Abstract
Taurolithocholate (TLC) acutely inhibits biliary excretion of Mrp2 substrates by inducing Mrp2 retrieval from the canalicular membrane, while cAMP increases plasma membrane MRP2. The effect of TLC may be mediated via protein kinase Cε (PKCε). Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) is a membrane bound F-actin cross linking protein and is phosphorylated by PKCs. MARCKS phosphorylation has been implicated in endocytosis and the underlying mechanism appears to be detachment of phosphorylated MARCKS from the membrane. The aim of the present study was to test the hypothesis that TLC-induced MRP2 retrieval involves PKCε mediated MARCKS phosphorylation. Studies were conducted in HuH7 cells stably transfected with NTCP (HuH-NTCP cells) and in rat hepatocytes. TLC increased plasma membrane (PM) PKCε and decreased PM-MRP2 in both HuH-NTCP cells and hepatocytes. Cyclic AMP did not affect PM-PKCε and increased PM-MRP2 in these cells. In HuH-NTCP cells, dominant negative (DN) PKCε reversed TLC-induced decreases in PM-MRP2 without affecting cAMP-induced increases in PM-MRP2. TLC, but not cAMP, increased MARCKS phosphorylation in HuH-NTCP cells and hepatocytes. TLC and PMA increased cytosolic phospho-MARCKS and decreased PM-MARCKS in HuH-NTCP cells. TLC failed to increase MARCKS phosphorylation in HuH-NTCP cells transfected with DN-PKCε suggesting PKCε mediated phosphorylation of MARCKS by TLC. In HuH-NTCP cells transfected with phosphorylation deficient MARCKS, TLC failed to increase MARCKS phosphorylation and to decrease plasma membrane MRP2.
Conclusion
Taken together, these results support the hypothesis that TLC-induced MRP2 retrieval involves TLC mediated activation of PKCε followed by MARCKS phosphorylation and consequent detachment of MARCKS from the membrane.
Keywords: DN-PKCε, Phosphorylation deficient MARCKS, PMA, cAMP, HuH-NTCP cells
Multidrug resistant associated protein 2 (MRP2; ABCC2), an ABC transporter located at the canalicular membrane of hepatocytes, is involved in biliary secretion of conjugated endogenous and exogenous organic anions {1, 2}. MRP2 has been shown to undergo both transcriptional and post-translational regulation in cholestasis. For example, transcription of MRP2 is down regulated in rodent models of cholestasis {3} and during liver regeneration {4}. Cholestatic agents, such as taurolithocholate (TLC) {5} or estradiol-17β-glucuronide (E217G) {6}, induce retrieval of MRP2 from the canalicular membrane. More recent studies suggest that PKCs may be involved in the retrieval of MRP2 by TLC and E217G. Based on studies with chemical inhibitors, it has been proposed that the effect of E217G may be mediated via classical PKC-induced endocytosis {7} and PI3K/Akt signaling pathway {8}. Similarly, the TLC-induced retrieval of Mrp2 has been suggested to be mediated via a PI3K- and PKCε-dependent mechanism {9, 10}. However, the role of PKCε in TLC-induced MRP2 retrieval has not been directly evaluated. Moreover, signaling pathways by which PKCε may induce MRP2 retrieval have not been investigated.
PKCs mediate effects by phosphorylating their substrates. MARCKS (Myristoylated Alanine-Rich C Kinase (PKC) Substrate) is one such substrate and plays a key role in cytoskeletal dynamics {11, 12}. MARCKS is an F-actin cross-linking protein and is phosphorylated by cPKCα, PKCδ and PKCε in vitro {13, 14}. Phosphorylation of MARCKS by PKCδ and PKCε has been shown to be involved in exocytosis and endocytosis in non-hepatic cells. Thus, MARCKS phosphorylation by PKCδ is involved in airway mucin secretion {15, 16} and gut peptide secretion {17}. MARCKS phosphorylation by PKCε has been shown to stimulate vesicle translocation in chromaffin cells {18} and basolateral fluid-phase endocytosis in T84 cells {19}. Phosphorylation of MARCKS by PKCs results in the retrieval of MARCKS from the plasma membrane to the cytosol and in F-actin disassembly {18}. It may be noted that actin plays an important role in hepatobiliary transporter translocation {20–22} and TLC induces F-actin accumulation around bile canaliculi {23}. Phosphorylation of MARCKS by PKCs requires translocation of PKCs to MARCKS located in the plasma membrane, and as a result, MARCKS phosphorylation and the consequent effect are dependent on subcellular targeting of PKC {24, 25}. These studies raise the possibility that TLC-induced endocytic retrieval of Mrp2 may result from PKCε-dependent MARCKS phosphorylation.
In the present study we determined whether TLC-induced MRP2 retrieval is mediated via PKCε and whether the effect of PKCε is mediated via MARCKS phosphorylation. Results of our studies with dominant negative (DN) PKCε and phosphorylation deficient (PD) MARCKS in HuH-NTCP cells are consistent with the following signaling pathway: TLC→PKCε→MARCKS phosphorylation→MRP2 retrieval.
Materials and Methods
Materials
8-(4-chlorophenylthio)-cAMP (CPT-cAMP), wortmannin and the antibody for human MRP2 were purchased from Sigma-Aldrich (St. Louis, MO). Commercial sources of other antibodies were Cell Signaling (pMARCKS & HA), Calbiochem (actin), Clontech (GFP), Upstate (PKCε), BD Transduction Laboratories (E-Cadherin). Sulfosuccinimidyl-6-(biotin-amido)hexanoate (Sulfo-NHS-LC-Biotin) was purchased from Pierce (Rockford, IL). Streptavidin beads were purchased from Novagen (Madison, WI). Lipofectamin 2000 was obtained from Invitrogen (Carlsbad, CA). Plasmid constructs for WT and phosphorylation deficient MARCKS (PD-MARCKS with the effector domain phosphorylation sites at S152, S156 and S163 replaced by alanine) were kind gifts from Dr. Saito {26}. Kinase dead dominant negative (DN) PKCε plasmids were purchased from Addgene (Cambridge, MA). HuH-NTCP cells (HuH-7 cells stably transfected with human NTCP) were generously provided by Dr. Gores {27}.
Rat Hepatocytes Preparation
Rat hepatocytes were isolated from male Wistar rats (200–250g) and cultured as previously described {28}, and used to determine the effect of TLC on PKCε, Mrp2 and phosphorylation of MARCKS.
HuH-NTCP Cell Culture and Transfections
HuH-NTCP cells were cultured in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, 1.2g/L G418, 100,000 units/liter penicillin, 100 mg/liter streptomycin and 25 μg/mL amphotericin B at 37 °C in a 5% CO2, 95% O2 air incubator. For transfection experiments involving DN-PKCε, WT-MARCKS and PD-MARCKS, the cells were cultured in 6-well plates for 24 h and then transiently transfected with 1–3 μg of the desired plasmid using Lipofectamine as previously described {29}. Following 24 h of incubation in the transfection medium, the cells were cultured for an additional 24 h in culture medium. The expression of these plasmids was confirmed by immunoblot analysis using anti-HA (for PKCε), anti-GFP (for WT-MARCKS and PD-MARCKS) antibodies. Cells were transfected at 70–80% confluence and non-transfected cells were at 80–90% confluence before treatments. For all experiments, cells were then incubated in serum-free media for 3 h at 37 °C before treatments as described under figure legends.
Plasma membrane MRP2 and PKCε
A cell surface protein biotinylation method as previously described by us {20, 30–32} was used to assess MRP2 and PKCε translocation to plasma membranes. Briefly, following various treatments cell surface proteins were biotinylated by exposing hepatocytes to sulfo-NHS-LC-Biotin followed by preparation of a whole cell lysate. Biotinylated proteins were isolated using streptavidin-agarose beads and then subjected to immunoblot analysis to determine plasma membrane MRP2, PKCε and E-cadherin. The amount of MRP2 and PKCε present at the plasma membrane was expressed as a relative value compared to E-cadherin (a plasma membrane protein), which was used as a loading control.
Other Methods
Phosphorylation of MARCKS was determined using phospho-MARCKS (Ser152/156) antibody. The Lowry method {33} was used to determine cell protein. The blots were scanned using Adobe Photoshop® (Adobe Systems, Incorporated, San Jose, CA), and the relative band densities were quantitated using Sigmal Gel® (Jandel Scientific Software, San Rafael, CA). All values were expressed as mean ± S.E. Analysis of variance followed by Fisher’s least significant difference (LSD) test was used to statistically analyze the data, with p<0.05 considered significant.
Results
TLC-induced MRP2 internalization is mediated via PKCε
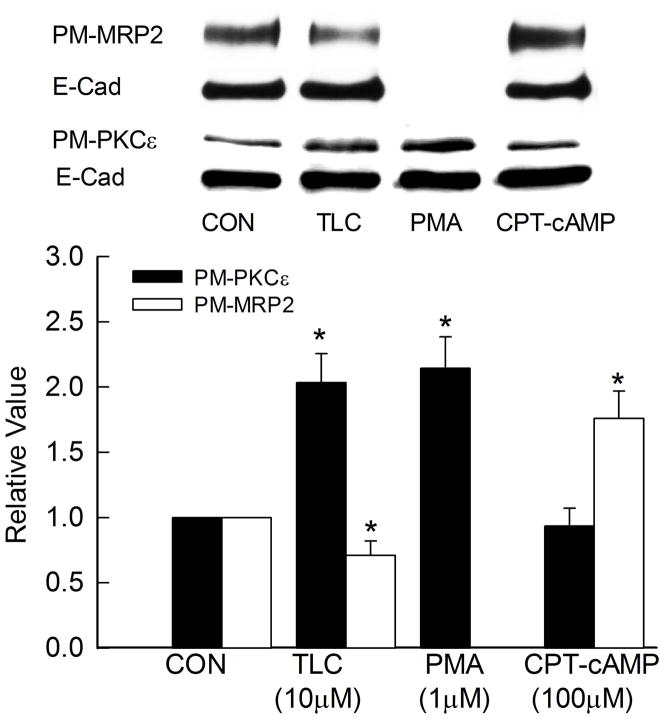
TLC has been shown to activate PKCε {9, 10} and internalize Mrp2 in rat hepatocytes {5}. This is further confirmed by our studies showing that TLC, but not cAMP, increased PM-PKCε in rat hepatocytes (Supplementary Fig. 1). Furthermore, TLC decreased and cAMP increased PM-MRP2 in rat hepatocytes (Supplementary Fig. 2). In the present study, we tested the hypothesis that this effect of TLC is mediated via PKCε using DN-PKCε in HuH-NTCP cells, which constitutively express MRP2 {32}. To ascertain that HuH-NTCP cells is a valid model, we first determined whether TLC activates PKCε and internalizes MRP2 in this cell line. To determine the effects of PKCε and MRP2, cells were treated with TLC for 15 min or 25 min, respectively. These time points are based on previous studies reporting the effect of TLC on PKCε activation in HuH-NTCP cells {34} and biliary excretion of Mrp2 substrate in perfused rat livers {5}. TLC increased plasma membrane translocation of PKCε and decreased PM-MRP2 (Fig. 1) in HuH-NTCP cells. PMA, used as a positive control, also increased plasma membrane PKCε. Cyclic AMP, used as a negative control, did not affect plasma membrane PKCε; cAMP does not activate PKCε in rat hepatocytes {31}. Cyclic AMP also increased PM-MRP2 in HuH-NTCP cells (Fig. 1). Thus, HuH-NTCP cells were considered a valid model to study the role of PKCε on TLC-induced MRP2 internalization.
Figure 1.
TLC induces plasma membrane translocation of PKCε and retrieval of MRP2. HuH-NTCP cells were incubated with 10 μM TLC, 1μM PMA or 100μM CPT-cAMP for 15 followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated PKCε (PM-PKCε, 88kD) and E-cadherin (E-Cad as loading control, 135kD). For MRP2 assay, cells were treated with 10μM TLC (25 min) or 100μM CPT-cAMP (15 min) followed by and immunoblot analysis of biotinylated MRP2 (PM-MRP2, 195kD) and E-cadherin. Typical PM-MRP2, PM-PKCε and E-Cad immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=5) are shown in the bar graph. *Significantly different (p<0.05) from respective control (CON) values.
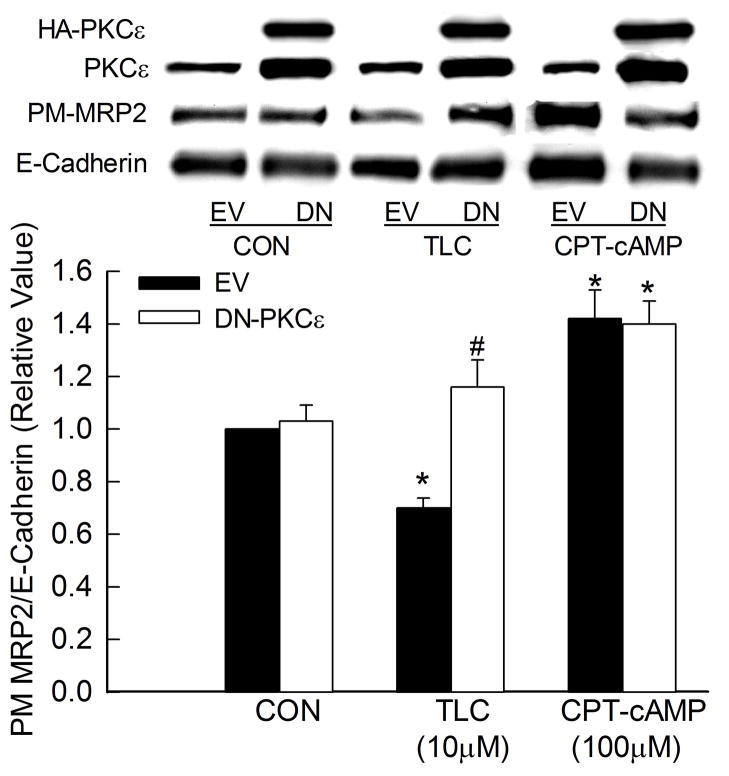
Transfection of HuH-NTCP cells with HA tagged DN-PKCε resulted in over-expression of total PKCε by 2–3 folds (Fig. 2). DN-PKCε did not affect basal expression of MRP2 in the plasma membrane when compared to empty vector. TLC decreased plasma membrane expression of MRP2 in cells transfected with empty vector. However, this effect was reversed in cells transfected with DN-PKCε. Cyclic AMP, which has been shown to increase plasma membrane expression of MRP2 by activating PKCδ {31, 32}, was used as a negative control. The ability of cAMP to increase plasma membrane MRP2 was not affected by DN-PKCε. These results support the hypothesis that TLC induced internalization of MRP2 is mediated via PKCε and that cAMP-mediated translocation of MRP2 to plasma membrane does not involve PKCε.
Figure 2.
DN-PKCε inhibits TLC-induced MRP2 retrieval. HuH-NTCP cell transfected with HA-tagged DN-PKCε were treated with TLC for 25 min or CPT-cAMP for 15 min followed by biotinylation of cell surface proteins and immunoblot analysis of HA, PKCε, biotinylated MRP2 (PM-MRP2) and biotinylated E-cadherin. Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=4) are shown in the bar graph. *Significantly different from respective control (CON) and #significantly different from respective empty vector (EV) values.
TLC phosphorylates and translocates MARCKS into cytosol
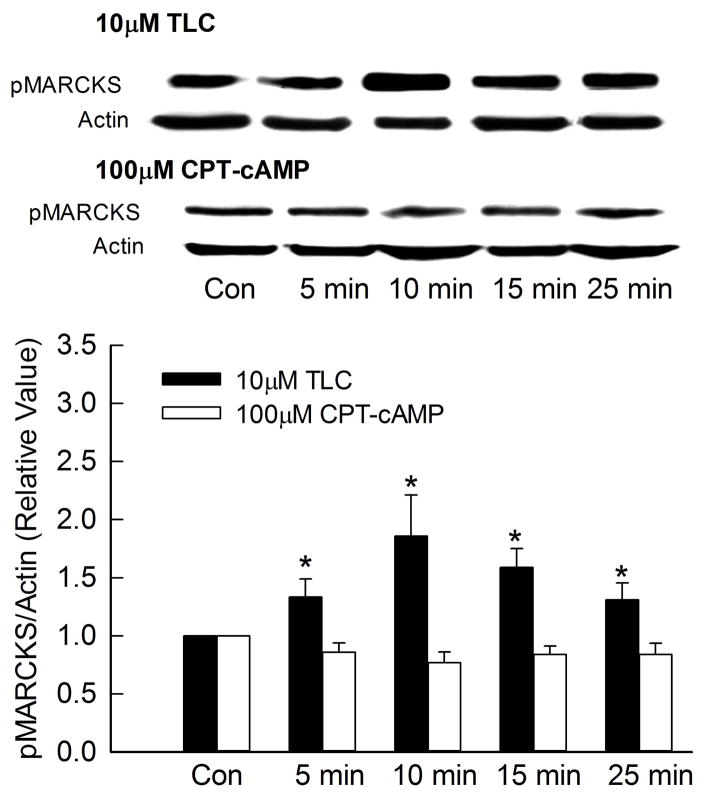
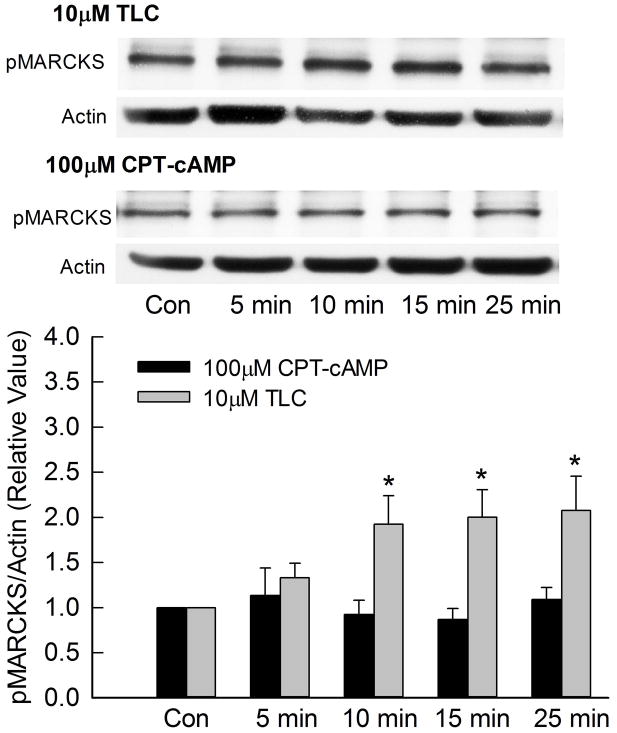
Since MARCKS is a substrate for PKC and has been implicated in endocytosis {19}, it is possible that TLC-induced MRP2 internalization involves TLC/PKCε mediated phosphorylation of MARCKS. To test this hypothesis, we first determined whether TLC can phosphorylate MARCKS. In these studies, actin instead of MARCKS was used as the loading control, since MARCKS antibody gave inconsistent results on stripped blots. A time dependent study showed that TLC increased MARCKS phosphorylation as early as 5 min with significant phosphorylation observed until 25 min (Fig. 3). On the other hand, cAMP, which stimulates MRP2 translocation to the plasma membrane, did not phosphorylate MARCKS during the same time period. Similar results were obtained in rat hepatocytes (Fig. 3B) indicating that this is not an effect specific to transformed cells. Thus, MARCKS phosphorylation may be involved in MRP2 retrieval and not MRP2 translocation to the membrane.
Figure 3.
TLC phosphorylates MARCKS. HuH-NTCP cells (A) or cultured rat hepatocytes (B) were treated with 10 μM TLC or 100 μM CPT-cAMP for the indicated time followed by determination of phosphorylated MARCKS (pMARCKS, 80kD) and actin (loading control). Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=5) are shown in the bar graph. *Significantly different (p < 0.05) from control (Con) values.
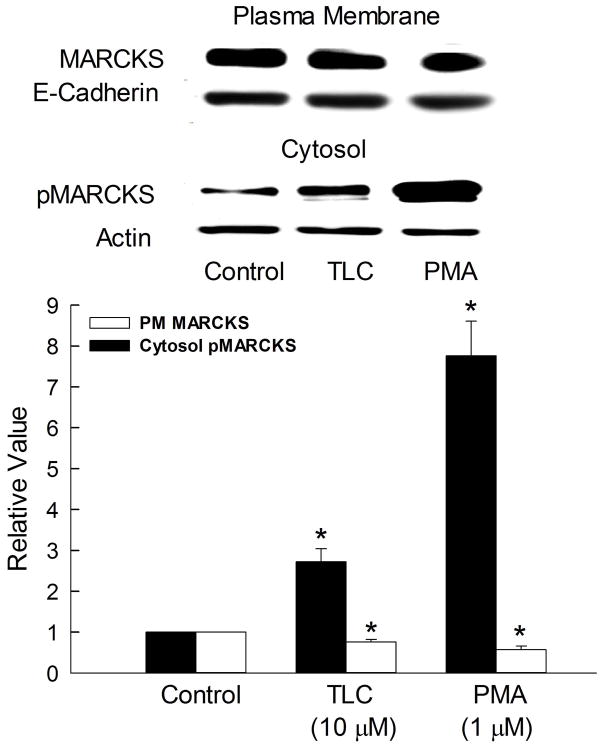
One of the consequences of MARCKS phosphorylation is the retrieval of MARCKS from the plasma membrane to the cytosol resulting in F-actin disassembly {18}. Thus, we determined whether TLC increases cytosolic phosphorylated MARCKS (pMARCKS). TLC increased cytosolic pMARCKS by 2.5 fold compared to controls (Fig. 4). PMA, used as a positive control, increased cytosolic pMARCKS by over 7 fold. The more pronounced effect of PMA is likely to be due to activation of other PKCs. The observed increases in cytosolic phospho-MARCKS were associated with decreases in PM-MARCKS (Fig. 4), indicating translocation of MARCKS from the membrane to the cytosol following phosphorylation. This result suggests that TLC-induced phosphorylation and subsequent removal of MARCKS from the plasma membrane may be related to MRP2 retrieval by TLC.
Figure 4.
TLC increases cytosolic pMARCKS. HuH-NTCP cells were treated with 10μM TLC or 1μM PMA for 15 min followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated plasma membrane MARCKS and E-cadherin or isolation of cytosol (100,000xg supernatant) and determination of phospho-MARCKS (pMARCKS). Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=3) are shown in the bar graph. *Significantly different (p < 0.05) from control (con) values.
TLC-induced MARCKS phosphorylation is mediated via PKCε
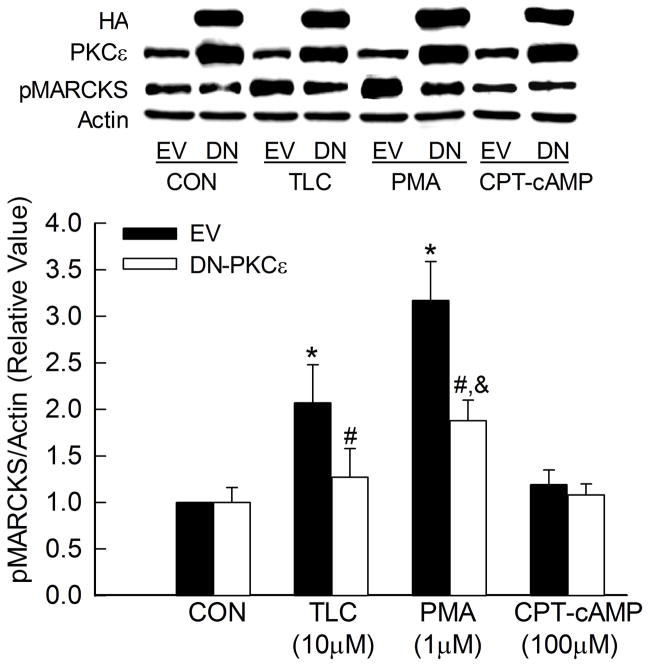
MARCKS is a substrate for PKCs and TLC may activate PKCs other than PKCε. To determine whether TLC-induced MARCKS phosphorylation is mediated via PKCε, we studied the effect of DN-PKCε on MARCKs phosphorylation (Fig. 5). DN-PKCε did not affect the basal level of MARCKS phosphorylation. TLC increased MARCKS phosphorylation in cell transfected with an empty vector (EV) but failed to do so in cells transfected with DN-PKCε. The effect of PMA, used as a positive control, on MARCKS phosphorylation was also significantly decreased by DN-PKCε. The residual MARCKS phosphorylation by PMA is likely due to activation of other PKCs. As expected, the effect of cAMP, used as a negative control, was not affected by DN-PKCε. These results are consistent with the hypothesis that TLC-induced MARCKS phosphorylation is mediated via PKCε.
Figure 5.
TLC-induced MARCKS phosphorylation is mediated via PKCε. HuH-NTCP cell transfected with HA-tagged DN-PKCε were treated with 10μM TLC, 1μM PMA or 100μM CPT-cAMP for 15 followed by immunoblot analysis of HA, PKCε, pMARCKS and actin. Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=5) are shown in the bar graph. *Significantly different from empty (EV) control (CON), &significantly different from DN-PKCε control and #significantly different from respective empty vector (EV) values.
Phosphorylation deficient (PD) MARCKS inhibits TLC-induced MRP2 retrieval
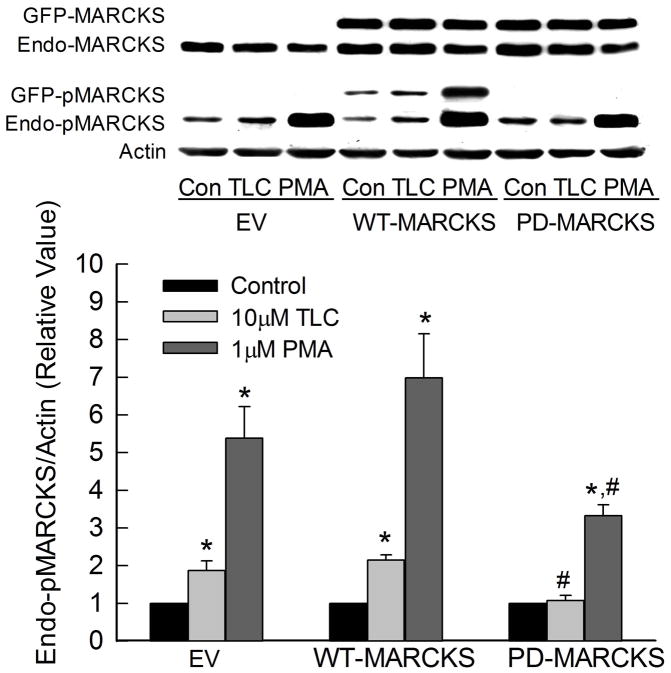
MARCKS phosphorylation has been implicated in fluid-phase endocytosis in T84 cells {19}. Thus, it is possible that MARCKS phosphorylation may be involved in TLC-induced MRP2 retrieval. We tested this hypothesis by determining the effect of TLC on plasma membrane MRP2 in cells transfected with GFP-tagged WT- and PD-MARCKS. First we determined the effect of WT- and PD-MARCKS on TLC-induced MARCKS phosphorylation (Fig. 6). Since transfected MARCKS were tagged with GFP (26.9 kD), this allowed us to distinguish between transfected (GFP-MARCKS) and endogenous MARCKS (Endo-MARCKS) at the same time when probed with MARCKS antibody; GFP-MARCKS (107 kD) appeared above Endo-MARCKS (80 kD). Transfection with GFP-MARCKS did not affect the level of endogenous MARCKS (Fig. 6), and GFP-MARCKS represented 50–80% of total MARCKS (GFP + endogenous MARCKS). Phosphorylation of GFP-MARCKS was detected in cells transfected with WT-MARCKS. In contrast, no phosphorylation of GFP-MARCKS was detected in cells transfected with PD-MARCKS confirming the inability of PD-MARCKS to be phosphorylated. TLC increased phosphorylation of endogenous and transfected MARCKS in cells transfected with empty vector or WT-MARCKS. However, TLC failed to increase phosphorylation of endogenous MARCKS in cells transfected with PD-MARCKS. The ability of PMA to increase MARCKS phosphorylation decreased significantly in cells transfected with PD-MARCKS.
Figure 6.
Phosphorylation deficient MARCKS (PD-MARCKS) inhibit TLC-induced phosphorylation of endogenous MARCKS. HuH-NTCP cell transfected with empty vector (EV) GFP-tagged WT- or PD-MARCKS (107kD) were treated with 10μM TLC or 1μM PMA for 15 min followed by immunoblot analysis of MARCKS, pMARCKS and actin. Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=5) are shown in the bar graph. Control values for EV, WT-MARCKS and PD-MARCKS were set at 1. *Significantly different from respective control values and #significantly different from respective empty vector (EV) value.
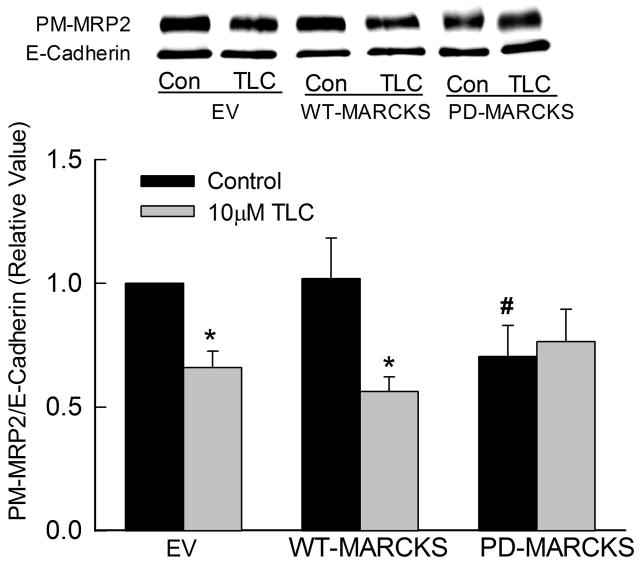
TLC also decreased plasma membrane MRP2 in cells transfected with empty vector or WT-MARCKS (Fig. 7). The basal level of plasma membrane MRP2 was not affected in cells transfected with WT-MARCKS. However, plasma membrane MRP2 decreased by 30% in cells transfected with PD-MARCKS raising the possibility that MRP2 may be stabilized in the membrane by unphosphorylated MARCKS (see discussion). In addition, TLC failed to further decrease plasma membrane MRP2 in cells transfected with PD-MARCKS. These results suggest that phosphorylation of MARCKS is necessary for TLC-induced retrieval of MRP2.
Figure 7.
PD-MARCKS decreases plasma membrane MRP2 and inhibits TLC-induced MRP2 retrieval. HuH-NTCP cell transfected with GFP-tagged WT- or PD-MARCKS were treated with 10μM TLC for 25 min followed by biotinylation of plasma membrane proteins and immunoblot analysis of PM-MRP2 and E-cadherein. Typical immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=5) are shown in the bar graph. *Significantly different from respective empty vector (EV) values and #significantly different from EV control (Con) values.
Discussion
The aim of the present study was to further define the mechanism by which TLC induces retrieval of MRP2. The present study showed that TLC increased plasma membrane localization of PKCε and a kinase dead DN-PKCε inhibited TLC-induced MRP2 retrieval. In addition, DN-PKCε inhibited TLC-induced increases in phosphorylation of MARCKS and phosphorylation deficient MARCKS inhibited TLC-induced MRP2 retrieval. These results suggest that TLC-induced MRP2 retrieval involves activation of PKCε followed by phosphorylation of MARCKS as discussed below.
PKCε has been suggested to be involved in TLC-induced cholestasis {9}. However, this conclusion is based on indirect evidence. Strongest evidence in favor of this hypothesis has been the reversal of TLC-induced membrane translocation of PKCε and cholestasis by tauroursodeoxycholate {9}. In the present study, we tested this hypothesis more directly by using DN-PKCε. Present study showed that, as previously reported in rat hepatocytes {5, 10}, TLC-induced translocation of PKCε to the plasma membrane and retrieval of MRP2 from the plasma membrane in HuH-NTCP cells as well as in rat hepatocytes. TLC failed to induce MRP2 retrieval when cells were transfected with kinase dead DN-PKCε, indicating that the PKCε kinase activity is needed for TLC-induced MRP2 retrieval. This is the first direct demonstration of a role for PKCε in MRP2 retrieval by TLC.
Our studies also provide evidence for PKCε-mediated phosphorylation of MARCKS by TLC. MARCKS is a protein kinase C substrate and binds non-covalently to plasma membrane {12}. MARCKS phosphorylation leads to its translocation to the cytosol in chromaffin cells {18}. A previous study reported that PMA translocated MARCKS from the plasma membrane to the cytosol in HepG2 cells and this effect, based on inhibition by chemical inhibitors of PKCs, appeared to be mediated via Ca2+-dependent as well as Ca2+-independent PKCs {35}. However, whether PMA phosphorylated MARCKS was not determined. In the present study we observed that TLC induced phosphorylation of MARCKS, increased the cytosolic levels of phospho-MARCKS and decreased PM-MARCKS. Thus, TLC mediated phosphorylation of MARCKS results in dissociation of MARCKS from the membrane. In addition, TLC-induced MARCKS phosphorylation was inhibited in cells transfected with DN-PKCε. These results would suggest that TLC acting via PKCε phosphorylates MARCKS resulting in dissociation of MARCKS from the plasma membrane.
The present study suggests that MARCKS phosphorylation by PKCε is involved in MRP2 retrieval by TLC. This is supported by results that TLC failed to induce MRP2 retrieval in cells transfected with PD-MARCKS (Fig. 7). While the role of MARCKS phosphorylation has been investigated in other cell types, little is known about its effect in hepatocytes. PMA has been shown to phosphorylate and translocate MARCKS to lysosome in rat hepatocytes {36}. Studies in most other cell types suggest a role of MARCKS in exocytosis and exocytotic insertion of membrane proteins. Thus, the phosphorylation of MARCKS has been implicated in neurotransmitter release {37}, glucose-induced secretion in isolated rat pancreatic islets {38}, release of ATCH in ovine anterior pituitary cells {39}, thrombin-induced serotonin release from platelets {40}, insulin-induced Glut4 translocation to the plasma membrane in rat skeletal muscle cells {41} and mucin secretion in bronchial epithelial cells {15}. However, MARCKS phosphorylation, most likely by PKCε, has also been suggested to be involved in basolateral fluid phase endocytosis in T84 cells {19}. MARCKS phosphorylation has also been suggested to be involved in abnormal endocytic pathway in Alzheimer disease {42}. Based on these studies and results of the present study we suggest that MARCKS phosphorylation leads to endocytic retrieval of MRP2 in hepatocytes. To our knowledge, this is the first study implicating MARCKS phosphorylation in membrane transporter retrieval in hepatocytes.
The precise intracellular mechanisms by which MARCKS regulates endocytosis and exocytosis have not been fully elucidated {12, 43}. The finding that MARCKS can bind directly to actin and crosslinks it to plasma membrane {44} has led to the suggestion that actin is essential to the overall functioning of MARCKS. The binding of MARCKS to the membrane requires electrostatic interaction of basic (serine) residues of MARCKS in its effector domain with acidic lipids of the membrane and hydrophobic insertion of myristate into the core of the membrane. Both of these two interactions are necessary for significant membrane binding {12, 43, 44}. When the serine residues in the effector domain of MARCKS is phosphorylated by PKC or replaced by alanine as in PD-MARCKS, the electrostatic interaction between MARCKS and the acidic lipids is abolished resulting in dissociation of MARCKS from the membrane. Because of the proximity of MARCKS phosphorylation sites to the actin binding site {45}, MARCKS phosphorylation also results in the release of actin and a local softening (disruption) of the actin cytoskeletal with increased plasticity and endocytosis {11, 12}. Thus, it can be speculated that unphosphorylated MARCKS by binding and tethering actin stabilizes MRP2 in the membrane as it is suggested for other actin crosslinking proteins, radixin {46, 47} and NHERF-1 {48}. Consistent with this hypothesis is a recent study in rats showing that taurochenodexoycholate-induced retrieval of MRP2 is associated with changes in actin cytoskeleton {49}. Since three serine residues are replaced by alanine in PD-MARCKS, such a mechanism can also explain decreased plasma membrane MRP2 in cells transfected with PD-MARCKS (Fig. 7) presumably due to the inability of PD-MARCKS to bind membrane and thereby crosslink actin resulting in actin cytoskeletal changes. It should however be noted that endocytic retrieval of a transporter is a complex process requiring participation of a number of regulatory proteins {42, 50} and MARCKS phosphorylation may also affect these regulators. Thus, further studies are needed to define the mechanism by which MARCKS phosphorylation leads to MRP2 retrieval.
In summary, results of the present study support the hypothesis that TLC-induced retrieval of MRP2 from plasma membrane involves activation of PKCε followed by PKCε-mediated phosphorylation of MARCKS. Unlike in most other cell types, MARCKS may be involved in endocytosis in hepatic cells.
Supplementary Material
Supplementary Figure 1. TLC induces plasma membrane translocation of PKCε in rat hepatocytes. Cultured hepatocytes cells were incubated with 10 μM TLC, 1μM PMA or 100μM CPT-cAMP for 15 followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated PKCε (PM-PKCε) and E-cadherin (E-Cad as loading control). Typical PM-PKCε and E-Cad immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=4) are shown in the bar graph. *Significantly different (p<0.05) from respective control values.
Supplementary Figure 2. TLC induces retrieval of Mrp2 in rat hepatocytes. Cultured hepatocytes cells were incubated with 10 μM TLC (25 min) or 100μM CPT-cAMP (15 min) followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated MRP2 (PM-MRP2) and E-cadherin. Typical PM-MRP2 and E-Cad immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=4) are shown in the bar graph. *Significantly different (p<0.05) from respective control values.
Acknowledgments
We thank Holly Jameson and Ariel Hobson for excellent technical assistance.
Financial Support:
This study was supported in part by National Institutes of Health Grants DK-33436 and DK-90010 (MSA) and DK-65975 (CRLW).
List of Abbreviations
- TLC
taurolithocholate
- TCDC
taurochenodeoxycholate
- PMA
phorbol myristate acetate
- MARCKS
Myristoylated Alanine-Rich C Kinase Substrate
- PKC
protein kinase C
- PD
phosphorylation deficient
- DN
dominant negative
- WT
wild type
- NTCP
Sodium Taurocholate Cotransporting Polypeptide
- HuH-NTCP cells
HuH7 cells stably transfected with NTCP
- HA
Hemagglutinin
Contributor Information
Christopher M. Schonhoff, Email: Christopher.Schonhoff@Tufts.edu.
Cynthia R. L. Webster, Email: cynthia.leveille-webster@tufts.edu.
M. Sawkat Anwer, Email: Sawkat.Anwer@tufts.edu.
References
- 1.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 2.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302:407–415. doi: 10.1124/jpet.102.035014. [DOI] [PubMed] [Google Scholar]
- 3.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang TH, Hakamada K, Toyoki Y, Tsuchida S, Sasaki M. Expression of MRP2 and MRP3 during liver regeneration after 90% partial hepatectomy in rats. Transplantation. 2004;77:22–27. doi: 10.1097/01.TP.0000089234.93366.6D. [DOI] [PubMed] [Google Scholar]
- 5.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, et al. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 6.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology. 2002;35:1409–1419. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 7.Crocenzi FA, Sanchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, et al. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48:1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boaglio AC, Zucchetti AE, Sanchez Pozzi EJ, Pellegrino JM, Ochoa JE, Mottino AD, et al. Phosphoinositide 3-kinase/protein kinase B signaling pathway is involved in estradiol 17beta-D-glucuronide-induced cholestasis: complementarity with classical protein kinase C. Hepatology. 2010;52:1465–1476. doi: 10.1002/hep.23846. [DOI] [PubMed] [Google Scholar]
- 9.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, et al. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol-3 kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem. 2003;278:17810–17818. doi: 10.1074/jbc.M209898200. [DOI] [PubMed] [Google Scholar]
- 10.Beuers U, Probst I, Soroka C, Boyer JL, Kullak-Ublick GA, Paumgartner G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology. 1999;29:477–482. doi: 10.1002/hep.510290227. [DOI] [PubMed] [Google Scholar]
- 11.Akita Y. Protein kinase Cepsilon: multiple roles in the function of, and signaling mediated by, the cytoskeleton. FEBS J. 2008;275:3995–4004. doi: 10.1111/j.1742-4658.2008.06557.x. [DOI] [PubMed] [Google Scholar]
- 12.Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujise A, Mizuno K, Ueda Y, Osada S, Hirai S, Takayanagi A, et al. Specificity of the high affinity interaction of protein kinase C with a physiological substrate, myristoylated alanine-rich protein kinase C substrate. J Biol Chem. 1994;269:31642–31648. [PubMed] [Google Scholar]
- 14.Heemskerk FM, Chen HC, Huang FL. Protein kinase C phosphorylates Ser152, Ser156 and Ser163 but not Ser160 of MARCKS in rat brain. Biochem Biophys Res Commun. 1993;190:236–241. doi: 10.1006/bbrc.1993.1036. [DOI] [PubMed] [Google Scholar]
- 15.Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol. 2007;171:1822–1830. doi: 10.2353/ajpath.2007.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol. 2005;167:651–661. doi: 10.1016/s0002-9440(10)62040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, O’Connor KL, Greeley GH, Jr, Blackshear PJ, Townsend CM, Jr, Evers BM. Myristoylated alanine-rich C kinase substrate-mediated neurotensin release via protein kinase C-delta downstream of the Rho/ROK pathway. J Biol Chem. 2005;280:8351–8357. doi: 10.1074/jbc.M409431200. [DOI] [PubMed] [Google Scholar]
- 18.Park YS, Hur EM, Choi BH, Kwak E, Jun DJ, Park SJ, et al. Involvement of protein kinase C-epsilon in activity-dependent potentiation of large dense-core vesicle exocytosis in chromaffin cells. J Neurosci. 2006;26:8999–9005. doi: 10.1523/JNEUROSCI.2828-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JC, Hrnjez BJ, Farokhzad OC, Matthews JB. PKC-epsilon regulates basolateral endocytosis in human T84 intestinal epithelia: role of F-actin and MARCKS. Am J Physiol. 1999;277:C1239–C1249. doi: 10.1152/ajpcell.1999.277.6.C1239. [DOI] [PubMed] [Google Scholar]
- 20.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–G1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- 21.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, et al. Short-term regulation of bile acid uptake by microfilament-dependent translocation of ntcp to the plasma membrane. Hepatology. 1999;30:223–229. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi Y, Kipp H, Arias IM. Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. J Biol Chem. 2006;281:27669–27673. doi: 10.1074/jbc.R600013200. [DOI] [PubMed] [Google Scholar]
- 23.Thibault N, Maurice M, Maratrat M, Cordier A, Feldmann G, Ballet F. Effect of tauroursodeoxycholate on actin filament alteration induced by cholestatic agents. A study in isolated rat hepatocyte couplets. J Hepatol. 1993;19:367–376. doi: 10.1016/s0168-8278(05)80544-6. [DOI] [PubMed] [Google Scholar]
- 24.Heidkamp MC, Iyengar R, Szotek EL, Cribbs LL, Samarel AM. Protein kinase Cepsilon-dependent MARCKS phosphorylation in neonatal and adult rat ventricular myocytes. J Mol Cell Cardiol. 2007;42:422–431. doi: 10.1016/j.yjmcc.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mammen JM, Song JC, Yoo J, Kim PS, Davis HW, Calvo MI, et al. Differential subcellular targeting of PKC-epsilon in response to pharmacological or ischemic stimuli in intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2005;288:G135–G142. doi: 10.1152/ajpgi.00139.2004. [DOI] [PubMed] [Google Scholar]
- 26.Shiraishi M, Tanabe A, Saito N, Sasaki Y. Unphosphorylated MARCKS is involved in neurite initiation induced by insulin-like growth factor-I in SH-SY5Y cells. J Cell Physiol. 2006;209:1029–1038. doi: 10.1002/jcp.20814. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El Deiry W, et al. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem. 2001;276:38610–38618. doi: 10.1074/jbc.M105300200. [DOI] [PubMed] [Google Scholar]
- 28.Johnston A, Ponzetti K, Anwer MS, Webster CR. cAMP-guanine exchange factor protection from bile acid induced hepatocyte apoptosis involves glycogen synthase kinase regulation of C-Jun-NH terminal kinase. Am J Physiol Gastrointest Liver Physiol. 2011;301:G385–G400. doi: 10.1152/ajpgi.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem. 2002;277:28578–28583. doi: 10.1074/jbc.M201937200. [DOI] [PubMed] [Google Scholar]
- 30.Webster CRL, Blanch CJ, Philips J, Anwer MS. Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem. 2000;275:29754–29760. doi: 10.1074/jbc.M002831200. [DOI] [PubMed] [Google Scholar]
- 31.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 32.Park SW, Schonhoff CM, Webster CR, Anwer MS. Protein kinase Cdelta differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane. Am J Physiol Gastrointest Liver Physiol. 2012;303:G657–G665. doi: 10.1152/ajpgi.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowry DH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Schonhoff CM, Yamazaki A, Hohenester S, Webster CR, Bouscarel B, Anwer MS. PKC{epsilon}-dependent and -independent effects of taurolithocholate on PI3K/PKB pathway and taurocholate uptake in HuH-NTCP cell line. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1259–G1267. doi: 10.1152/ajpgi.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubitz R, Saha N, Kuhlkamp T, Dutta S, vom DS, Wettstein M, et al. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]
- 36.Larocca MC, Ochoa EJ, Rodriguez Garay EA, Marinelli RA. Protein kinase C-dependent inhibition of the lysosomal degradation of endocytosed proteins in rat hepatocytes. Cell Signal. 2002;14:641–647. doi: 10.1016/s0898-6568(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 37.Robinson PJ. The role of protein kinase C and its neuronal substrates dephosphin, B-50, and MARCKS in neurotransmitter release. Mol Neurobiol. 1991;5:87–130. doi: 10.1007/BF02935541. [DOI] [PubMed] [Google Scholar]
- 38.Calle R, Ganesan S, Smallwood JI, Rasmussen H. Glucose-induced phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in isolated rat pancreatic islets. J Biol Chem. 1992;267:18723–18727. [PubMed] [Google Scholar]
- 39.Liu JP, Engler D, Funder JW, Robinson PJ. Arginine vasopressin (AVP) causes the reversible phosphorylation of the myristoylated alanine-rich C kinase substrate (MARCKS) protein in the ovine anterior pituitary: evidence that MARCKS phosphorylation is associated with adrenocorticotropin (ACTH) secretion. Mol Cell Endocrinol. 1994;101:247–256. doi: 10.1016/0303-7207(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 40.Elzagallaai A, Rose SD, Brandan NC, Trifaro JM. Myristoylated alanine-rich C kinase substrate phosphorylation is involved in thrombin-induced serotonin release from platelets. Br J Haematol. 2001;112:593–602. doi: 10.1046/j.1365-2141.2001.02642.x. [DOI] [PubMed] [Google Scholar]
- 41.Chappell DS, Patel NA, Jiang K, Li P, Watson JE, Byers DM, et al. Functional involvement of protein kinase C-betaII and its substrate, myristoylated alanine-rich C-kinase substrate (MARCKS), in insulin-stimulated glucose transport in L6 rat skeletal muscle cells. Diabetologia. 2009;52:901–911. doi: 10.1007/s00125-009-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su R, Han ZY, Fan JP, Zhang YL. A possible role of myristoylated alanine-rich C kinase substrate in endocytic pathway of Alzheimer’s disease. Neurosci Bull. 2010;26:338–344. doi: 10.1007/s12264-010-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green TD, Crews AL, Park J, Fang S, Adler KB. Regulation of mucin secretion and inflammation in asthma: a role for MARCKS protein? Biochim Biophys Acta. 2011;1810:1110–1113. doi: 10.1016/j.bbagen.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 45.Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31:320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, et al. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem. 2010;285:19299–19307. doi: 10.1074/jbc.M109.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rost D, Kloeters-Plachky P, Stiehl A. Retrieval of the rat canalicular conjugate export pump Mrp2 is associated with a rearrangement of actin filaments and radixin in bile salt-induced cholestasis. Eur J Med Res. 2008;13:314–318. [PubMed] [Google Scholar]
- 50.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. TLC induces plasma membrane translocation of PKCε in rat hepatocytes. Cultured hepatocytes cells were incubated with 10 μM TLC, 1μM PMA or 100μM CPT-cAMP for 15 followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated PKCε (PM-PKCε) and E-cadherin (E-Cad as loading control). Typical PM-PKCε and E-Cad immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=4) are shown in the bar graph. *Significantly different (p<0.05) from respective control values.
Supplementary Figure 2. TLC induces retrieval of Mrp2 in rat hepatocytes. Cultured hepatocytes cells were incubated with 10 μM TLC (25 min) or 100μM CPT-cAMP (15 min) followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated MRP2 (PM-MRP2) and E-cadherin. Typical PM-MRP2 and E-Cad immunoblots are shown in the upper panel and results of densitometric analysis (Mean±SEM, n=4) are shown in the bar graph. *Significantly different (p<0.05) from respective control values.