Abstract
BACKGROUND & AIMS
Disordered defecation is attributed to pelvic floor dyssynergia. However, clinical observations indicate a spectrum of anorectal dysfunctions. The extent to which these disorders are distinct or overlap is unclear; anorectal manometry might be used in diagnosis, but healthy persons also can have abnormal rectoanal pressure gradients during simulated evacuation. We aimed to characterize phenotypic variation in constipated patients through high-resolution anorectal manometry.
METHODS
We evaluated anorectal pressures, measured with high-resolution anorectal manometry, and rectal balloon expulsion time in 62 healthy women and 295 women with chronic constipation. Phenotypes were characterized by principal components analysis of high-resolution anorectal manometry.
RESULTS
Two healthy persons and 71 patients had prolonged (>180 s) rectal balloon expulsion time. A principal components logistic model discriminated healthy people from patients with prolonged balloon expulsion time with 75% sensitivity and a specificity of 75%. Four phenotypes discriminated healthy people from patients with abnormal balloon expulsion times; 2 phenotypes discriminated healthy people from those with constipation but normal balloon expulsion time. Phenotypes were characterized based on high anal pressure at rest and during evacuation (high anal), low rectal pressure alone (low rectal) or low rectal pressure with impaired anal relaxation during evacuation (hybrid), and a short anal high-pressure zone. Symptoms were not useful for predicting which patients had prolonged balloon expulsion times.
CONCLUSIONS
Principal components analysis of rectoanal pressures identified 3 phenotypes (high anal, low rectal, and hybrid) that can discriminate among patients with normal and abnormal balloon expulsion time. These phenotypes might be useful to classify patients and increase our understanding of the pathogenesis of defecatory disorders.
Keywords: Anorectal Test, Dyssynergic Defecation, Anismus, Pelvic Floor Dysfunction
Although symptoms and results of a digital rectal examination may suggest a defecatory disorder (DD), anorectal tests are necessary to confirm the diagnosis.1–3 Diagnostic guidelines recommend anorectal manometry and a rectal balloon expulsion test (BET), which should be supplemented by barium or magnetic resonance defecography when necessary.1,4 Currently, a negative rectoanal pressure gradient (ie, anal pressure greater than rectal pressure) measured by manometry or impaired rectal evacuation during defecography or rectal BET are considered indicative of DD.5,6
However, several questions exist about the use of anorectal manometry to diagnose DD and identify clinical phenotypes. First, the utility of an negative rectoanal pressure gradient (ie, anal pressure is greater than rectal pressure) as a marker of DD is unclear because this gradient among healthy persons and constipated patients with and without a defecatory disorder overlap considerably.6 Although high-resolution manometry (HRM) increasingly is used to evaluate anorectal functions in clinical practice, all 30 asymptomatic women younger than 50 years in one study had greater anal pressures than rectal pressures during simulated evacuation.7 Rectoanal dyssynergia does not predict an abnormal rectal BET in healthy people.8
Second, investigators in one study suggested that anorectal pressures during simulated evacuation are useful for classifying patients with DD into 3 types: dyssynergia (type 1), impaired rectal propulsion with paradoxic contraction (type 2), and increased intrarectal pressure without anal relaxation (type 3).9 Because all participants in that study had symptoms of DD, it is unclear whether these patterns can discriminate among healthy people, constipated patients without DD, and constipated patients with DD. Moreover, because these subtypes were defined a priori through pattern recognition by an expert rather than by formal analysis (eg, factor or principal components [PCs] analysis), it is unclear whether these subtypes are distinct or uncorrelated to each other. Also, this classification is based on the impaired rectoanal gradient during simulated evacuation, but it does not incorporate other features of DD (eg, high anal resting pressure).4 Finally, clinical observations suggest that not all patients with DD can be classified into 1 of these 3 types.
Therefore, the aims of this study were to use anorectal parameters to discriminate among healthy people with normal balloon expulsion, constipated patients with normal balloon expulsion, and persons with abnormal balloon expulsion using a PC analysis that integrated original HRM variables into phenotypes. Similar approaches have been used to identify symptom phenotypes in irritable bowel syndrome,10 colonic motor disturbances in chronic constipation,11 and phenotypes incorporating anorectal manometry and magnetic resonance proctography in DD.4 However, the present study was limited to anorectal manometry, which is used more widely than magnetic resonance proctography to diagnose DD. The aims of this study were as follows: (1) to evaluate the utility of anorectal HRM for diagnosing DD, (2) to assess whether anorectal HRM can identify phenotypes and facilitate the classification of DD, and (3) to evaluate the utility of symptoms for predicting abnormal balloon expulsion.
Materials and Methods
Study Subjects
Between August 2010 and September 2011 there were 670 women referred for anorectal HRM to our institution who completed questionnaires and 655 patients authorized review of their medical records for research (Supplementary Figure 1). Because our objectives were to better understand the utility of anorectal manometry and a rectal balloon expulsion test in women presenting with primary constipation, 360 patients with other conditions were excluded, providing 295 patients who had Rome III symptom criteria for functional constipation or constipation-predominant irritable bowel syndrome for this analysis (Supplementary Figure 1). Although patients who had major anorectal procedures or colonic surgery were excluded, patients who had undergone other abdominal surgeries (eg, right hemi-colectomy, 1 patient; and appendectomy, cholecystectomy, and hysterectomy, 93 patients) were included. Permission to review these studies was obtained from the Mayo Clinic Institutional Review Board. Also, 62 healthy, asymptomatic women were recruited for this study through public advertisement.
All participants had a clinical interview and physical examination. In healthy participants, exclusion criteria were significant cardiovascular, respiratory, neurologic, psychiatric, or endocrine disease, irritable bowel syndrome as assessed by a validated bowel disease questionnaire,12 medications (with the exception of oral contraceptives or thyroid supplementation), and abdominal surgery (other than appendectomy, cholecystectomy, or hysterectomy).
Procedures
Anorectal manometry
Anal pressures were assessed by an HRM catheter (4.2-mm outer diameter; Sierra Scientific Instruments, Los Angeles, CA), which has 10 circumferential sensors: 8 sensors at 6-mm intervals along the anal canal and 2 sensors in the rectal balloon. At each level, 36 circumferentially oriented, pressure-sensing elements detect pressure using proprietary pressure transduction technology (TactArray; Pressure Profile Systems, Inc, Los Angeles, California) over 2.5 mm. Then, these 36 sector pressures are averaged to obtain a single value. The response characteristics of each sensing element are such that they can record pressure transients in excesses of 6000 mm Hg/s and are accurate to within 1 mm Hg of atmospheric pressure for measurements obtained for at least the final 5 minutes of the study, immediately before thermal recalibration. The data-acquisition frequency is 35 Hz for each sensor.
Each study contained, in the following chronologic order, an assessment of anorectal pressures at rest, during squeeze (3 attempts), and in simulated evacuation with an empty rectal balloon. Thereafter, the rectoanal inhibitory reflex and rectal sensation were evaluated simultaneously by progressively distending the rectal balloon until patients reported severe urgency, in 20-mL increments from 0 to 200 mL, and thereafter in 40-mL increments, until a maximum volume of 400 mL. The data were analyzed as described elsewhere.7
Rectal BET
A 4-cm–long latex balloon filled with 50 mL of warm water, tied to 2-mm diameter plastic tubing, was inserted into the rectum, with the study participant lying in the left lateral decubitus position. The participant then was asked to sit on a commode and expel the balloon in privacy. The BET was noted. The balloon was removed if participants could not expel the balloon within 3 minutes.13
Colonic transit
Colonic transit was assessed with established scintigraphic techniques in 114 patients.14 Colonic transit was summarized as the colonic geometric center (GC), which is the weighted average of counts in the different colonic regions. A higher GC reflects faster colonic transit. Based on the 10th percentile value for healthy participants in our laboratory, delayed colonic transit was defined by a GC value for 24 hours of less than 1.5.
Statistical Analysis
Because only 2 healthy women had a prolonged BET, healthy women and patients with a prolonged BET were combined for analysis, providing 3 groups: healthy women with a normal BET (<180 sec) (the reference group), DD with normal BET, and DD or healthy women with a prolonged BET. Univariate associations between variables and subject group status were assessed by the Fisher exact test for bowel symptoms categorized as significant or as not according to Rome II criteria, and by the Kruskal–Wallis rank test for anorectal manometry variables.
The next step was to discriminate among these 3 groups using a logistic regression discriminant analysis of anorectal variables (ie, anal resting and squeeze pressures, length of anal high-pressure zone, anal squeeze duration, and rectal and anal pressures and anal relaxation during simulated evacuation). However, correlations among anorectal parameters (eg, between anal resting and squeeze pressure) can limit the interpretation of this analysis. Hence, a PC analysis was used to transform anorectal variables into uncorrelated factors as described later. In essence, a PC analysis identifies linear combinations of the anorectal variables, which are weighted (loading factor) sums of the variables. Each linear combination is chosen to explain the most between-subject variation subject to the constraint that they are uncorrelated with other combinations. Thus, the first PC score (or PC1) was a weighted linear combination of the 7 HRM variables that accounted for the maximum between-subject variation. The weight (loading) for a specific variable is the coefficient multiplier used for that variable in the given PC score. The second linear combination (PC2) explained the maximum possible remaining variation and was uncorrelated with PC1. The PC analysis included 7 HRM variables, therefore a total of 7 PC scores were computed. Because correlations between anorectal parameters and age and between anorectal parameters and body mass index (BMI) can affect the discriminant analysis, the PC analysis partial out age and BMI (ie, only that portion of anorectal variables that was not explained by age and BMI were incorporated in the PCs used in the discriminant model). The correlations of PC scores with the original HRM variables were computed.15
The initial multiple predictor variable logistic regression model used PC scores, age, and BMI to predict group status, yielding estimates of the odds for DD with normal BET vs the reference group and odds for DD with abnormal BET vs the reference group.
The generalizing of discriminant models is limited by differences in study populations. Hence, this initial model was validated by a bootstrap process that randomly generated 300 samples from this cohort.16 Every sample contained 357 observations, which is the total number of healthy participants and patients in this cohort. Within each sample, some subjects were represented multiple times and others were not represented at all. The logistic discriminant model described earlier was applied to each bootstrap sample. The coefficients were averaged over the 300 samples to obtain a final model that was applied to the 357 unique participants in the original set. The predicted probabilities from this final model were used to assess the sensitivity and specificity of anorectal parameters for discriminating patients with DD and a normal BET from the reference group and, separately, patients with DD and an abnormal BET from the reference group (receiver operating characteristic [ROC] curves).
Finally, the predicted probabilities from initial and final (bootstrap) models were compared with the actual group status for each subject.
Results
Demographic Features and Clinical Characteristics
Age and BMI were comparable in asymptomatic women and patients (Table 1). In addition, 71 patients and 2 asymptomatic participants had a prolonged rectal BET. Sixteen patients had symptoms of constipation-predominant irritable bowel syndrome and 279 patients had symptoms of functional constipation. Sixty-five percent to 80% of patients had each of the following symptoms: hard stools, excessive straining or a sense of anorectal blockage during defecation, and a sensation of incomplete evacuation after defecation. Twenty-six patients also had slow colonic transit.
Table 1.
Demographic Variables, Symptoms, and Colonic Transit of Study Participants
| Rectal BET of healthy participantsa |
Rectal BET of patients with chronic constipationa |
|||
|---|---|---|---|---|
| Normal (n = 60) |
Prolonged (n = 2) |
Normal (n = 224) |
Prolonged (n = 71) |
|
| Demographic variables, mean ± SEM | ||||
| Age, y | 44.1 ± 2.1 | 45 ± 21 | 48 ± 1.2 | 46 ± 1.8 |
| BMI, kg/m2 | 25.6 ± 0.6 | 24.5 ± 3.8 | 25.1 ± 0.4 | 25.7 ± 0.8 |
| Symptomsb | ||||
| Reduced stool frequency | 0 | 0 | 97 (43) | 35 (49) |
| Hard stools | 6 (10) | 0 | 171 (76) | 58 (82) |
| Excessive straining | 0 | 0 | 166 (74) | 48 (68) |
| Sense of incomplete evacuation | 0 | 0 | 152 (68) | 48 (68) |
| Anal digitation | 0 | 0 | 98 (44) | 40 (56)c |
| Anorectal blockage | 0 | 0 | 166 (74) | 57 (80) |
| Colonic transit (GC24)d Delayed/assessed, % | NA | NA | 18/85 (21) | 8/29 (28) |
| Colonic transit (GC24)d | 2.0 ± 0.1 | 1.8 ± 0.1 | ||
NA, not applicable; SEM, standard error of the mean; GC24, geometric center for colonic transit at 24 hours.
Values are presented as the number (percentage) of patients unless specified otherwise.
Frequent symptoms (≥25% of the time).
P = .048 (the Fisher exact test) vs constipation with normal BET.
Colonic transit was assessed by scintigraphy in 114 patients.
Anorectal Pressures and Rectal Sensation
Anal squeeze pressures were lower (P < .001) in constipated patients regardless of the BET (Table 2). During simulated evacuation, anal pressures were higher than rectal pressures (ie, the rectoanal gradient was negative) even in healthy participants. Indeed, only 11 participants had a positive gradient during evacuation. However, the gradient was more negative (P < .001) in healthy participants (mean [standard error of the mean], −25 [5] vs −82 [27] mm Hg) and patients (mean [standard error of the mean], −39 [2] vs −61 [4] mm Hg) with prolonged BET than those with normal BET. The volume threshold for the desire to defecate also was higher (P < .02) in healthy participants and patients with prolonged BET.
Table 2.
Comparison of Anorectal Sensorimotor Functions in Healthy Controls and in Chronic Constipation
| Rectal balloon expulsion |
||||
|---|---|---|---|---|
| Healthy controls (N = 62) |
Patients with chronic constipation (N = 295) |
|||
| Valuesa | Normal (n = 60) |
Abnormal (n = 2) |
Normal (n = 224) |
Abnormal (n = 71) |
| Anorectal pressures, mm Hg | ||||
| Anal pressure at restb | 79 (3) | 76 (27) | 72 (2) | 83 (2) |
| Anal pressure at squeezec | 167 (7) | 187 (8) | 140 (4) | 145 (7) |
| Anal squeeze duration, s | 12 (1) | 14 (9) | 13 (0.4) | 14 (1) |
| Rectal pressure: simulated evacuation | 26 (3) | 21 (7) | 21 (1) | 16 (2) |
| Anal pressure: simulated evacuationc | 53 (4) | 104 (22) | 60 (2) | 78 (3) |
| Rectoanal gradient: simulated evacuationc | −25 (5) | −82 (27) | −39 (2) | −61 (4) |
| Anal relaxation: simulated evacuation, % | 31 (6) | −44 (21) | 14 (2) | 4 (3) |
| Balloon expulsion time, median (IQR), s | 8 (5–14) | 180 (180–180) | 11 (7–22) | 180 (180–180) |
| Rectal sensory thresholds, mL | ||||
| First sensation | 33 (1) | 30 (10) | 33 (2) | 35 (2) |
| Desire to defecated | 58 (3) | 60 (0) | 58 (2) | 77 (7) |
| Urgency | 91 (4) | 105 (15) | 87 (3) | 117 (9) |
IQR, interquartile range.
Values are presented as mean (standard error of the mean) unless specified otherwise.
P = .004.
P < .001.
P = .016, Kruskal–Wallis test.
Relative to the 10th–90th percentile values in healthy participants, 36 patients (12%) had low and 12 patients (4%) had high anal resting pressures. Similarly, the anal high-pressure zone was short (60 patients [20%]) or long (23 patients [8%]). Anal squeeze pressures were low (104 patients [35%]) or high (32 patients [11%]), and the squeeze duration was reduced in 18 patients (6%). During simulated evacuation, 45 patients (15%) had low rectal pressures, 13 patients (4%) had diminished anal relaxation, and 23 patients (8%) had high anal pressures. Among patients with an abnormal balloon expulsion test, 4 patients (6%) had high anal resting pressure, 14 patients (20%) had low rectal pressures, 4 patients (6%) had high anal pressures, and 2 patients (3%) had diminished anal relaxation during simulated evacuation. In total, 65 (29%) patients with normal BET and 28 (39%) patients with prolonged BET had either high anal resting pressure or had high anal or low rectal pressure during evacuation.
Can Anorectal Parameters Identify Clusters in Patients?
The PC analysis generated 7 composite scores (or PCs) that explained 27%, 20%, 19%, 14%, 13%, 6%, and 1%, respectively (a sum of 100%), of the total between participant variation in the 7 response variables. The loading (or weighting) factors used to derive these PCs from anorectal parameters are shown in Supplementary Table 1.
PC1, PC3, and PC5 were correlated with BET (Table 3). Correlation coefficients between the PCs and anorectal variables are useful for identifying the latent dimensions represented by these PCs (Table 3). PC1, PC3, and PC5 were correlated with rectal and anal pressures during evacuation. Specifically, greater values of PC1 were associated with higher anal pressures (overall: r = 0.88; P < .001) during evacuation (Figure 1). A high PC1 score also was correlated with high anal resting pressure (r = 0.53; P < .001). Lower PC5 scores were associated (r = 0.73; P < .001) with lower rectal pressures during evacuation. Lower PC3 scores were associated with lower rectal pressures (r = 0.46; P < .001) and less anal relaxation during (r = 0.43; P < .001) evacuation and also with lower anal squeeze pressures and squeeze duration. Finally, lower scores of PC4 were associated most strongly with a shorter high-pressure zone.
Table 3.
Correlation Coefficients for Principal Component (PC) Scores vs Anorectal Variables and Symptoms
| Correlation coefficientsa |
|||||||
|---|---|---|---|---|---|---|---|
| *Variable | PC1 (high-anal) | PC2 | PC3 (hybrid) | PC4 | PC5 (low-rectal) | PC6 | PC7 |
| Anal resting pressureb | 0.53 c | 0.63 c | 0.15d | −0.05 | −0.15d | −0.22c | −0.09 |
| Length of HPZb | 0.17d | 0.07 | −0.25c | 0.89 c | 0.27c | −0.08 | −0.001 |
| Anal (squeeze – resting) pressureb | 0.29c | −0.33c | 0.59 c | 0.26c | −0.08 | 0.53c | 0.04 |
| Anal squeeze durationb | 0.17d | 0.57 c | −0.57 c | −0.21c | 0.36c | −0.36c | −0.0005 |
| Simulated evacuation: anal relaxationb |
−0.50 c | 0.71 c | 0.43 c | 0.08 | 0.006 | −0.05 | 0.12c |
| Simulated evacuation: rectal pressureb |
0.27c | −0.17d | 0.46 c | −0.2c | 0.73 c | −0.12e | 0.003 |
| Simulated evacuation: anal pressureb |
0.88 c | −0.09 | −0.24c | −0.1e | −0.14d | −0.12e | 0.13d |
| Balloon expulsion timef | 0.28c | 0.49 | −0.20c | 0.04 | −0.25c | −0.05 | 0.07 |
| Stool frequencyf,g | −0.01 | −0.01 | −0.04 | 0.01 | 0.04 | −0.23e | −0.03 |
| Hard stoolsf,g | −0.04 | −0.008 | −0.08 | 0.07 | 0.04 | 0.05 | 0.02 |
| Straining during defecationf,g | 0.72 | 0.02 | 0.82 | 0.19 | 0.73 | 0.68 | 0.79 |
| Sense of incomplete evacuationf,g | −0.04 | −0.01 | −0.009 | −0.04 | 0.027 | −0.07 | 0.01 |
| Anorectal blockage during defecationf,g |
−0.02 | −0.12 | −0.07 | −0.01 | −0.13c | 0.03 | 0.02 |
| Anal digitationf,g | 0.03 | 0.04 | −0.03 | −0.03 | −0.005 | 0.2e | −0.01 |
HPZ, high-pressure zone.
Correlation coefficients with an absolute value of 0.4 or greater, which indicates that the variable is correlated substantially with the factor, are shown in bold.
Pearson correlation coefficients.
P < .001.
P < .01.
P < .05.
Spearman correlation coefficient.
Patients only.
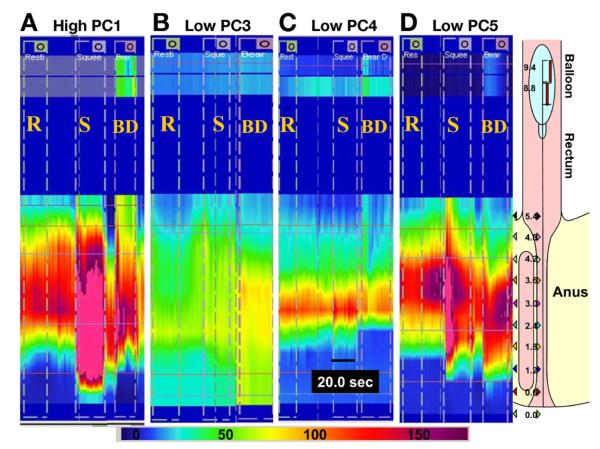
Figure 1.
Representative high-resolution manometry tracings in constipated patients with (A) high PC1, (B) low PC3, (C) low PC4, or (D) low PC5 scores. (A) Higher PC1 scores were associated with 3 higher anal resting and squeeze pressures and higher anal pressure during simulated evacuation (dyssynergia). Rectal balloon pressure during simulated evacuation increased with (A) but not with (B) low PC3 score or (D) low PC5 score. Relative to rest, anal pressure increased during simulated evacuation in the patient with a (B) low PC3 score but not in the patient with a (D) low PC5 score. (C) A short high-pressure zone is the distinguishing feature in patients with low PC4 scores. The profile on the far right shows the location of the sensors in the anorectum for the example in panel D. BD, bear down (simulated evacuation); R, rest; and S, squeeze. Color scale shows pressure in mmHg
Because the PC scores are continuous variables, the 10th–90th percentile values in healthy participants were used to identify the proportion of patients with a prolonged BET who had abnormal PC scores. Thirty-five of 71 (49%) patients had one or more abnormal PC scores, that is, high PC1 (6%), low PC3 (13%), low PC4 (11%) or low PC5 (4%), and 15% had abnormal values for 2 or more PC scores.
Initial and Bootstrap Discrimination Models
In the initial model and the final bootstrap model, high values for PC3 and PC4 were associated individually with decreased odds for constipation with normal BET and, separately, with decreased odds of prolonged BET compared with the reference group (Table 4). While PC1 was associated with increased odds for a prolonged BET, PC5 was associated with decreased odds for prolonged BET. Neither PC1 nor PC5 were associated with constipation and normal BET.
Table 4.
Odds Ratios for PCs to Discriminate Healthy Control Subjects With Normal BET (Reference Group) From Patients With Normal BET and, Separately, Versus Control Subjects or Patients With Prolonged BET (Estimates From Final Bootstrap Model)
| Principal component | Patients with normal BET vs reference group, OR (95% CI) |
Control subjects or patients with abnormal BET vs reference group, OR (95% CI) |
|---|---|---|
| PC1 | 1.09 (0.85–1.41) | 1.85 (1.35–2.54) |
| PC2 | 0.77 (0.50–1.17) | 0.77 (0.47–1.28) |
| PC3 | 0.54 (0.38–0.78) | 0.31 (0.20–0.49) |
| PC4 | 0.64 (0.48–0.86) | 0.53 (0.35–0.81) |
| PC5 | 0.79 (0.52–1.19) | 0.43 (0.27–0.70) |
| PC6 | 1.14 (0.63–2.08) | 0.74 (0.36–1.53) |
| PC7 | 1.23 (0.13–11.6) | 3.57 (0.22–58.1) |
CI, confidence interval; OR, odds ratio.
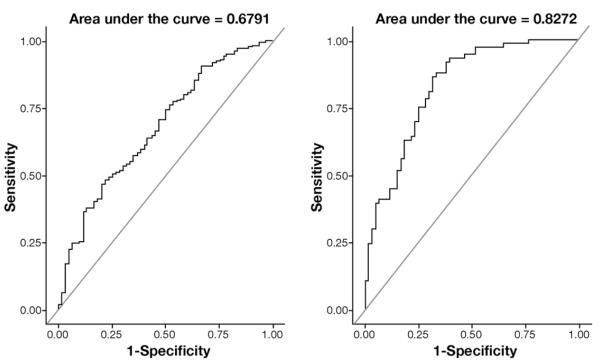
In the final bootstrap model, age, BMI, and anorectal variables yielded an area under the ROC curve of 0.68 for discriminating between constipated patients with normal BET and the reference group and an area of 0.83 under the ROC curve for discriminating the reference group from constipated patients with prolonged BET (Figure 2). At a specificity of 75%, anorectal manometry parameters were 50% sensitive for discriminating between healthy persons and patients with normal BET and 75% sensitive for discriminating between healthy persons with normal BET from control subjects and patients with prolonged BET.
Figure 2.
ROC curves showing the utility of anal manometric features for discriminating between: (1) control subjects and patients with normal balloon expulsion time (left panel) and (2) control subjects with normal balloon expulsion and controls or patients with prolonged balloon expulsion time.
Supplementary Figure 2 shows the relation between the actual probabilities (observed proportions) and the (mean) predicted probabilities for the initial logistic model and the final bootstrap model. The initial and final (bootstrap) models are close to the line of equality for discriminating the reference group from control subjects or patients with abnormal BET (Supplementary Figure 2, right panel). In contrast, the initial and final (bootstrap) models for discriminating control subjects with normal BET from patients with normal BET deviate a bit more from the line of equality. This deviation implies that the model is less reliable for discriminating control subjects vs patients with normal BET, which also is evident from the corresponding ROC curve (Figure 2).
Relation Between Anorectal Variables and Symptoms
Among constipated patients, anal digitation (P = .048) was more common in patients with prolonged BET than without it (Table 1). However, the sense of anal blockage during defecation was the only bowel symptom that was correlated (r =−0.16; P = .009), albeit weakly, with an anorectal manometry parameter (ie, with rectal pressure during simulated evacuation). Likewise, correlations between PCs and bowel symptoms were very weak (Table 3); PC5 was the only PC associated with a symptom (ie, anorectal blockage during defecation).
Discussion
Anorectal manometry and a rectal BET are recommended for diagnosing DD.1 An abnormal rectal BET predicts which patients are likely to benefit from pelvic floor biofeedback therapy.17 It is intuitive that defecation may be impaired because of weak propulsive forces, increased resistance to evacuation, or both. However, it is not clear whether these disturbances reflect similar or different pathophysiological mechanisms. The present PC analysis did not identify a single factor underlying the correlation structure in these variables. Rather, it identified 3 PCs (high-anal, low-rectal, and high anal–low rectal [or hybrid], PCs), of which each is interpretable as representative of a proposed component of patients with DD and prolonged BET. This interpretation suggests that the 7 HRM variables are best described as representing these 3 independent physiological domains.
Anorectal Phenotypes Identified by PC Analysis
High PC1 scores represent a phenotype, which we refer to as the high-anal phenotype, characterized by high anal pressures at rest and during evacuation, which is similar to dyssynergia or the type 1 pattern described by Rao et al.9 Low PC3 scores were associated with inadequate rectal propulsive forces and less anal relaxation during evacuation (ie, a hybrid phenotype). This pattern resembles the type 2 pattern described by Rao et al.9 Low PC5 scores were associated with inadequate rectal propulsive forces during evacuation (ie, low-rectal phenotype). Low PC4 scores identified a phenotype characterized by a short high-pressure zone. None of the PCs resembled the type 3 pattern of Rao et al9 (ie, high rectal pressures without anal relaxation). PC3 was associated not only with constipated patients who had a prolonged BET but also with those who had a normal BET. Indeed, an association between irritable bowel syndrome and pelvic floor dysfunction is recognized.18,19 Moreover, impaired anal relaxation, which is a feature of the low-PC3 phenotype, was also the most common anorectal disturbance in another study of irritable bowel syndrome, affecting 91% of patients.18
Because these PCs were, by design, uncorrelated to each other, they reflect distinct underlying pathophysiological mechanisms in patients with DD. Perhaps the high-anal phenotype is attributable to excessive straining during defecation against a background of high anal resting pressure. Indeed, excessive straining is the most frequently implicated mechanism for DD.20 It is more challenging to interpret the mechanism for reduced rectal propulsive forces in the low-rectal and hybrid phenotypes because the contribution of visceral (ie, rectal contraction) and somatic (ie, increased intra-abdominal pressure) mechanisms to increased rectal pressure during normal defecation are not understood. To speculate, the presence of rectal and anal disturbances in the hybrid phenotype may suggest that reduced rectal forces are caused by a somatic disturbance, such as inappropriate abdominal contraction. In contrast, low rectal pressures with preserved anal relaxation in PC5 may reflect a visceral disturbance (rectal sensorimotor dysfunction). Although plausible and rational, these mechanisms need to be confirmed by studies evaluating the impact of modulating these PCs on symptoms of DD. The significance of the phenotype represented by low PC4 scores is unclear. Moreover, a short anal high-pressure zone (PC4) would not be anticipated to obstruct defecation. Conceivably, the shortened anal high-pressure zone phenotype may reflect anal structural damage caused by obstetric injury, excessive straining, or other risk factors.
Utility of PCs for Discriminating Between Healthy Participants and Patients
In regression models based on PCs, age, and BMI, there were 4 PCs (PC1, PC3, PC4, and PC5) associated with a prolonged rectal BET; PC3 and PC4 also were associated with constipated patients who had a normal BET. At comparable specificity (75%), anorectal variables were 75% sensitive for distinguishing between healthy participants with a normal BET and controls or patients with a prolonged BET, but were only 50% sensitive for distinguishing between healthy participants and patients with a normal BET. The utility of PCs for discriminating between healthy participants and constipated patients with a normal BET or subjects with a prolonged BET was similar when 1 instead of 3 minutes was used as the normal value for BET (data not shown). These observations underscore the limited utility of anorectal manometry for discriminating between healthy subjects and constipated patients with an abnormal BET, perhaps in part because there is considerable overlap in anorectal pressures between healthy participants and constipated patients with a normal or prolonged BET. Because the generalization of PC analysis is limited by differences in study populations, a bootstrap process was used to assess reproducibility in different samples. The original (1 sample of 357 study subjects) and bootstrap models (300 samples, with 357 study subjects each) were comparable for discriminating between controls with a normal BET and controls or patients with an abnormal BET, suggesting that this model is reliable. In comparison, the model for distinguishing between control subjects and patients with a normal BET was less reliable.
Clinical Utility of PCs
The clinical utility of these PCs is unclear pending future studies to evaluate whether they predict the response to pelvic floor retraining by biofeedback therapy. Approximately 50% of constipated patients with an abnormal BET had abnormal values for 1 or more of 4 PCs that discriminated between healthy participants and constipated patients with an abnormal BET. There are several possible explanations as to why the remaining 50% had normal PC scores. The PC analysis only incorporated a finite number of manometric variables, which are of presumed importance and are available in the commercial program. Manometry is performed in the left lateral position whereas rectal balloon expulsion is evaluated in the seated position. Some subjects may find it challenging to replicate the process of defecation during manometry.
Comparison of PC Analysis With Alterative Approaches to Identify Phenotypes
Four differences were detected with use of the PC analysis to classify DD in the present study and the approach used by Rao et al.9 First, phenotypes were identified by an objective, data-driven approach (PC analysis) rather than the expert-based, pattern-recognition process in the study by Rao at al.9 Second, although the phenotypes identified through the PC analysis and the Rao et al9 classification are based primarily on anorectal pressures during simulated evacuation, the PC analysis also included anal resting and squeeze pressures. Third, in contrast to the Rao et al9 study, this study also includedhealthy persons, which is useful because anorectal pressures during simulated evacuation overlap between asymptomatic patients with normal balloon expulsion and patients with abnormal balloon expulsion.6 Finally, this study used HRM whereas the Rao et al9 study used conventional solid-state pressure sensors. Other than requiring less time, because HRM pressure sensors straddle the entire anal canal obviating the need for a station pull-through maneuver, there are no documented advantages of HRM over conventional manometry.
Multiple-variable predictor models are an alternative approach to understanding the relation between various predictors such as anorectal manometry variables and a dependent variable such as BET. However, the results of multiple-variable predictor models are influenced by correlations among predictor variables (eg, between anal resting pressure and anal squeeze pressure), which adversely can affect forward or backward elimination variable selection methods. The PC analysis mitigates these effects by transforming variables to uncorrelated dimensions, which may represent independent physiological domains.
Utility of Symptoms for Identifying DD
Previous studies have suggested that symptoms have limited utility in discriminating between DD and other causes of chronic constipation. Yet, some of these studies included only patients with symptoms of difficult defecation.9 Symptoms were assessed retrospectively21 or not22 using Rome criteria. In general, our findings confirmed these observations. Although anal digitation was significantly more common in patients with prolonged than normal BET, 44% of patients with normal BET reported anal digitation. Because symptoms were correlated weakly with individual anorectal manometry parameters, it is not surprising that they also were correlated weakly with abnormal PC scores. Because defecatory symptoms vary over time, partly because of variations in stool consistency, bowel diaries may provide a more refined characterization of defecatory symptoms.23 However, symptoms evaluated through bowel diaries also were not useful for distinguishing between patients with normal and prolonged BET.5
Limitations
In addition to the limitations stated earlier, colonic transit was evaluated in less than 50% of patients, and the contribution of colonic transit to these phenotypes could not be assessed. Consistent with previous studies, some patients with DD had slow colonic transit,11 reinforcing the need to evaluate anorectal functions before colonic transit in chronic constipation.24 Although DD has been associated with rectal hyposensitivity,25 sensory thresholds were not incorporated in the PC analysis because 18 subjects who did not report one or more rectal sensations would have to be excluded completely from the PC analysis. Second, thresholds for first sensation and desire to defecate or for the desire to defecate and urgency were identical in many subjects. Third, values for thresholds were rather discrete and hence less amenable for inclusion in a principal components analysis. Fourth, if the sensory thresholds were included in the PCs, the relationship between PCs defined by HRM variables and sensory thresholds could not be examined. In addition, other factors including prior pelvic surgery may contribute to disordered defecation. Of 295 patients, 93 (32%) reported a hysterectomy. Although several cross-sectional and uncontrolled prospective studies suggested that bowel symptoms are more common after a hysterectomy, a prospective controlled study observed no increase in bowel symptoms after a hysterectomy,26 and a prospective uncontrolled study identified relatively minor effects on anorectal sensorimotor functions after a hysterectomy.27
In summary, a PC analysis of various anorectal variables uncovered phenotypic heterogeneity in DD. Some phenotypes were associated only with abnormal balloon expulsion (PC1 and PC5) whereas others also were associated with constipation and normal BET (PC3 and PC4). The 3 phenotypes that discriminated between a patient with normal BET and one with prolonged BET were characterized predominantly by dyssynergia (PC1 or high-anal phenotype) and low rectal propulsive pressure without (PC5 or low-rectal phenotype) or with impaired anal relaxation during evacuation (PC3 or hybrid phenotype). Symptoms assessed by questionnaire were not particularly useful for discriminating between constipated patients with normal BET and those with prolonged BET. Future studies should confirm these phenotypes, integrate them with assessments of colonic transit, and determine whether they affect the response to pelvic floor retraining with biofeedback therapy.
Supplementary Material
Supplementary Table 1. Variable Loading Factors for PC Scores
Supplementary Figure 1. CONSORT diagram. IBS, irritable bowel syndrome.
Supplementary Figure 2. Calibration plot for predicting which patients have a normal balloon expulsion test (left panel) and which control subjects (Controls) and patients have an abnormal balloon expulsion test (right panel). The x-axis corresponds to the predicted proportion of patients by decile of predicted probability, and the y-axis corresponds to the actual observed proportion of patients in each decile. The black dotted line represents the line of equality (ie, the predicted and actual proportions of patients in each decile are identical). The red and blue lines represent the initial model and final bootstrap models, respectively.
Acknowledgments
Funding Supported in part by United States Public Health Service National Institutes of Health grant R01 DK78924.
Abbreviations used in this paper
- BET
balloon expulsion test
- BMI
body mass index
- DD
defecatory disorder
- GC
geometric center
- HRM
high-resolution manometry
- PC
principal component
- ROC
receiver operating characteristic
Footnotes
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2012.10.049.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Bharucha AE, Wald A, Enck P, et al. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Wald AM. Anorectal disorders. Am J Gastroenterol. 2010;105:786–794. doi: 10.1038/ajg.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 7.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SSC, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006;101:2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 9.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead WE, Drossman DA. Validation of symptom-based diagnostic criteria for irritable bowel syndrome: a critical review. Am J Gastroenterol. 2010;105:814–820. doi: 10.1038/ajg.2010.56. quiz, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2009;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharucha AE, Locke GR, Seide B, et al. A new questionnaire for constipation and fecal incontinence. Aliment Pharmacol Ther. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao SS, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 14.Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 15.Morrison DF. The structure of multivariate observations: I. Principal components. In: Blackwell D, Solomon H, editors. Multivariate statistical methods. 2nd ed McGraw-Hill Book Company; New York: 1976. pp. 266–301. [Google Scholar]
- 16.Barrett TW, Martin AR, Storrow AB, et al. A clinical prediction model to estimate risk for 30-day adverse events in emergency department patients with symptomatic atrial fibrillation. Ann Emerg Med. 2011;57:1–12. doi: 10.1016/j.annemergmed.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Prott G, Shim L, Hansen R, et al. Relationships between pelvic floor symptoms and function in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:764–769. doi: 10.1111/j.1365-2982.2010.01503.x. [DOI] [PubMed] [Google Scholar]
- 19.Suttor VP, Prott GM, Hansen RD, et al. Evidence for pelvic floor dyssynergia in patients with irritable bowel syndrome. Dis Colon Rectum. 2010;53:156–160. doi: 10.1007/DCR.0b013e3181c188e8. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead WE, Bharucha AE. Diagnosis and treatment of pelvic floor disorders: what’s new and what to do. Gastroenterology. 2010;138:1231–1235. doi: 10.1053/j.gastro.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotz RL, Pemberton JH, Talley NJ, et al. Discriminant value of psychological distress, symptom profiles, and segmental colonic dysfunction in outpatients with severe idiopathic constipation. Gut. 1994;35:798–802. doi: 10.1136/gut.35.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glia A, Lindberg G, Nilsson LH, et al. Clinical value of symptom assessment in patients with constipation. Dis Colon Rectum. 1999;42:1401–1408. doi: 10.1007/BF02235036. discussion, 1408–1410. [DOI] [PubMed] [Google Scholar]
- 23.Bharucha AE, Seide BM, Zinsmeister AR, et al. Insights into normal and disordered bowel habits from bowel diaries. Am J Gastroenterol. 2008;103:692–698. doi: 10.1111/j.1572-0241.2007.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharucha AE, Locke GR, Pemberton JH. AGA Practice Guideline on Constipation: technical review. Gastroenterology. 2012 In press. [Google Scholar]
- 25.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 26.Sperber AD, Morris CB, Greemberg L, et al. Constipation does not develop following elective hysterectomy: a prospective, controlled study. Neurogastroenterol Motil. 2009;21:18–22. doi: 10.1111/j.1365-2982.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Klingele CJ, Seide BM, et al. Effects of vaginal hysterectomy on anorectal sensorimotor functions–a prospective study. Neurogastroenterol Motil. 2012;24:235–241. doi: 10.1111/j.1365-2982.2011.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Variable Loading Factors for PC Scores
Supplementary Figure 1. CONSORT diagram. IBS, irritable bowel syndrome.
Supplementary Figure 2. Calibration plot for predicting which patients have a normal balloon expulsion test (left panel) and which control subjects (Controls) and patients have an abnormal balloon expulsion test (right panel). The x-axis corresponds to the predicted proportion of patients by decile of predicted probability, and the y-axis corresponds to the actual observed proportion of patients in each decile. The black dotted line represents the line of equality (ie, the predicted and actual proportions of patients in each decile are identical). The red and blue lines represent the initial model and final bootstrap models, respectively.