Abstract
Objective
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, with overall 5-year survival rate of only 3-5%. As the current therapies offer very limited survival benefits, novel therapeutic strategies are urgently required to treat this disease. Here, we determined whether metformin administration inhibits the growth of PANC-1 and MiaPaCa-2 tumor xenografts in vivo.
Methods
Different xenograft models, including orthotopic implantation, were used to determine whether intraperitoneal or oral administration of metformin inhibits the growth of pancreatic cancer in vivo.
Results
We demonstrate that metformin given once daily intraperitoneally at various doses (50-250 mg/kg) to nude mice inhibited the growth of PANC-1 xenografts in a dose-dependent manner. A significant effect of metformin was obtained at 50 mg/Kg and maximal effect at 200 mg/Kg. Metformin administration also caused a significant reduction in the phosphorylation of ribosomal S6 protein and ERK in these xenografts. Metformin also inhibited the growth of pancreatic cancer xenografts when administered orally (2.5 mg/ml) either prior or after tumor implantation. Importantly, oral administration of metformin also inhibited the growth of MiaPaCa-2 tumors xenografted orthotopically.
Conclusion
The studies presented here provide further evidence indicating that metformin offers a potential novel approach for PDAC prevention and therapy.
Keywords: Pancreatic ductal adenocarcinoma, PANC-1 cells, MiaPaCa-2 cells, Orthotopic tumor xenografts, mTORC1, ERK
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, with overall 5-year survival rate of only 3-5%. The estimated incidence of PDAC in the US has increased to 44,000 new cases in 2011 and is now the fourth leading cause of cancer mortality in both men and women (1). As the current therapies offer very limited survival benefits, novel therapeutic strategies are urgently required to treat this aggressive but under-studied disease.
Many epidemiological studies have linked obesity and long-standing type 2 diabetes mellitus (T2DM) with increased risk and worse clinical outcomes for developing PDAC and other clinically aggressive cancers (2-8). Indeed, a recent analysis of a large, pooled set of studies included in the National Cancer Institute (NCI) Pancreatic Cancer Cohort Consortium (PanScan) has provided strong support for a positive association between obesity and increased risk of PDAC (9) and a consensus report substantiated a link between T2DM and PDAC (10). These metabolic disturbances (obesity, metabolic syndrome and early stages of T2DM), which are reaching alarming rates in the western world (11-14), are characterized by peripheral insulin resistance, compensatory overproduction of insulin by the β cells of the islet and increased bioavailability of IGF-1 (15). Given the complexity of the pancreatic microcirculation (16) and the close topographical relationship between the islets, small ducts and centroacinar cells (17), locally overproduced insulin is thought to act directly on ductal pancreatic cancer cells. We identified a novel crosstalk between insulin/IGFI receptors and G protein–coupled receptors (GPCRs) signaling systems in pancreatic cancer cells, leading to enhancement of GPCR-induced signaling (18). Crosstalk between insulin/IGF receptor and GPCR signaling systems, is mediated through the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR complex 1 (mTORC1) signaling module (18), a key pathway in insulin/IGF action (19) and pancreatic cancer proliferation (20).
Metformin (1,1-dimethylbiguanide hydrochloride) is the most widely prescribed drug for treatment of T2DM, worldwide (21, 22). Mounting epidemiological studies are linking administration of metformin with reduced incidence, recurrence and mortality of cancer in T2DM patients (20, 23-32). Strikingly, T2DM patients who had taken metformin had a 62% lower adjusted incidence of PDAC compared with those who had not taken metformin (30), a result recently substantiated in a different patient population (32). In contrast, diabetic patients who received insulin or insulin secretagogues (e.g. sulfonylureas) had a significantly higher risk of PDAC compared with diabetic patients who had not taken these drugs (30, 31). Although epidemiological associations do not establish causation, they provide an important line of evidence that supports the need of further preclinical and clinical studies.
We previously demonstrated that metformin inhibits mitogenic signaling induced by the crosstalk between insulin/IGF and GPCR signaling systems in pancreatic cancer cells in vitro (33). In this study, we extend our previous results showing that metformin administration inhibits the growth of PANC-1 and MiaPaca-2 tumor xenografts in vivo. We demonstrate that the inhibitory effect of metformin on the growth of these pancreatic cancer models is dose-dependent and also produced by oral administration of the drug. Importantly, metformin also inhibited the growth of pancreatic cancer xenografted orthotopically.
Materials and Methods
Mice xenografts
All animal studies were approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Subcutaneous tumor xenografts
Early passage PANC-1 or MIAPaCa-2 cells were harvested, and various number of cells, as specified in the individual experiments, were implanted into the right flanks of male nu/nu mice. The male nu/nu mice were maintained in a specific pathogen–free facility at the University of California at Los Angeles. The animals were randomized into control and treated groups (10 mice per group). Tumor volume (V) was measured with an external caliper every 4 d and it was calculated as V = 0.52 (length × width2). Treatments were initiated as indicated in the individual experiments. All treatments were continued until any of the subcutaneous tumors reached 2 cm in length, when all the animals were sacrificed and the tumors removed. The volume of the excised tumors was calculated as V = 0.52 (length × width × depth).
Orthotopic tumor xenografts
The orthotopic xenograft was performed as described earlier (34). Briefly, 2×106 Mia PaCa-2 pancreatic cancer cells were injected subcutaneously into the flank of anesthetized donor nude mice. After 4weeks, the donor tumor was harvested and minced to fragments of approximately 1 mm3.Only macroscopically viable tissue from the periphery of the subcutaneous tumor was used for the orthotopic transplantation. The recipient nude mice were anesthetized with isoflurane and opened by a left longitudinal laparotomy. The spleen, together with the pancreatic tail, was gently exteriorized, and a tissue pocket was created in the pancreatic parenchyma. A tumor fragment was placed into the tissue pocket so it was entirely surrounded by normal pancreas. After careful relocation of the pancreas and spleen into the abdominal cavity, the abdominal wall was closed in two layers. The second postoperative day the animals were randomly selected into control or treated groups (10 mice/group). After 8weeks of treatment the animals were sacrificed and the abdominal, retroperitoneal and chest cavities explored. The primary tumors in the pancreas were removed and local invasion and macroscopic dissemination into the liver, spleen, lymph nodes, kidneys, small bowel and lungs were evaluated.
Metformin administration
Intraperitoneal injections
Metformin (obtained from Sigma-Aldrich) was dissolved in sterile saline and given once daily at the doses described in the individual experiments. All injections were calculated for a 50 μL/mouse volume. The control group received vehicle only (50 μL saline).
Oral administration
Metformin was dissolved in the drinking water at a final concentration of 2.5 mg/ml. The mice were allowed to consume water (treated or un-treated) ad libidum, for the entire duration of the experiment. The medicated drinking water was replenished every other day. Animals did not display any clinical symptoms of metformin-induced toxicity, including overt changes in behavior, and body weight.
Western blot analysis
Equal portions (by volume) of tumor samples were homogenized in a buffer containing 50 mM Tris-HCl, pH 7.6, 2 mM EGTA, 2 mM EDTA, 1 mM dithiothreitol, 100 μg/ml leupeptin, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, hydrochloride (Pefabloc), 1% Triton X-100 and 0.1% SDS. The homogenates were then centrifuged at 18,000 g and the protein concentration of the supernatants was then determined with the BCA Protein Assay kit (Pierce). An equal volume of 4× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (40 mM Tris/HCl, pH 6.8, 6% SDS, 4 mM EDTA, 8% 2-mercaptoethanol, 20% glycerol) was then added to each supernatant and boiled for 10 min, followed by SDS-PAGE on 4-16% gradient gels and transfer to Immobilon-P membranes (Millipore, Billerica, MA). Western blots were then performed on membranes incubated overnight with the specified antibodies in PBS containing 0.1% Tween-20. The immunoreactive bands were detected with ECL (enhanced chemiluminescence) reagents (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). The phosphospecific antibodies used, a polyclonal antibody to S6 Ser235/236 and a monoclonal antibody ERK1/2 Thr202 and Tyr204 were purchased from Cell Signaling Technology (Danvers, MA). Equal loading of the gels was verified using a tubulin polyclonal purchased from Santa Cruz, CA.
Statistical analysis
The values obtained are presented as the mean ± SE and analyzed with Student's t test, using SigmaPlot 2000 (SPSS).
Results and Discussion
Metformin inhibits the growth of PANC1 xenografts in a dose-dependent manner
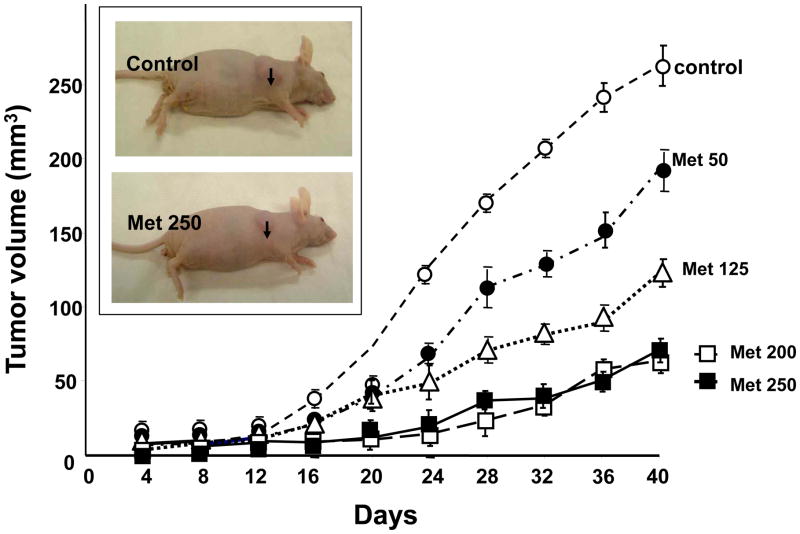
Initially, we determined the inhibitory effects of different doses of metformin (50-250 mg/Kg) on the growth of PDAC cells implanted subcutaneously in immunosuppressed mice. The xenografts were derived by injection of 2×106 PANC-1 cells into the right flanks of male nu/nu mice (33, 35). The animals were randomized into control and metformin-treated groups (10 mice per group). Treatment was initiated when the tumors reached a mean diameter of 2 mm, and the 1st day of treatment was designated as day 0. Metformin, dissolved in saline solution, was given once daily intraperitoneally at various doses (50, 125, 200 and 250 mg/kg) for the duration of the experiment. As shown in Fig. 1, administration of metformin strikingly decreased the rate of growth of PANC-1 cells xenografted in nude mice, even at the lowest dose tested (50 mg/kg).
Figure 1. Metformin dose-dependently inhibits the rate of growth of PANC-1 tumor xenografts.
Xenografts were generated by implantation of 2 × 106 cells of PANC-1 cells into the right flanks of male nu/nu mice. When the tumors reached a mean diameter of 2 mm, the animals were randomized into control and treated groups (10 mice per group). Metformin was given once daily intraperitoneally at various doses (50, 125, 200 and 250 mg/kg) for the duration of the experiment. The first day of treatment was designated as day 0. Control animals received saline. Tumor volumes were measured every 4 days as described in Materials and Methods. Insert: Representative subcutaneous tumors of control and metformin-treated (250 mg/kg), as indicated by the arrows.
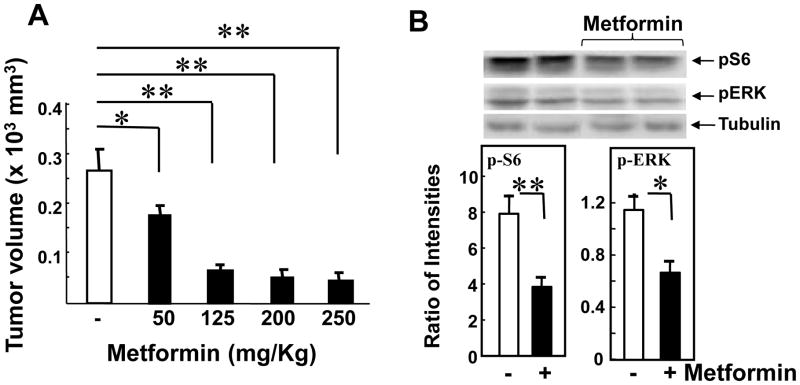
The tumor volumes at the end of the experiment (Day 44) are presented in Fig. 2, A. Maximal inhibitory effect on the increase in tumor volume (∼80%) was achieved by administration of metformin at 200 mg/kg (51 ± 10mm3 vs. 268 ± 65 mm3 in control), and increasing the dose of the drug to 250 mg/kg did not produce any additional inhibitory effect (60 ± 12mm3). A statistically significant decrease of tumor volume at the end of the experiment (p<0.05) was achieved by administration of metformin at 50 mg/Kg (Fig. 1, A).
Figure 2. Metformin decreases the final volume and the phosphorylation of S6 and ERK in the excised tumors.
A. Metformin decreases tumor volume at the end of the experiment in a dose-dependent manner. On day 44 the tumors generated in Fig. 1 were removed, measured, and tumor volumes estimated as V = 0.52 (length × width × depth). The results are shown are means ± SE. [Points, mean; bars, SE (*, p < 0.05; **, p < 0.01 versus control, Student's t test]. B. Metformin decreases the phosphorylation of S6K and ERK in extracts of the excised tumors. Tumors samples of were prepared for SDS-PAGE as described in “Materials and Methods”. The samples were analyzed by SDS-PAGE and immunoblotting with the following phospho antibodies: S6 Ser235/236 (pS6), ERK1/2 Thr202 and Tyr204 (pERK). Immunoblotting with total tubulin was used to verify equal loading. Shown here are representative immunoblots (two controls and two metformin-treated mice). Similar results were obtained in 4 additional tumors Quantification was performed using Multi Gauge V3.0. Results are expressed as the ratio of the intensities of the phosphosphorylated S6 or ERK immune-reactive bands to the intensity of the tubulin band. P values were determined using the t-test (Sigma Plot 12) n=6, **, P = 0.01, * P = 0.03.
We also found a significant decrease in the phosphorylation of S6 ribosomal protein at Ser235/236, a site targeted by S6K, downstream of mTORC1, and in the phosphorylation of ERK (Fig. 2, B). There was no toxicity detected in any of the metformin-treated groups during the experiment and, accordingly, there was no significant difference in the body weight of the animals in the different groups. These results demonstrate that metformin inhibits the growth of PANC-1 cells xenografted into nude mice in a dose-dependent manner and cause a marked decrease in mTORC1 and ERK signaling in the xenografts.
Metformin administered orally markedly inhibits the tumor growth
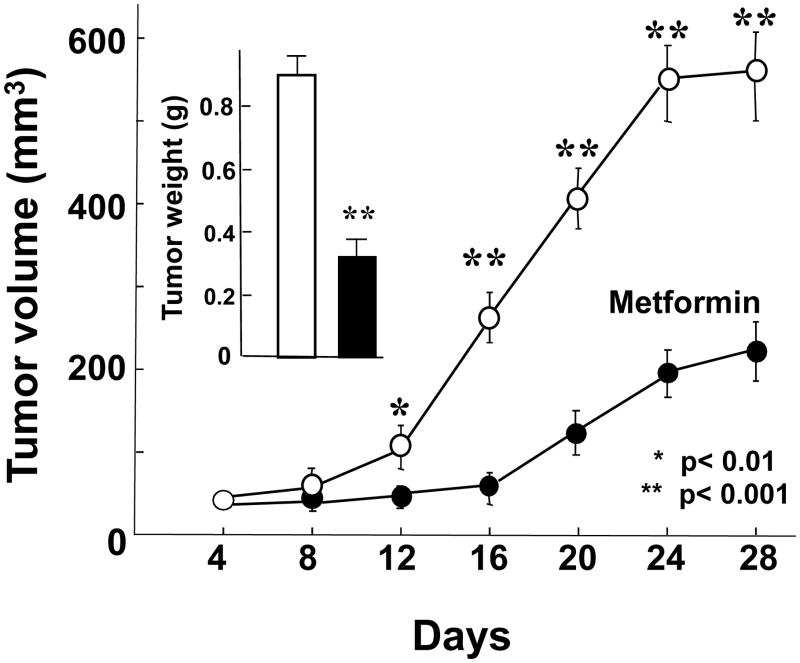
Since metformin is given orally to patients with T2DM, we tested whether the inhibitory effect of metformin on PDAC xenografts in nude mice could be also demonstrated when metformin is administered in the drinking water. We used two distinct models to analyze the growth-inhibitory effect of metformin administered orally. Initially, we used an established tumor xenograft model to emulate the clinical scenario usually observed in pancreatic cancer. Metformin was dissolved in the drinking water at a final concentration of 2.5 mg/ml. This dose was based on preliminary range-finding studies and previous work published by others (36-38). In addition, a recent study demonstrated that oral administration of metformin at 1-5 mg/ml doses achieved circulating levels in mice that were consistent with the steady-state values in patients with T2DM who are treated with this anti-diabetic agent (39). As shown in Fig. 3, oral administration of metformin significantly inhibited the rate of growth of MiaPaca-2 xenografts and reduced the volume of the tumors at the end of the experiment by 67% inhibition, as shown in the insert of Fig. 3. Orally administered metformin also markedly inhibited the tumor growth of established PANC-1 xenografts (results not shown). During these experiments, metformin was well tolerated and did not significantly affect the body weight of the mice.
Figure 3. Orally administered metformin inhibits the growth of Mia PaCa-2 xenografts.
Xenografts were generated by implantation of 2 × 106 cells of Mia PaCa-2 cells into the right flanks of male nu/nu mice. When the tumors reached a mean diameter of 2 mm, the animals were randomized into control and treated groups (10 mice per group). Metformin was dissolved in the drinking water in the final concentration of 2.5mg/ml. Animals were allowed to drink ad libidum and the drinking water (with or without metformin) was replenished every other day. The tumor measurements and removal were performed as described in Materials and Methods. In the inset shown are the tumor volumes after the removal of the tumors from the animals on the last day of the experiment. [Points, mean; bars, SE (*, P < 0.05; **, P < 0.01 versus control, Student's t test].
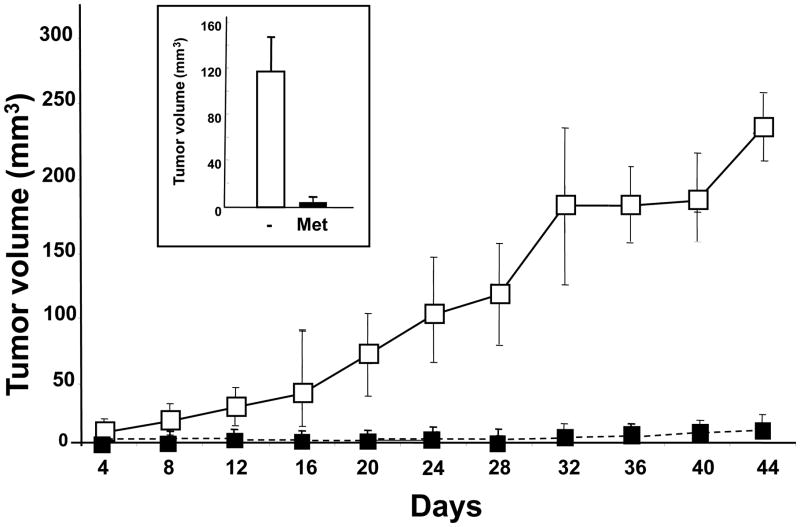
In a second protocol, oral metformin administration was started 5 days prior to inoculation of 106 PANC-1 cells and continued for 44 additional days. This administration of metformin to mice preceding inoculation with lower number of cells simulates an in vivo model for tumors initiating metastatic processes. As shown in Fig. 4, orally administered metformin prior to tumor implantation strikingly inhibited the growth of the PANC-1 xenografts and markedly reduced the tumor volume at the end of the experiment (insert, Fig. 4). The results shown in Figs. 3 and 4 demonstrate that oral metformin inhibited PDAC tumor growth when administered either prior or after tumor implantation but the drug was very effective when it was administered to mice preceding inoculation with a low number of cells.
Figure 4. Prior administration of metformin prevents tumor development and growth in nu/nu mice.
Animals received drinking water or metformin containing drinking water (2.5mg/ml, as described in Materials and Methods) for 5 days. Then, the animals were divided into groups for implantation of PANC-1 cells (either 4×105 or 106) into the right flanks in nu/nu mice (Day 0). The animals continued receiving control or metformin-treated drinking water for the duration of the experiment. The development and growth of the tumors in the flanks were measured in every 4 days, as described in Materials and Methods. On the last day of the experiment (Day 49) the animals were sacrificed, the tumors removed, measured and weighted (inset). [Points, mean; bars, SE (*, P < 0.05; **, P < 0.01 versus control, Student's t test].
Metformin inhibits the growth of pancreatic tumor xenografts implanted in nu/nu mice orthotopically
Next, we examined whether the growth of pancreatic tumors, implanted in their native environment in the pancreatic parenchyma, would also be affected by metformin treatment. Orthotopic tumor xenografts were generated as described in Materials and Methods. On the second postoperative day the animals were randomly selected into metformin-treated or control groups (10 animals/group). Metformin was administered in the drinking water (2.5 mg/kg), as described in the previous experiments. After 8 weeks of treatment the animals were sacrificed, the primary tumors in the pancreas removed and local invasions and disseminations into the liver, spleen, lymph nodes, kidneys, small bowel and lungs were evaluated.
At the end of the 8-week long treatment, the weight and volume of the tumors were markedly reduced (by 73%) in the animals that received metformin, as shown in Fig. 5. In the control group, some of tumors derived from MiaPaCa-2 cells demonstrated local invasion and lymph nodes metastases at the end of the experiment. We detected metastases into multiple lymph nodes in two animals and extended local invasions of the tumors in other two animals. (In one of these mice the local invasion even propagated to the chest cavity). In the metformin-treated group, a single metastasis was found in only one animal in a lymph node in the retroperitoneal area. As in the other protocols, we verified that administration of metformin did not produce a significant change in the body weight of the animals (Fig. 5).
Figure 5. Metformin inhibits the growth of MIA PaCa-2 tumor xenografts implanted in nu/nu mice orthotopically.
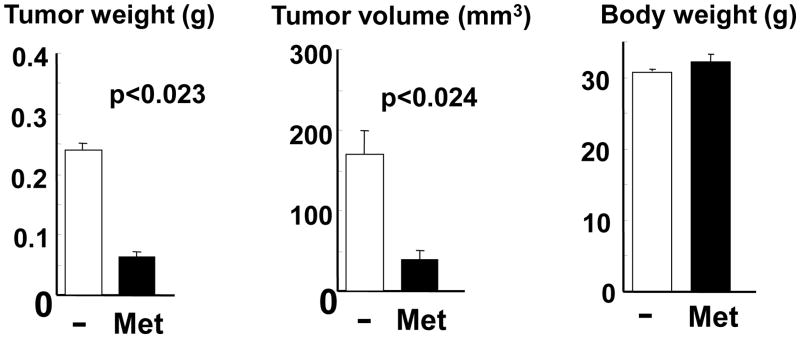
Small (1mm3) fragments of MiaPaCa-2 tumors (developed in donor nu/nu mice) were implanted into microsurgicaly prepared tissue pockets within the parenchyma of the body/tail of the pancreas in nu/nu mice. Next day the animals were randomly selected into metformin-treated or control groups (10 animals/group). Metformin was administered orally in the drinking water (2.5 mg/kg), as in previous experiments. After 8 weeks of treatment the animals were sacrificed and the abdominal, retroperitoneal and chest cavities explored. The primary tumors in the pancreas were removed and local invasion and macroscopic dissemination into the liver, spleen, lymph nodes, kidneys, small bowel and lungs was assessed.
Concluding Remarks
PDAC is a devastating disease, with overall 5-year survival rate of only 3-5%. As the current therapies offer very limited survival benefits, novel therapeutic strategies are urgently required to prevent and treat this aggressive but under-studied disease. We previously demonstrated that metformin, the most widely prescribed drug for treatment of T2DM (21, 22), inhibits mitogenic signaling, including mTORC1 activity, induced by crosstalk between insulin/IGF and GPCR signaling systems in pancreatic cancer cells in vitro (33). Epidemiological studies are linking administration of metformin with reduced incidence of pancreatic cancer in T2DM patients (30, 32). In contrast, diabetic patients who received insulin or insulin secretagogues (e.g. sulfonylureas) had a significantly higher risk of PDAC compared with diabetic patients who had not taken these drugs (30, 31).
In the present study, we extend our previous results showing that metformin administration inhibits the growth of PANC-1 and MiaPaca-2 tumor xenografts in vivo. We demonstrate that the inhibitory effect of metformin on the growth of these pancreatic cancer models is dose-dependent and associated with inhibition of mTORC1 activity in the xenograft. Metformin was also effective in inhibiting pancreatic cancer growth when it was administered orally either prior or after tumor implantation. In particular, metformin was very effective when given before inoculation of a smaller number of pancreatic cancer cells. Importantly, we also demonstrated, for the first time, that oral administration of metformin inhibited the growth of pancreatic cancer xenografted orthotopically. The doses of metformin used in our experiments were shown to achieve circulating levels of this drug in mice that were consistent with the steady-state values in patients with T2DM who are treated with this anti-diabetic agent (39). FDA-approved drugs that advanced to clinical testing have been extensively studied and have usually favorable pharmacological properties and safety profiles (40). The studies presented here provide further evidence indicating that metformin offers a potential novel approach for PDAC prevention and therapy and thus, warrant further experimental work to elucidate its precise mechanism of anticancer action.
Acknowledgments
This work was supported by the National Institutes of Health Grants R21CA137292, RO1DK55003 and P30DK41301 (to ER), P01AT003960 (to Go VLW and GE), P01 CA163200 (to GE) and funds from the endowed Hirshberg Chair of Pancreatic Cancer Research (ER).
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011. CACancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud DS, Wolpin B, Giovannucci E, et al. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:2101–9. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 5.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and Cancer. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsugane S, Inoue M. Insulin resistance and cancer: Epidemiological evidence. Cancer Science. 2010;101:1073. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric Measures, Body Mass Index, and Pancreatic Cancer: A Pooled Analysis From the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and Cancer: A Consensus Report. CA: A Cancer Journal for Clinicians. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 11.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 12.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 13.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 14.Muoio DM, Newgard CB. Obesity-Related Derangements in Metabolic Regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 15.Tam CS, Viardot A, Clement K, et al. Short-Term Overfeeding May Induce Peripheral Insulin Resistance Without Altering Subcutaneous Adipose Tissue Macrophages in Humans. Diabetes. 2010;59:2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 17.Bertelli E, Regoli M, Orazioli D, et al. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia. 2001;44:575–584. doi: 10.1007/s001250051663. [DOI] [PubMed] [Google Scholar]
- 18.Kisfalvi K, Rey O, Young SH, et al. Insulin Potentiates Ca2+ Signaling and Phosphatidylinositol 4,5-Bisphosphate Hydrolysis Induced by Gq Protein-Coupled Receptor Agonists through an mTOR-Dependent Pathway. Endocrinology. 2007;148:3246–3257. doi: 10.1210/en.2006-1711. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 20.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn BB, Alquier T, Carling D, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 25.Libby G, Donnelly LA, Donnan PT, et al. New Users of Metformin Are at Low Risk of Incident Cancer. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong CR, Chabner BA. Mysterious Metformin. Oncologist. 2009;14:1178–1181. doi: 10.1634/theoncologist.2009-0286. [DOI] [PubMed] [Google Scholar]
- 27.Ben Sahra I, Le Marchand-Brustel Y, Tanti JFo, et al. Metformin in Cancer Therapy: A New Perspective for an Old Antidiabetic Drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 28.Landman GWD, Kleefstra N, van Hateren KJJ, et al. Metformin Associated With Lower Cancer Mortality in Type 2 Diabetes. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeCensi A, Puntoni M, Goodwin P, et al. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-analysis. Cancer Prev Res. 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Yeung SCJ, Hassan MM, et al. Anti-diabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisfalvi K, Eibl G, Sinnett-Smith J, et al. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–6545. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 35.Guha S, Eibl G, Kisfalvi K, et al. Broad-spectrum G protein-coupled receptor antagonist, [D-Arg1,D-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005;65:2738–2745. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 36.Bergheim I, Guo L, Davis MA, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 38.Ma TC, Buescher JL, Oatis B, et al. Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Memmott RM, Mercado JR, Maier CR, et al. Metformin Prevents Tobacco Carcinogen-Induced Lung Tumorigenesis. Cancer Prev Res. 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]