Abstract
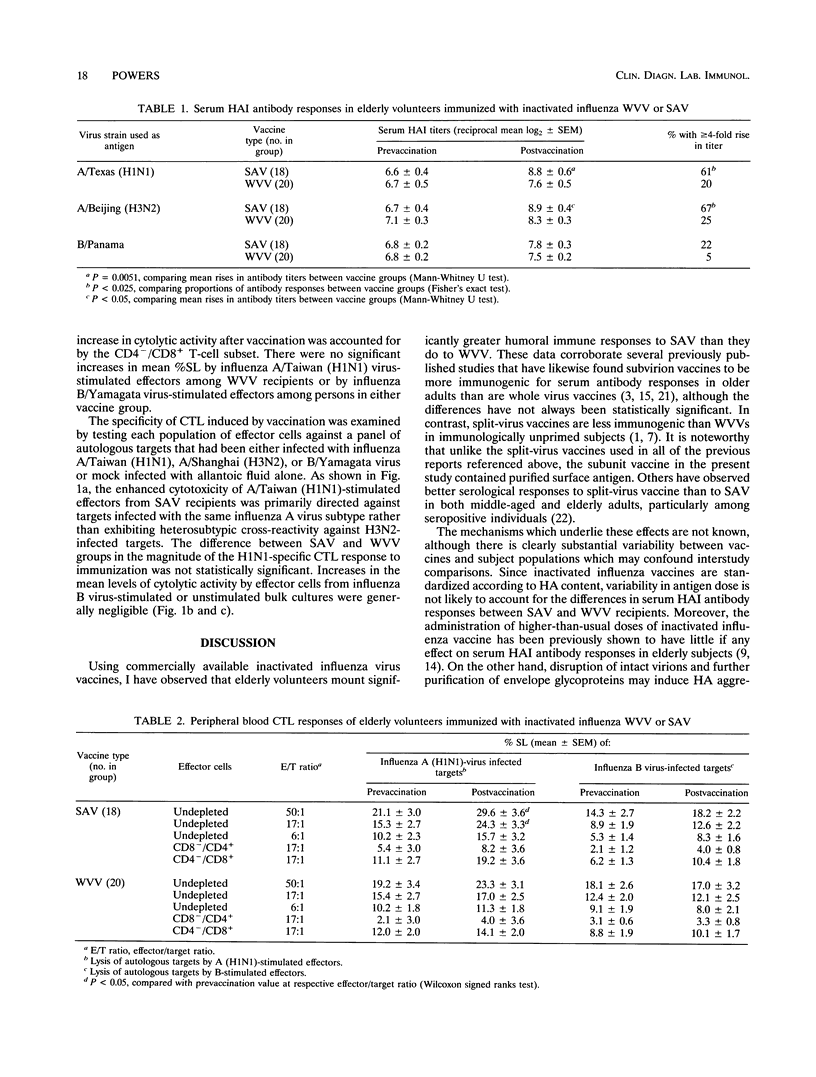
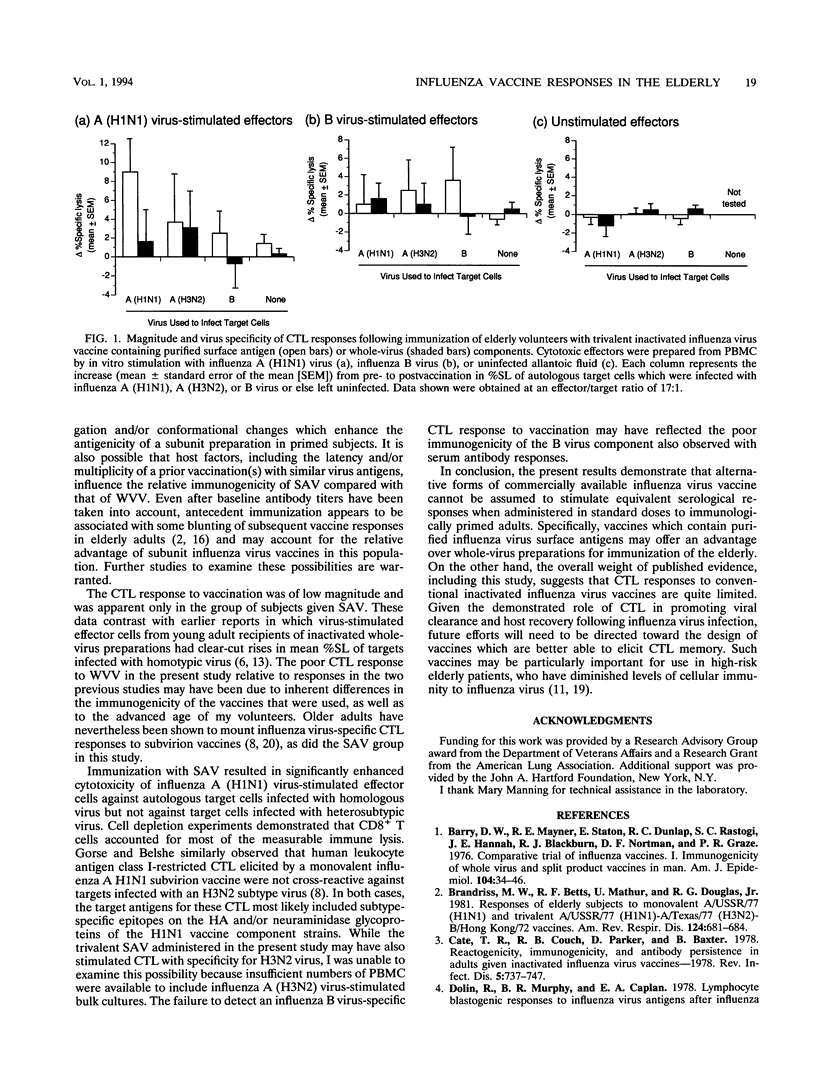
Thirty-eight elderly female subjects (aged 80 +/- 7 years, mean +/- standard deviation) were randomized to immunization with trivalent inactivated influenza virus vaccine containing either purified surface antigen (n = 18) or whole virus (n = 20) components from A/Texas/36/91 (H1N1), A/Beijing/353/89 (H3N2), and B/Panama/45/90 strains. Humoral and cellular immune responses were assessed by measuring serum hemagglutination inhibition antibodies and cytotoxic T lymphocyte (CTL) activity at 0 and 3 weeks postvaccination. Serological responses to both of the type A vaccine strains following immunization with surface antigen vaccine (SAV) were significantly more frequent and greater in magnitude than those induced by whole-virus vaccine. Antibody responses to the B/Panama component were modest and did not differ significantly between the two vaccines. Persons given SAV, but not those given whole-virus vaccine, had a small ¿ but significant increase in mean percent specific lysis of influenza A (H1N1) virus-infected autologous targets by peripheral blood mononuclear cells which were stimulated in vitro with influenza A (H1N1) virus. The H1N1-stimulated cytotoxic effectors induced by SAV were CD8+ and were not cross-reactive against H3N2-infected targets. Influenza B virus-specific CTL responses were not observed with either vaccine. These results suggest that currently available subunit influenza virus vaccines may offer an advantage over inactivated whole-virus preparations for inducing humoral and cellular immune responses in the elderly, although the CTL response may be too limited to be of physiological significance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry D. W., Mayner R. E., Staton E., Dunlap R. C., Rastogi S. C., Hannah J. E., Blackburn R. J., Nortman D. F., Graze P. R. Comparative trial of influenza vaccines. I. Immunogenicity of whole virus and split product vaccines in man. Am J Epidemiol. 1976 Jul;104(1):34–46. doi: 10.1093/oxfordjournals.aje.a112272. [DOI] [PubMed] [Google Scholar]
- Brandriss M. W., Betts R. F., Mathur U., Douglas R. G., Jr Responses of elderly subjects to monovalent A/USSR/77 (H1N1) and Trivalent A/USSR/77 (H1N1)-A/TEXAS/77 (H3N2)-B/Hong Kong/72 vaccines. Am Rev Respir Dis. 1981 Dec;124(6):681–684. doi: 10.1164/arrd.1981.124.6.681. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Couch R. B., Parker D., Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines - 1978. Rev Infect Dis. 1983 Jul-Aug;5(4):737–747. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Rook A. H., Qi Y. H., Schild G. C., Riley D., Pratt R., Potter C. W. HLA restricted virus-specific cytotoxic T-lymphocyte responses to live and inactivated influenza vaccines. Lancet. 1981 Oct 24;2(8252):887–891. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- Frank A. L., Webster R. G., Glezen W. P., Cate T. R. Immunogenicity of influenza A/USSR (H1N1) subunit vaccine in unprimed young adults. J Med Virol. 1981;7(2):135–142. doi: 10.1002/jmv.1890070207. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B. Enhancement of anti-influenza A virus cytotoxicity following influenza A virus vaccination in older, chronically ill adults. J Clin Microbiol. 1990 Nov;28(11):2539–2550. doi: 10.1128/jcm.28.11.2539-2550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Quinnan G. V., Jr, Weksler M. E., Gaerlan P. F., Denning C. R. Immunization of elderly people with high doses of influenza vaccine. J Am Geriatr Soc. 1988 Mar;36(3):209–212. doi: 10.1111/j.1532-5415.1988.tb01802.x. [DOI] [PubMed] [Google Scholar]
- McElhaney J. E., Beattie B. L., Devine R., Grynoch R., Toth E. L., Bleackley R. C. Age-related decline in interleukin 2 production in response to influenza vaccine. J Am Geriatr Soc. 1990 Jun;38(6):652–658. doi: 10.1111/j.1532-5415.1990.tb01424.x. [DOI] [PubMed] [Google Scholar]
- McElhaney J. E., Meneilly G. S., Beattie B. L., Helgason C. D., Lee S. F., Devine R. D., Bleackley R. C. The effect of influenza vaccination on IL2 production in healthy elderly: implications for current vaccination practices. J Gerontol. 1992 Jan;47(1):M3–M8. doi: 10.1093/geronj/47.1.m3. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F., Cullen P., Askonas B., Webster R. G. The human cytotoxic T cell response to influenza A vaccination. Clin Exp Immunol. 1981 Feb;43(2):276–284. [PMC free article] [PubMed] [Google Scholar]
- Palache A. M., Beyer W. E., Sprenger M. J., Masurel N., de Jonge S., Vardy A., Charpentier B., Noury J., van Beek W. C., Borst R. J. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11(1):3–9. doi: 10.1016/0264-410x(93)90333-s. [DOI] [PubMed] [Google Scholar]
- Parkman P. D., Hopps H. E., Rastogi S. C., Meyer H. M., Jr Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis. 1977 Dec;136 (Suppl):S722–S730. doi: 10.1093/infdis/136.supplement_3.s722. [DOI] [PubMed] [Google Scholar]
- Peters N. L., Meiklejohn G., Jahnigen D. W. Antibody response of an elderly population to a supplemental dose of influenza B vaccine. J Am Geriatr Soc. 1988 Jul;36(7):593–599. doi: 10.1111/j.1532-5415.1988.tb06152.x. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S. Determinants of immunity to influenza infection in man. Br Med Bull. 1979 Jan;35(1):69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- Powers D. C., Belshe R. B. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993 Mar;167(3):584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- Powers D. C. Immunological principles and emerging strategies of vaccination for the elderly. J Am Geriatr Soc. 1992 Jan;40(1):81–94. doi: 10.1111/j.1532-5415.1992.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Powers D. C. Influenza A virus-specific cytotoxic T lymphocyte activity declines with advancing age. J Am Geriatr Soc. 1993 Jan;41(1):1–5. doi: 10.1111/j.1532-5415.1993.tb05938.x. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Schooley R., Dolin R., Ennis F. A., Gross P., Gwaltney J. M. Serologic responses and systemic reactions in adults after vaccination with monovalent A/USSR/77 and trivalent A/USSR/77, A/Texas/77, B/Hong Kong/72 influenza vaccines. Rev Infect Dis. 1983 Jul-Aug;5(4):748–757. doi: 10.1093/clinids/5.4.748. [DOI] [PubMed] [Google Scholar]
- Zei T., Neri M., Iorio A. M. Immunogenicity of trivalent subunit and split influenza vaccines (1989-90 winter season) in volunteers of different groups of age. Vaccine. 1991 Sep;9(9):613–617. doi: 10.1016/0264-410x(91)90184-8. [DOI] [PubMed] [Google Scholar]



