Abstract
Small GTPases of the Rab family are master regulators of membrane trafficking, responsible for coordinating the sorting, packaging and delivery of membrane-bound vesicles to specific sites within eukaryotic cells. The contribution of these proteins to the biology of the human pathogenic fungus Aspergillus fumigatus has not been explored. In this study, we characterized the srgA gene, encoding a Rab GTPase closely related to Sec4. We found that a GFP-SrgA fusion protein accumulated preferentially at hyphal tips and mature condiophores. The radial growth of a ΔsrgA mutant was impaired on both rich and minimal medium, consistent with a role for SrgA in filamentous growth. In addition, the ΔsrgA mutant revealed dysmorphic conidiophores that produced conidia with heterogeneous morphology. The ΔsrgA mutant was hypersensitive to brefeldin A-mediated inhibition of vesicular trafficking and showed increased temperature sensitivity relative to wild type A. fumigatus. However, the most striking phenotype of this mutant was its phenotypic heterogeneity. Individual colonies isolated from the original ΔsrgA mutant showed variable morphology with colony sectoring. In addition, each isolate of the ΔsrgA mutant displayed divergent phenotypes with respect to thermotolerance, in vitro stress response and virulence in a Galleria mellonella infection model. Taken together, these results indicate that SrgA contributes to the asexual development and filamentous growth of A. fumigatus. However, the discordant phenotypes observed among individual isolates of the ΔsrgA mutant suggest that the absence of srgA exerts selective pressure for the acquisition of compensatory changes, such as second-site suppressor mutations.
Introduction
Filamentous fungi elongate and branch by apical extension, a mode of growth that involves the establishment of a stable axis of polarity, followed by the maintenance of growth in the same direction [1]. The ability to sustain polarization requires a constant stream of new cell wall and plasma membrane material to the hyphal apex [2]. This is accomplished by packaging components required for membrane and cell wall biogenesis into membrane-enclosed vesicles of the secretory system and delivering them to the growing tip cell [3]. The secretory pathway is also exploited for the transport of hydrolytic enzymes to the hyphal apex, where they are exocytosed into the surrounding substrate to assist with nutrient acquisition [4], [5]. Current evidence suggests that both exocytosis and cell growth are concentrated at the hyphal tips of filamentous fungi, although not exclusively [6]. The Spitzenkörper is an apical cluster of vesicles and cytoskeletal components that assists in this process by providing a vesicle supply center for the rapid delivery of enzymes into and across the apical cell membrane [7]. This contrasts the budding yeast Saccharomyces cerevisiae, where the continual delivery of vesicles across the entire cell surface promotes spherical rather than polarized growth [8].
Members of the Rab family of GTPases have pivotal functions in the regulation of vesicular trafficking in eukaryotes. By cycling between inactive (GDP-bound) and active (GTP-bound) states the Rab GTPases, in coordination with their many effector proteins, are able to orchestrate precise spatial targeting of secretory vesicles [9]. The Rab GTPase Sec4 is central to this process, contributing to the transport of vesicles from the trans-Golgi to the plasma membrane [10]. Loss of sec4 results in the accumulation of secretory vesicles and disruption of protein secretion, which is incompatible with viability in a number of fungal species [10], [11], [12], [13], [14]. Additionally, other Sec4 homologues have been linked to functions that contribute to fungal pathogenesis, such as the formation of specialized infection structures [15] or the extracellular release of vesicles containing virulence-related factors [13].
Very little is known about Rab GTPases in Aspergillus fumigatus, an opportunistic human mold pathogen that causes a life-threatening infection known as invasive aspergillosis [16]. In this study, we characterized the A. fumigatus srgA gene, encoding a Sec4 homolog that was initially annotated in Aspergillus niger as secretion-related GTPase A (SrgA) [17]. An A. fumigatus ΔsrgA mutant was constructed and shown to be associated with abnormal colony morphology, attenuated conidiation, reduced hyphal growth, and hypersensitivity to environmental stress. However, there was surprising phenotypic heterogeneity among independent isolates of this mutant with respect to in vitro phenotypes and virulence, suggesting that the consequences of losing SrgA function is modified by the activation of different compensatory responses.
Results
Identification of the Sec4 Homolog SrgA in A. fumigatus
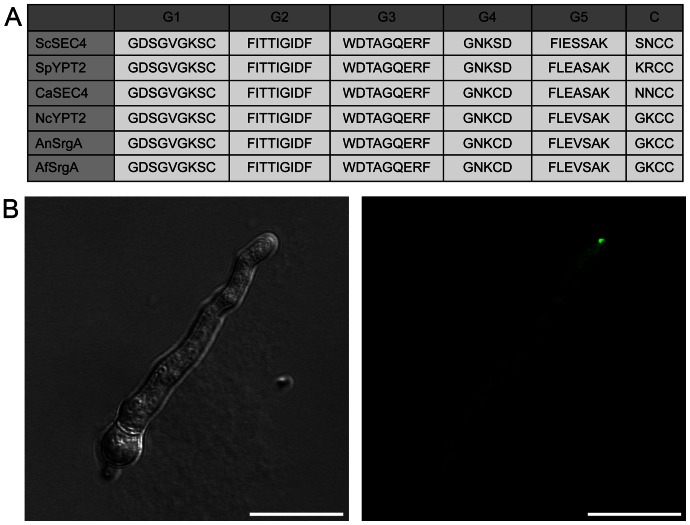
SrgA was previously identified in A. niger as one of five different secretion-related GTPases thought to be involved in mediating different stages of vesicle transport [17]. The corresponding gene in A. fumigatus (AFUA_4G04810), encodes a 206 amino acid protein in which multiple Rab-family motifs are found. Included within these shared motifs are the five “G box” sequences, which are present in all small GTPase families [18]. As shown in Figure 1A, there is high sequence homology within these G box motifs between A. fumigatus SrgA and other previously characterized fungal Sec4 proteins. Conservation within the G2 domain is particularly noteworthy, as this region is the effector domain, responsible for functional specificity within the Rab GTPase family [17]. Also contributing to Rab GTPase function are two conserved C-terminal cysteine residues, which are posttranslationally modified to allow for, and stabilize, the protein's association with vesicle membranes [19].
Figure 1. Relationship between A. fumigatus SrgA and Sec4 homologs.
A: Comparison of G-box motifs (G1–G5) and C-termini (C) from fungal Sec4 homologs in Saccharomyces cerevisiae (Sc), Schizosaccharomyces pombe (Sp), Candida albicans (Ca), Neurospora crassa (Nc)), Aspergillus niger (An), and Aspergillus fumigatus (Af). B: Intracellular localization of A. fumigatus SrgA. The SrgA protein was tagged at its N-terminus with GFP and expressed in A. fumigatus under the control of the gpdA promoter. Scale bar = 10 µm.
To determine the intracellular localization of SrgA, the protein was tagged at its N-terminus with green fluorescent protein (GFP) and expressed in wild type (wt) A. fumigatus under the control of the gpdA promoter. As shown in Figure 1B, the GFP-SrgA fusion protein accumulated preferentially at hyphal tips, similar to what has been described for Sec4 and related Sec proteins in Candida albicans [20], [21]. This localization is consistent with the putative role for SrgA in the regulation of apical vesicle transport in filamentous fungi.
Loss of SrgA Generates Phenotypic Heterogeneity in Colony Morphology
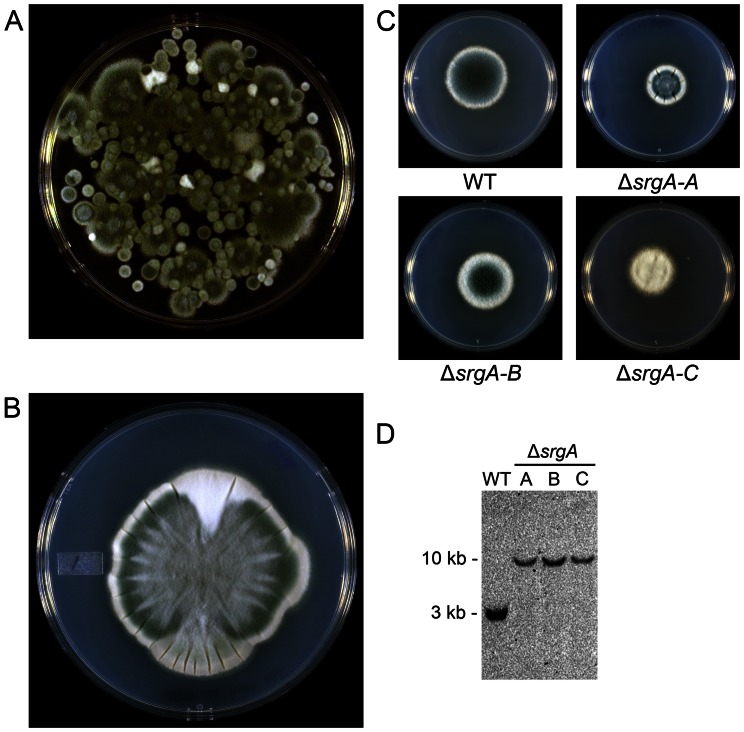
A ΔsrgA strain was constructed by replacing the entire srgA coding region with a phleomycin-resistance cassette. The expected deletion was identified by probing HindIII-digested genomic DNA with a srgA 5′ flanking probe (probe A, Figure 2), revealing the loss of the wt 2.8 kb HindIII fragment and the appearance of the expected 10.3 kb fragment.
Figure 2. Deletion of srgA from A. fumigatus.
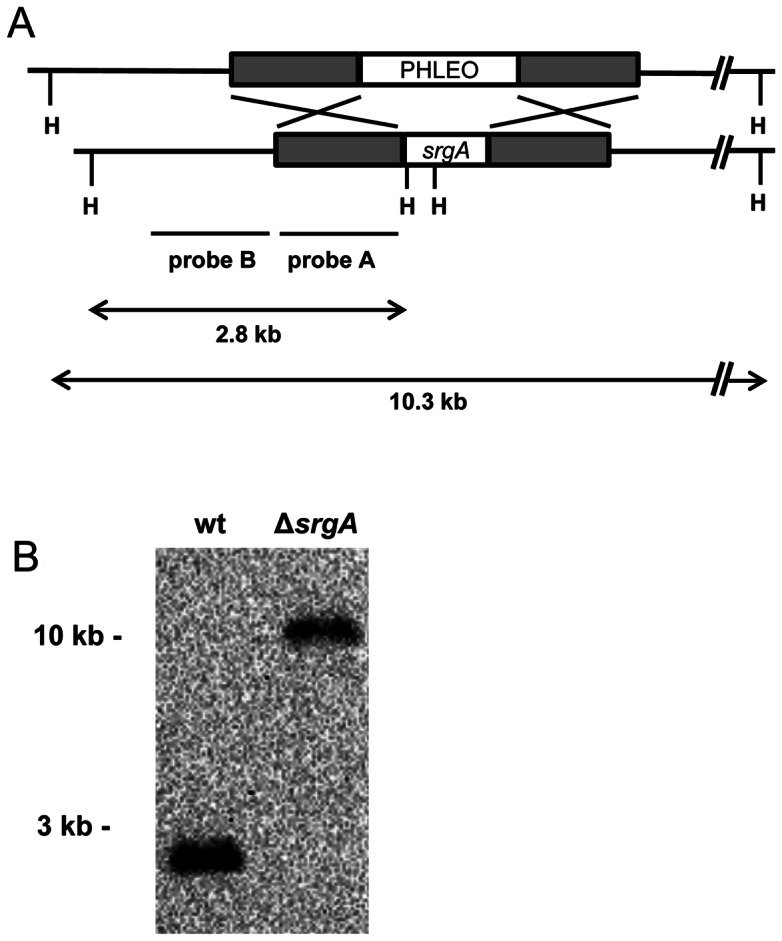
Southern blot analysis of HindIII-digested genomic DNA using a probe located upstream of the srgA coding region (probe A) identified the predicted 2.8 kb fragment in wt A. fumigatus, which was lengthened to 10.3 kb in the ΔsrgA mutant due to replacement of srgA with the phleomycin-resistance cassette (PHLEO).
The ΔsrgA mutant showed surprising phenotypic heterogeneity when plated for isolation on solid media, manifested by differences in colony size, the level of conidiation and colony sectoring (Figure 3A and 3B). Three distinct colonial morphologies were arbitrarily selected for further phenotypic analysis, using size and conidiation as a crude measure of individuality, hereafter referred to as ΔsrgA isolates A, B, and C (Figure 3C). Genotypic analysis by Southern blot, using a probe that is upstream of the srgA open-reading frame (probe B, Figure 2) confirmed that each ΔsrgA isolate lacked the srgA gene (Figure 3D). Moreover, no wt conidia were recovered by plating the mutant onto non-selective media, suggesting that the mutants are not heterokaryons that are protected by a small population of wt nuclei. The presence of the phleomycin resistance cassette, in the absence of any detectable srgA gene was also confirmed by PCR in each of the ΔsrgA isolates (data not shown). Together, these findings suggest that deletion of srgA generates phenotypic diversity in colony morphology, possibly due to the activation of compensatory changes that were selected for based on their ability to improve fitness.
Figure 3. Loss of SrgA is associated with diverse colony morphology.
A: Conidia harvested from the initial monoconidial ΔsrgA mutant produced a heterogeneous population of colonies when spread for isolation. B: Colony sectoring was observed in ΔsrgA isolates (shown here, isolate-A). C: Three individual ΔsrgA isolates (A–C) were selected from the heterogeneous population shown in panel A. D: Southern blot analysis of HindIII-digested genomic DNA using a probe located upstream of the srgA coding region (Figure 2, probe B) identified the predicted 2.8 kb fragment in wt A. fumigatus, which was lengthened to 10.3 kb in the ΔsrgA isolates due to replacement of srgA with the phleomycin-resistance cassette.
Loss of SrgA Impairs Conidiation
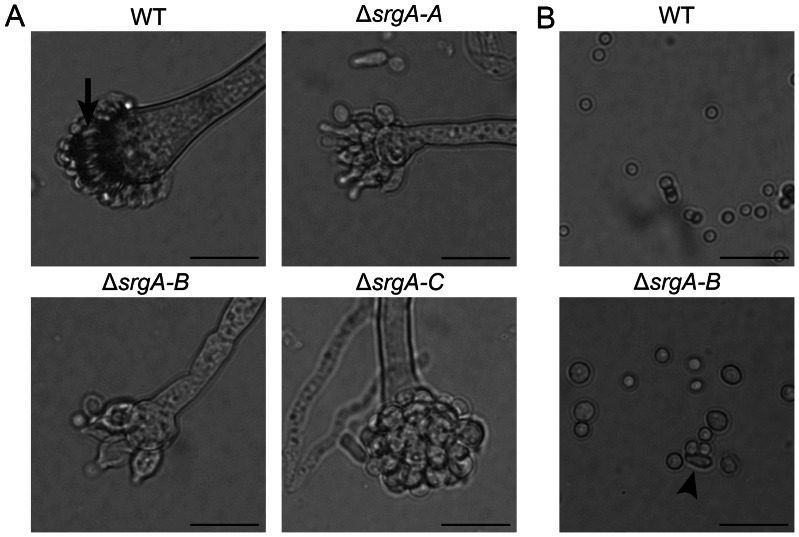
The decreased pigmentation of all ΔsrgA colonies suggested that loss of SrgA reduces the efficiency of asexual development. Consistent with this, dysmorphic conidiophores were observed in all three of the ΔsrgA mutant isolates; the vesicle was attenuated in size and the phialides were irregularly shaped, often appearing swollen at the base (Figure 4A). In contrast to wt conidia, which formed uniform spheres approximately 2 µm in diameter, the conidia that were released from ΔsrgA colonies were heterogeneous in size, ranging from 2–5 µm in diameter (Figure 4B). In addition, all three mutant isolates produced oval and tear-drop shaped conidia, some of which may represent the abnormal release of phialides from the mutant conidiophores rather than true conidia (Figure 4B, arrow). Despite this aberrant morphology, all of the mutant conidia were viable and germinated normally in liquid culture (data not shown). The abnormal conidiation observed in ΔsrgA colonies could not be rescued by osmotic stabilization of the medium with sorbitol (data not shown). Moreover, none of the ΔsrgA isolates showed increased sensitivity to the cell wall-targeting antifungal agent, caspofungin (data not shown), suggesting that the reduced conidiation is not due to a major defect in cell wall integrity.
Figure 4. Loss of SrgA impairs conidiation.
A: All three ΔsrgA isolates have attenuated conidophores and dysmorphic phialides (normal phialides are shown by the arrow in wt). B: All three ΔsrgA isolates release conidia that are heterogeneous in both size and shape. Some of the elongated conidia may be abnormal phialides that are released along with the conidia (arrow) (Scale bar = 20 µm).
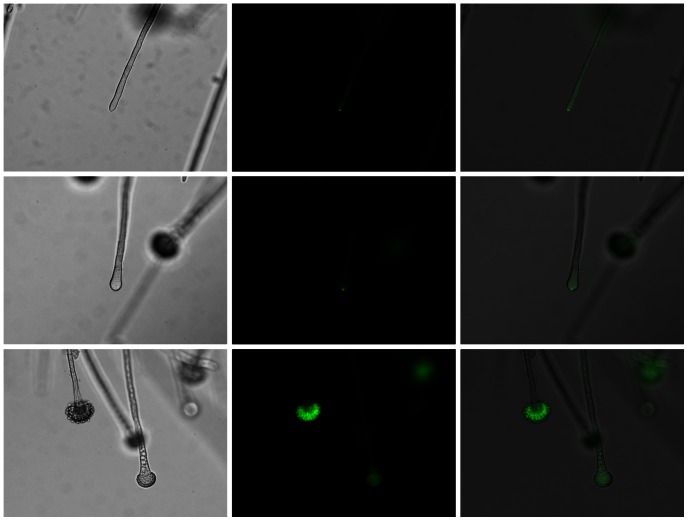
The aberrant conidiophores in the ΔsrgA mutant suggested that SrgA may be localized at the site of conidia production. This was confirmed by analysis of GFP-SrgA localization during sporulation. As shown in Figure 5, the GFP-SrgA fusion protein localized to a distinct spot at the apex of young developing conidiophores, which progressively expanded to include the entire vesicle in mature conidiophores. Taken together, these findings suggest that SrgA plays a role in the developmental program, presumably by maximizing the efficiency of vesicle delivery to the developing condiophore.
Figure 5. GFP-SrgA localizes to conidiophores.
GFP-SrgA localizes to the apex of both hyphae and conidiophores. A punctate accumulation at the tip is seen in both hyphae and the early stages of vesicle swelling (top and middle rows, respectively), but a more diffuse localization is evident in mature conidiophores (bottom row). Left column: brightfield; middle column: GFP fluorescence; bottom column: Merged image.
Loss of SrgA Impairs Hyphal Growth
In A. niger, the ΔsrgA mutant displayed a two-fold increase in hyphal diameter, as well as unusual apical branching [17]. By contrast, hyphal morphology was normal in the A. fumigatus ΔsrgA mutant, with no evidence of increased hyphal thickness or hyperbranching (data not shown). However, all three ΔsrgA isolates were growth impaired at temperatures ranging from 30°C to 45°C. The extent of growth inhibition was variable between strains (Figure 6). For example, isolate C grew more slowly than the other two isolates at 30°C. However, at 37°C, isolate C grew at the same rate as isolate A, and only slightly slower than isolate B. At 45°C, all three strains grew at distinctly different rates, with isolate A being the most growth impaired. This phenotypic heterogeneity is consistent with the notion that each mutant harbors a different compensatory response to the loss of srgA, which impacts the ability of the organism to grow at different temperatures.
Figure 6. Loss of SrgA impairs hyphal growth.
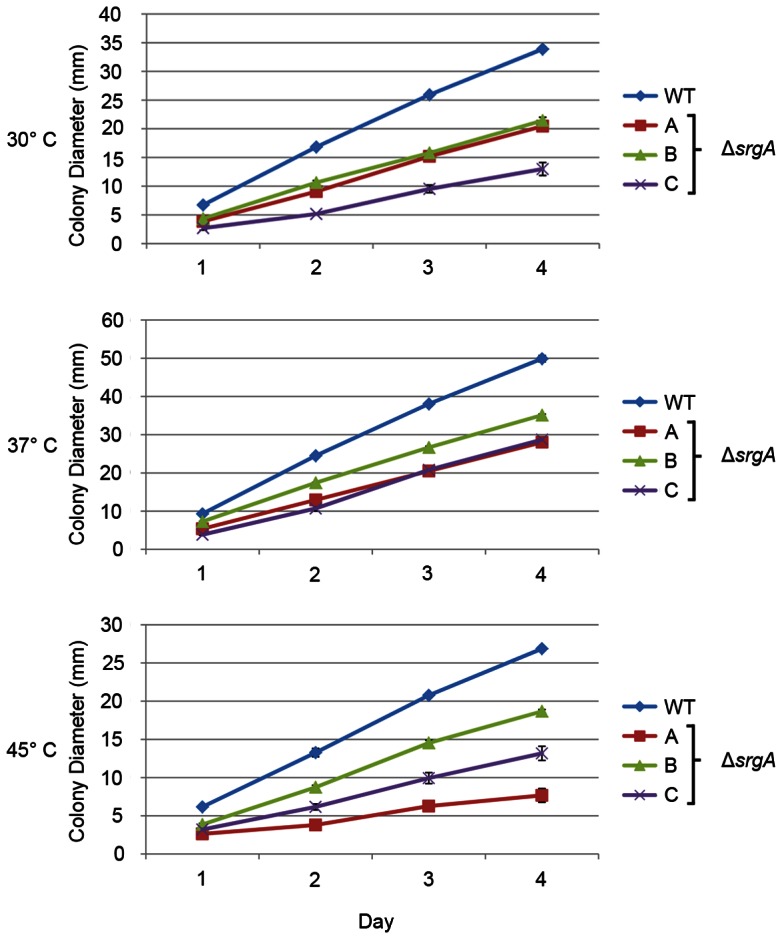
Equal numbers of conidia were plated on the center of a plate of solid AMM and colony diameter was measured every day during a four-day incubation period at the indicated temperatures. The experiment was performed in triplicate and the values represent the mean ± SEM.
Loss of SrgA Alters Susceptibility to ER stress
Mutations that adversely affect homeostasis of the secretory pathway are often associated with heightened sensitivity to agents that cause endoplasmic reticulum (ER) stress, such as dithiothreitol (DTT) and tunicamycin (TM) [22]. We found that loss of srgA was associated with hypersensitivity to DTT, but only in isolate C (Figure 7A). Similarly, isolates A and C were hypersensitive to TM, but isolate B was not (Figure 7B). However, all three isolates were hypersensitive to the ER stress-inducing agent brefeldin A (BFA) (Figure 8). It is likely that the shared hypersensitivity to BFA, relative to the more variable responses to DTT and TM, reflects differences in the mechanism by which each agent disrupts ER homeostasis. DT and TM induce generalized protein folding stress by interfering with disulfide bonds and N-linked glycosylation, respectively [22]. Thus, the divergent responses of the three ΔsrgA isolates to DTT and TM may be due to the different compensatory changes that each isolate has undergone in order to mitigate the loss of SrgA function. By contrast, BFA disrupts vesicle trafficking between the ER and the Golgi [23]. Since Sec4 homologs also regulate vesicular trafficking [10], we speculate that BFA treatment in the absence of srgA is a more difficult obstacle to overcome by compensatory mechanisms because it induces a critical defect in vesicle trafficking homeostasis that is incompatible with growth.
Figure 7. Sensitivity of ΔsrgA to ER stress.
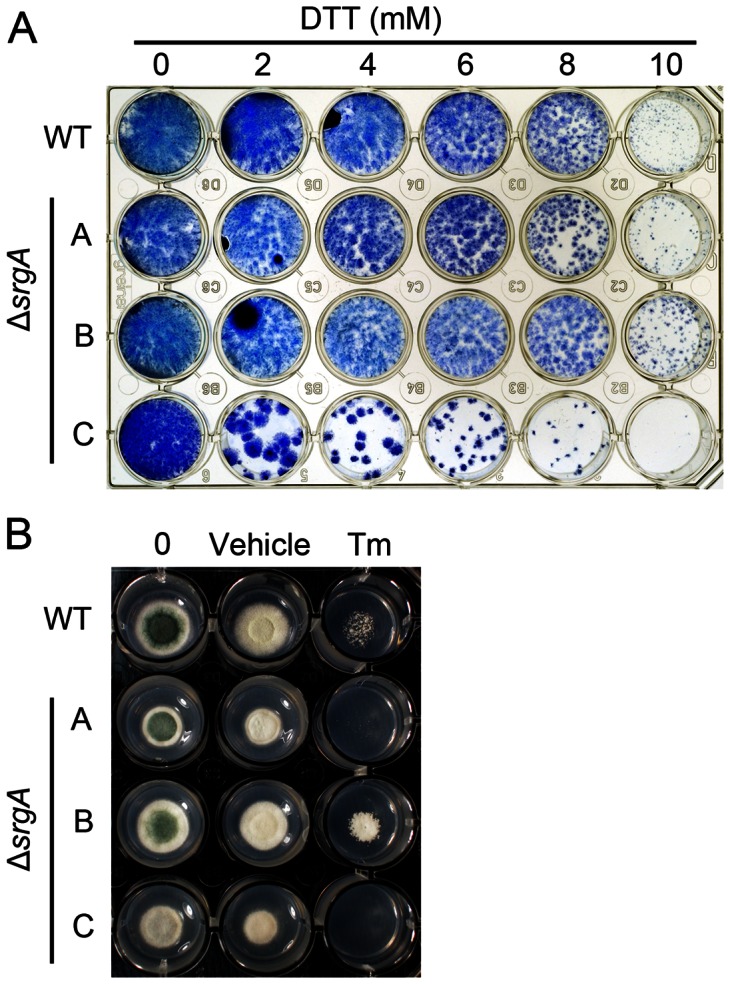
A: Equal numbers of conidia were added to individual wells of a 24-well plate containing liquid AMM media and the indicated concentrations of dithiothreitol (DTT). Plates were incubated at 37°C for three days, after which the mycelial biomass that was adhered to the plate surface was stained with methylene blue and photographed. B: Equal numbers of conidia were inoculated onto solid AMM media containing either the vehicle control (DMSO) or 100 µg/ml tunicamycin (TM) and incubated for three days at 37°C.
Figure 8. Sensitivity of ΔsrgA to brefeldin A.
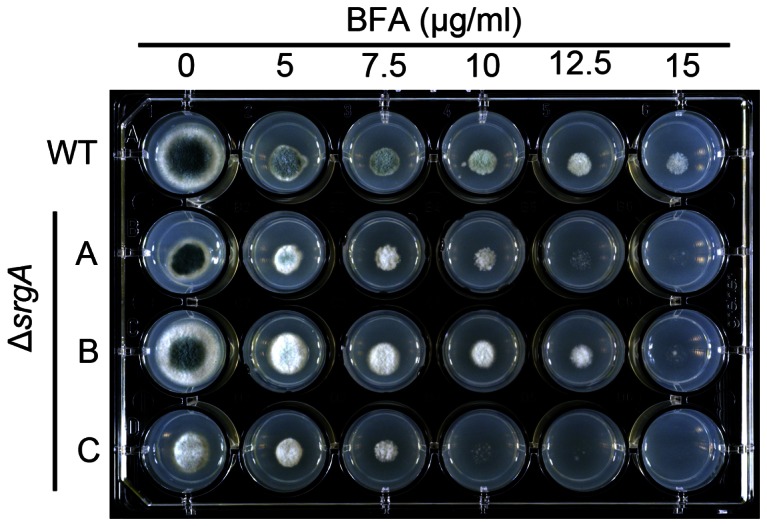
Equal numbers of conidia were inoculated onto solid AMM media containing the indicated concentrations of brefeldin A (BFA) and incubated for three days at 37°C.
Loss of SrgA Alters Virulence
The virulence of each ΔsrgA isolate was tested in a Galleria mellonella infection model. G. mellonella larvae do not have to be immunosuppressed in order to allow initiation of an A. fumigatus infection, which allows fungal pathogenesis to be studied in the context of an intact immune system [24], [25], [26]. Conidia were injected into the last pro-leg of sixth instar G. mellonella larvae and survival was monitored over five days. As shown in Figure 9, the ΔsrgA isolate C had attenuated virulence relative to wt A. fumigatus. However, isolates A and B were statistically indistinguishable from wt, indicating there is also diversity among ΔsrgA isolates with respect to virulence.
Figure 9. Analysis of ΔsrgA virulence in an insect model of A. fumigatus infection.
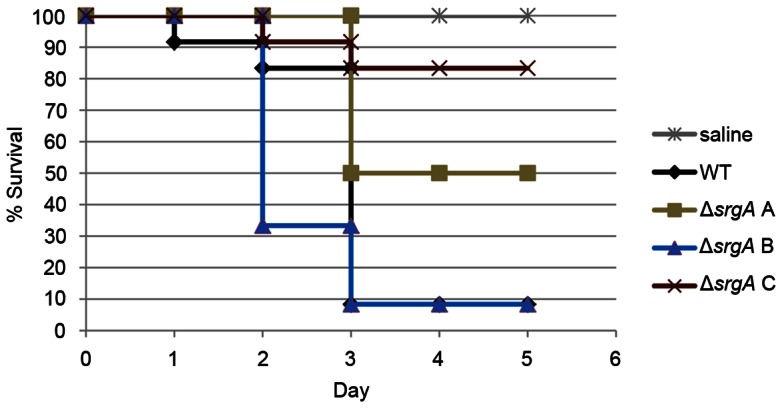
Groups of 12 G. mellonella larvae were infected with conidia from the indicated strains. Larvae were incubated at 37°C and mortality was monitored over a five day period. Kaplan-Meier survival curves were compared using a log-rank test, followed by a pairwise multiple comparison test (Holm-Sidak). The ΔsrgA isolate C survival curve is statistically different from wt, but isolates A and B were indistinguishable from wt.
Reproducibility of Phenotypic Heterogeneity Among ΔsrgA Isolates
The discordant phenotypes observed between individual isolates of the A. fumigatus ΔsrgA mutant suggested that the deletion of srgA selects for the acquisition of compensatory changes, such as second-site mutations. This complicates the interpretation of complementation studies, since the reconstitution of srgA into the three ΔsrgA isolates is unlikely to correct the phenotypic heterogeneity because each isolate would still harbor unknown and potentially unique mutations in related pathways. When gene reconstitution is unsuitable for genetic deletion experiments, the isolation of a second, independently derived mutant strain can be used as alternative way to confirm a phenotype [27]. Thus, we performed a separate transformation experiment with the srgA knockout construct and obtained a second ΔsrgA strain, designated ΔsrgA-2. Similar to the original ΔsrgA strain (ΔsrgA-1), ΔsrgA-2 revealed colony heterogeneity (Figure S1, A). Based on morphological similarities to the previous ΔsrgA-1 isolates, three ΔsrgA-2 isolates were selected (A, B, and C) and tested under in vitro growth conditions. All ΔsrgA-2 isolates were growth impaired to the same extent as the ΔsrgA-1 isolates (Figure S1, B). In addition, we identified increased sensitivity of all three ΔsrgA-2 isolates to BFA compared to wt, but phenotypic divergence between the three ΔsrgA-2 isolates in their sensitivity to DTT, similar to what was observed in the ΔsrgA-1 isolates (Figure S1, C and D). The observation that phenotypic heterogeneity occurs in two independently isolated ΔsrgA mutants suggests that loss of srgA is the predisposing factor for A. fumigatus to undergo additional alterations to mitigate the effects of srgA deficiency.
Discussion
In this study we deleted the A. fumigatus srgA gene, encoding a Rab GTPase homologue that is closely related to Sec4. The most striking finding was that srgA deletion was associated with phenotypic heterogeneity, which was manifested by distinct colony morphologies and variable responses to both in vitro and in vivo stress conditions. Phenotypic variability was not observed in the corresponding mutant in A. niger, [17] suggesting fundamental differences between the two species with respect to the response to SrgA deficiency. This phenotypic variation was also not observed in the A. fumigatus parental strain used in this study, nor in other mutants that have been generated on the same genetic background [28], [29], [30], which implicates the loss of srgA as the predisposing factor for these diverse phenotypes. It is worth noting that the frequency of homologous targeting was very low for this gene; only two ΔsrgA mutants were identified in a screen of approximately 100 transformants from two genetic backgrounds (kuA and CBS 144.89). This is consistent with the notion that the loss of srgA creates a severe phenotypic defect, possibly lethality, which selects for suppressor mutations to compensate for the defect. We speculate that one or more such mutations have occurred within each of the ΔsrgA isolates, which improves the fitness of the fungus beyond that of the original ΔsrgA strain. These could be multi-copy suppressors derived from other members of the Rab GTPase family, or mutations in genes in related pathways that can partially compensate for the absence of SrgA. Unfortunately, while genetic models to identify suppressor mutations are well established in yeast, and have been previously used to discover suppressors of Rab GTPase mutants [31], [32], [33], [34], [35], [36], [37], [38], such techniques are poorly developed in A. fumigatus. Therefore, secondary mutations that may be contributing to the phenotypic heterogeneity of the ΔsrgA isolates remain to be identified.
Despite the heterogeneity among ΔsrgA isolates, all of them shared the same phenotype of reduced radial growth rate and abnormal conidiation. This finding is consistent with the defects in polarized growth and sporulation reported for srgA-disruption mutants in A. niger [17]. Interestingly, only one of the three A. fumigatus ΔsrgA isolates had attenuated virulence, making it unclear whether it is the loss of srgA or associated compensatory mutations that contribute to reduced pathogenicity in this model. However, since the three isolates grow at the same rate in vitro, the observed reduction in pathogenicity is not simply due to a slower growth rate. Rather, attenuated virulence correlated more closely with stress response: the ΔsrgA isolates that exhibited a superior ability to adapt to in vitro stress showed wt virulence, whereas the isolate with the least resistance to in vitro stress had attenuated virulence.
The findings from the current study demonstrate that A. fumigatus is capable of surviving without SrgA-specific functions. However, the unexpected phenotypic heterogeneity that accompanies the loss of SrgA suggests that a variety of mechanisms are triggered to compensate for the absence of SrgA, some of which may be suppressor mutations. Future studies to elucidate these compensatory changes may provide important insight into networks that support homeostasis of the secretory pathway in this important fungal pathogen.
Materials and Methods
Culture Conditions
Strains used in this study are listed in Table 1. Conidia were harvested from strains grown on OSM plates [29] (Aspergillus minimal media (AMM) containing 10 mM ammonium tartrate and osmotically stabilized with 1.2 M sorbitol). Unless otherwise noted, all experiments were conducted at 37°C. For analysis of dithiothreitol (DTT) susceptibility, 5,000 conidia were inoculated into each well of a 24-well plate containing liquid AMM supplemented with increasing concentrations of DTT. Plates were incubated at 37°C for three days without shaking. The medium was then aspirated, and the hyphae adhering to the base of the well were stained with 0.5% (w/v) methylene blue for one hour at 37°C. After removing the methylene blue solution, the adherent hyphae were rinsed with sterile water and dried prior to photographing. Sensitivity to tunicamycin (100 µg/ml) and brefeldin A (5–15 µg/ml) was determined by spotting conidia into each well of a 24-well plate containing AMM with increasing concentrations of the compound and incubating for 2–3 days at 37°C.
Table 1. Strains used in this study.
| Name | Description | Source |
| wt (AfS28) | ΔakuA::ptrA | S. Krappmann |
| ΔsrgA-1 and ΔsrgA-2 | (AfS28), ΔakuA::ptrA, ΔsrgA::ble | This study |
| CBS144.89 | Wild type | R. Cramer |
| GFP-SrgA | (CBS144.89), gfp- srgA/ble | This study |
For analysis of hyphal growth, conidia were spot-plated onto the surface of a plate containing AMM agar and radial growth was monitored over a four-day incubation period at 37°C. The rate of radial growth was calculated as the colony diameter on day four minus the initial colony diameter after the first 24 hours of incubation divided by the incubation period.
Analysis of Intracellular Localization by GFP-Tagging
PCR primers used to construct a GFP-srgA expression cassette are listed in Table S1. Total DNA was extracted from overnight cultures of wt A. fumigatus and srgA was PCR amplified using primers 824 and 825. The PCR product was then inserted into the NdeI and NotI sites of p538, a GFP-fusion cassette driven by the Aspergillus nidulans gpdA promoter [39], thus generating p626. Plasmid 626 was then ectopically introduced into the wt strain CBS144.89. The intracellular localization of the fusion protein was then determined by inoculating conidia from the GFP-SrgA A. fumigatus strain onto a glass coverslip submerged in liquid AMM and incubating overnight at 37°C. Coverslips, with adhered germlings on the surface, were then inverted and mounted on a glass slide. Images were acquired with a Zeiss LSM710 confocal with an Axio Observer Z1 set for GFP detection. Images of developing conidiophores were acquired using an Olympus IX71 inverted microscope set for GFP detection.
Deletion of A. fumigatus srgA
The gene encoding A. fumigatus SrgA (AFUA_4G04810) was replaced with the phleomycin resistance gene using the split-marker method [40]. The first two-thirds of the phleomycin resistance cassette were amplified from pAN7-1 using primers 398 and 408, creating PCR product #1. The second two-thirds of phleomycin were then amplified with primers 409 and 410, creating PCR Product #2. The left arm of the srgA gene was amplified from wt DNA using primers 694 and 695, and the right arm was amplified with primers 696 and 697, generating PCR products #3 and #4, respectively. PCR products #1 and #3 were then combined in an overlap PCR reaction with primers 398 and 695 to generate PCR product #5 and PCR products #2 and #4 were combined in an overlap reaction with primers 696 and 410 to generate PCR product #6. PCR products #5 and #6 were then cloned into pCR-Blunt II-TOPO (Invitrogen) to create plasmids p599 and p600, respectively. The p599 and p600 plasmids were linearized with XhoI/BamHI and EcoRI, respectively, and transformed into AfS28 (referred to here as wt) A. fumigatus protoplasts as previously described [39]. For each transformation, phleomycin-resistant colonies were plucked from the original selection plate and transferred to secondary plates containing phleomycin. Monoconidial strains were obtained after two passages, in which conidia were spread on selection-free media and individual colonies were isolated. Conidia from monoconidial colonies were then used to create the final 10% glycerol stocks.
Analysis of Conidiophore Development
For analysis of conidiophore morphology, conidia were inoculated onto the edge of an OSM agar plug. A glass coverslip was placed on top of the plug and incubated for three days at 37°C. The coverslips were removed, mounted on a glass slide, and condiophores were observed using bright-field microscopy. For analysis of conidia morphology, wt and the ΔsrgA isolates were incubated on OSM plates for ten days at 37°C in tissue culture flasks; the flasks were then removed and incubated at room-temperature (RT) for seven days (RT incubation facilitated the conidiation of ΔsrgA isolate C). Conidia were then harvested from the plates and analyzed microscopically.
G. mellonella Infection Model
G. mellonella larvae in the final instar stages were obtained from Vanderhorst, Inc (St. Marys, OH). Twelve larvae per group, weighing between 250–350 milligrams, were inoculated with conidia from either wt A. fumigatus or one of the ΔsrgA isolates. Five microliters of a 1×108 conidia/ml saline suspension (5×105 conidia) were injected into the last left pro-leg of each larva using a Hamilton syringe (Hamilton Company, Nevada). Six larvae were included in a control group, with each larva receiving an inoculum of five microliters of saline. The larvae were placed in petri dishes and incubated in the dark at 37°C for five days. Larvae were examined daily and mortality was defined as lack of movement upon physical stimulation. Survival rates were recorded using a Kaplan-Meier survival curve and analyzed using a log-rank test, followed by a Holm-Sidak test for pairwise multiple comparisons (Sigma Stat 3.5).
Supporting Information
Phenotypic heterogeneity is a reproducible phenotype associated with srgA deletion. A second transformation was performed in order to obtain another, independently isolated ΔsrgA mutant (ΔsrgA-2). A: The ΔsrgA-2 mutant showed the same colony heterogeneity as the original ΔsrgA shown in Fig. 3. B: Three different isolates of ΔsrgA-2 were spotted onto AMM and incubated at 37°C for four days. Radial growth rate was determined by measuring colony diameter after the first 24 hours of incubation [*statistically significant by Student's T-test (p<0.001)]. C: Equal numbers of conidia were inoculated onto solid AMM media containing increasing concentrations of brefeldin A (BFA) and incubated for two days at 37°C. D: Equal numbers of conidia from the three isolates of ΔsrgA-2 were added to individual wells of a 24-well plate containing liquid AMM media and the indicated concentrations of dithiothreitol (DTT). Plates were incubated at 37°C for three days, after which the mycelial biomass that was adhered to the plate surface was stained with methylene blue and photographed.
(TIF)
PCR primers used in this study. M13-derived sequences used for overlap PCR are underlined.
(DOCX)
Acknowledgments
The authors thank Tim Stephens and Stephanie White for technical assistance and Jay Card for photography and illustration.
Funding Statement
Supported by National Institutes of Health grant R01AI072297 to DSA and a University of Cincinnati Summer Research Fellowship to MPF (URL: NIH.gov/grants). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Momany M (2002) Polarity in filamentous fungi: establishment, maintenance and new axes. Curr Opin Microbiol 5: 580–585. [DOI] [PubMed] [Google Scholar]
- 2. Steinberg G (2007) Hyphal growth: a tale of motors, lipids, and the Spitzenkorper. Eukaryot Cell 6: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riquelme M, Yarden O, Bartnicki-Garcia S, Bowman B, Castro-Longoria E, et al. (2011) Architecture and development of the Neurospora crassa hypha – a model cell for polarized growth. Fungal Biol 115: 446–474. [DOI] [PubMed] [Google Scholar]
- 4. Wosten HA, Moukha SM, Sietsma JH, Wessels JG (1991) Localization of growth and secretion of proteins in Aspergillus niger . J Gen Microbiol 137: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 5. Moukha SM, Wosten HA, Asther M, Wessels JG (1993) In situ localization of the secretion of lignin peroxidases in colonies of Phanerochaete chrysosporium using a sandwiched mode of culture. J Gen Microbiol 139: 969–978. [DOI] [PubMed] [Google Scholar]
- 6. Read ND (2011) Exocytosis and growth do not occur only at hyphal tips. Mol Microbiol 81: 4–7. [DOI] [PubMed] [Google Scholar]
- 7. Sudbery P (2011) Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus. Fungal Genet Biol 48: 849–857. [DOI] [PubMed] [Google Scholar]
- 8. Arkowitz RA, Bassilana M (2011) Polarized growth in fungi: symmetry breaking and hyphal formation. Semin Cell Dev Biol 22: 806–815. [DOI] [PubMed] [Google Scholar]
- 9. Kabcenell AK, Goud B, Northup JK, Novick PJ (1990) Binding and hydrolysis of guanine nucleotides by Sec4p, a yeast protein involved in the regulation of vesicular traffic. J Biol Chem 265: 9366–9372. [PubMed] [Google Scholar]
- 10. Salminen A, Novick PJ (1987) A ras-like protein is required for a post-Golgi event in yeast secretion. Cell 49: 527–538. [DOI] [PubMed] [Google Scholar]
- 11. Haubruck H, Engelke U, Mertins P, Gallwitz D (1990) Structural and functional analysis of ypt2, an essential ras-related gene in the fission yeast Schizosaccharomyces pombe encoding a Sec4 protein homologue. EMBO J 9: 1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craighead MW, Bowden S, Watson R, Armstrong J (1993) Function of the ypt2 gene in the exocytic pathway of Schizosaccharomyces pombe . Mol Biol Cell 4: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoneda A, Doering TL (2006) A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell 17: 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mao Y, Kalb VF, Wong B (1999) Overexpression of a dominant-negative allele of SEC4 inhibits growth and protein secretion in Candida albicans . J Bacteriol 181: 7235–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siriputthaiwan P, Jauneau A, Herbert C, Garcin D, Dumas B (2005) Functional analysis of CLPT1, a Rab/GTPase required for protein secretion and pathogenesis in the plant fungal pathogen Colletotrichum lindemuthianum . J Cell Sci 118: 323–329. [DOI] [PubMed] [Google Scholar]
- 16. Segal BH (2009) Aspergillosis. N Engl J Med 360: 1870–1884. [DOI] [PubMed] [Google Scholar]
- 17. Punt PJ, Seiboth B, Weenink XO, van Zeijl C, Lenders M, et al. (2001) Identification and characterization of a family of secretion-related small GTPase-encoding genes from the filamentous fungus Aspergillus niger: a putative SEC4 homologue is not essential for growth. Mol Microbiol 41: 513–525. [DOI] [PubMed] [Google Scholar]
- 18. Jiang SY, Ramachandran S (2006) Comparative and evolutionary analysis of genes encoding small GTPases and their activating proteins in eukaryotic genomes. Physiol Genomics 24: 235–251. [DOI] [PubMed] [Google Scholar]
- 19. Calero G, Gupta P, Nonato MC, Tandel S, Biehl ER, et al. (2003) The crystal structure of palmitoyl protein thioesterase-2 (PPT2) reveals the basis for divergent substrate specificities of the two lysosomal thioesterases, PPT1 and PPT2. J Biol Chem 278: 37957–37964. [DOI] [PubMed] [Google Scholar]
- 20. Jones LA, Sudbery PE (2010) Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryot Cell 9: 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bishop A, Lane R, Beniston R, Chapa-y-Lazo B, Smythe C, et al. (2010) Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. EMBO J 29: 2930–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ (2005) ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods 35: 395–416. [DOI] [PubMed] [Google Scholar]
- 23. Nebenfuhr A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slater JL, Gregson L, Denning DW, Warn PA (2011) Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol 49 Suppl 1S107–113. [DOI] [PubMed] [Google Scholar]
- 25. Mylonakis E (2008) Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 165: 1–3. [DOI] [PubMed] [Google Scholar]
- 26. Fallon J, Kelly J, Kavanagh K (2012) Galleria mellonella as a model for fungal pathogenicity testing. Methods Mol Biol 845: 469–485. [DOI] [PubMed] [Google Scholar]
- 27. Joubert A, Simoneau P, Campion C, Bataille-Simoneau N, Iacomi-Vasilescu B, et al. (2011) Impact of the unfolded protein response on the pathogenicity of the necrotrophic fungus Alternaria brassicicola . Mol Microbiol 79: 1305–1324. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan K, Feng X, Powers-Fletcher MV, Bick G, Richie DL, et al. (2013) Effects of a defective endoplasmic reticulum-associated degradation (ERAD) pathway on the stress response, virulence and antifungal drug susceptibility of the mold pathogen Aspergillus fumigatus. Eukaryot Cell. [DOI] [PMC free article] [PubMed]
- 29. Richie DL, Hartl L, Aimanianda V, Winters MS, Fuller KK, et al. (2009) A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus . PLoS Pathog 5: e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng X, Krishnan K, Richie DL, Aimanianda V, Hartl L, et al. (2011) HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus . PLoS Pathog 7: e1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoo JS, Grabowski R, Xing L, Trepte HH, Schmitt HD, et al. (1999) Functional implications of genetic interactions between genes encoding small GTPases involved in vesicular transport in yeast. Mol Gen Genet 261: 80–91. [DOI] [PubMed] [Google Scholar]
- 32. Gerassimenko OG (1994) Yeast ts secretory mutation rgs1 is suppressed by the SEC4 gene of Saccharomyces cerevisiae . Curr Genet 25: 178–179. [DOI] [PubMed] [Google Scholar]
- 33. Ortiz D, Medkova M, Walch-Solimena C, Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Warner JR (1996) Mutation of the Rab6 homologue of Saccharomyces cerevisiae, YPT6, inhibits both early Golgi function and ribosome biosynthesis. J Biol Chem 271: 16813–16819. [DOI] [PubMed] [Google Scholar]
- 35. Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG (1996) Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol 132: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frigieri MC, Joao Luiz MV, Apponi LH, Zanelli CF, Valentini SR (2008) Synthetic lethality between eIF5A and Ypt1 reveals a connection between translation and the secretory pathway in yeast. Mol Genet Genomics 280: 211–221. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto K, Jigami Y (2002) Mutation of TRS130, which encodes a component of the TRAPP II complex, activates transcription of OCH1 in Saccharomyces cerevisiae . Curr Genet 42: 85–93. [DOI] [PubMed] [Google Scholar]
- 38. Georgiev A, Leipus A, Olsson I, Berrez JM, Mutvei A (2008) Characterization of MYR1, a dosage suppressor of YPT6 and RIC1 deficient mutants. Curr Genet 53: 235–247. [DOI] [PubMed] [Google Scholar]
- 39. Bhabhra R, Miley MD, Mylonakis E, Boettner D, Fortwendel J, et al. (2004) Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun 72: 4731–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Catlett NL, Lee B-N, Yoder OC, Turgeon BG (2002) Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet News 50: 9–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic heterogeneity is a reproducible phenotype associated with srgA deletion. A second transformation was performed in order to obtain another, independently isolated ΔsrgA mutant (ΔsrgA-2). A: The ΔsrgA-2 mutant showed the same colony heterogeneity as the original ΔsrgA shown in Fig. 3. B: Three different isolates of ΔsrgA-2 were spotted onto AMM and incubated at 37°C for four days. Radial growth rate was determined by measuring colony diameter after the first 24 hours of incubation [*statistically significant by Student's T-test (p<0.001)]. C: Equal numbers of conidia were inoculated onto solid AMM media containing increasing concentrations of brefeldin A (BFA) and incubated for two days at 37°C. D: Equal numbers of conidia from the three isolates of ΔsrgA-2 were added to individual wells of a 24-well plate containing liquid AMM media and the indicated concentrations of dithiothreitol (DTT). Plates were incubated at 37°C for three days, after which the mycelial biomass that was adhered to the plate surface was stained with methylene blue and photographed.
(TIF)
PCR primers used in this study. M13-derived sequences used for overlap PCR are underlined.
(DOCX)