotine gum (15, 16) and smokeless tobacco (15, 17) d) Fagerstrom Test for Nicotine Dependence (FTND, 18); e) Centers for Epidemiological Studies 20-item scale (CES-D) assessing current symptoms of depression (19) and f) Perceived Health Risk, a ladder involving rating risk for addiction to a product on a scale ranging from 1 to 10 (6). All of these measures were assessed at baseline. Cigarette or product use was assessed daily, the Tobacco Use Questionnaire and Minnesota Nicotine Withdrawal Scale at each clinic visit and Perceived Health Risk at weeks 2 and 6.
Statistical Analysis
Subjects’ baseline characteristics including demographics and smoking history were described and compared among three intervention groups. Discrete variables were analyzed using Pearson's χ2 test or Fisher's exact test. Continuous variables were analyzed using either one-way analysis of variance (ANOVA) or Kruskal-Wallis test.
We conducted an intention-to-treat analysis. Biomarkers including TNE, cotinine and NNAL were adjusted for creatinine and analyzed on the natural log scale to ensure normality; geometric means in original units are presented. Abstinence during the first 6-week treatment period was calculated as point prevalence abstinence from usual brand cigarettes for the past 7 days (by self-report). After this period, biochemically verified 7-day point prevalence abstinence and continuous abstinence rates during weeks 12, 16, 24, and 36 were calculated. Differences between treatment groups were evaluated using χ2 tests. Drop-outs were considered to have relapsed at the date of their last follow-up visit. Time to smoking relapse was calculated from the start of treatment to date of first relapse (defined as smoking of >4 usual brand cigarettes during treatment period or any tobacco use during follow-up). Those who remained abstinent throughout the study were censored at the time of last follow-up. Kaplan-Meier methods were used to determine the median time to relapse and 95% confidence interval (CI) for each group.
All continuous outcomes with repeated measures from baseline through treatment were analyzed using mixed effects ANOVA models with fixed effects for site, treatment, visit, interaction between treatment and visit, and a random effect for subject. Least squares (LS) means and 95% CI are presented unless otherwise noted. The p-values reported were adjusted for multiple comparisons as appropriate using a Bonferroni correction. P-values <0.05 were considered statistically significant.
RESULTS
Subjects
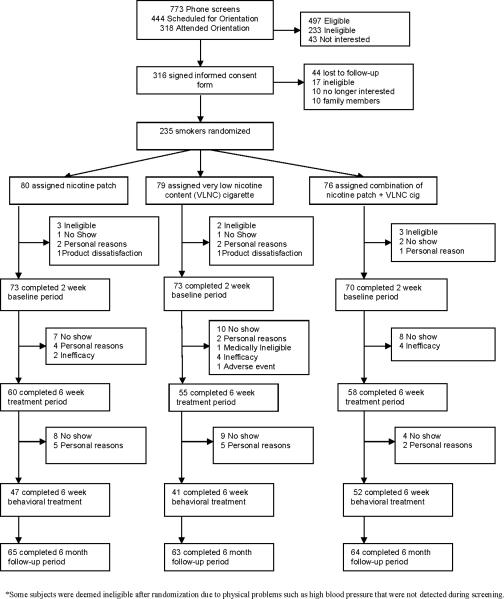
Figure 1 shows the consort diagram outlining the disposition of the subjects. Of the 316 signed the informed consent form, 235 smokers (N=203 from Minneapolis, Minnesota and N=32 from Duluth, Minnesota) were randomly assigned to treatment (80 to NP, 79 to VLNC, and 76 to VLNC + NP).
Figure 1.
Flow of subjects through study
No significant differences in demographics and smoking history, or biomarkers of exposure were observed across the treatment conditions at baseline (see Tables 1 and 2). Of those subjects who enrolled in the study, 173 subjects completed treatment (N=60 in NP, N=55 in VLNC, and N=58 in VLNC + NP). The number of drop-outs in each group at various stages throughout the study with reasons for drop-outs is indicated in Figure 1. All subjects were contacted for follow-up, accounting for the higher numbers during follow-up compared to end of treatment. There were no significant differences in baseline characteristics between subjects who dropped out of the study after randomization and those who completed the entire study.
Table 1.
Baseline demographics and smoking history of subjects by treatment group (N=235).1
| Overall | VLNC | NP | VLNC+NP | |
|---|---|---|---|---|
| Age (years) | 47.0±11.7 | 46.5±12.2 | 47.3±11.0 | 47.0±11.9 |
| Female | 57.9% | 59.5% | 57.5% | 56.6% |
| Non-Hispanic Whites | 82.0% | 85.9% | 84.8% | 75.0% |
| Education | ||||
| 8th grade or less | 0.9% | 2.6% | 0.0% | 0.0% |
| Some high school | 2.6% | 3.9 | 1.3% | 2.7% |
| High school graduate | 22.8% | 18.0% | 26.3% | 24.0% |
| Some college/2-year | 56.7% | 53.9% | 58.8% | 57.3% |
| College graduate | 13.7% | 18.0% | 11.3% | 12.0% |
| Graduate | 3.4% | 3.9% | 2.5% | 4.0% |
| Marital Status | ||||
| Never Married | 26.8% | 25.3% | 28.9% | 25.3% |
| Currently Married | 38.7% | 41.8% | 36.3% | 41.8% |
| Currently Not Married | 34.5% | 32.9% | 35.0% | 32.9% |
| Cigarettes per day | 18.9±7.2 | 19.4±6.2 | 19.5±8.6 | 17.7±6.3 |
| Duration of having smoked at this rate (yrs) | 29.1±12.0 | 29.2±11.6 | 29.6±11.7 | 28.4±12.8 |
| Age becoming a regular smoker (yrs) | 17.9±4.6 | 17.3±3.6 | 17.7±4.9 | 18.8±5.0 |
| Number of quit attempts | ||||
| 0-2 | 23.5% | 21.6% | 22.4% | 26.9% |
| 3-5 | 39.6% | 37.8% | 40.8% | 40.3% |
| 6-10 | 22.6% | 23.0% | 22.4% | 22.4% |
| 11-20 | 11.1% | 12.2% | 11.8% | 9.0% |
| 20+ | 3.2% | 5.4% | 2.6% | 1.5% |
| Motivation to quit (0-10 scale) | 8.5±1.4 | 8.5±1.4 | 8.3±1.5 | 8.6±1.4 |
| FTND | 5.4±1.9 | 5.6±1.7 | 5.3±2.1 | 5.1±2.0 |
| CES-D (0-60 scale) | 10.6±7.4 | 11.1±7.7 | 10.9±7.7 | 9.6±6.7 |
Due to missing values, the Ns were 217 for quit attempts, 224 for motivation to quit, 227 for FTND and 223 for CES-D. Otherwise, all other variables had data from 233 to 235 subjects. FTND: Fagerstrom Test for Nicotine Dependence; CES-D: Centers for Epidemiological Studies Depression scale.
Table 2.
Geometric means of biomarkers at baseline and week 6 of treatment period by treatment groups. Values are for all subjects from whom data were collected at the visit in question.
| Biomarkers | Baseline | Week 6 | ||
|---|---|---|---|---|
| N | Geometric Mean (95% CI) | N | Geometric Mean (95% CI) | |
| Total TNE 1 | ||||
| VLNC Cigarette | 54 | 55.70 (48.42, 64.07) | 54 | 6.89 (4.26, 11.02) |
| Patch | 59 | 49.90 (43.82, 57.40) | 58 | 23.10 (14.59, 36.60) |
| VLNC Cigarette + Patch | 58 | 53.52 (46.99, 61.56) | 58 | 27.39 (17.29, 43.38) |
| Total Cotinine 1 | ||||
| VLNC Cigarette | 54 | 17.12 (14.44, 20.09) | 54 | 2.03 (1.20, 3.45) |
| Patch | 59 | 16.78 (14.30, 19.69) | 59 | 5.50 (3.31, 9.13) |
| VLNC Cigarette + Patch | 58 | 17.99 (15.33, 21.12) | 58 | 7.65 (4.59, 12.76) |
| Total NNAL 2 | ||||
| VLNC Cigarette | 53 | 1.20 (1.00, 1.43) | 52 | 0.40 (0.29, 0.55) |
| Patch | 59 | 1.29 (1.09, 1.53) | 59 | 0.25 (0.18, 0.34) |
| VLNC Cigarette + Patch | 57 | 1.09 (0.92, 1.30) | 55 | 0.26 (0.19, 0.36) |
nmol/mg creatinine
pmol/mg creatinine
Product use during treatment
The number of assigned cigarettes smoked per day during the first 6-week treatment period is illustrated in Figure 2A. There were significant treatment (F(2,876)=130.88, p<0.0001), time (F(5,876)=36.75, p<0.0001) and treatment by time (F(10,876)=9.81, p<0.0001) effects observed. Significant differences in number of assigned cigarettes smoked were observed at each time point between the VLNC and VLNC + NP conditions (p ≤ 0.01), with the exception of week 1 (p=0.063); those assigned to the VLNC + NP smoked fewer cigarettes. At week 6, the mean ± S.D. number of VLNC cigarettes smoked in the VLNC condition was 16.2±10.2 and in the VLNC + NP condition was 11.4±7.6. Among subjects assigned to the two conditions with the NP, 100% of the NP group and 95.6% of the VLNC+NP group reported daily use of the patch at week 6.
Figure 2.
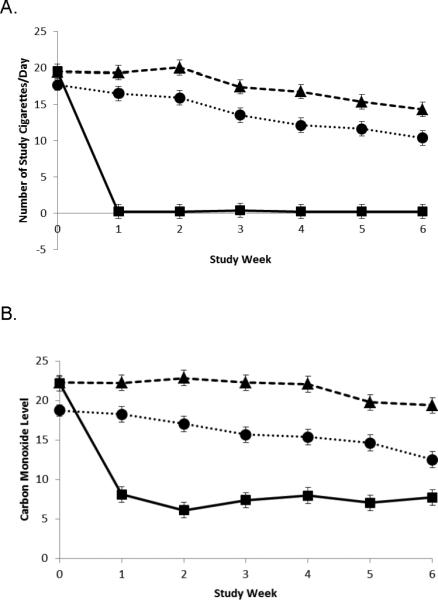
Least squares (LS) mean (±SE) of number of study cigarettes smoked per day (Panel A) and exhaled carbon monoxide (Panel B). Diamond represents very low nicotine content (VLNC) cigarette alone; square represents nicotine patch (NP) alone; circle represents VLNC + NP.
Across treatment groups, significant differences in abstinence from non-study cigarettes (e.g., usual brand cigarettes) were observed during the 6-week product assignment period (p=0.0001). In particular, those subjects in the VLNC + NP group were significantly more likely to be abstinent than either the NP or VLNC only groups (p=0.004 and p=0.009, respectively). Subjects were most likely to smoke usual brand cigarettes during the first week of treatment: 54.2% of those assigned to VLNC cigarettes, 63.8% of those assigned NP alone and 41.5% assigned to the combined products group. After week 1, the percentage who reported using usual brand cigarettes ranged from 25.4-38.2% (during weeks 2 through 6) in the VLNC cigarette group, 35.6-50.8% in the NP group and 8.3-21.7% in the VLNC + NP group. Significant differences between groups were observed during each of these weeks (all p < 0.02). At the week 6 visit, 32.7% in the VLNC cigarettes group, 43.3% in the NP group and 13.8% in the combined products group reported using such products (p=0.002). Among those reporting smoking non-study cigarettes during the treatment period, the mean number of self-reported usual brand cigarettes smoked ranged from 2.8 to at most 4.4 per week.
Effects of products on biomarkers of exposure during treatment
Exhaled CO during the treatment period is shown in Figure 2B. Urinary TNE, total cotinine, and total NNAL adjusted for creatinine are presented in Table 2. As illustrated in Figure 2B, exhaled CO concentrations followed a similar pattern as seen for number of cigarettes smoked per day. There were significant treatment (F(2,847)=53.99, p<0.0001), time (F(5,847)=4.88, p=0.0002) and treatment by time (F(10,847)=2.10, p=0.022) effects. All comparisons between CO levels for each treatment pair at each visit are significantly different from each other (p ≤ 0.05).
Baseline TNE, total cotinine and total NNAL levels were significantly higher than levels assessed at Week 6 for each of the products (all p-values < 0.007). Compared to subjects assigned to the VLNC condition, subjects assigned to the NP and VLNC + NP conditions had significantly higher TNE levels (p=0.0005 and p=0.0001, respectively) and total cotinine levels (p=0.021 and p=0.002, respectively) at week 6. Subjects assigned to NP had significantly lower total NNAL than VLNC users at week 6 (p=0.024), but no significant differences were observed between subjects assigned to VLNC + NP vs. VLNC cigarettes (p=0.276).
Effects of products on subjective responses during treatment
Dependence
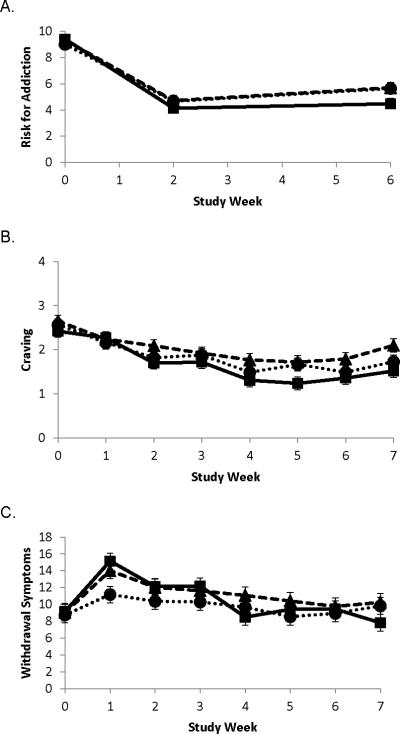
Perceived Heath Risk score for addiction during treatment is illustrated in Figure 3A. Significant decreases were observed across all treatments compared to baseline (p<0.0001); no differences were observed across treatments. Nicotine craving and withdrawal symptoms during the 6-week product assignment period and 1 week after this period are illustrated in Figure 3B and 3C. Upon cessation of usual brand cigarettes and switching to the products (week 1 compared to baseline), there was a significant decrease in craving (p=0.0002) and increase in withdrawal symptoms (p<0.0001) across all three treatment groups. For craving, no significant differences were observed between treatments. Increase in nicotine withdrawal scores upon cessation of usual brand cigarettes was significant by treatment group (p=0.008); those assigned to VLNC + NP had significantly lower withdrawal symptoms than NP alone (p=0.008), but only borderline significantly lower than VLNC alone (p=0.092). No differences were observed between NP vs. VLNC alone. Upon cessation of the product (week 7 compared to week 6), a significant increase in craving was observed (p<0.0001), but no differences among treatments. For withdrawal symptoms, a significant change was observed (p<0.0001), with withdrawal symptoms lower in week 7 compared to week 6 for those assigned to the NP and slightly higher in week 7 for those assigned to the VLNC or VLNC + NP groups. These differences were not quite statistically significant among treatments (p=0.110).
Figure 3.
Least squares (LS) mean (±SE) of Perceived Health Risk addiction score at baseline, week 2 and week 6 (Panel A). Least squares (LS) mean (±SE) of craving and withdrawal symptoms (Panels B and C) at baseline through week 7. Diamond represents very low nicotine content (VLNC) cigarette alone; square represents nicotine patch (NP) alone; circle represents VLNC + NP.
Abstinence
After completion of the assigned product treatment period, biochemically verified (CO < 6 ppm to rule out cigarette use) point prevalence rates of abstinence from cigarettes at each of the follow-up visits and continuous abstinence rates (at weeks 12, 24 and 36) showed no significant differences across treatment groups (Table 3). Similar results were observed for abstinence from all nicotine containing products (CO < 6 ppm, cotinine < 35 ng/ml). If subjects who never received the product were excluded from the analysis, the rates of point prevalence abstinence at week 36 across the conditions would range from 19.2% to 21.4%. The median time to relapse (95% CI) since treatment onset was 7.1 (6.7 to 7.7) weeks for VLNC + NP, 2.6 (1.7 to 5.9) weeks for VLNC, and 2.1 (1.6 to 3.9) weeks for NP.
Table 3.
Continuous (since week 6) and point-prevalence (past 1 week) post treatment abstinence rates.
| CO and Cotinine Verified Continuous Abstinence | |||||||
|---|---|---|---|---|---|---|---|
| Treatments |
|||||||
| VLNC cigarette (n=79) | Nicotine Patch (n=80) | VLNC + NP (n=76) | |||||
| Week | # abstinent | % | # abstinent | % | # abstinent | % | p-value |
| 12 | 11 | 13.9 | 11 | 13.8 | 8 | 10.5 | 0.776 |
| 24 | 9 | 11.4 | 10 | 12.5 | 6 | 7.9 | 0.625 |
| 36 | 8 | 10.1 | 8 | 10.0 | 6 | 7.9 | 0.867 |
| CO Verified Point Prevalence Abstinence | |||||||
|---|---|---|---|---|---|---|---|
| Treatments |
|||||||
| VLNC cigarette (n=79) | Nicotine Patch (n=80) | Combination (n=76) | |||||
| Week | # abstinent | % | # abstinent | % | # abstinent | % | p-value |
| 12 | 21 | 26.6 | 24 | 30.0 | 22 | 29.0 | 0.888 |
| 16 | 21 | 26.6 | 20 | 25.0 | 23 | 30.3 | 0.752 |
| 24 | 18 | 22.8 | 17 | 21.3 | 16 | 21.1 | 0.959 |
| 36 | 15 | 19.0 | 19 | 23.8 | 16 | 21.1 | 0.763 |
| CO and Cotinine Verified Point Prevalence Abstinence | |||||||
|---|---|---|---|---|---|---|---|
| Treatments |
|||||||
| VLNC cigarette (n=79) | Nicotine Patch (n=80) | Combination (n=76) | |||||
| Week | # abstinent | % | # abstinent | % | # abstinent | % | p-value |
| 12 | 19 | 24.1 | 19 | 23.8 | 18 | 23.7 | 0.998 |
| 24 | 15 | 19.0 | 16 | 20.0 | 13 | 17.1 | 0.896 |
| 36 | 14 | 17.7 | 15 | 18.8 | 15 | 19.7 | 0.950 |
DISCUSSION
The combination of VLNC + NP led to significantly lower rate of smoking assigned cigarettes and hence lower CO levels compared to VLNC cigarettes alone. As expected, both NP and VLNC + NP conditions resulted in higher levels of cotinine and TNE than VLNC alone condition. All treatment conditions showed a significant reduction in biomarkers of exposure compared to baseline. The combination condition also resulted in lower severity of withdrawal when switching from usual brand cigarettes to the assigned products (although only nearly significant different from VLNC alone), with no difference between NP and VLNC only conditions. Cessation from product use led to an increase in craving with no differences across groups and a change in withdrawal symptoms. Although not significantly different across groups, withdrawal symptom severity decreased with patch but increased in the conditions that used VLNC. Most importantly, the amount of usual brand smoking was lowest in the combination condition during the product assignment period. Thus, in general, the combination approach performed significantly better on many outcome variables than the conditions alone. However, exploratory analysis showed that after the product assignment, higher rates of usual brand cigarette abstinence in the combination condition were not sustained and no differences were observed across conditions.
The results from the VLNC cigarettes observed in this study are concordant with findings from a prior study that we conducted in which a VLNC cigarette (Quest 3) was compared with a higher reduced nicotine content cigarette and with the nicotine lozenge (6). The VLNC cigarettes led to reduced rates of smoking and reduced levels of carbon monoxide, cotinine and total NNAL levels compared to baseline, and no greater withdrawal symptoms or differences in treatment outcome compared to nicotine lozenge alone.
Five other studies have examined the use of the NP in combination with the VLNC cigarettes. In a 10-day laboratory study conducted by Donny and Jones (20), subjects (N=68) were randomly assigned one of four conditions: a) placebo patch plus nicotine-containing cigarettes (Quest 1, 0.6 mg nicotine yield, 0/NC); b) placebo patch plus VLNC cigarettes (Quest 3, 0.05 mg nicotine yield, 0/VLNC); c), 7 mg NP plus VLNC cigarettes (7/VLNC), and d) 21 mg NP plus VLNC (21/VLNC). Consistent with our findings, subjects assigned to the 7 or 21/VLNC compared to 0/VLNC showed a greater decrease in the number of VLNC cigarettes smoked and a greater decrease in total volume of VLNC cigarette smoke inhaled. Similarly, there was a trend towards participants in the 21/VLNC to demonstrate a greater decrease in CO relative to baseline and significantly less increase in CO boost after smoking the VLNC cigarette than participants in the 0/VLNC. Finally, greater withdrawal symptom relief was observed in the 7 or 21 /VLNC compared to the 0/VLNC during a required abstinence period when subjects used their assigned products in a laboratory setting.
In another small, pilot treatment study (N=16-17 in each condition), two weeks prior to quit date, smokers were assigned to nicotine or placebo patch in each of three cigarette conditions containing different levels of nicotine (21). Relevant to our study, during 2 weeks before the quitting date, subjects assigned to VLNC cigarettes (0.08 mg nicotine) reported smoking 3 usual brand cigarettes in NP condition as opposed to 46 usual brand cigarettes in the placebo condition. In addition, in the VLNC condition, NP compared to placebo patch treatment was associated with a lower number of total cigarettes smoked per day but no differences were observed in CO levels, or effects on craving or withdrawal symptoms.
Walker and associates in 2012 (3) conducted a large randomized controlled trial to determine the effects of VLNC cigarettes (Quest 3) plus usual Quitline care (nicotine replacement therapy [NRT] and behavioral support) vs. Quitline care alone on smoking abstinence. Smokers randomized to VLNC cigarettes were instructed to use these cigarettes whenever they had an urge to smoke for up to six weeks after their quit date. The results showed that more subjects withdrew in the usual care group compared to the group assigned the VLNC cigarettes (32 vs. 11 at 6 months). Furthermore, the group assigned the VLNC cigarettes had higher 7-day point prevalence abstinence rate at the 6 month follow-up compared to usual care (33% vs. 28%, RR=1.18, 95% CI 1.01, 1.39) and higher continuous abstinence rates (23% vs. 15%, RR=1.50, 95% CI 1.20, 1.87). Median time to relapse in the group assigned VLNC cigarettes was 2 months compared to 2 weeks in the usual care. Thus, unlike the results from the current study, the combination approach appeared to improve long-term cessation rate.
Two other studies were conducted that did not directly examine the effects of adding nicotine patch to VLNC cigarettes, but demonstrated the principle that providing nicotine through the use of nicotine patch but dissociating the direct delivery of nicotine with cigarette smoking through the use of VLNC cigarettes may ease craving (22) or facilitate cessation (23) compared to continued use of higher level nicotine containing cigarettes prior to cessation (24).
The results from our studies and other studies may have implications both for treatment and for a potential national policy measure. For treatment, studies support the notion that targeting both the sensory aspects of smoking and nicotine addiction through the slow delivery nicotine (thereby dissociating smoking with the delivery of nicotine) provides greater withdrawal relief and may minimize use of usual brand cigarettes while on treatment or, based on the findings of other studies, at follow-up.
A national policy measure to reduce the levels of nicotine to non-addicting levels will undoubtedly require access to different pharmacotherapies for tobacco cessation. The availability of the pharmacotherapies may not only reduce the discomfort associated with the reduced nicotine content cigarettes but may lead to substantial reductions in cigarette smoking and possibly to eventual cessation of all products.
There are several limitations to this study. First, no placebo patch was provided so it is unclear as to whether smoking reduction was due to subject concern about smoking and using the patch at the same time. On the other hand, this study represents more naturalistic comparisons of the effects combining both products. Furthermore, Donny and Jones (20) incorporated placebo patch in their study and observed similar results. Second, the short duration on product may have led to insignificant differences in treatment outcome during follow-up. Additionally, for a clinical trial, the sample size was quite small. Third, VLNC cigarettes were switched in the middle of the study because the manufacturer had stopped making the initial cigarettes that were used. Therefore, the level of nicotine was increased. However, prior studies showed that significant reduction in cigarette smoking occur when cigarettes reach less than 0.1 mg nicotine yield (5, 25). Additionally, when analyzing only those who received the Xodus product, the results were similar. Finally, we were unable to verify self-reported abstinence from usual cigarettes during product assignment. However, the patterns of biomarkers of exposures across the groups did not indicate that one group was more likely to report inaccurate data than another group.
In summary, the results from this study suggest that combining nicotine replacements with VLNC cigarettes may improve any acute effects resulting from switching to VLNC compared to VLNC alone and lead to a greater reduction in withdrawal discomfort or use of usual brand cigarette during treatment compared to the nicotine patch alone.
Acknowledgments
Financial support: National Institutes of Health, R01 DA025598 and U54DA031659. Sources of funding for alll authors are NIH and their respective departments.
Footnotes
Conflict of interest: Dorothy Hatsukami had received funding from Nabi Biopharmaceuticals to conduct a trial on the nicotine vaccine. No other conflicts of interests.
References
- 1.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 2.Hatsukami D, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, et al. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19:e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction. 2012;107:1857–67. doi: 10.1111/j.1360-0443.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3rd Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8:309–13. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 8.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- 9.Jacob P, III, Byrd GD. Use of chromatographic and mass spectrometric techniques for the determination of nicotine and its metabolites. In: Gorrod JW, Jacob P III, editors. Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Elsevier; Amsterdam: 1999. pp. 191–224. [Google Scholar]
- 10.Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:907–13. [PubMed] [Google Scholar]
- 11.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–61. [PubMed] [Google Scholar]
- 12.Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H. Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. J Subst Abuse. 1991;3:427–40. doi: 10.1016/s0899-3289(10)80024-0. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991;48:52–9. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 15.Hatsukami D, Anton D, Keenan R, Callies A. Smokeless tobacco abstinence effects and nicotine gum dose. Psychopharmacology (Berl) 1992;106:60–6. doi: 10.1007/BF02253589. [DOI] [PubMed] [Google Scholar]
- 16.Hatsukami D, Huber M, Callies A. Physical dependence on nicotine gum: effect of duration on use. Psychopharmacology. 1993;111:449–56. doi: 10.1007/BF02253535. [DOI] [PubMed] [Google Scholar]
- 17.Hatsukami D, Gust SW, Keenan RM. Physiologic and subjective changes from smokeless tobacco withdrawal. Clin Pharmacol Ther. 1987;41:103–7. doi: 10.1038/clpt.1987.17. [DOI] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104:23–33. doi: 10.1016/j.drugalcdep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- 22.Rezaishiraz H, Hyland A, Mahoney MC, O'Connor RJ, Cummings KM. Treating smokers before the quit date: Can nicotine patches and denicotinized cigarettes reduce cravings? Nicotine Tob Res. 2007;9:1139–46. doi: 10.1080/14622200701684172. [DOI] [PubMed] [Google Scholar]
- 23.Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–48. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- 24.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–85. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz N. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]