Summary
Biomarkers for active tuberculosis (TB) are urgently needed. Mycobacteria produce membrane vesicles (MVs) that contain concentrated immune-modulatory factors that are released into the host. We evaluated the human immune responses to BCG and M. tuberculosis MVs to characterize the antibody responses and identify potentially novel TB biomarkers. Serological responses to MVs were evaluated by ELISAs and immunoblots with sera from 16 sputum smear-positive, 12 smear-negative HIV uninfected pulmonary TB patients and 16 BCG vaccinated Tuberculin skin-test positive controls with and without latent tuberculosis infection. MVs from both BCG and M. tuberculosis induced similar responses and were strongly immunogenic in TB patients but not in controls. Several MV-associated antigens appear to induce robust antibody responses, in particular the arabinomanan portion of the cell wall glycolipid lipoarabinomannan. Three proteins at ~36, 25, and 23 kDa were simultaneously recognized by sera from 16/16 smear-positive, 9/12 smear-negative TB patients and 0/16 controls. These results provide promise and encouragement that antibody responses to proteins enriched in MVs of pathogenic mycobacteria may constitute a novel TB biomarker signature that could have diagnostic information.
Keywords: Serology, immunoglobulins, infection, human studies, diagnostics
Introduction
The identification of easily detectable biomarkers for active tuberculosis (TB) is a global health priority 1, 2. TB remains a worldwide public health problem underscored by an estimated 8.7 million new cases in 2011 with almost one million TB-associated deaths among HIV− and ~0.43 million among HIV+ people 3. Rapid TB diagnosis and treatment leads to reduced transmission, morbidity and mortality but is often delayed, especially in resource-limited settings where the vast majority of people with TB reside. Thus, TB biomarkers that can lead to simple rapid point-of-care (POC) tests are urgently needed.
The gold standard test for TB diagnosis remains the detection of Mycobacterium tuberculosis in culture 4. However, culture methods necessitate a laboratory infrastructure and entail incubation times of weeks to months. Molecular methods for detecting M. tuberculosis-specific nucleic acids, especially the recently WHO endorsed GeneXpert M.TB/RIF, have revolutionized the rapid diagnosis of drug-sensitive and resistant TB 5–8. However, they are costly and require technological investment. Therefore, although limited by a sensitivity of around 50% 9–11, microscopy remains the most widely used method for rapid TB diagnosis, and often is the only test available in resource-limited settings. Despite ongoing research efforts a simple inexpensive POC test, applicable in all settings, is still not available 8, 12.
Serum antibodies (Abs) can be detected by rapid “dip-stick” formats suitable for POC testing 13–15, but no accurate serodiagnostic tests for TB have been developed to date 16–18. We have recently reported that pathogenic mycobacteria produce membrane vesicles (MVs) that are released into the extracellular space and contribute to mycobacterial virulence in mice 19. These MVs vary in diameter between 60 to 300 nm and their composition includes glycolipids and a large number of lipoproteins. MVs provide an effective way for intra-cellular bacteria to release concentrated immune-modulatory factors into the host. Hence, the assessment of the host immune response to MVs provides a unique opportunity for identification of novel biomarkers. The objective of this study was to evaluate the serological responses to mycobacterial MVs in human TB cases and controls. We demonstrate that MVs from M. tuberculosis and M. bovis Bacillus Calmette-Guérin (BCG) elicit strong Ab responses in humans that include reactivity with a set of MV proteins to produce a serological profile that is highly sensitive and specific for TB and thus potentially constitutes a new TB biomarker.
Subjects, Materials and Methods
Subjects and Study Design
Subjects were 21 – 80 years old and enrolled at 4 public hospitals in New York City from 2007–2010. All subjects were HIV uninfected and either had pulmonary TB (n=28) or were healthy asymptomatic controls with a positive tuberculin skin-test (TST+; n=16). TB cases were confirmed by a positive respiratory culture for M. tuberculosis (gold standard) and enrolled prior to, or within the first 7 days, of antituberculous treatment. They were further categorized by sputum smear microscopy results and considered smear-positive if one of the initial three sputum smears were positive regardless of number of acid-fast bacilli (AFB) detected. Controls were asymptomatic TST+ health care providers who were all BCG vaccinated and reported a positive exposure history to patients with TB. TST+ controls had no abnormalities on chest X-ray and were further categorized based on results for an interferon-gamma release assay (IGRA; QuantiFERON®-TB Gold, Celestis, Australia). Nine/16 controls had a negative IGRA result and were considered TST+ due to a history of BCG vaccination. Seven/16 had a positive IGRA result and were considered to have latent tuberculosis infection (LTBI). All subjects provided written informed consent prior to enrollment. Approval for human subjects’ research was obtained from the Internal Review Boards at the New York University School of Medicine, NY, NY, and the Albert Einstein College of Medicine, Bronx, NY.
Mycobacterial MV Preparation
Vesicles were isolated through a series of gradient filtration and centrifugation steps as previously described 19. Essentially, M. tuberculosis (strain H37Rv), obtained from the Trudeau Institute (Saranac Lake, NY), or M. bovis BCG (Pasteur strain), obtained from the Statens Serum Institute (Copenhagen, Denmark), were grown in mid-logarithmic phase at 37°C in roller bottles containing minimal media. Mycobacteria were harvested after 10 days of growth and pelleted to remove cell fractions. The supernatant was then filtered through a 0.45 μm polyvinylidene difluoride membrane filter (Millipore, MA) and concentrated using a 100-kDa exclusion filter with an Amicon Ultrafiltration System (Millipore, MA). The concentrate was ultracentrifuged at 60,000 rpm for 1 h at 4°C to sediment the vesicular fraction into a pellet which was resuspended in PBS. The protein concentration of the MV preparation was determined using a BCA Protein Assay Kit (Thermo Scientific, IL).
Antibody Detection Assays
Antibody reactivity to MVs was determined via enzyme-linked immunosorbent assay (ELISA) as described 20, 21. Briefly, 96-well microtiter plates (Immulon 2HB, Fisher Scientific, NY) were coated with either 4 μg/ml protein concentration of MVs, 10 μg/ml of lipoarabinomannan (LAM) or arabinomannan (AM), or 4 μg/ml of antigen 85B (Ag 85B) for 1 h and then blocked with 3% BSA/0.1% PBST over night. LAM prepared from the Mtb strain H37Rv and Ag85B prepared from culture filtrates of H37Rv were obtained from the Biodefense and Emerging Infectious Disease Research Resources Repository (BEI Resources; Manassas, VA). AM was prepared from the Mtb strain H37Rv and the BCG Pasteur strain as described 21. Serum samples diluted at 1:50 were added in duplicates to the coated wells and Abs were detected via either Protein A-alkaline phosphatase (AP) (Sigma, MO) for immunoglobulin (Ig) G, goat anti-human IgA-AP or goat anti-human IgM-AP (Southern Biotech, AL). IgG subclass reactivity was detected using anti-human IgG1-AP, IgG2-AP, IgG3-AP, or IgG4-AP (Southern Biotech, AL). All secondary Abs were used at a concentration of 1 μg/ml (1:1000). The positive control consisted of a serum sample from a TB patient with known high Ab reactivity to a wide range of mycobacterial antigens. The negative controls consisted of wells treated in the same manner as described above without the addition of serum.
Antibody response profiles to MV proteins were analyzed by immunoblotting. A solution with 50 μg of MVs was electrophoresed in 4% stacking and 12% SDS polyacrylamide resolving gel as described 22. Briefly, the gel proteins were transferred to a membrane via the Invitrogen iBlot® Dry Blotting System (Life Technologies, NY) after which the membranes were blocked for 1h using 5% milk in TBS + 0.01% Tween 20. Serum samples diluted at 1:100 were incubated at 4°C over night using the multi-channel blotting system Surf-Blot 7.5 (Idea Scientific, MN). The membranes were washed and incubated for 45 min with Protein A-AP (1:1000; Sigma, MO) and the bands were visualized using SIGMAFAST BCIP®/NBT substrate (Sigma, MO). Band intensity was analyzed by densitometry using ImageQuant TL, Version 7.0 (GE Healthcare, PA) 23, 24. Backgrounds were determined for each lane individually and set as a straight line at the lowest point of the histological profile of the lane. Bands were considered positive if the band volume (pixel3), representing the bands signal intensity as well as thickness, was greater than the cut-off determined by receiver operating characteristics (ROC) curve analysis.
Monoclonal antibodies (mAbs)
Mtb and BCG MV-associated proteins have been previously identified via proteomic analysis 19. To further identify antigens reactive with TB+ sera in Western blots we obtained available mAbs against MV proteins in the molecular weight range of our target antigens. These included two different mAbs against the Ag85 complex, clones CS-90 and IT-44, and an mAb against the lipoprotein LprG, clone α-RV1411c (BEI Resources, Manassas, VA). An mAb against the MTP64 protein is commercially available (SunnyLab, Sittingbourne, UK) but was not available at the time of the study, and was not tested. The vesicle blots were processed as above with mAbs diluted 1:50 (1 ug/ml) and developed with anti-mouse IgG (H+L) (Southern Biotech, Birmingham, AL) at a concentration of 1:1000 (1 ug/ml).
Statistical Analysis
Statistical analysis was done using the Prism software, version 5.02 (GraphPad Inc., CA) and STATA software, version 9.2 (StataCorp, TX). We used non-parametric tests, such as Mann-Whitney U test and Spearman rank correlation test, for all comparisons because Ab responses to some MV antigens were not normally distributed. Optimal cut-off value for band intensity in immunoblots was determined by ROC analysis as described 25.
Results
Our overall objective was to evaluate the serological responses to MVs from pathogenic mycobacteria in TB patients compared to TST+ controls. We initially evaluated the Ab responses to whole MVs, followed by the delineation of Ab responses to MV-associated glycolipids and protein antigens.
Antibody responses to entire mycobacterial MVs
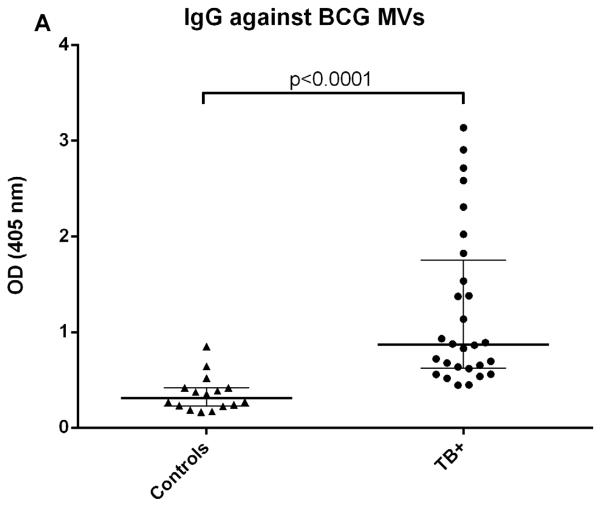
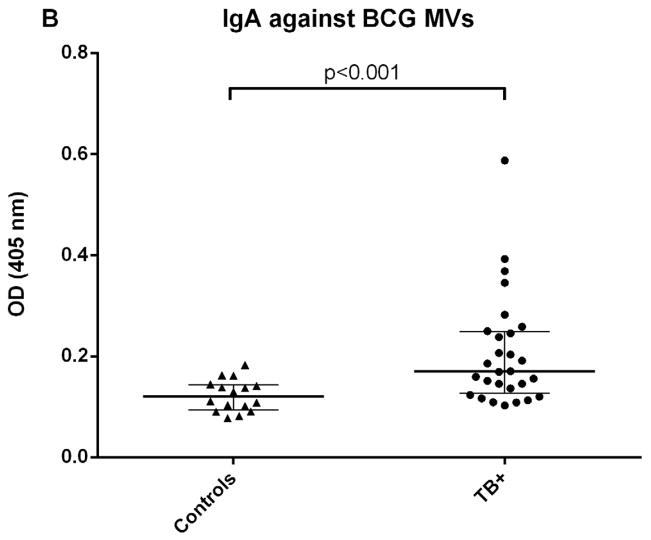
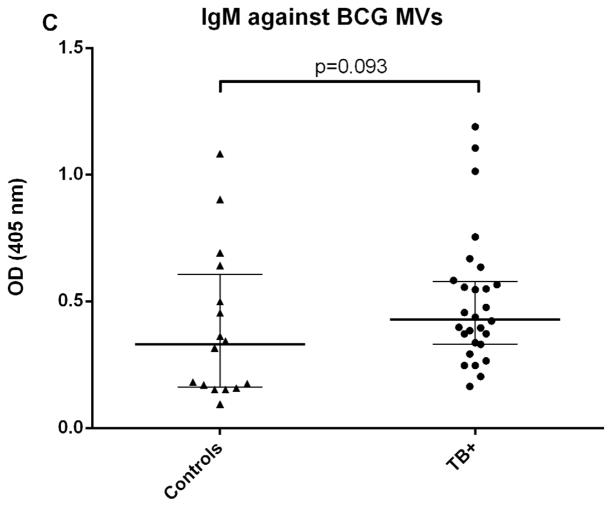
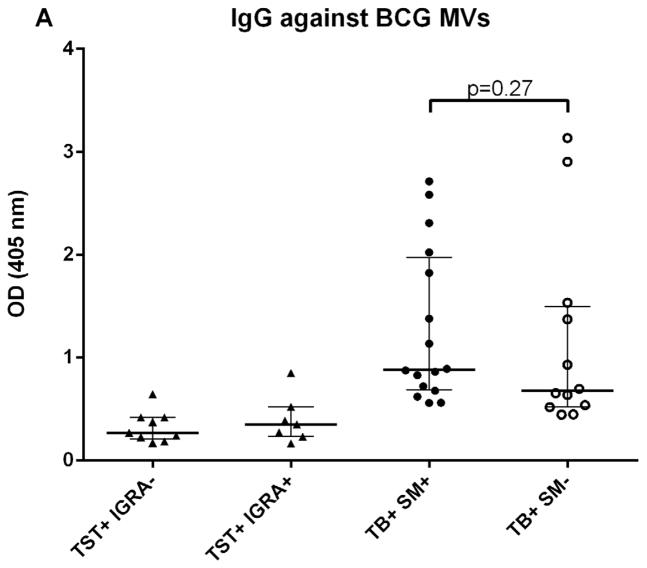
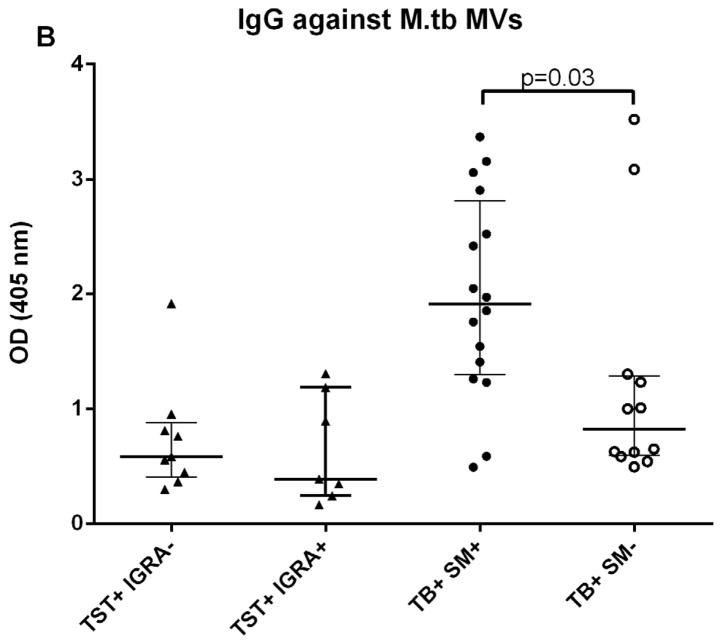
We initially determined Ab responses to whole MVs via ELISA. Although there were some differences between patient groups, overall Ab isotype responses against M. tuberculosis MVs correlated strongly and significantly with those against BCG MVs for both TB+ cases and controls (r=0.90, p<0.0001 for IgG; r=0.81, p<0.0001 for IgM; and r= 0.78, p<0.0001 for IgA). The predominant isotype response against MVs was IgG which was significantly higher in TB cases than TST+ BCG vaccinated controls (p < 0.0001; Fig. 1A). IgA and IgM responses were generally lower, and the difference between TB cases and controls remained only significant for IgA (p < 0.001 for IgA and p=0.09 for IgM; Fig. 1B&C). None of the isotype responses were significantly different between TST+ IGRA− and TST+ IGRA+ healthy controls. Of note, IgG responses against M. tuberculosis MVs were significantly higher in smear-positive compared to smear-negative TB cases (p=0.03; Fig. 2A), a difference that was not observed for IgG responses against BCG MVs (Fig. 2B). IgG responses were mostly due to subtypes IgG1 and IgG2 with little to no contribution from IgG3 and IgG4.
Fig. 1.
Antibody isotype responses against BCG MVs in TB patients and TST+ controls. A. IgG responses against BCG MVs; B. IgA responses against BCG MVs; C. IgM responses against BCG MVs. Statistical analysis with Mann-Whitney U test. Bars show median values with interquartile range.
Fig. 2.
A–B. IgG responses to BCG (A) and M. tuberculosis MVs (B), demonstrating significantly higher IgG titers in smear-positive compared to smear-negative TB patients against M. tuberculosis MVs but not against BCG MVs. Statistical analysis with Mann-Whitney U test. Bars show median values with interquartile range.
Antibody responses to specific mycobacterial MV antigens
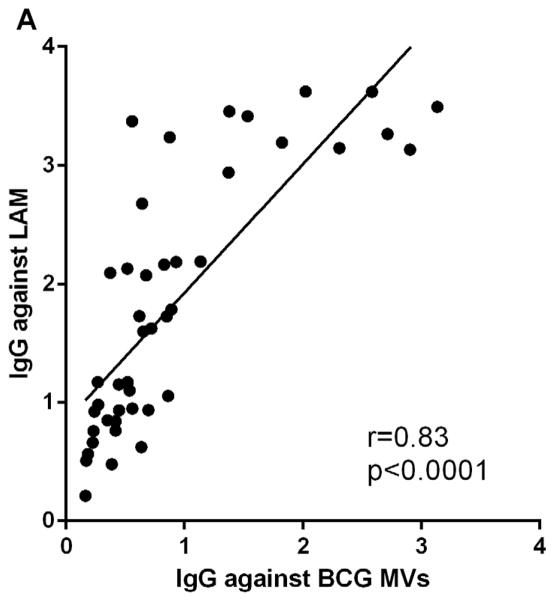
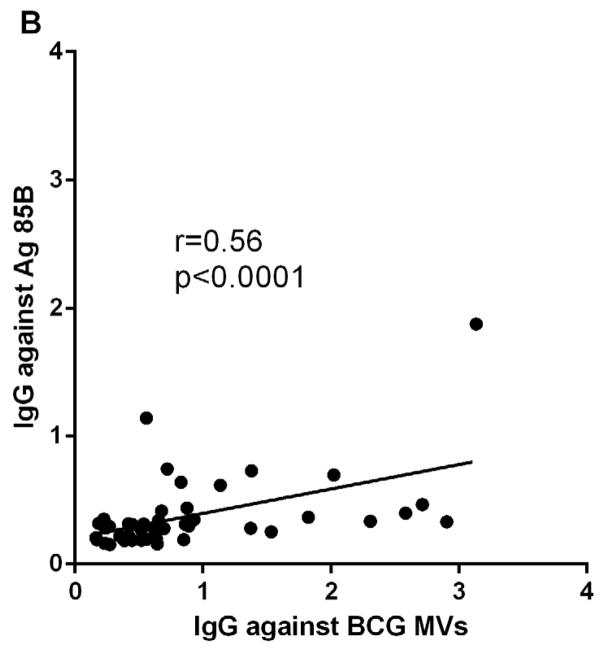
To identify antigens that contributed to MV immunogenicity, we correlated Ab responses to whole MVs with those to one of the major MV antigens, LAM, as well as to the protein antigen Ag 85B. M. tuberculosis and BCG MVs are comprised of almost 40% of cell wall and cell processes-associated proteins and contain an abundance of the glycolipid cell wall antigen LAM 19. The Ag 85B has cell wall mycolyltransferase activity and has been identified in mycobacterial MVs and exosomes 19, 26, 27. IgG reactivity to whole MVs and LAM correlated strongly and significantly in TB cases and controls (r=0.83, p<0.0001; Fig. 3A). In addition, we evaluated IgG reactivity to AM and found a similarly strong correlation (r=0.80, p<0.0001), suggesting that Ab responses were elicited by this highly immunogenic portion of LAM. This correlation was similarly strong regardless whether MVs and AM were isolated from BCG or M. tuberculosis. The correlation between IgG responses to MVs and Ag 85B was weaker but also highly significant (r=0.56, p<0.0001; Fig. 3B).
Fig. 3.
Correlations of IgG responses between A. BCG MVs and cell wall glycolipid lipoarabinomannan (LAM), and B. BCG MVs and protein antigen Ag 85B. Statistical analysis with Spearman rank correlation test.
Antibody profiles to mycobacterial MV proteins
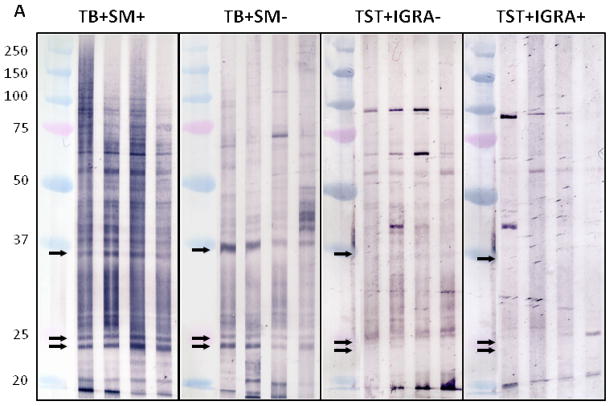
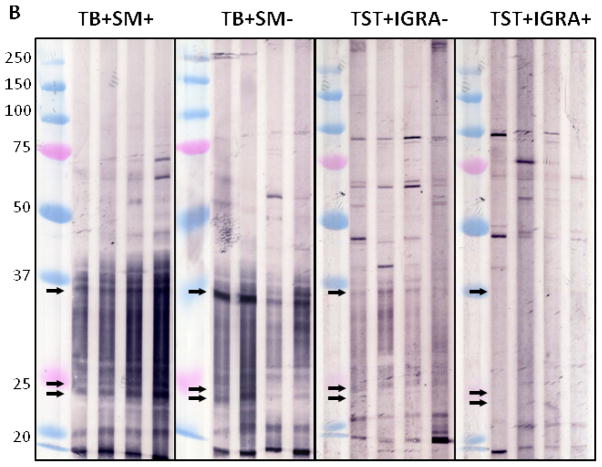
Immunoblot profiles with M. tuberculosis and BCG MVs revealed a reactive IgG pattern with sera from TB patients that was absent with sera from TST+ BCG vaccinated controls (Fig. 4A+B). Compared to BCG MV immunoblots, an overall higher background intensity was observed with M. tuberculosis MV immunoblots but no additional bands were identified.. This is consistent with the results of proteomic analysis, identifying 66 proteins in BCG MVs in contrast to 48 in M. tuberculosis MVs 19. Therefore, BCG MV immunoblots were utilized for visual and quantitative analysis of band intensity. Of note is that band intensities varied according to culture duration of mycobacteria prior to MV harvest. Thus, a culture protocol with MV harvest after 10 days was implemented to assure reproducibility of the antigen profiles. Overall, several more bands were recognized with smear-positive compared to smear-negative TB samples in BCG MV immunoblots. This is consistent with other serological studies, demonstrating Ab reactivity to many mycobacterial antigens in a higher proportion of smear-positive compared to smear-negative TB patients (reviewed in 28). We identified three bands at ~36, 25, and 23 kDa that were simultaneously recognized by 16/16 sputum smear-positive, 9/12 smear-negative TB patients and 0/16 TST+ controls, of which 9/16 had a negative and 7/16 a positive IGRA result indicative of LTBI. Optimal cut-off for intensity of all bands was 30,000 pixel3 as determined by ROC (Fig. 4C&D), a setting that demonstrated > 90% sensitivity (95% CI 72–98%) with 100% specificity (95% CI 80–100%) for all samples combined. Of note, the 3 smear-negative TB patients without Abs to the 3 MV proteins had evidence of disseminated TB. We also identified a band at ~95 kDa with 11/16 TST+ control sera (regardless of IGRA result) which was not seen with any of the sera from the 28 TB cases. These findings are suggestive of the notion that some of the Abs against mycobacterial MVs could have protective function.
Fig. 4.
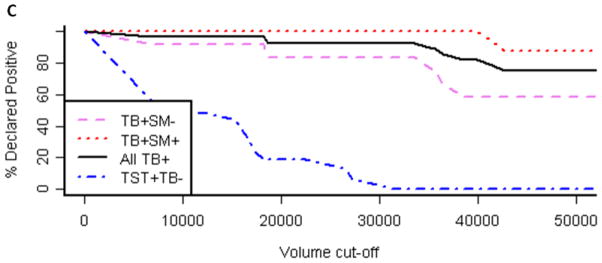
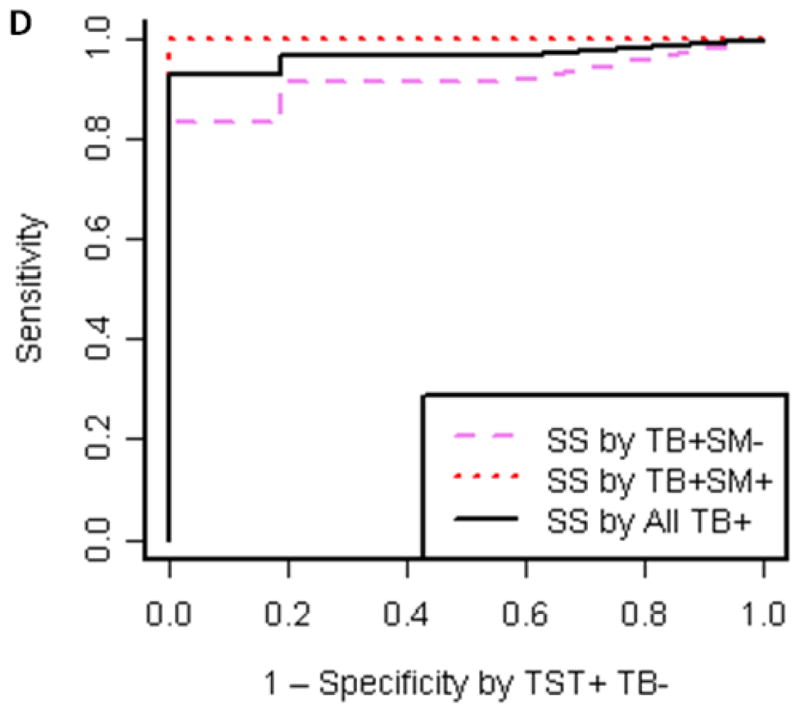
A–B. Reactive IgG pattern to BCG MV (A) and M. tuberculosis MV (B) proteins in immunoblots with 4 representative sera from smear-positive and smear-negative TB patients and from TST+ TB− BCG vaccinated controls categorized by interferon-gamma release assay (IGRA) result demonstrating 3 bands ~36, 25 and 23 kDa that are simultaneously recognized with sera of TB+ cases but not BCG vaccinated TST+ controls without or with latent M. tuberculosis infection (LTBI). Due to higher background with M. tuberculosis MV immunoblots, BCG MV immunoblots were used for further analysis; C. Proportion of positive immunoblots (y-axis) for smear-positive, smear-negative, and all TB+ cases as well as TST+ controls according to band intensity in pixel3 (volume; x-axis) indicating that optimal cut-off values can range between about 30,000 and 35,000 pixel3; and D. Receiver-operating characteristics (ROC) curve for optimal cut-off at 30,000 pixel3
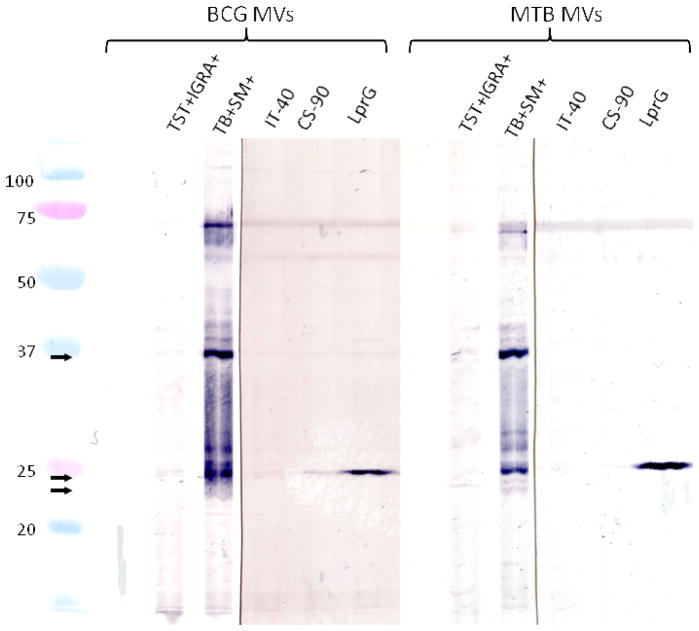
To further identify the major bands reactive with TB+ sera, MV blots were probed with available mAbs against MV proteins with a corresponding molecular mass, Ag85 (~34–37 kDa) and the lipoprotein LprG (~25 kDa). The band ~25 kDa was strongly reactive with the mAb α-RV1411c suggesting that this band contained LprG (Fig. 5). No reactivity was observed with the two mAbs CS-90 and IT-44 indicating that the band recognized at ~36 kDa did not consist of Ag85 complex components.
Fig. 5.
BCG and M. tuberculosis MV immunoblots probed with sera from a TST+ IGRA+ control and smear-positive TB patients, and mAbs against the Ag 85 complex (IT-40 and CS-90) and the lipoprotein LprG. The mAb against LprG is reactive, while no reactivity is observed with mAbs against Ag 85.
Discussion
This is the first study demonstrating that MVs from pathogenic mycobacteria are strongly immunogenic in HIV uninfected patients with pulmonary TB. Our results show that certain proteins enriched in M. tuberculosis and BCG MVs elicit serum IgG responses in both smear-positive and smear-negative TB patients but not in TST+ BCG vaccinated healthy controls with or without LTBI. We identified 3 bands at ~36, 25, and 23 kDa, that, when recognized simultaneously in immunoblots, may constitute a highly sensitive and specific Ab biomarker signature for TB that has diagnostic potential.
Historically, MVs have largely been studied in Gram negative and lately also in Gram positive bacteria where their release facilitates the transportation of virulence factors into the host 29, 30. MVs differ significantly from exosomes in that MVs are directly released by bacteria, while exosomes are secreted by (infected) mammalian cells. Immunogenicity of MVs was demonstrated for several organisms such as N. meningitides, S. typhimurium, V. cholera and B. anthracis by immunization studies, which, except for N. meningitides were mostly performed in mice 31–40. We now show that MVs released by pathogenic mycobacteria induce a strong serological response in TB patients which, in accordance with the studies of bacterial MVs as well as prior serological studies with other M. tuberculosis antigens, is based predominantly on IgG with lower IgM and IgA responses (34, 36 and reviewed in 28).
Our results reveal that the serological responses are induced by several M. tuberculosis and BCG MV-associated antigens, and suggest that a considerable portion might be elicited by AM, a capsular polysaccharide antigen and the highly immunogenic fraction of the cell wall glycolipid LAM. In agreement with one of our prior studies, demonstrating significantly higher IgG titers against AM in smear-positive than in smear-negative patients, we also found significantly higher IgG titers against M. tuberculosis MVs in smear-positive compared to smear-negative TB patients 21. Interestingly, while Ab responses to M. tuberculosis MVs were significantly different between smear-positive and smear-negative TB, Ab responses to BCG MVs were not. This indicates that some of the MV-associated antigens, especially those that are cell wall associated, might differ in composition and be more immunogenic in M. tuberculosis compared to BCG. Ab responses were significantly lower in controls than in TB cases and not different between TST+ IGRA− and TST+ IGRA+ controls, indicating that neither BCG vaccination nor LTBI induce considerable Abs against whole MVs.
For a variety of infectious diseases immunoblotting is more accurate and discriminating than other Ab-detection methods 41–43. In line with these studies, we identified a signature pattern rather than reactivity with individual proteins. We demonstrated that the simultaneous presence of Abs to 3 MV proteins at ~36, 25, and 23 kDa constituted a highly sensitive and specific TB Ab biomarker, detecting 100% of smear-positive and 75% of smear-negative TB but none of the TST+ controls. Given that we can achieve comparable serological specificity with both M. tuberculosis and BCG MVs, we believe that the Ab signature is in response to a sufficient antigen concentration resulting from a large M. tuberculosis burden releasing MVs (and causing disease) because it was absent in asymptomatic individuals with a history of exposure to other mycobacteria (e.g. BCG vaccination) or with LTBI. These are particularly promising results as TB serodiagnostic assays can lead to simple POC tests but currently available serologic tests, most of which are ELISA-based, lack sufficient sensitivity and specificity, especially in patients at early disease stages 16, 18, 20, 28. We feel that a sensitivity of around 75% in smear-negative TB patients has considerable clinical value as these patients would not be detected by sputum smear microscopy which is often the only diagnostic test available in resource-limited settings.
Our data indicate that certain antigens enriched in mycobacterial MVs, many of them lipoproteins which are membrane and cell-wall associated 19, are highly immunogenic. Despite the recent possibility to screen for Abs to the entire M. tuberculosis proteome 44, the identification of MV proteins via immunoblots can provide additional diagnostic information because the array of membrane proteins on chips is challenging 45. Furthermore, currently available chips utilize proteins expressed in E. coli with limited post-translational modifications. Hence, our MV-based approach could identify Ab responses that might not be detectable with currently available microarrays.
Three smear-negative TB patients without Abs to the 3 MV proteins had evidence of disseminated TB. We also identified a band at ~95 kDa with 11/16 TST+ control sera (regardless of IGRA result) which was not seen with any of the sera from the 28 TB cases. Although cell-mediated immunity undoubtedly plays the main role in the defense against TB, these findings raise the possibility that Abs against some MV proteins might also be involved in the protection against TB. Many studies have provided evidence that protective and non-protective Abs exist. For example, monoclonal Abs to mycobacterial antigens, ranging from surface proteins to polysaccharides, can protect mice against experimental infection 46–53, and three recent serum transfer studies within the same species (mice) or from humans to mice have shown protection against TB 54–56. In contrast, the absence of Abs has been associated with susceptibility to TB as well as disseminated TB (reviewed in 57). Thus, although the role of Abs in the protection against TB remains uncertain, our findings warrant further investigation.
The accuracy of diagnostic tests is often overestimated when comparing patients with the disease to healthy controls 58. Furthermore, our study was limited by small sample size. Thus, larger studies including patients with respiratory diseases other than TB as well as HIV-associated TB are warranted to further validate our MV-based TB biomarker signature. This will require identification and expression of our candidate MV proteins. We have previously identified ~50–60 Mtb and BCG MV-associated proteins (19 and supplemental material). About 20 of these proteins have a molecular mass that corresponds to that of our major immunoblot bands. Five of these 20 proteins, namely Ag 85-A, B & C (~34–37 kDa), MPT64 (~25 kDa), and LprG (~25 kDa) were previously associated with TB 16, 18, 28, 44. Our results suggest that the band ~25 kDa represents LprG, a lipoprotein that was recently identified as a serologic target 44. Further studies to evaluate the accuracy of this protein for the serodiagnosis of TB are lacking. On the other hand, the band ~36 kDa was not reactive with two different mAbs against the Ag85 complex. Therefore, it appears that both new and known Ab targets are among the candidate MV proteins. Nevertheless, we stress that the novelty of our approach includes signature pattern rather than Ab reactivity with individual proteins.
In conclusion, we demonstrate a strong humoral immune response in TB patients against MVs of pathogenic mycobacteria. Our results indicate that Ab responses to certain proteins enriched in mycobacterial MVs may constitute a novel TB Ab biomarker signature that has the potential for diagnostic information. Larger studies are needed to validate these findings.
Acknowledgments
Funding
This work was supported by funds from the National Institute of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID; AI-067665 to J.M.A. and AI-033774, AI-052733, AI-033142 to A.C.) and the National Heart, Lung, and Blood Institute (NHLBI; HL-059842 to A.C.), the Center for AIDS Research (CFAR) at the Albert Einstein College of Medicine (AI-51519; J.M.A.), the Aeras TB vaccine foundation (R. P.-R.) and the Gates Foundation (J.M.A. and A.C.).
Footnotes
Conflict of interest
None of the authors have any commercial or other association that might pose a conflict of interest.
Ethical approval
Approval for human subjects’ research was obtained from the Internal Review Boards at the New York University School of Medicine, NY, NY (IRB# 12590), and the Albert Einstein College of Medicine, Bronx, NY (CCI# 2006-428).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rylance J, Pai M, Lienhardt C, Garner P. Priorities for tuberculosis research: a systematic review. Lancet Infect Dis. 2010;10:886–892. doi: 10.1016/S1473-3099(10)70201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maertzdorf J, Weiner J, 3rd, Kaufmann SH. Enabling biomarkers for tuberculosis control. Int J Tuberc Lung Dis. 2012;16:1140–1148. doi: 10.5588/ijtld.12.0246. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report 2012. Geneva, Switzerland: 2012. www.who.int/tb/publications/global_report/ [Google Scholar]
- 4.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 5.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-Lopez EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahid P, Kim PS, Evans CA, Alland D, Barer M, Diefenbach J, Ellner J, Hafner R, Hamilton CD, Iademarco MF, Ireton G, Kimerling ME, Lienhardt C, MacKenzie WR, Murray M, Perkins MD, Posey JE, Roberts T, Sizemore C, Stevens WS, Via L, Williams SD, Yew WW, Swindells S. Clinical research and development of tuberculosis diagnostics: moving from silos to synergy. J Infect Dis. 2012;205(Suppl 2):S159–168. doi: 10.1093/infdis/jis194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Reported tuberculosis in the United States, 2008. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. [Google Scholar]
- 10.Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins M, Aziz MA, Pai M. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 11.2006 Annual TB Summary. New York, NY: New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control; 2008. http://www.nyc.gov/html/doh/downloads/pdf/tb/tb2006.pdf. [Google Scholar]
- 12.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, Zumla A. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–1937. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 13.Clavijo E, Diaz R, Anguita A, Garcia A, Pinedo A, Smits HL. Comparison of a dipstick assay for detection of Brucella-specific immunoglobulin M antibodies with other tests for serodiagnosis of human brucellosis. Clin Diagn Lab Immunol. 2003;10:612–615. doi: 10.1128/CDLI.10.4.612-615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. Validation of a rapid and reliable test for diagnosis of chagas’ disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol. 2005;43:5065–5068. doi: 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smits HL, Eapen CK, Sugathan S, Kuriakose M, Gasem MH, Yersin C, Sasaki D, Pujianto B, Vestering M, Abdoel TH, Gussenhoven GC. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin Diagn Lab Immunol. 2001;8:166–169. doi: 10.1128/CDLI.8.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, Ramsay A, Hopewell PC, Pai M. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8:e1001062. doi: 10.1371/journal.pmed.1001062. PMEDICINE-D-10-00633 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, Cunningham J, Weldingh K, Pai M. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Postgrad Med J. 2007;83:705–712. doi: 10.1136/thx.2006.075754. 83/985/705 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, Cunningham J, Weldingh K, Pai M. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. 06-PLME-RA-0909 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Jr, Porcelli SA, Casadevall A. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121:1471–1483. doi: 10.1172/JCI44261. 44261 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achkar JM, Jenny-Avital E, Yu X, Burger S, Leibert E, Bilder PW, Almo SC, Casadevall A, Laal S. Antibodies against immunodominant antigens of Mycobacterium tuberculosis in subjects with suspected tuberculosis in the United States compared by HIV status. Clin Vaccine Immunol. 2010;17:384–392. doi: 10.1128/CVI.00503-09. CVI.00503-09 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by HIV status. Clin Vaccine Immunol. 2012;19:198–208. doi: 10.1128/CVI.05550-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Deeg CA, Raith AJ, Amann B, Crabb JW, Thurau SR, Hauck SM, Ueffing M, Wildner G, Stangassinger M. CRALBP is a highly prevalent autoantigen for human autoimmune uveitis. Clin Dev Immunol. 2007:39245. doi: 10.1155/2007/39245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimazaki K, Jirawuthiworavong GV, Heckenlively JR, Gordon LK. Frequency of anti-retinal antibodies in normal human serum. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2008;28:5–11. doi: 10.1097/WNO.0b013e318167549f. [DOI] [PubMed] [Google Scholar]
- 25.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 26.Kremer L, Maughan WN, Wilson RA, Dover LG, Besra GS. The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Letters in applied microbiology. 2002;34:233–237. doi: 10.1046/j.1472-765x.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010;78:5116–5125. doi: 10.1128/IAI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P, Hopewell PC, Ramsay A, Pai M, Laal S. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16:260–276. doi: 10.1128/CVI.00355-08. CVI.00355-08 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes & development. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 30.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan JI, Rijpkema SG, Durrani Z, Roake JA. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol. 2003;36:199–205. doi: 10.1016/S0928-8244(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 32.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 33.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008;76:4554–4563. doi: 10.1128/iai.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConnell MJ, Rumbo C, Bou G, Pachon J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine. 2011;29:5705–5710. doi: 10.1016/j.vaccine.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010;107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine. 2011;29:8381–8389. doi: 10.1016/j.vaccine.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camacho AI, de Souza J, Sanchez-Gomez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–8229. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- 38.Jackson C, Lennon D, Wong S, Yan J, Stewart J, Reid S, Oster P, Ypma E, Martin D. Antibody persistence following MeNZB vaccination of adults and children and response to a fourth dose in toddlers. Arch Dis Child. 2011;96:744–751. doi: 10.1136/adc.2009.180596. [DOI] [PubMed] [Google Scholar]
- 39.Nokleby H, Aavitsland P, O’Hallahan J, Feiring B, Tilman S, Oster P. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine. 2007;25:3080–3084. doi: 10.1016/j.vaccine.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Wedege E, Bolstad K, Aase A, Herstad TK, McCallum L, Rosenqvist E, Oster P, Martin D. Functional and specific antibody responses in adult volunteers in new Zealand who were given one of two different meningococcal serogroup B outer membrane vesicle vaccines. Clinical and Vaccine Immunology. 2007;14:830–838. doi: 10.1128/cvi.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yera H, Andiva S, Perret C, Limonne D, Boireau P, Dupouy-Camet J. Development and evaluation of a Western blot kit for diagnosis of human trichinellosis. Clin Diagn Lab Immunol. 2003;10:793–796. doi: 10.1128/CDLI.10.5.793-796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engstrom SM, Shoop E, Johnson RC. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol. 2007;49:13–21. doi: 10.1111/j.1574-695X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 44.Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, Felgner PL, Liang X, Gennaro ML. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107:14703–14708. doi: 10.1073/pnas.1009080107. 1009080107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 46.Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, van Egmond M, Challacombe S, Woof JM, Ivanyi J. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186:3113–3119. doi: 10.4049/jimmunol.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers MA, Gavier-Widen D, Hewinson RG. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 2004;41:93–100. doi: 10.1016/j.femsim.2004.01.004. S0928824404000239 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. CEI2593 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez Y, Yero D, Falero-Diaz G, Olivares N, Sarmiento ME, Sifontes S, Solis RL, Barrios JA, Aguilar D, Hernandez-Pando R, Acosta A. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16kDa protein in a model of progressive pulmonary infection. International journal of medical microbiology: IJMM. 2009;299:447–452. doi: 10.1016/j.ijmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, Menozzi FD. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. 35084083 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, Challacombe S, Marsh PD, Ivanyi J. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. 1809 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buccheri S, Reljic R, Caccamo N, Meraviglia S, Ivanyi J, Salerno A, Dieli F. Prevention of the post-chemotherapy relapse of tuberculous infection by combined immunotherapy. Tuberculosis (Edinb) 2009;89:91–94. doi: 10.1016/j.tube.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Guirado E, Amat I, Gil O, Diaz J, Arcos V, Caceres N, Ausina V, Cardona PJ. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Olivares N, Leon A, Lopez Y, Puig A, Cadiz A, Falero G, Martinez M, Sarmiento ME, Farinas M, Infante JF, Sierra G, Solis RL, Acosta A. The effect of the administration of human gamma globulins in a model of BCG infection in mice. Tuberculosis (Edinb) 2006;86:268–272. doi: 10.1016/j.tube.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, Dagg B, Tascon RE, Lowrie DB, Colston MJ, Jolles S. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–6109. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell host & microbe. 2013 doi: 10.1016/j.chom.2013.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM. Empirical evidence of design-related bias in studies of diagnostic tests. Jama. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061. joc90732 [pii] [DOI] [PubMed] [Google Scholar]