Abstract
BACKGROUND
Angiosarcoma of bone is a rare high-grade malignant vascular tumor. The literature regarding treatment and outcome of patients with this tumor is limited.
We performed a two-institutional retrospective study to analyze treatment and survival of patients with angiosarcoma of bone.
PATIENTS AND METHODS
We reviewed patients with the histological diagnosis of primary angiosarcoma of bone treated from 1980 to 2009. Demographic details, histology, treatment and survival were reviewed.
RESULTS
38 men, 22 women (median age 54 years). Most lesions occurred in the femur and the pelvis. Metastatic disease at presentation was diagnosed in 24 patients (40%). Forty-three patients underwent surgery, with 30 of them achieving surgical complete remission (SCR). Radiotherapy (RT) was applied to 17 patients, and chemotherapy (CT) to 13/35 and 15/22 patients with localized and metastatic disease, respectively.
The 5-year overall survival (OS) was 20%: 33% for patients with localized disease and 0% for metastatic patients. Higher 5-year OS was reported for patients who achieved SCR (46%) than for those who did not (0%). In non-metastatic patients, a trend towards improved survival was observed after SCR and adjuvant chemotherapy based on cisplatin, doxorubicin and ifosfamide.
Fifteen patients received chemotherapy for metastases. Two RECIST partial responses of 13 evaluable patients were documented (paclitaxel [n=1] and doxorubicin [n=1]). Stable disease was observed in 2 patients.
CONCLUSIONS
Complete surgical resection is essential for outcome. Survival of patients with metastatic or unresectable disease is very poor. Activity of taxanes and anthracycline was observed in the metastatic setting and merits further evaluation.
Keywords: Angiosarcoma of bone, Surgery, Chemotherapy
INTRODUCTION
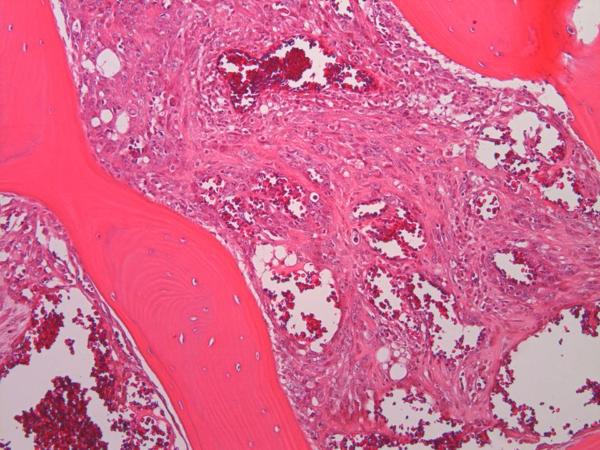
Angiosarcoma is at the high grade, malignant end of the spectrum of vascular tumors, spanning diagnoses including hemangiomas, hemangioendotheliomas, well-differentiated and poorly-differentiated angiosarcomas, among others (1). Angiosarcoma of bone is rare, accounting for less than 1% of all primary bone sarcomas and it is associated with a poor prognosis (1, 2). Although any age can be affected, the incidence is highest between 50–70 years of age (1, 3). Angiosarcoma of bone may present as unifocal or multifocal disease (2, 3–7). The most common locations of unifocal tumor are the long and short tubular bones, followed by the pelvis, and trunk (3). Histologically, angiosarcomas of bone are composed of anastomosing vascular channels lined by atypical endothelial cells with enlarged nuclei, prominent nucleoli, and increased mitoses. Inflammatory cells, mostly eosinophils, may be present (Fig 1) (2).
Figure 1.
Microscopic anatomy of bone angiosarcoma; irregular and haphazard blood-filled cavities are rimmed by highly malignant atypical cells diffusely permeating the host trabeculae.
Given the rarity of angiosarcoma of bone, the literature is limited regarding treatment and outcome of patients with this tumor. Most information is currently obtained from small series and case reports, and treatments are based on guidelines for other types of primary bone sarcomas (1, 3–8). The role of chemotherapy and prognostic factors for these patients remain unclear. The largest series included either patients with both low and high grade vascular tumors of bone (9, 10) or focused on the histological characteristics of these tumors rather than on their treatments (3).
We examined the registry of two institutions for malignant vascular tumors of bone, reviewed the histology of the patients, and retrospectively studied the medical files of all patients with histologically proven diagnosis of primary bone angiosarcoma, to evaluate the treatment and survival of these patients.
PATIENT AND METHODS
Patient Selection
After obtaining Institutional Review Board approval, we searched the registry of two referral centers for patients with a diagnosis of bone tumors: the Istituto Ortopedico Rizzoli (IOR), Bologna, Italy (1980–2009) and Memorial Sloan-Kettering Cancer Center (MSKCC), New York, NY, USA (1996–2009). Tumors were classified by expert pathologists (F.B., M.A., C.R.A.); low-grade lesions and consultation-only cases were excluded.
Demographic details, clinical symptoms, imaging at presentation, treatment and outcome were recorded from patient files. Staging included computed tomography (CT) and/or magnetic resonance (MR) imaging of the lesion, CT of the chest and bone scan in all patients. Prior to 1986, radiographs of the lesion and the thorax were performed.
The pattern of metastases was classified as lung only, multivisceral, and bone only. The latter, included the so-called “multi-focal” or “multi-centric” angiosarcoma, namely lesions that involve several bones separated by one joint (11).
A surgical complete remission (SCR) was defined as the surgical removal of the primary tumor and, for the metastatic patients, of all sites of metastatic disease.
The surgical margins were considered adequate if wide or radical, and inadequate when intralesional, marginal or contaminated (12). In metastatic patients who received chemotherapy, the response rate was assessed according to RECIST 1.0. (13).
Follow-up examinations were performed most commonly every 3 months for the first 3 years after diagnosis, every 4 months for the following two years and then every 6 months.
Overall (OS) and event-free (EFS) survival was evaluated using the Kaplan-Meier analysis. OS was calculated from the date of diagnosis to death or the last follow-up while EFS was calculated from date of diagnosis to the occurrence of an event such as local or distant recurrence, or death. OS and EFS were analyzed with respect to potentially prognostic variables including gender, age (<26 years, 26–65 years, and >65 years), stage (localized vs. metastatic), administration of chemotherapy, achievement of complete surgical resection and tumor size (>10 cm vs. ≤10 cm).
RESULTS
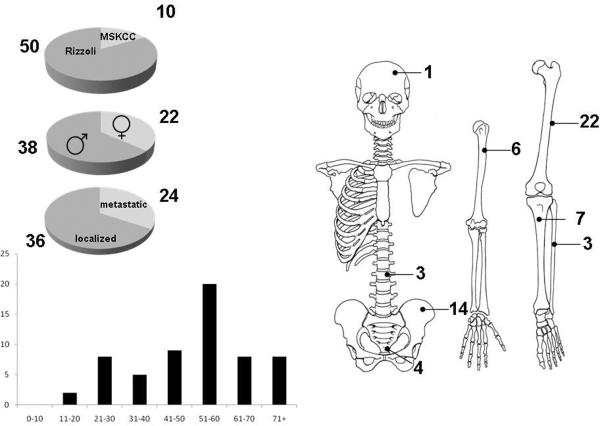
Sixty consecutive patients with a histologic diagnosis angiosarcoma of bone made between 1980 and 2009 were identified, 50 (83%) patients from the Istituto Ortopedico Rizzoli (IOR) and 10 (17%) from Memorial Hospital (Figure 2). Thirty-four (68%) of the IOR patients were part of a prior pathology manuscript in which clinical outcomes were not detailed (3). The median age was 54 years (range 18–82 years); 38 (63%) of patients were male and 22 (37%) female (37%). Over 50% of primary tumors were located in the lower limbs (Figure 2). Thirty-six (60%) patients presented with localized disease and 24 (40%) patients with metastatic disease (Figure 2). Multivisceral metastases were identified in 12 (50%) patients, bone only metastases (multicentric angiosarcoma of bone) in 8 (33%) patients, lung only metastases in 3 (13%) patients, and in 1 (4%) patient the metastasis was located in the spleen. Primary lesion size was available in 51 (85%) patients: 29 patients (57%) presented with tumors >10 cm.
Figure 2.
Clinical and demographic characteristics of 60 patients with bone angiosarcoma
Surgical Treatment
Forty-three patients (72%) underwent surgery of the primary lesion: 17 (40%) patients had an amputation, while 26 (60%) patients had limb sparing surgery. Surgical margins were available in 35 (81%) cases; the margins were adequate in 29 (83%) patients, and inadequate in 6 (17%) patients (4 marginal/intralesional; 2 contaminated). A surgical complete remission (SCR) was achieved in 30 (50%) patients: 1/24 (4%) patients with metastatic disease at presentation and 29/36 (80.5%) patients with localized disease.
Radiotherapy and Chemotherapy
Overall, radiotherapy was administered to 17 (28%) patients, in most of the cases (12 patients, 71%), with palliative intent. Five (29%) patients with localized disease were administered adjuvant radiation therapy, in 4 cases following surgery with inadequate surgical margins.
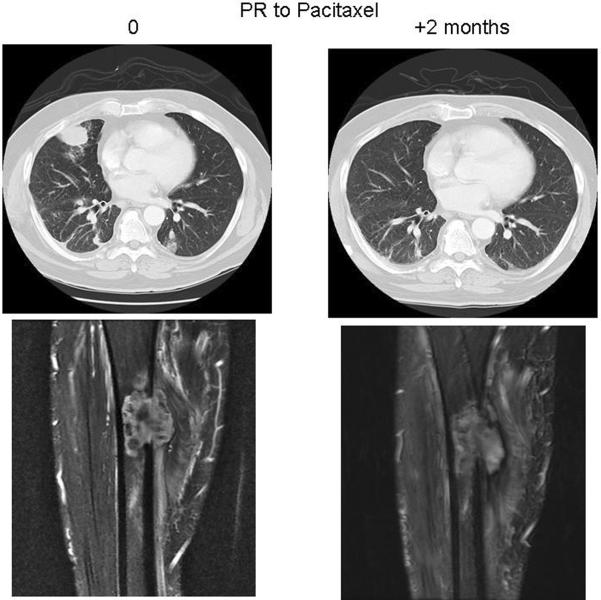
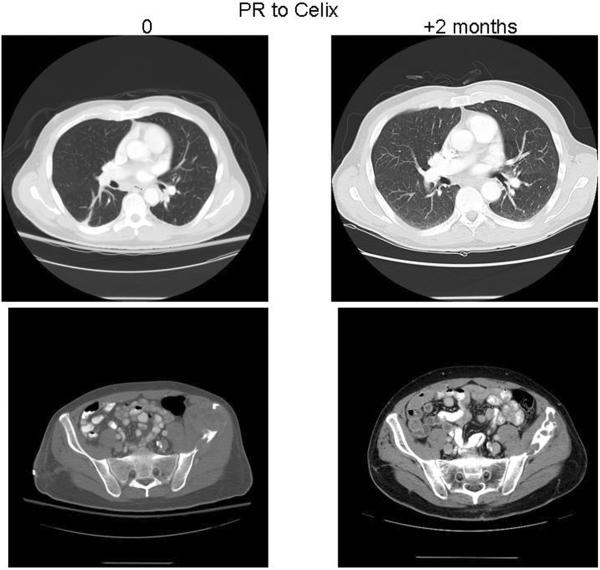
Chemotherapy was administered to 13 (37%) of 35 patients with localized disease and to 15 (65%) of 22 patients with metastatic disease (Table 1, 2). In 3 cases (two metastatic and one localized angiosarcoma of bone) complete detail regarding their chemotherapy regimens were not available. Patients with localized disease mainly received an osteosarcoma-like regimen (doxorubicin, cisplatin, and ifosfamide ± methotrexate), whereas 2 patients were administered paclitaxel (Table 1). In the metastatic setting, several chemotherapy regimens were adopted. First line treatments are described in table 2; in the second line setting, paclitaxel was used in 5 patients, in combination with bevacizumab in 1 case; sorafenib was given to 1 patient. Thirteen patients with metastatic disease were evaluable for response: partial responses (PR), stable disease (SD) and progression of disease (PD) as best responses were documented in 2 patients (PR), 2 patients (SD) and 9 patients (PD) respectively (Table 2). The two patients achieving PR had been given paclitaxel (Figure 3) or pegylated liposomal doxorubicin (Figure 4).
Table 1.
Chemotherapy delivered to patients in localized patients
| Regimen | Pts | % |
|---|---|---|
| Osteosarcoma-like (DOX, MTX, CDDP, IFO) | 10 | 77 |
| Taxanes | 2 | 15 |
| Unknown | 1 | 8 |
Abbreviations: DOX: doxorubicin, MTX: methotrexate, CDDP: cisplatin, IFO: ifosfamide.
Table 2.
Best response in patients with metastatic disease receiving chemotherapy (RECIST 1.0).
| Best Result (RECIST 1.0) | ||||
|---|---|---|---|---|
| Regimen | N° of pts | PR | SD | PD |
| Osteosarcoma-like | 6 | 1 | 5 | |
| Anthracycline monotherapy* | 4 | 1 | 1 | 2 |
| Paclitaxel | 2 | 1 | 1 | |
| Cisplatin-etoposide | 1 | 1 | ||
| Unknown | 2 | |||
Standard doxorubicin or pegylated liposomal doxorubicin
Figure 3.
A patient with femur angiosarcoma metastatic to the lungs. Chest and thigh CT scan shows a partial response (PR) after 2 months on paclitaxel treatment, with disappearance of one right lung nodule.
Figure 4.
A patient with lung metastases of the left iliac wing angiosarcoma. After 2 months on pegylated liposomal doxorubicin, the pelvic CT scan demonstrated a decrease of the soft tissue component of the primary tumor and bone calcification.
Overall Survival (OS)
At the time of writing this manuscript, 9 (15%) patients were alive and free of their disease, 47 (78%) patients were dead of disease, two patients were dead of other causes (thyroid cancer and acute cerebrovascular accident) and two were alive with disease.
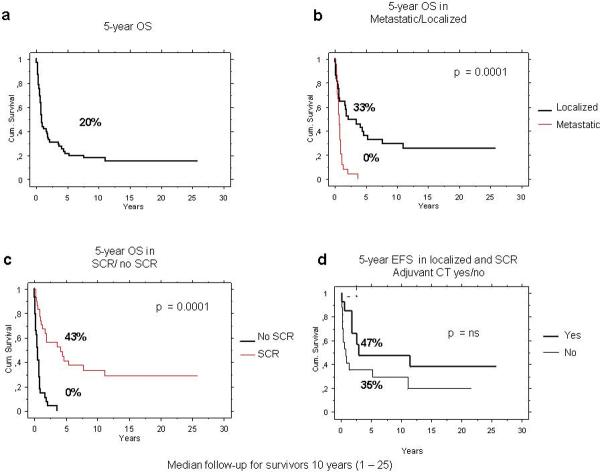
At a median follow-up of 10 years (range 1–25 years), the 5-year OS rate was 20% (95%CI 9–30%): 33% (95%CI 17–50%) for patients with localized disease and 0 for patients with metastatic disease, P<0.0001 (Figure 4). Patient achieving a complete surgical resection had 43% 5-year OS rate (95%CI 29–63%), whereas all patients who did not have complete surgical resection had died at 5 years (P<0.0001) (Figure 4). A trend towards inferior OS was observed in older patients, larger and central located tumors (Table 3).
Table 3.
Univariate analysis for Overall Survival (OS) in all patients
| Variable | Pts n° | 5-year 0S | 95% CI | p | |
|---|---|---|---|---|---|
| Age | < 26 yrs | 4 | 25 | 0–67 | 0.6 |
| 26 – 65 yrs | 42 | 23 | 10–36 | ||
| > 65 yrs | 14 | 11 | 0–32 | ||
| Gender | M | 38 | 25 | 11–39 | 0.4 |
| F | 22 | 13 | 0–28 | ||
| Stage | localized | 36 | 33 | 17–50 | 0.0001 |
| metastatic | 24 | 0 | |||
| Size* | < 10 cm | 22 | 33 | 13–54 | 0.09 |
| ≥ 10 cm | 29 | 11 | 0–23 | ||
| Site | extremity | 38 | 29 | 14–44 | 0.1 |
| central | 22 | 6 | 0–17 | ||
| Chemotherapy** | yes | 28 | 22 | 6–38 | 0.11 |
| no | 29 | 22 | 7–39 | ||
| SCR | yes | 30 | 46 | 29–63 | 0.0001 |
| no | 30 | 0 |
M: male; F: Female; SCR: surgical complete remission
Size was known in 51 patients;
Chemotherapy information was available for 57 patients
The median survival time of the study population was 0.9 years (range 0.1–25 years); the median survival time was 1.9 years (0.1–25 years) for the patients with localized disease and 0.7 years (range 0.1–3.7 years) for those with metastases. When a complete surgical remission was achieved, the median survival time was 3.3 years (range 0.2–25 years), whereas it was 0.7 years (0.1–3.7) in patients who were not rendered surgically free of disease.
In the 30 patients in SCR, the 5-year EFS was 41% (95%CI 23–59). On univariate analysis, no differences according to patient gender, age (<26 years; 26–65%; >65 years) or tumor size (>10 cm vs. ≤10 cm) were observed.
The use of adjuvant chemotherapy was associated with a 5-year EFS of 47% (95%CI 17–77%) compared with 35% (95%CI 13–58%) for those who did not (Figure 5). None of the patients with localized disease who received adjuvant radiotherapy following a surgery with inadequate margins were alive at 5 years.
Figure 5.
Five-year OS of 60 patients with bone angiosarcoma (panel a), by stage at presentation (panel b) and by surgical complete remission status (panel c). Five-year EFS in patients with localized disease and who achieved a surgical complete remission, according to the use of adjuvant chemotherapy (panel d).
Pattern of relapse
Overall, 19 (63%) patients in SCR experienced a subsequent disease recurrence. Details on the pattern of relapse were available in 14 patients. Local recurrence was documented in 7 (50%) patients (in 5 associated with distant metastases). Four (28%) patients had lung only metastases, 2 (14%) patients had multivisceral lesions and 1 patient had bone-only metastases.
DISCUSSION
Due to their rarity, there has not been a general consensus on the incidence, classification, and nomenclature of angiosarcoma of bone. The terms hemangiosarcoma, high-grade hemangioendothelioma, and high-grade hemangioendothelial sarcoma have been used interchangeably for the same entity (4, 14, 15)
Most published series of bone vascular tumors include both low and high grade lesions. The proportion of high grade tumors in these studies has been variable. In a study by Tsuneyoshi et al. (14), about half of the cases were angiosarcomas, whereas the frequency of high grade bone vascular tumors was significantly lower (7%) in a study by Kleer et al. (4). In the first retrospective series presented by the Istituto Rizzoli, 52% of the tumors were high grade (15). The authors of this paper reported a discrepancy between histological grade and biological behavior and concluded that the study of large and multiple sections (“tumor mapping”) might help for the diagnosis of angiosarcoma of bone (15).
In the current study, after histological review of all patients with a diagnosis of primary vascular tumor of bone from 1980 to 2009, we excluded those with low grade lesions, aiming to study the outcome of those with angiosarcoma of bone specifically. The median age for these patients was 54 years (range 18–82 years). This age spectrum is similar to that of soft tissue angiosarcoma (16) rather than that of bone osteosarcomas (17).
Furthermore, unlike osteosarcomas, angiosarcomas are more frequently metastatic at presentation; in the present series 40% of the patients presented with metastases compared to approximately. 20%, in osteosarcoma series (18).
Survival is poor for patients with angiosarcoma. In this series patient with localized disease had a 33% (95%CI 17–50) 5-year OS rate. This is worse than the 5-year OS of localized osteosarcoma, which usually ranges from 60% to 75% (17, 19). Nevertheless, studies including patients older than 40 years with localized osteosarcoma (20, 21), showed survival rates similar to those of localized angiosarcoma of bone, which reached about 40% at 5-year in our series.
The comparison of the survival of patients with angiosarcoma of the bone with that of patients with angiosarcoma arising in other locations such as soft tissue or skin is likely flawed. Patient characteristics vary, such as the proportion of metastatic patients at presentation or of radiation-induced tumors or anatomic primary site, and the rarity of all these entities in the published series contribute to their heterogeneity (8, 22). A retrospective study that assessed the survival of 161 patients with angiosarcomas in different sites showed an inferior outcome for bone (36% 5-year OS), liver, heart, and splenic angiosarcomas, compared with angiosarcomas arising in the soft tissues, skin and breast (8).
The data from this retrospective analysis underscores that surgical treatment remains the treatment of choice for bone angiosarcoma; there were no cures for this tumor without a surgical complete resection. (5-year OS for patients in SCR 43% vs. 0% for those without SCR). The high incidence (50%) of local recurrence observed in our series emphasizes the importance of adequate local treatment for this tumor. Multidisciplinary care with both surgery and radiation would appear prudent to attempt to achieve the highest degree of local control. Also, radiation therapy should be considered for those patients with multifocal and/or surgically inaccessible tumors since it has been shown effective for patients with low or intermediate grade malignant vascular tumors of bone (9).
Although chemotherapy may be useful for these lesions, its role has not been assessed prospective in bone angiosarcoma (1, 9). The high local and distant relapse rate in the present analysis begs the question of adjuvant chemotherapy. A discussion of the relative risks and potential benefits of systemic therapy is required on a case by case basis, but at least in principle adjuvant chemotherapy can be considered for a patient with complete surgical resection, given the short median survival even for patients resected completely. It is important to emphasize that the analysis on adjuvant chemotherapy in the present study is exploratory. Our series showed that patients in which SCR can be achieved and who received adjuvant chemotherapy had a 5-year OS of 43%.
Given to the heterogeneity of the agents examined in this retrospective series, we cannot draw definitive conclusions on the type of chemotherapy for bone angiosarcoma. We observed that most chemotherapeutic agents employed in bone angiosarcomas were similar to those for osteosarcomas (doxorubicin, cisplatin, methotrexate and ifosfamide). Only more recently a few patients underwent treatments with taxanes, which is more commonly used for patients with soft tissue angiosarcomas. In the metastatic setting we documented 1 PR out of 4 patients treated with doxorubicin, and 1 out of 2 patients treated with paclitaxel. The relative lack of activity of the multi-drugs combination used in osteosarcoma therapy is consistent with greater similarity of bone angiosarcomas to soft tissue angiosarcomas rather than osteogenic sarcomas per se. This notion would require prospective evaluation. Many studies have addressed the activity of taxanes in soft tissue angiosarcoma, reporting a RR ranging from 18 to 89% (16, 23, 24, 25). Our results, far of being conclusive because of the small sample size, suggest that taxanes may be beneficial in patients with bone angiosarcoma. Unaddressed is the role of antiangiogenic agents for the treatment of patient with angiosarcoma of bone, whereas in soft tissue angiosarcomas there appears to be a ~15% RECIST response rate with antiangiogenic agents such as sorafenib, bevacizumab, and brivanib (26–29).
There are significant limitations of this series. First, this is a retrospective study with its attendant limitations such as recall bias. Second, the number of the patients is small. Nonetheless, the present study represents the largest series specifically evaluating histologically confirmed primary angiosarcoma of bone and give some guidance as to the management of this very rare condition.
Given the poor survival even for patients with resected disease, new agents clearly are required for patients with this rare diagnosis. The role of taxanes in bone angiosarcoma deserves further evaluation. Anthracycline-based therapy represents a regimen to consider in the adjuvant setting in those patients in whom aggressive therapy is desired. The outcomes for patients with overt metastatic disease, unresectable disease, or those with inadequate resections are poor, making such patients candidates for studies of novel antiangiogenic agents, with a hope of improving these outcomes.
Footnotes
All authors declare no financial disclosures nor funding sources for this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roessner A, Boehling T. Angiosarcoma. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. World Health Organization Classification of Tumours. IARC Press; Lyon: 2002. pp. 243–45. [Google Scholar]
- 2.Wenger DE, Wold LE. Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29:619–31. doi: 10.1007/s002560000261. [DOI] [PubMed] [Google Scholar]
- 3.Verbeke SLJ, Bertoni F, Bacchini P, et al. Distinct Histologic Features Characterize Primary Angiosarcoma of Bone. Histopathology. 2011;58:254–64. doi: 10.1111/j.1365-2559.2011.03750.x. [DOI] [PubMed] [Google Scholar]
- 4.Kleer CG, Unni KK, McLeod RA. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20:1301–11. doi: 10.1097/00000478-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bruegel M, Waldt S, Weirich G, et al. Multifocal epithelioid hemangioendothelioma of the phalanges of the hand. Skeletal Radiol. 2006;35:787–92. doi: 10.1007/s00256-005-0943-6. [DOI] [PubMed] [Google Scholar]
- 6.Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: A study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol. 2003;34:680–89. doi: 10.1016/s0046-8177(03)00249-1. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande V, Rosenberg AE, O'Connell JX, et al. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27:709–16. doi: 10.1097/00000478-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030–36. doi: 10.1093/annonc/mdm381. [DOI] [PubMed] [Google Scholar]
- 9.Wold LE, Unni KK, Beabout JW, et al. Hemangioendothelial sarcoma of bone. Am J Surg Pathol. 1982;6:59–70. doi: 10.1097/00000478-198201000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Unni KK, Ivins JC, Beabout JW, et al. Hemangioma,hemangiopericytoma, and hemangioendothelioma (angiosarcoma) of bone. Cancer. 1971;27:1403–14. doi: 10.1002/1097-0142(197106)27:6<1403::aid-cncr2820270621>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Mirra JM. Angiosarcoma of bone: solitary and multifocal variants. In: Mirra JM, Picci P, Gold R, editors. Bone turnours. Clinical, radiologic and pathologic correlations. Lea and Febiger; Philadelphia: PA: 1989. pp. 1382–17. [Google Scholar]
- 12.Enneking WF, Spanier SS, Goodman MA. Current concepts review. The surgical staging of musculoskeletal sarcoma. J Bone Joint Surg. 1980;62-A:1027–30. [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Ins. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Tsuneyoshi M, Dorfman HD, Bauer TW. Epithelioid hemangioendothelioma of bone: a clinicopathologic, ultrastructural, and immunohistochemical study. Am J Surg Pathol. 1986;10:754–64. doi: 10.1097/00000478-198611000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Campanacci M, Boriani S, Giunti A. Hemangioendothelioma of bone: a study of 29 cases. Cancer. 1980;46:804–14. doi: 10.1002/1097-0142(19800815)46:4<804::aid-cncr2820460427>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and and chemotherapy. Ann Surg Oncol. 2009;16:250–59. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Smeland S, Mercuri M, et al. the Italian and Scandinavian Sarcoma Groups Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 18.Kager L, Zoubek A, Pötschger U, et al. the Cooperative German-Austrian-Swiss Osteosarcoma Study Group Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–18. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 19.Bielack S, Jürgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289–308. doi: 10.1007/978-1-4419-0284-9_15. [DOI] [PubMed] [Google Scholar]
- 20.Grimer RJ, Cannon SR, Taminiau AM, et al. Osteosarcoma over the age of forty. Eur J Cancer. 2003;39:157–63. doi: 10.1016/s0959-8049(02)00478-1. [DOI] [PubMed] [Google Scholar]
- 21.Bacci G, Ferrari S, Donati D, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity in patients in the fourth and fifth decade of life. Oncol Rep. 1998;5:1259–63. doi: 10.3892/or.5.5.1259. [DOI] [PubMed] [Google Scholar]
- 22.Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983–91. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 23.Fata F, O'Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034–37. [PubMed] [Google Scholar]
- 24.Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433–36. doi: 10.1016/j.ejca.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–74. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 26.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller CK, Charlson JA, Dankle SK, et al. Dramatic improvement of inoperable angiosarcoma with combination paclitaxel and bevacizumab chemotherapy. J Am Acad Dermatol. 2010;63:83–84. doi: 10.1016/j.jaad.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Agulnik M, Okuno SH, Von Mehren M, et al. Journal of Clinical Oncology. 2009;27(15S):10522. ASCO Annual Meeting Proceedings. [Google Scholar]
- 29.Schwartz GK, Maki RG, Ratain MJ, et al. Brivanib (BMS-582664) in advanced soft-tissue sarcoma (STS): Biomarker and subset results of a phase II randomized discontinuation trial. Journal of Clinical Oncology. 2011;29(15S):10000. ASCO Annual Meeting Proceedings. [Google Scholar]