Abstract
The calcium-binding protein S100P is expressed in a variety of human cancer cells and is important in cancer cell growth and invasion. Using differential display, we found S100P is overexpressed in human hepatocellular carcinoma (HCC). We examined the expression of 305 unifocal, primary HCC tumors using immunohistochemistry. The S100P protein was expressed in 173 of the 305 (56.7%) HCC tumors. The expression of S100P correlated with female sex (P = 0.0162), high serum α-fetoprotein level (P = 0.0001), high tumor grade (P = 0.0029), high tumor stage (P = 0.0319), the presence of the p53 mutation (P = 0.0032), and the absence of the β-catenin mutation (P = 0.0489). Patients with HCC tumors that expressed S100P were more likely to have early tumor recurrence (ETR) (P = 0.0189) and lower 5-year survival (P = 0.0023). The multivariate analysis confirmed that S100P expression was an independent prognostic factor in HCC. The combinatorial analysis showed an additive unfavorable prognostic interaction between S100P expression and the p53 mutation. In contrast, the β-catenin mutation was associated with better prognosis in both S100P-positive and -negative HCCs. Furthermore, S100P expression was a predictor of survival in HCC patients with high tumor stage or ETR (P = 0.0026 and P = 0.0002, respectively). Our study indicates the expression of the S100P protein is a novel independent predictor for poor prognosis in HCC, and it is also an unfavorable prognostic predictor in HCC patients with high tumor stage or ETR.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, particularly in Taiwan, southern China, Southeast Asia, and sub-Saharan Africa, and the incidence of HCC is increasing in Western countries [1]. The major risk factors for HCC are hepatitis B and C, cirrhosis, and exposure to environmental carcinogens, such as aflatoxin [2]. Although surgical resection and various methods of tumor ablation can be curative or prolong survival, the outcome for patients with HCC remains grave. This is particularly true in advanced-stage HCC because the tumor has often spread throughout the liver via the intrahepatic portal venous system, and a considerable number of HCC patients develop early intrahepatic and/or extrahepatic recurrence postoperatively [3].
Early tumor recurrences (ETRs) arise primarily from intrahepatic metastases, and a significantly poorer prognosis [4]. However, the molecular factors related to tumor progression and ETR in HCC remain unclear. Therefore, the identification of molecular markers that correlate with tumor progression, ETR, and poor prognosis would aid efforts to establish better treatment plans for HCC patients.
One way to elucidate the pathogenesis of cancers is to identify the genes that are up- and down-regulated during the disease course. Methods for detecting such altered expression include mRNA differential display (DD), serial analysis of gene expression, subtractive hybridization, proteomics, and cDNA microarray. Using DD, we identified several upregulated genes in HCC that have clinical significance with regard to tumor proliferation and metastasis [5]–[11]. These include KIAA0101, stathmin, Reg1A/PAP, and CK19. We also identified a cDNA clone that is identical to S100P, which is preferentially expressed in tumors.
The S100 proteins are small, dimeric members of the EF-hand superfamily of Ca2+-binding proteins that contain 2 Helix-E and Helix-F loop-hand Ca2+-binding motifs that mediate Ca2+-dependent signal transduction. They play important roles in many intracellular and extracellular processes, including the regulation of protein phosphorylation, enzyme activation, gene transcription, the assembly of cytoskeleton components, cell proliferation and differentiation [12]. The S100P protein has been reported to be located in the nucleus, in the cytoplasm, at the cell membrane, and in the extracellular space, depending on the experiment conditions [13]–[15]. The S100P protein functions as both an extracellular and intracellular signaling molecule. In the extracellular space, S100P interacts with the receptor for advanced-glycation end products to activate signal transduction pathways, including the mitogen-activated protein kinase, serine protein kinase, extracellular-regulated kinase, and nuclear factor pathways [16]–[18], and promote tumor development [19]. Intracellular S100P interacts with the cytoskeletal multidomain protein ezrin through a Ca2+-dependent mechanism [20]. Ezrin links the plasma membrane and the actin cytoskeleton to regulate cell migration and cell proliferation [21]. Another binding partner of S100P is CacyBP/SIP. The interaction of S100P and CacyBP/SIP leads to the degradation of β-catenin [22]. Thus, S100P contributes to cancer progression by promoting cell proliferation, cell survival, angiogenesis, and metastasis.
The S100P protein was first identified in the human placenta [23], and has been reported to be overexpressed in multiple types of cancer cells, including breast cancer [13], [24], esophageal cancer [15], colon cancer [19], lung cancer [25], pancreatic cancer [26], [27], prostatic cancer [28], ovarian cancer [29], cholangiocarcinoma [30]–[32], and HCC [33]. However, the clinical and pathological significance of S100P expression in human HCC remains unclear. Moreover, HCC harbors frequent genetic mutations in p53 and β-catenin, which have opposing roles in tumor progression, ETR, and prognosis [4], [34], [35]. The mutations of p53 are associated with more advanced HCC and poor prognosis [4], [35], whereas the mutations of β-catenin are associated with less invasive tumors and better prognosis [34]. The expression of S100P in conjunction with these critical gene mutations requires further investigation to better understand the role of S100P in HCC progression. The aims of our study were to elucidate the role of S100P in vascular invasion, ETR, and HCC prognosis, to investigate the relationships between S100P expression and the p53 and β-catenin mutations, and to evaluate S100P as a predictive biomarker for survival in HCC patients with high tumor stage or ETR.
Materials and Methods
Tissue Samples
A total of 305 unifocal, primary HCC tumors surgically resected from patients at National Taiwan University Hospital from 1982 to 1998 were used in this study. All resected tumors underwent detailed pathological assessment, and all patients received regular follow-up examinations, as described previously [5], [6]. Our study was approved by the Ethics Committee of National Taiwan University Hospital (approval no. 201107042RC), and all study procedures were conducted therein. All study participants provided written informed consent, which was approved by the Ethics Committee of National Taiwan University Hospital. One teenaged male was included in our study, for whom written, informed consent was obtained from his parents. There were no other minor participants in our study. The anonymity of all patients was maintained, and all specimens were analyzed in a blinded manner. The HCC patients included 239 males and 66 females, with a mean age of 55.09 years (range, 15 to 88 years). Serum hepatitis B surface antigen (HBsAg) was detected in 202 of 305 (66.2%) cases, and hepatitis C antibody (anti-HCV) was detected in 97 of 281 (34.5%) cases, 20 of which were positive for both. All patients had adequate liver function reserve at the time that they received curative liver resection, and their records contained complete clinical, histopathological, and follow-up data. No patients had distant metastasis, nor had they received anticancer treatments before undergoing surgery, such as transhepatic arterial embolization, percutaneous ethanol injection, radiofrequency ablation, or chemotherapy.
Histology and Tumor Staging
Surgically resected specimens were formalin fixed and paraffin embedded. Histological sections were cut at 5-µm thickness and stained with hematoxylin-eosin. All specimens were reviewed by the same investigator (HCH) to determine tumor grade and stage. The tumor grading was based on the criteriae proposed by Edmonson and Steiner [36]. The tumors were staged according to the American Joint Committee on Cancer system [37]. Because the aim of our study was to evaluate the prognostic value of resectable HCCs, patients classified with stages IVA and IVB were excluded. The margins of the surgical specimens were inked and checked microscopically. Only completely resected specimens were included in our study.
Differential Display
For differential display, paired mRNA samples from tumor cells and non-cancerous liver parenchyma cells from 3 low-stage (Stage I) and 6 high-stage HCCs were examined. Total RNA (2 µg) was reverse transcribed to produce cDNA using 500 units of Moloney murine leukemia virus reverse transcriptase (Bethesda Research Laboratories, Gaithersburg, MD, USA), the H-T11C primer (5'- AAGCTTTTTTTTTTTC-3'), 16 µM deoxynucleotide triphosphates (dNTPs), 16 µM DTT, 20 units of RNasin, and reverse transcription buffer in a 30-µL reaction volume for 60 min at 35°C. Polymerase chain reaction (PCR) was performed using 0.1 mL of reverse transcription reaction mixture and 1 unit DNA polymerase in a solution containing 2.5 µM H-T11C, 0.5 µM random primer HAP6 (5'-AAGCTTGCACCAT-3'), 1× PCR buffer, 8 mM dNTPs, and 10 µCi [-S35]dATP. The PCR procedure was performed using 40 cycles of 94°C for 30 s, 37°C for 1 min, and 72°C for 30 s, followed by a final elongation at 72°C for 5 min. After adding loading buffer to the reaction, the PCR products were heated at 70°C for 10 min, and separated by electrophoresis on a 6% polyacrylamide gel. Kodak XAR-5 film was exposed on the gels for 48 h. Bands that indicated the differential expression of mRNA were excised from the dried gel, eluted, re-amplified, cloned, and sequenced as previously described [3], [38], [39].
RNA Isolation and Reverse Transcription–polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from tissue samples and cell lines using the Trizol reagent (Life Technologies, Invitrogen, Carlsbad, CA. USA), according to the manufacturer’s instructions. RT-PCR was used to determine the mRNA levels of S100P in the paired HCC and non-tumorous liver samples. S26 ribosomal protein mRNA, a housekeeping gene, was used as an internal control. PCR was arrested during the exponential phase for each gene (i.e. 28 cycles for S100P and 22 for S26). PCR was performed in an automatic DNA thermal cycler (PerkinElmer, Wellesley, MA, USA) with initial heating at 94°C for 2 min followed by cycles at 94°C for 30 s, 58°C for 1 min, 72°C for 1 min and finally, 72°C for 10 min. The primers for S100P were S100P-F (5′-CTCAAGGATCTGATGGAGAA -3′) and S100P-R (5′- CCAGGGCATCATTTGAGTCC-3′). The primers for S26 were S26-F (5′- CCGTGCCTCCAAGATGACCAAAG-3′) and S26-R (5′-GTTCGGTCCTTGCGGGCTTCAC-3′).
Immunohistochemical Analysis of S100P Protein Expression
The S100P protein was detected in formalin-fixed, paraffin-embedded sections of HCC and liver tissues using a labeled streptavidin-biotin method after antigen retrieval, as previously described [6], [11]. The tissue sections were dewaxed and rehydrated. The antigen was retrieved by incubating the slides in 0.01 M citrate buffer at 100°C for 10 min. After blocking with 3% H2O2 and 5% fetal bovine serum (FBS), the slides were incubated in a 1∶200 dilution of a goat anti-S100P polyclonal antibody (R&D Systems, Minneapolis, MN, USA) at 4°C overnight. The slides are then incubated with N-Histofine Simple Stain Mouse MAX PO (G) reagent (Cosmo Bio, Carlsbad, CA, USA). The peroxidase activity was visualized using a diamino-benzidine tetrahydroxychloride solution (BioGenex, San Ramon, CA, USA), and the sections were counterstained with hematoxylin. We replaced the primary antibody with 5% FBS as a negative control. One pathologist who was blinded to patients' outcomes calculated the percentage of positive cells based on 5 independent microscopic fields (400× magnification) for each slide. For data presentation, the proportion of the tumor cells that were positive for S100P immunostaining were categorized as diffuse S100P expression (>50%), focal or heterogeneous S100P expression (10% to 50%), or S100P expression in a small number of tumor cells (1% to 10%). In the nontumorous liver, S100P protein was detected only in very few isolated liver cells. Hence, HCC with more than 1% of tumor cells showing immunostaining for S100P was regarded as positive [32].
Analysis of p53 and β-catenin Mutations
Mutations of the p53 tumor suppressor gene were analyzed in 187 tumors by direct sequencing of the chromosomal region spanning exon 2 to exon 11, as described previously [40]. Mutations of the β-catenin gene were analyzed in 214 cases by direct sequencing of exon 3 in the chromosomal DNA as described previously [34].
Follow-up Examination and Early Tumor Recurrence
All patients had been followed up for more than 5 years or until death. Among the 305 study patients, 106 (34.6%) survived longer than 5 years, and 259 (84.9%) were eligible for the evaluation of ETR. The follow-up periods for survivors ranged from 30 to 236 months (median, 136 months). Following surgery, all patients received laboratory examinations, including assessment of serum α-fetoprotein (AFP) level, at 1- to 6-month intervals, and ultrasonography of the liver at 3- to 12-month intervals. Computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to confirm and differentiate intrahepatic recurrence and/or distal metastasis in patients with clinical signs of recurrence. Cases of CT/MRI confirmed intrahepatic tumor recurrence or distant metastasis within 12 months of tumor resection were defined as ETR events, as previously described [6], [41].
Depending on the tumor site, the tumor size, the number of tumors, the level of liver function, and the patient's condition, tumor recurrence was treated by a second resection, percutaneous ethanol injection, transhepatic arterial chemo-embolization, radiofrequency ablation, or chemotherapy. All patients had an equal opportunity to access all therapeutic modalities supported by the Bureau of National Health Insurance, Taiwan.
Statistical Analysis
The data analyses were performed using the Epi Info computer software, version 7.1.0.6 (Centers for Disease Control and Prevention, Atlanta, GA, USA). A univariate analysis was used to examine whether the immunohistochemical markers correlated with the gene mutations and the clinical and pathological parameters using the χ2 test. The survival rates after tumor resection were calculated using the Kaplan-Meier method, and the difference in the survival curves was analyzed using the log rank test. A multivariate survival analysis of all the parameters that were found to be significantly correlated in the univariate analysis was performed using a Cox proportional-hazards regression model. A two-tailed P value of less than 0.05 was considered to indicate a statistically significant relationship.
Results
Expression of S100P Protein in HCC and Liver Cells
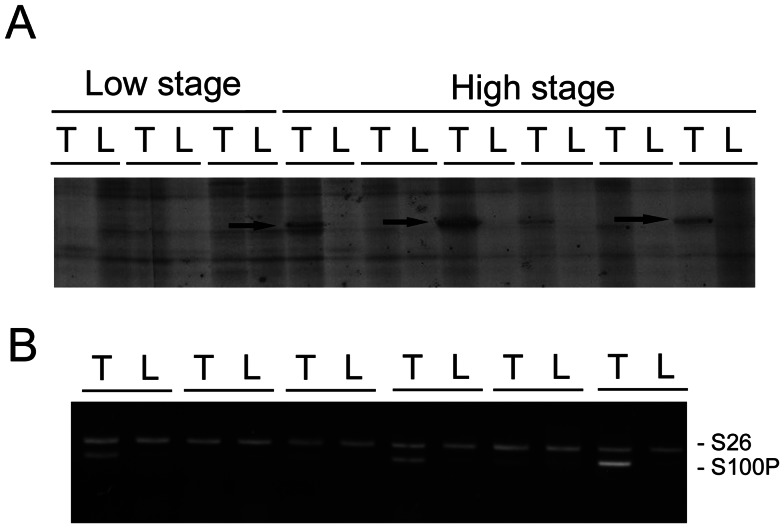
Using differential display, we identified a band overexpressed in high-stage HCC. The bands were excised from the gel and confirmed to be S100P by cloning and sequencing (Figure 1A). To prove S100P was overexpressed in HCC, the mRNA levels were measured in paired HCC and non-cancerous liver parenchyma. As shown in Figure 1B, S100P mRNA was overexpressed in 3 of 6 HCCs.
Figure 1. Identification and confirmation of overexpression of S100P in HCC.
(A) Differential display showed that S100P expression was upregulated in high-stage HCCs. The arrows indicate bands of S100P, which was confirmed by subsequent cloning and sequencing. T: hepatocellular carcinoma; L: non-cancerous liver parenchyma. (B) Expression of S100P mRNA in paired HCC (T) and non-cancerous liver parenchyma (N). RT-PCR measurement identified S100P overexpression in 3 of 6 HCC specimens.
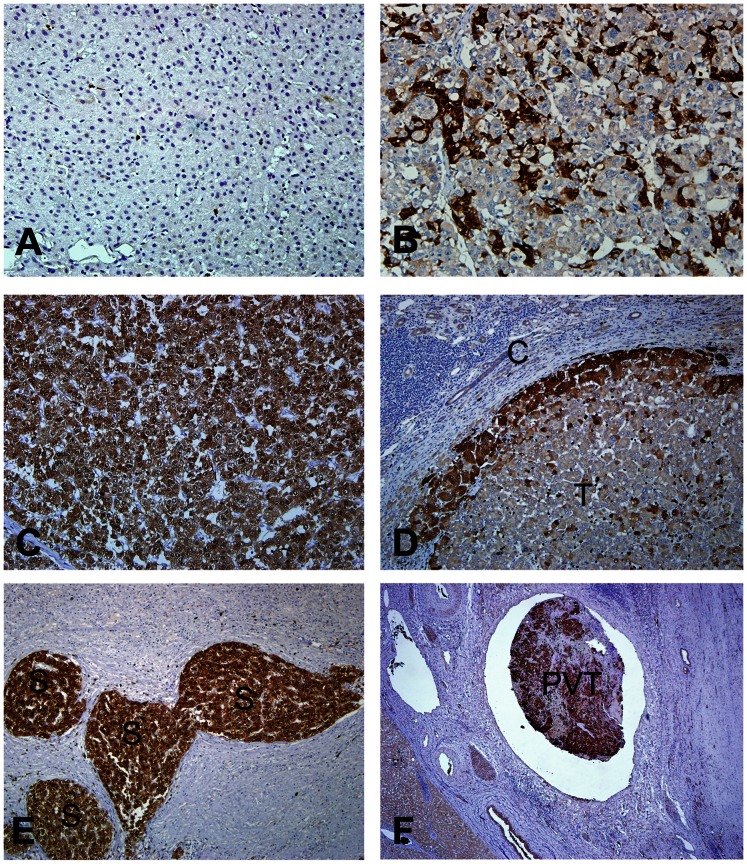
The immunohistochemical staining was used to screen 305 HCCs to determine the frequency of S100P expression and the clinical and pathological significance of S100P expression in HCC. S100P protein was not detected or detected only in very few isolated liver cells (Figure 2A), but was detected in the tumor cell cytoplasm and/or nuclei in 173 of 305 HCCs (56.7%). The level of S100P protein expression in HCC cells varied considerably, and displayed a heterogeneous distribution. S100P was expressed in a small number of tumor cells in 131 cases. Heterogeneous to focal S100P expressions in 10∼50% tumor cells were seen in 33 cases (Figure 2B). Diffuse S100P expressions in more than 50% tumor cells were seen in 9 cases (Figure 2C). In cells that exhibited heterogeneous to focal or trace S100P expression, S100P was predominantly expressed at the periphery of the tumor masses (Figure 2D) and satellite nodules (Figure 2E). Notably, the intravascular tumor thrombi in large and small portal vein branches often showed more intense and diffuse immunoreactivity than the main tumor mass (Figure 2F). In about 10% of all cases, the S100P protein was detected in a small subset of hepatocytes that usually comprised less than 1% of the total hepatocytes. Therefore, those that did not express S100P or expressed S100P in less than 1% of the tumor cells were defined as the negative group (132 cases). In some specimens, S100P was also expressed in inflammatory cells that were located within both tumors and non-cancerous liver tissues.
Figure 2. Expression of S100P in HCC and non-cancerous liver parenchyma.
(A) No immunostaining of S100P in non-cancerous liver parenchyma. (B and C) Heterogeneous and diffuse expression of S100P in HCC. (D) The expression of S100P was more prominent at the periphery of the tumor mass near the tumor capsule. (E) Strong expression of S100P in satellite nodules. (F) A portal vein tumor embolus exhibiting strong S100P expression. T: tumor, C: capsule, S: satellite nodule, PVT: portal vein tumor embolus.
Clinical and Pathological Significance of S100P Expression in HCC
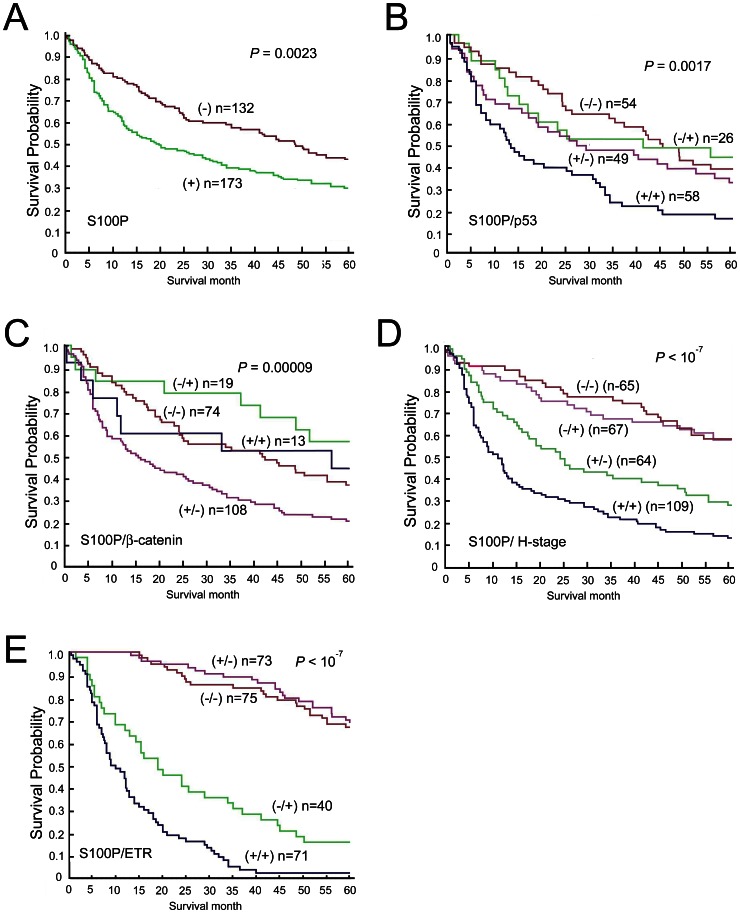
To elucidate the significance of S100P expression in HCC, we examined possible correlations between S100P protein expression and major clinical and pathological features of HCC. As shown in Table 1, S100P protein expression tended to occur in female patients (P = 0.0162), and correlated with high serum AFP (>200 ng/mL; P = 0.0001). However, it did not correlate with other clinical parameters, such as age, serum HBsAg, Anti-HCV status, or tumor size. S100P expression closely correlated with high tumor grade (grade 2 to 4; OR, 2.12; 95% CI, 1.25–3.62; P = 0.0029) and high tumor stage (stages II and III; OR, 1.65; 95% CI, 1.02–2.69; P = 0.0319). The Kaplan–Meier survival analysis showed S100P expression was associated with lower 5-year survival in HCC patients (Figure 3A).
Table 1. Univariate analysis of S100P protein expression with various clinicopathological features and aberrant gene expression in 305 patients with surgically removed unifocal primary hepatocellular carcinoma.
| S100P protein expression | ||||
| Variables | Total | Yes n (%) | Odds Ratio | P value |
| Age | ||||
| >56 | 136 | 75 (55) | 1.0 | |
| ≤56 | 169 | 98 (58) | 1.12 (0.69–1.82) | 0.6186 |
| Sex | ||||
| Male | 239 | 127 (53) | 1.0 | |
| Female | 66 | 46 (70) | 2.03 (1.09–3.79) | 0.0162 |
| HBsAg | ||||
| Negative | 103 | 61 (59) | 1.0 | |
| Positive | 202 | 112 (55) | 1.17 (0.80–1.53) | 0.5889 |
| Anti-HCV | ||||
| Negative | 184 | 97 (53) | 1.0 | |
| Positive | 97 | 59 (61) | 1.39 (0.82–1.32) | 0.1935 |
| α-fetoprotein (ng/ml) | ||||
| ≤200 | 167 | 78 (44) | 1.0 | |
| >200 | 138 | 95 (69) | 2.52 (1.53–4.16) | 0.0001 |
| Tumor size (cm) | ||||
| ≤5 | 149 | 77 (52) | 1.0 | |
| >5 | 156 | 96 (62) | 1.50 (0.92–1.54) | 0.0823 |
| Tumor grade | ||||
| 1 | 203 | 103 (51) | 1.0 | |
| 2∼4 | 102 | 70 (69) | 2.12 (1.25–3.62) | 0.0029 |
| Tumor stage | ||||
| I | 129 | 64 (50) | 1.0 | |
| II∼III | 176 | 109 (62) | 1.65 (1.02–2.69) | 0.0319 |
| p53 mutation | ||||
| No | 103 | 49 (48) | 1.0 | |
| Yes | 84 | 58 (69) | 2.46 (1.29–4.71) | 0.0032 |
| β-catenin mutation | ||||
| No | 182 | 108 (59) | 1.0 | |
| Yes | 32 | 13 (41) | 0.46 (0.20–1.07) | 0.0489 |
| Early tumor recurrence | ||||
| No | 115 | 40 (35) | 1.0 | |
| Yes | 144 | 71 (49) | 1.82 (1.10–3.02) | 0.0189 |
Figure 3. Kaplan–Meier analysis of overall survival in 305 patients with HCC.
(A) The expression of S100P protein in HCC tumor cells was associated with a significantly lower 5-year survival rate than that of HCC tumors in which the S100P protein was not expressed. (B) Patients with S100P-positive, p53-mutated HCCs had a lower 5-year survival rate than the other groups. (C) Patients with S100P-positive, β-catenin-wild type HCCs had the lower 5-year survival rate than the other groups. (D) High tumor stage and concurrent S100P expression in HCC patients were associated with the lower 5-year survival than that of HCC patients with high stage but S100P negative tumors. (E) ETR and concurrent S100P expression in HCC patients were associated with lower 5-year survival than that of HCC patients with ETR but S100P negative tumors. (+): The presence of S100P expression, p53 mutation, β-catenin mutation, high tumor stage, or ETR. (−): The absence of S100P expression, p53 mutation, β-catenin mutation, high tumor stage, or ETR.
S100P Expression Predicts ETR and Poor Prognosis
The ETR is the most critical clinical factor that is predictive of poor prognosis in HCC after hepatectomy [6], [41]. In our series, ETR occurred in 111 of 259 patients (42.9%). ETR occurred at a rate that was approximately 2 times higher in patients with S100P-positive HCCs than those with S100P-negative HCCs. (P = 0.0198; Table 1). To further elucidate the factors associtaed with ETR, we investigated its relationship with the major clinical and pathological factors. We found that serum HBsAg (P = 0.0045), tumor size (P = 1.2×10−6), tumor grade (P = 0.0456), and tumor stage (P<1×10−7) were important clinical and histopathological risk factors for ETR (Table 2). Four molecular markers were also analyzed. A high serum level of AFP (P = 2.3×10−6), the presence of the p53 mutation (P = 0.0032), and the absence of the β-catenin mutation (P = 0.0011) predicted ETR. Notably, S100P expression was also a significant risk factor for ETR (P = 0.0189) (Table 2).
Table 2. Univariate analysis of clinicopathological variables and S100P protein expression with early tumor recurrence (ETR) in patients with surgical removed unifocal primary hepatocellular carcinoma.
| ETR | ||||
| Variables | Total | Yes n (%) | Odds Ratio | P value |
| Clinical features | ||||
| Age | ||||
| >56 | 114 | 41 (43) | 1.0 | |
| ≤56 | 145 | 70 (48) | 1.24 (0.73–2.09) | 0.3961 |
| Sex | ||||
| Male | 201 | 85 (42) | 1.0 | |
| Female | 58 | 26 (45) | 1.11 (0.59–2.08) | 0.7307 |
| HBsAg | ||||
| Negative | 88 | 27 (31) | 1.0 | |
| Positive | 171 | 84 (49) | 2.18 (1.22–3.90) | 0.0045 |
| Anti-HCV | ||||
| Negative | 163 | 74 (45) | 1.0 | |
| Positive | 80 | 30 (38) | 1.39 (0.77–2.49) | 0.2422 |
| Histopathological features | ||||
| Tumor size (cm) | ||||
| ≤5 | 130 | 34 (26) | 1.0 | |
| >5 | 129 | 77 (60) | 4.18 (2.47–7.07) | 1.2×10−6 |
| Tumor grade | ||||
| 1 | 176 | 68 (38) | 1.0 | |
| 2∼4 | 83 | 43 (52) | 1.70 (1.01–2.89) | 0.0456 |
| Tumor stage | ||||
| I | 114 | 19 (17) | 1.0 | |
| II∼III | 145 | 92 (63) | 8.68 (4.78–15.77) | <1×10−7 |
| Molecular markers | ||||
| α-fetoprotein (ng/ml) | ||||
| ≤200 | 144 | 43 (30) | 1.0 | |
| >200 | 115 | 68 (59) | 3.40 (1.97–5.89) | 2.3×10−6 |
| p53 mutation | ||||
| No | 89 | 30 (34) | 1.0 | |
| Yes | 74 | 42 (57) | 2.58 (1.30–5.14) | 0.0032 |
| β-Catenin mutation | ||||
| No | 158 | 79 (50) | 1.0 | |
| Yes | 26 | 4 (15) | 5.50 (1.81–16.69) | 0.0011 |
| S100P expression | ||||
| No | 115 | 40 (35) | 1.0 | |
| Yes | 144 | 71 (49) | 1.82 (1.07–3.12) | 0.0189 |
To elucidate whether S100P is an independent factor for predicting patient survival, high tumor stage, and ETR, multivariate analyses using a Cox proportional-hazards model were performed (Table 3). We found that ETR (P<0.0001), high tumor stage (P = 0.0256), and S100P expression (P = 0.0044) were independent risk factors for poor survival in HCC patients.
Table 3. Multivariate analyses of prognostic factors in hepatocellular carcinoma patients.
| Covariate | Coefficient | S.E. | Z-Statistic | H.R. (95% C.I.) | P- value |
| Sex (M/F) | 0.0143 | 0.2479 | 0.0577 | 1.0144 (0.6241–1.6489) | 0.9540 |
| AFP (L/H) | 0.0628 | 0.2262 | 0.2777 | 1.0648 (0.6834–1.6591) | 0.7813 |
| Grade (L/H) | −0.2358 | 0.2227 | −1.0586 | 0.7895 (0.5106–1.2223) | 0.2898 |
| Stage (L/H) | −0.6438 | 0.2883 | −2.2329 | 0.5253 (0.2985–0.9243) | 0.0256 |
| p53 mutation (P/N) | −0.0743 | 0.2263 | −0.3282 | 0.9284 (0.5958–1.4467) | 0.7427 |
| β-catenin mutation (P/N) | −0.0149 | 0.3779 | −0.0393 | 0.9853 (0.4698–2.0663) | 0.9686 |
| ETR(P/N) | 2.1589 | 0.2690 | 8.0264 | 8.6620 (5.1128–14.6748) | <0.0001 |
| S100P expression (P/N) | 0.6481 | 0.2277 | 2.8459 | 1.9118 (1.2235–2.9873) | 0.0044 |
Abbreviations: S.E., Standard error; H.R., Hazard ratio; C.I., Confidence interval; M, male; F, female; AFP,
α-fetoprotein; ETR, early tumor recurrent; P, presence; N, absence; L: low; H: high.
Correlation of S100P Expression with p53 and β-catenin Mutations
The p53 and β-catenin genes are commonly mutated in HCCs [34], [40]. In our HCC series, the p53 mutation was detected in 84 of 187 cases (44.9%), and the β-catenin mutation was identified in 32 of 214 tumors (15.0%). As shown in Table 1, S100P protein expression correlated with the p53 mutation (P = 0.0032), and the absence of the β-catenin mutation (P = 0.0489). To better understand the role of S100P expression in the progression of HCC, we stratified HCC patients according to S100P, p53 and β-catenin gene status. As shown in Table 4, HCCs with S100P expression and p53 mutation had the highest frequencies of high-stage tumor (stage II and III) and ETR (88% and 69% of cases, respectively), which were approximately 2-fold higher than the rates of high-stage tumor and ETR in HCC cases without either S100P expression or the p53 mutation (44% and 35%; P = 1×10−6 and P = 0.00068; respectively). Hence, HCC patients with S100P expression and p53 mutation had the lowest 5-year survival (P = 0.0017 (Figure 3B), even worse than those with the p53 mutation (+)/S100P (-) (P = 0.01) or p53 mutation (-)/S100P (+) tumors (P = 0.01 and P = 0.035, respectively).
Table 4. Interaction between S100P expression with p53 mutation or β-catenin mutation in the tumor progression of hepatocellular carcinoma.
| Feature | S100P expression/p53 mutation | ||||
| Yes/Yes | Yes/No | No/Yes | No/No | P value | |
| Stage | |||||
| I | 7 (12%)a | 23 (47%) | 9 (35%) | 30 (56%)a | 0.0000115 |
| II–III | 51 (88%)b, | 26 (53%)b | 17 (65%) | 24 (44%) | |
| ETR† | |||||
| Presence | 35 (69%)c | 13 (33%)c | 7 (32%) | 17 (35%) | 0.00051 |
| Absence | 16 (31%)d | 27 (67%) | 15 (68%) | 32 (65%)d | |
| S100P expression/β-catenin mutation | |||||
| Yes/Yes | Yes/No | No/Yes | No/No | P value | |
| Stage | |||||
| I | 7 (54%)e | 27 (25%)e | 15 (79%) | 28 (38%) | 0.0000446 |
| II–III | 6 (46%) | 81 (75%)f | 4 (21%)f | 46 (62%) | |
| ETR† | |||||
| Presence | 2 (22%) | 53 (57%)g | 2 (12%)g | 26 (40%) | 0.00142 |
| Absence | 7 (78%)h | 40 (43%)h | 15 (88%) | 39 (60%) | |
Abbreviations: NS, not significant; ETR, early tumor recurrence.
Tumor recurrence within 12 months after hepatectomy.
a, b, c, d, e, f, g and h designate comparison between the indicated two groups.
P values: a0.000001; b0.00006; c0.0006; d0.00068; e0.0288; f0.000004; g0.0015; h0.0457.
In contrast to the p53 mutation, the β-catenin mutation is associated with low tumor grade, low tumor stage, and better 5-year survival in HCC [34]. Consistent with such findings, our data showed a correlation between S100P expression and the absence of the β-catenin mutation in HCC tumors (Table 1, P = 0.0489). In addition, as shown in Table 4, β-catenin-wild type HCCs with S100P expression had the highest frequencies of high tumor stage (75%) and ETR (57%), followed by HCC without S100P expression or the β-catenin mutation (62% and 40%, respectively). HCC with the β-catenin mutation but negative for S100P expression had lowest risk of high-stage tumor and ETR (21%, P = 0.0000446 and 12%, P = 0.00142, respectively). Hence, HCC patients with S100P expression and absence of β-catenin mutation had the lowest 5-year survival (Figure 3C), than those with β-catenin-mutated HCC or S100P (+), β-catenin-wild type HCC.
S100P Expression Predicts Poor Prognosis in Patients with High-stage Tumors or ETR
Because tumor stage and ETR are most important predictive factors for poor prognosis in HCC, we further analyzed the prognostic role of S100P expression in patients with high tumor stage or ETR. The combinatorial analysis showed that HCC patients with high tumor stage and S100P expression had a significantly lower -5-year survival rate (Figure 3D) than high-tumor-stage HCC patients without S100P expression (P = 0.0026). Similarly, HCC patients with ETR and S100P expression had a significantly lower 5-year survival rate (Fig. 3E) than HCC patients with ETR alone (P = 0.0002).
Discussion
Although S100P was reported to be expressed in various types of human cancer cells [13], [15], [19], [24]–[32], including human HCC [33], the clinical and pathological significances of S100P expression in HCC remains largely unclear. We found that S100P expression correlated with high tumor grade, high serum AFP level (>200 ng/mL), and large tumor size (>5cm). Dowen et al. reported that S100P expression correlated significantly with increasing grade of pancreatic intraepithelial neoplasia [42]. In addition, the down-regulation of S100P expression by small interfering RNA treatment suppressed growth and increased cellular apoptosis in Hep3B cells [33]. The knockdown of S100P expression decreased the S-phase fraction of cisplatin sensitive cell lines and slow cell proliferation [43]. These findings suggest that S100P plays an important role in facilitating tumor cell proliferation and differentiation, and S100P expression in HCC facilitates tumor cell growth and contributes to large tumor size, poor differentiation, and high AFP level.
Intrahepatic tumor spread through the portal vein system is the most crucial histological feature for high-stage HCC and is associated with poor prognosis [3]. However, the molecular markers related to vascular invasion of HCC are poorly understood. We demonstrated that high tumor stage (stage II and III) in HCC, which had vascular invasion and various degrees of intrahepatic spread, was more frequently associated with S100P expression, compared with HCCs of low tumor stage (stage I). In addition, patients with S100P-negative HCCs had better 5-year survival than patients with S00P-positive HCCs.
We also showed that S100P protein immunoreactivity was more intense at the tumor border, and that it was more diffuse and intense in satellite nodules and portal vein tumor emboli than in the main tumor mass. Arumugam et al. reported that S100P expression correlated with cell proliferation, migration, and invasion in pancreatic cancer [44]. Du et al. reported that the induction of S100P expression resulted in significantly reduced cellular adhesion and enhanced cell migration [45]. Chandramouli et al. demonstrated that the knockdown of S100P expression compromised invadopodia formation, colony growth, and cellular motility in colon cancer [46], and Zhou et al. showed that S100P plays a critical role in conferring tamoxifen resistance and enhancing cell motility [47]. These findings suggest that S100P expression is an important factor for tumor invasiveness and the metastatic potential of HCC, and may thus be associated with high tumor stage and poor prognosis.
To further elucidate the impact of the expression of S100P on the incidence of ETR, we investigated possible relationships with the major clinical and pathological factors. We found that large tumor size, high tumor grade, and high tumor stage were significant histopathological predictors of ETR, and that high AFP, the presence of the p53 mutation, and the absence of the β-catenin mutation were significant molecular factors for ETR. In addition, we demonstrated for the first time that HCCs with S100P expression were associated with a higher risk for ETR than HCCs that did not express S100P. The expression of S100P has also been shown to be a potential prognostic biomarker in colorectal cancer [48], breast cancer [49], and diffuse large B cell lymphoma [50]. These findings suggest that HCCs with S100P expression may have enhanced invasion/metastasis potential, thus contributing to more frequent ETR and poor prognosis.
In addition, the association of S100P with other molecular features, especially the p53 and β-catenin mutations [4], [34], [40], requires clarification. Mutations of the p53 and β-catenin genes contribute to two distinct pathways of hepatocarcinogenesis [51]. Inactivation of p53 leads to aberrant mitosis, chromosome instability, more aggressive tumorigenesis, and poor prognosis in HCC [4], [35], whereas the β-catenin mutation is associated with low tumor grade and low tumor stage, and better patient survival [34]. We showed that S100P expression correlated with the presence of the p53 mutation and the absence of the β-catenin mutation. Therefore, we conducted a combinatorial analysis to further elucidate the clinical significance of the mutations with regard to S100P expression. Our findings suggest that S100P expression is associated with HCC progression, and it interacts positively with the p53 mutation, contributing to more advanced disease. This observation is similar to our previous report that stathmin expression interacts positively with the p53 mutation, and contributes to advanced HCC [6]. Thus, S100P expression is an important molecular prognosticator of HCC, and contributes to poor prognosis when concurrent with the p53 mutation.
We also showed that HCC with S100P expression alone had 3- and 5-fold higher frequencies of vascular invasion (75%) and ETR (57%), than those observed for HCC with the β-catenin mutation and absence of S100P expression, which had the lowest frequency of vascular invasion (21%) and ETR (12%). Although HCC with S100P expression and the β-catenin mutation was rare in our series, occurring in only 6% of our cases, we found that the incidences of vascular invasion and ETR were higher in HCCs with S100P expression and wild type β-catenin. Further analysis showed that the majority of HCC cases with the β-catenin mutation had low tumor stage and a lower incidence of ETR, regardless of the presence or absence of S100P expression (7 of 13 versus 15 of 19 and 2 of 9 versus 2 of 17, respectively, both P>0.05). These findings suggest that mutant β-catenin exerts a strong negative effect on tumor progression that is not abolished by S100P expression. Taken collectively, these findings indicate that S100P expression and the β-catenin mutation play opposing roles in vascular invasion and ETR in HCC. These data highlight the importance of combinatorial analysis to better understand the significance of interactions between different molecular factors in disease processes, particularly in tumor progression.
Our previous studies have shown that tumor stage and ETR are independent prognostic factors in HCC [7], [11]. These findings encouraged us to investigate possible prognostic factors in HCC patients with high tumor stage and ETR. We found that S100P expression is an independent prognostic factor in HCC patients with high tumor stage and ETR. We found that HCC patients with high tumor stage and S100P expression had a lower 5-year survival rate than high tumor stage HCCs without S100P expression. Similarly, HCC patients with ETR and S100P expression had a lower 5-year survival rate than patients with S100P-negative HCC and ETR. These findings indicated that the expression of S100P exerted additional adverse effects on survival in HCC patients with high tumor stage or ETR. Thus, the stratification of patients into groups based on clinical and pathological factors may lead to a more accurate prediction of patient survival, and may aid in the development of better management strategies. Moreover, our findings suggest that the expression of S100P augments the metastatic potential of HCCs, resulting in high tumor stage, and increases the severity of the disease course, contributing to poor prognosis in HCC patients with high tumor stage or ETR.
In conclusion, our study revealed in vivo evidence that the expression of S100P is an important molecular factor for vascular invasion and intrahepatic spread, and may represent a predictive biomarker for ETR, and thus a prognosticator of unfavorable outcome. In addition, our combinatorial analysis revealed an additive unfavorable prognostic interaction of S100P expression and the p53 mutation. These findings also highlight the potential importance of combinatorial analyses for the molecular features of HCC. The expression of S100P may identify HCC patients with high tumor stage or ETR that are at increased risk for reduced survival, aiding in the development of improved management strategies.
Acknowledgments
We thank Miss Eunice J. Yuan for her secretarial assistance.
Funding Statement
This research was supported by a grant from National Taiwan University Hospital, Yun-Lin Branch (NTUHYL101.N004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Befeler AS, Di Bisceglie AM (2002) Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 122: 1609–1619. [DOI] [PubMed] [Google Scholar]
- 2. Schafer DF, Sorrell MF (1999) Hepatocellular carcinoma. Lancet 353: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 3. Pan HW, Ou YH, Peng SY, Liu SH, Lai PL, et al. (2003) Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 98: 119–127. [DOI] [PubMed] [Google Scholar]
- 4. Hsu HC, J TH, Lai PL, Lee PH, Peng SY (1993) Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res 53: 4691–4694. [PubMed] [Google Scholar]
- 5. Yuan RH, Jeng YM, Chen HL, Hsieh FJ, Yang CY, et al. (2005) Opposite roles of human pancreatitis-associated protein and REG1A expression in hepatocellular carcinoma: association of pancreatitis-associated protein expression with low-stage hepatocellular carcinoma, β-catenin mutation, and favorable prognosis. Clin Cancer Res 11: 2568–2575. [DOI] [PubMed] [Google Scholar]
- 6. Yuan RH, Jeng YM, Chen HL, Lai PL, Pan HW, et al. (2006) Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 209: 549–558. [DOI] [PubMed] [Google Scholar]
- 7. Yuan RH, Jeng YM, Pan HW, Hu FC, Lai PL, et al. (2007) Overexpression of KIAA0101 predicts high stage, early tumor recurrence, and poor prognosis of hepatocellular carcinoma. Clin Cancer Res 13: 5368–5376. [DOI] [PubMed] [Google Scholar]
- 8. Pan HW, Chou HY, Liu SH, Peng SY, Liu CL, et al. (2006) Role of L2DTL, cell cycle-regulated nuclear and centrosome protein, in aggressive hepatocellular carcinoma. Cell Cycle 5: 2676–2687. [DOI] [PubMed] [Google Scholar]
- 9. Lee YC, Pan HW, Peng SY, Lai PL, Kuo WS, et al. (2007) Overexpression of tumour-associated trypsin inhibitor (TATI) enhances tumour growth and is associated with portal vein invasion, early recurrence and a stage-independent prognostic factor of hepatocellular carcinoma. Eur J Cancer 43: 736–744. [DOI] [PubMed] [Google Scholar]
- 10. Peng SY, Lai PL, Pan HW, Hsiao LP, Hsu HC (2008) Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep 19: 1045–1053. [PubMed] [Google Scholar]
- 11. Yuan RH, Jeng YM, Hu RH, Lai PL, Lee PH, et al. (2011) Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. J Gastrointest Surg 15: 321–329. [DOI] [PubMed] [Google Scholar]
- 12. Jiang H, Hu H, Tong X, Jiang Q, Zhu H, et al. (2012) Calcium-binding protein S100P and cancer: mechanisms and clinical relevance. J Cancer Res Clin Oncol 138: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerreiro Da Silva ID, Hu YF, Russo IH, Ao X, Salicioni AM, et al. (2000) S100P calcium-binding protein overexpression is associated with immortalization of human breast epithelial cells in vitro and early stages of breast cancer development in vivo. Int J Oncol 16: 231–240. [PubMed] [Google Scholar]
- 14. Parkkila S, Pan PW, Ward A, Gibadulinova A, Oveckova I, et al. (2008) The calcium-binding protein S100P in normal and malignant human tissues. BMC Clin Pathol 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato N, Hitomi J (2002) S100P expression in human esophageal epithelial cells: Human esophageal epithelial cells sequentially produce different S100 proteins in the process of differentiation. Anat Rec 267: 60–69. [DOI] [PubMed] [Google Scholar]
- 16. Hsieh HL, Schäfer BW, Weigle B, Heizmann CW (2004) S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun 316: 949–959. [DOI] [PubMed] [Google Scholar]
- 17.Heizmann CW, Ackermann GE, Galichet A (2007) Pathologies involving the S100 proteins and RAGE. Subcell Biochem 45. [DOI] [PubMed]
- 18. Arumugam T, Simeone DM, Schmidt AM, Logsdon CD (2004) S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE). J Biol Chem 279: 5059–5065. [DOI] [PubMed] [Google Scholar]
- 19. Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, et al. (2007) RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum 50: 1230–1240. [DOI] [PubMed] [Google Scholar]
- 20. Koltzscher M, Neumann C, König S, Gerke V (2003) Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P. Mol Biol Cell 14: 2372–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austermann J, Nazmi AR, Müller-Tidow C, Gerke V (2008) Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem 283: 29331–29340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filipek A, Jastrzebska B, Nowotny M, Kuznicki J (2002) CacyBP/SIP, a calcyclin and Siah-1-interacting protein, binds EF-hand proteins of the S100 family. J Biol Chem 277: 28848–28852. [DOI] [PubMed] [Google Scholar]
- 23. Becker T, Gerke V, Kube E, Weber K (1992) S100P, a novel Ca2+-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. Eur J Biochem 207: 541–547. [DOI] [PubMed] [Google Scholar]
- 24. Schor AP, Carvalho FM, Kemp C, Silva ID, Russo J (2006) S100P calcium-binding protein expression is associated with high-risk proliferative lesions of the breast. Oncol Rep 15: 3–6. [PubMed] [Google Scholar]
- 25. Bartling B, Rehbein G, Schmitt WD, Hofmann HS, Silber RE, et al. (2007) S100A2-S100P expression profile and diagnosis of non-small cell lung carcinoma: impairment by advanced tumour stages and neoadjuvant chemotherapy. Eur J Cancer 43: 1935–1943. [DOI] [PubMed] [Google Scholar]
- 26. Crnogorac-Jurcevic T, Missiaglia E, Blaveri E, Gangeswaran R, Jones M, et al. (2003) Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol 201: 63–74. [DOI] [PubMed] [Google Scholar]
- 27. Ohuchida K, Mizumoto K, Egami T, Yamaguchi H, Fujii K, et al. (2006) S100P is an early developmental marker of pancreatic carcinogenesis. Clin Cancer Res 12: 5411–5416. [DOI] [PubMed] [Google Scholar]
- 28. Basu GD, Azorsa DO, Kiefer JA, Rojas AM, Tuzmen S, et al. (2008) Functional evidence implicating S100P in prostate cancer progression. Int J Cancer 123: 330–339. [DOI] [PubMed] [Google Scholar]
- 29. Surowiak P, Maciejczyk A, Materna V, Drag-Zalesińska M, Wojnar A, et al. (2007) Unfavourable prognostic significance of S100P expression in ovarian cancers. Histopathology 51: 125–128. [DOI] [PubMed] [Google Scholar]
- 30. Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, et al. (2011) Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol 35: 590–598. [DOI] [PubMed] [Google Scholar]
- 31. Hamada S, Satoh K, Hirota M, Kanno A, Ishida K, et al. (2011) Calcium-binding protein S100P is a novel diagnostic marker of cholangiocarcinoma. Cancer Sci 102: 150–156. [DOI] [PubMed] [Google Scholar]
- 32. Tsai JH, Huang WC, Kuo KT, Yuan RH, Chen YL, et al. (2012) S100P immunostaining identifies a subset of peripheral-type intrahepatic cholangiocarcinomas with morphological and molecular features similar to those of perihilar and extrahepatic cholangiocarcinomas. Histopathology 61: 1106–1116. [DOI] [PubMed] [Google Scholar]
- 33. Kim JK, Jung KH, Noh JH, Eun JW, Bae HJ, et al. (2009) Targeted disruption of S100P suppresses tumor cell growth by down-regulation of cyclin D1 and CDK2 in human hepatocellular carcinoma. Int J Oncol 35: 1257–1264. [PubMed] [Google Scholar]
- 34. Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, et al. (2000) β-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 157: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsu HC, Peng SY, Lai PL, Chu JS, Lee PH (1994) Mutations of p53 gene in hepatocellular carcinoma (HCC) correlate with tumor progression and patient prognosis. Int J Oncol 4: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 36. Edmonson HA, Steiner PE (1954) Primary carcinoma of the liver: A study of 100 among 489,000 necropsies. Cancer (Phila) 7: 462–503. [DOI] [PubMed] [Google Scholar]
- 37.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al.. (2010) AJCC cancer staging manual (7th ed). New York, NY: Springer.
- 38. Huang LR, Hsu HC (1995) Cloning and expression of CD24 gene in human hepatocellular carcinoma: a potential early tumor marker gene correlates with p53 mutation and tumor differentiation. Cancer Res 55: 4717–4721. [PubMed] [Google Scholar]
- 39. Hsu HC, Cheng W, Lai PL (1997) Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res 57: 5179–5184. [PubMed] [Google Scholar]
- 40. Hsu HC, Huang AM, Lai PL, Chien WM, Peng SY, et al. (1994) Genetic alterations at the splice junction of p53 gene in human hepatocellular carcinoma. Hepatology 19: 122–128. [PubMed] [Google Scholar]
- 41. Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, et al. (2004) High α-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and β-catenin mutations. Int J Cancer 112: 44–50. [DOI] [PubMed] [Google Scholar]
- 42. Dowen SE, Crnogorac-Jurcevic T, Gangeswaran R, Hansen M, Eloranta JJ, et al. (2005) Expression of S100P and its novel binding partner S100PBPR in early pancreatic cancer. Am J Pathol 166: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang X, Monitto CL, Demokan S, Kim MS, Chang SS, et al. (2010) Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res 70: 2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arumugam T, Simeone DM, Van Golen K, Logsdon CD (2005) S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res 11: 5356–5364. [DOI] [PubMed] [Google Scholar]
- 45. Du M, Wang G, Ismail TM, Gross S, Fernig DG, et al. (2012) S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem 287: 15330–15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chandramouli A, Mercado-Pimentel ME, Hutchinson A, Gibadulinová A, Olson ER, et al. (2010) The induction of S100p expression by the Prostaglandin E2 (PGE2)/EP4 receptor signaling pathway in colon cancer cells. Cancer Biol Ther 10: 1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou C, Zhong Q, Rhodes LV, Townley I, Bratton MR, et al. (2012) Proteomic analysis of acquired tamoxifen resistance in MCF-7 cells reveals expression signatures associated with enhanced migration. Breast Cancer Res 14: R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Q, Zhang YN, Lin GL, Qiu HZ, Wu B, et al. (2012) S100P, a potential novel prognostic marker in colorectal cancer. MOncol Rep 28: 303–310. [DOI] [PubMed] [Google Scholar]
- 49. Wang G, Platt-Higgins A, Carroll J, de Silva Rudland S, Winstanley J, et al. (2006) Induction of metastasis by S100P in a rat mammary model and its association with poor survival of breast cancer patients. Cancer Res 66: 1199–1207. [DOI] [PubMed] [Google Scholar]
- 50. Abd El, All H (2007) Smooth muscle actin and s100p on non germinal centre diffuse large B cell lymphoma are adverse prognostic factors: pilot study. Diagn Pathol 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, et al. (2001) Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 120: 1763–1773. [DOI] [PubMed] [Google Scholar]