Abstract
Cocaine exposure causes enduring neuroadaptations in ventral striatum, or nucleus accumbens (NAc), an area critically involved in reward learning and relapse of drug seeking. Medium spiny neurons (MSNs) in striatum are dichotomous in their expression of either D1 or D2 dopamine receptors, along with other receptors and neuropeptides. In dorsal striatum, these two subpopulations show non-overlapping innervation of distinct terminal fields via the direct or indirect pathways. However, NAc D1-MSNs and D2-MSNs are not fully segregated in this manner, with both cell types innervating ventral pallidum. Recent studies show that D1-MSNs and D2-MSNs play opposing roles in cocaine-associated behaviors. Further, cocaine induces differential adaptations in these two subpopulations in NAc, including changes to synaptic plasticity, glutamatergic signaling, and spine morphology.
Introduction
Long-lasting drug-induced neuroadaptations in ventral parts of striatum, or nucleus accumbens (NAc), are thought to contribute to the development of addiction and enduring relapse vulnerability that characterizes the disease[1]. Recent studies have focused on identifiying cocaine-induced adaptations that are distinct between the two classes of striatal medium spiny neurons (MSNs) defined by differential expression of D1 and D2 dopamine receptors. These subpopulations of D1-MSNs and D2-MSNs were originally characterized in dorsal striatum as belonging to either the direct pathway, with projections to substantia nigra (SN) and internal globus pallidus (entopeduncular nucleus in rodents), or the indirect pathway, with projections to external globus pallidus (globus pallidus in rodents)[2]. However, projections from NAc may not be segregated in this manner, as both cell types have overlapping projections to ventral pallidum (VP). VP functions as both an output and intrinsic structure of the basal ganglia, so NAc afferents are not easily characterized as direct or indirect in the classical sense of the terminology. We will review recent studies on cocaine-induced adaptations in NAc D1-MSNs and D2-MSNs, and discuss how these cell populations might contribute to cocaine seeking.
Role for striatum-based learning in cocaine seeking
Learning mechanisms within the basal ganglia are governed by parallel striatal systems, along with their cortical, limbic, and dopaminergic inputs, and the persistent relapse vulnerability plaguing drug addicts has been attributed to dysregulated prefrontal cortical control over basal ganglia-based learning processes[3]. The dorsomedial striatum (DMS) and dorsolateral striatum (DLS) are critical for goal-directed and habitual responding, respectively, and continued training on an instrumental task, such as drug self-administration, is thought to transition behavioral strategies from goal-directed to habitual[4]. As such, DLS is necessary for cocaine seeking following extinction or abstinence[5-6]. The ventral striatum, or NAc, is composed of shell and core compartments and plays a critical role in Pavlovian and instrumental learning and modulating task performance. Medial prefrontal cortex (mPFC) glutamatergic afferents to NAc core regulate reinstated cocaine seeking, especially after extinction training[1,7]. Although the NAc shell is involved in initial cocaine reinforcement, its role in subsequent drug seeking is unclear, as pharmacological manipulation of this structure can promote or suppress reinstated cocaine seeking[8-9]. Altogether, these studies indicate that different striatal regions play diverse and critical roles in cocaine-related behaviors.
D1-MSNs and D2-MSNs in relation to direct and indirect pathways
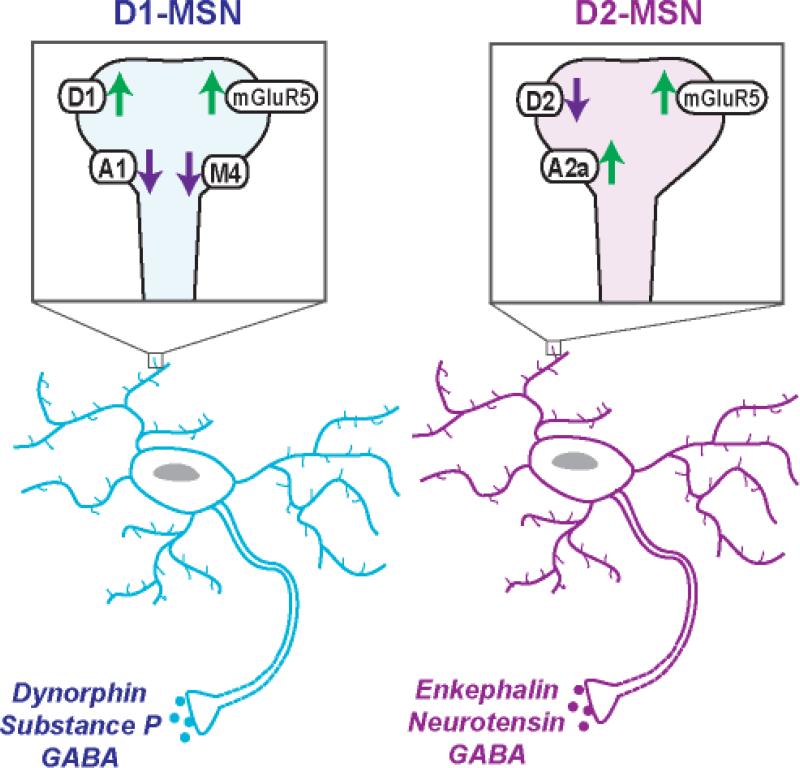
GABAergic projection neurons, or MSNs, comprise 90-95% of the striatal neuronal population. MSN projections to basal ganglia nuclei that innervate non-basal ganglia regions are classified as the direct pathway, whereas MSNs projecting to nuclei that innervate other basal ganglia structures compose the indirect pathway because they employ a multisynaptic circuit before leaving the basal ganglia[2]. MSNs are canonically divided into two subpopulations based on their projections, peptide co-transmitters and dopamine receptors[2]. D1-MSNs also express M4 cholinergic receptors, dynorphin, and substance P, whereas D2-MSNs co-express A2a adenosine receptors, enkephalin, and neurotensin (Figure 1)[2,10]. In dorsal striatum, D1-MSN axons course a direct pathway to output nuclei of the basal ganglia, including the entopeduncular nucleus (EP) and SN. D2-MSNs project to globus pallidus (GP) and reach output nuclei via an indirect, multisynaptic circuit that includes subthalamic nucleus[2].
Figure 1.
D1 and D2 dopamine receptor-expressing medium spiny neurons (MSNs) in nucleus accumbens (NAc) with co-localized receptors and neuropeptides. For each, a single dendritic spine is shown in the magnified box to indicate which receptors are present and their overall effect on neuronal excitability. In addition to dopamine receptors (D1, D2), MSNs express metabotropic glutamate receptors (mGluR5), adenosine receptors (A1, A2a) and cholinergic receptors (M4).
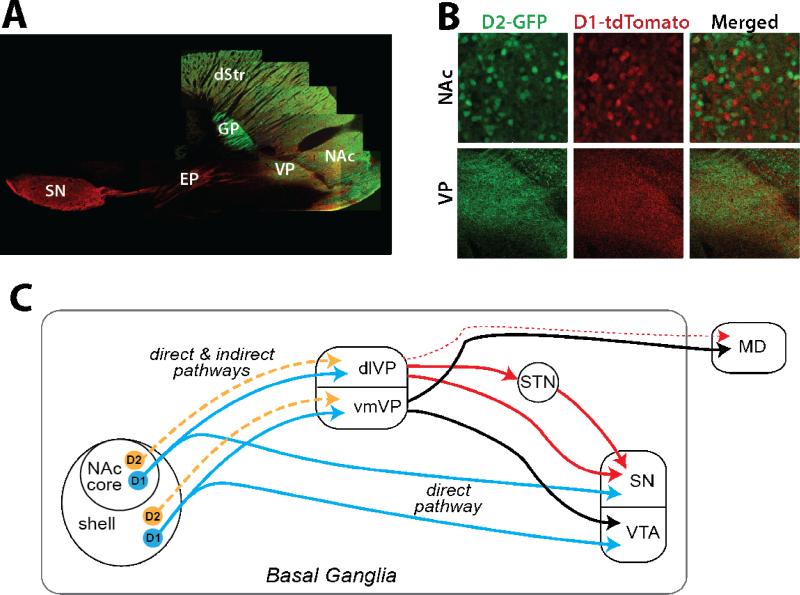
This discrete separation of D1-MSNs in the direct pathway and D2-MSNs in the indirect pathway does not necessarily apply in NAc. Retrograde tracer studies show that both D1-MSNs and D2-MSNs in NAc project to VP, whereas D1-MSNs alone project to ventral mesencephalon output structures (SN and ventral tegmental area, VTA)[11-12]. NAc pathways are segregated in that NAc core projects to dorsolateral VP (dlVP) and SN, and NAc shell to ventromedial VP (vmVP) and VTA (Figure 2c)[12-14], and Figure 2a,b shows these segregated pathways are rich in both D1-MSN and D2-MSN fibers. Although some neuropeptides show differential densities in these VP regions, the VP contains overlapping neuropeptide-expressing fibers from D2-MSNs (enkephalin higher in dlVP and neurotensin in vmVP) and D1-MSNs (substance P and dynorphin in dlVP and vmVP), unlike the GP[12,14-15]. MSN projections to VP may constitute both a direct and indirect pathway because VP functions as both an intrinsic and output structure of the basal ganglia (Figure 2c). Akin to the indirect pathway associated with GP, vmVP projects to VTA while dlVP projects to SN and subthalamic nucleus[13,16]. However, like the direct pathway associated with EP, vmVP (and to a lesser extent dlVP) also projects to mediodorsal thalamus (MD)[16-18]. Although the topography of D1 and D2 afferents relative to these projecting neurons in VP decreases the likelihood, it remains possible that D1-MSNs, and not D2-MSNs, synapse exclusively onto VP neurons that project to MD, thereby retaining NAc D1-MSNs and D2-MSNs synonymy with the direct and indirect pathways. Future studies are necessary to test this possibility.
Figure 2.
Output pathways of NAc D1-MSNs and D2-MSNs. A. Micrograph of projections from D1-(red) and D2-expressing cells (green) in D1-tdTomato x D2-GFP transgenic mice demonstrates that both MSN types in nucleus accumbens (NAc) project to ventral pallidum (VP), while D1-MSNs also terminate in midbrain (substantia nigra, SN, shown in this section, and ventral tegmental area, VTA, not shown). In contrast, dorsal striatal (dStr) D1-MSNs and D2-MSNs send distinct projections through the direct and indirect pathways. Output to the entopeduncular nucleus (EP) and SN is entirely from D1-MSNs, whereas the indirect projection to the globus pallidus (GP) is from D2-MSNs. B. While minimal co-localization is observed in NAc D1-MSNs and D2-MSNs, both MSNs terminate directly in VP. C. Diagram illustrating the overlap of D1- and D2-MSN projections to VP, with the NAc core projecting to dorsolateral (dlVP) and the shell innervating the ventromedial compartment (vmVP). The dlVP projects to the subthalamic nucleus (STN) and SN (solid red lines), with minor contributions to mediodorsal thalamus (MD, dashed red line). The vmVP projects within the basal ganglia to VTA and out of the basal ganglia to MD (solid black lines). Unlike the NAc projection to VP, NAc innervation of the ventral mesencephalon is entirely a direct pathway and contains axons from D1-MSNs only.
Roles for D1-MSNs and D2-MSNs in drug-associated behaviors
Recent investigations have begun exploring the contributions of D1-MSN and D2-MSN cell populations in NAc to cocaine-associated behaviors, and how cocaine-induced neuroadaptations specific to the cell types might underlie long-lasting relapse vulnerability. Acute or chronic administration of cocaine causes a host of cellular responses that are often specific to D1-MSNs and D2-MSNs, as reviewed recently[19]. While it is known that these cell types act in opposing directions to control movement, their roles in learning have only recently been explored. In a visual cue-guided plus maze, NAc (core+shell) D1-MSNs are critical for acquiring reward-based learning, and D2-MSNs needed for updating a response when the learning strategy is shifted[20**]. Opposing roles for the cell types in learning is further supported by the finding that selective optogenetic stimulation of D1-MSNs in DMS induces persistent reinforcement, while stimulating D2-MSNs causes transient punishment[21].
In terms of drug-associated behaviors, intra-NAc core injections of the A2a receptor agonist CGS 21680, which specifically targets D2-MSNs, reduces reinstatement of cocaine seeking elicited by cocaine or the D2 agonist quinpirole[22]. Loss of M4 cholinergic receptors selectively in D1 receptor-expressing cells causes enhanced hyperlocomotion and sensitization to psychostimulants, due to enhanced excitability when M4 receptors are unable to oppose D1-mediated cAMP stimulation[23]. In contrast, loss of the NR1 subunit of NMDA receptors in D1-MSNs attenuates amphetamine sensitization, and this effect is reversed by restoring NR1 largely in NAc core or by removing NR1 from D2-MSNs as well[24]. Finally, knockdown of mGluR5 only in D1-expressing neurons is sufficient to reduce cue-induced reinstatement in mice, while having no effect on cocaine intake[25**]. This reveals that a balance between the two subpopulations is likely necessary for normal behavior.
Differential involvement of these two cell types in drug-related behaviors is also seen using cell-specific promoters to drive expression of targeted toxins, light-activated channels (optogenetics), or designer receptors exclusively activated by designer drugs (DREADDs). These techniques have certain advantages over knockout models, including selective induction in adulthood, use in rats, and the fact that many manipulations are transient and reversible. Relevant studies show that D1-MSNs in NAc core, shell, or DMS are involved in the development of cocaine or amphetamine place preference and sensitization, whereas D2-MSNs oppose these behaviors[26*,27*,28*,29*]. These studies indicate that NAc D1-MSNs play an important role in acquisition of drug-related learning, and that D2-MSNs need to be simultaneously inhibited. However, there are few investigations to date with selective manipulation of D1-MSNs or D2-MSNs in animals models of drug relapse, and given the prepotent role of cortical and allocortical glutamatergic inputs to NAc in these models, as well as differences in glutamatergic influence on D1-MSNs and D2-MSNs, it is difficult to predict specific roles played by the two types[1,30].
Synaptic plasticity in D1-MSNs and D2-MSNs
Cocaine induces long-term adaptations in plasticity at glutamatergic synapses including long-term potentiation (LTP) or long-term depression (LTD), which are proposed to be the cellular substrates of learning and memory[31]. The exact magnitude of dopamine's influence on corticostriatal synaptic plasticity remains unclear, as some studies indicate that dopamine simply modulates plasticity while others point to an important role for dopamine receptor signaling in bidirectional plasticity[32*]. Recent investigations using BAC transgenic mice that express GFP specifically in D1- or D2-expressing neurons find that, in D1-MSNs, D1 receptor signaling appears to be involved in initiating LTP or mGluR5-dependent LTD[33-34,35*]. In contrast, synaptic plasticity in D2-MSNs is controlled by a balance between D2 and A2a receptor signaling[33,35-36].
Transgenic D1-GFP and D2-GFP mice have also been used to study cocaine-induced neuroplasticity. Following noncontingent cocaine administration, one study found impaired LTP selectively in D1-MSNs of NAc core and shell, and enhanced LTD in all neurons in shell[37]. However, other studies not distinguishing between D1-MSNs and D2-MSNs find that loss of LTD in PFC-NAc core synapses is associated with loss of mGluR5 function, and is observed following extinction but not abstinence in the self-administration paradigm[38-39,40**]. In a more rigorous self-administration model that classifies rats by addiction liability, only addiction vulnerable rats, and not resistant rats, show a long-lasting loss of NMDAR-dependent LTD in NAc core after extended self-administration (>50 days)[41]. Vulnerable rats also show reduced expression in dorsal and ventral striatum for genes encoding synaptic plasticity-associated proteins (e.g., mTOR, Arc) and D1 and D2 receptors[42*]. Therefore, continued loss of LTD may be critical for addiction vulnerability, or it may be that loss of LTD accompanies the milieu of long-lasting adaptations that contribute to impulsive drug seeking in vulnerable rats.
More studies have examined selective involvement of D1-MSNs and D2-MSNs in the long-lasting increases to dendritic complexity, spine density, and/or spine head diameter observed following cocaine exposure[43]. A majority of studies finds increased spine density throughout striatum specifically in D1-MSNs following cocaine withdrawal[44-47]. Cocaine-induced morphological alterations in D1-MSNs in NAc shell are accompanied by decreased membrane excitability, increased frequency of excitatory currents, and decreased frequency of inhibitory currents, while D2-MSNs show decreased frequency of excitatory currents[47]. However, future studies are needed to investigate morphological plasticity in D1-MSNs and D2-MSNs after cocaine self-administration, and to determine the functional implications to electrophysiology and behavior. For example, it has recently been shown that Rho signaling and actin polymerization modulate both morphological and behavioral changes induced by cocaine, and it is unknown if these molecular mechanisms occur predominantly in D1-MSNs or D2-MSNs[48-50].
Cocaine-induced alterations in glutamate transmission
Most of the recent studies investigating cocaine-induced adaptations in NAc glutamate transmission, such as increases in AMPA receptors, synaptic release probability, or glial regulation of extracellular glutamate at mGluRs, have not focused on the relative contribution of D1-MSNs and D2-MSNs and have been reviewed elsewhere[51-52]. However, one set of new data may be linked to D1-MSNs. Glutamatergic synapses where only NMDAR-mediated currents can be detected, or silent synapses, are fertile substrates for experience-dependent synaptic plasticity[53]. During early withdrawal from noncontingent cocaine, silent synapses form in NAc shell via insertion of GluN2B-containing NMDARs [54]. GluN2B insertion is CREB-dependent, and selective antagonism of GluN2B in NAc shell prevents the acquisition of locomotor sensitization[55]. In Fos-GFP mice, MSN ensembles in NAc shell that are strongly activated by cocaine (GFP+) display enhanced silent synapses that persist following 6-11 days of withdrawal[56*]. These silent synapses in GFP+ cells are not associated with increased NR2B expression, indicating that they were instead formed either via AMPAR removal or synaptogenesis[56]. Strongly activated GFP+ cells are critical for cocaine sensitization, indicating that silent synapse formation is an important mechanism underlying contextual learning for cocaine[57]. Given that cocaine-induced expression of Fos occurs predominantly in D1-MSNs, it seems likely that these silent synapses are located on D1-MSNs[58].
Conclusions and future directions
Several cellular and molecular adaptations have been found to contribute to cocaine-induced neuroplasticity, including changes in NAc glutamatergic transmission, synaptic plasticity, and dendritic spine morphology. In some instances, cocaine-induced adaptations have been shown to selectively occur in either D1- or D2-type MSNs, and in this regard, adaptations induced in D1-MSNs appear more frequent than in D2-MSNs. However, the majority of studies to date have measured adaptations following noncontingent cocaine. While it remains unknown if cell type-selective effects exist in models of relapse, D2-MSN adaptations are likely to be equally important because enkephalin release in VP critically regulates reinstatement-associated cocaine seeking and changes in GABA release[59]. Thus, a history of cocaine self-administration most likely causes distinct adaptations in D1-MSNs and D2-MSNs, and this imbalance disrupts the translation of cortical and allocortical glutamatergic inputs into motivated drug seeking. Importantly, with the advent of D1-MSN and D2-MSN selective transgenic mice and viral transfection of DREADDs and opsins, the field is beginning a thorough examination of the differential and overlapping roles played by these two cell types in relapse. These technologies will also help to describe the importance of different output pathways of NAc, so that the contributions of D1-MSN and D2-MSN subpopulations to direct and indirect pathways can be fully characterized.
Highlights.
Cocaine-induced adaptations in nucleus accumbens (NAc) contribute to relapse.
Projection neurons in NAc are D1 or D2 dopamine receptor-expressing.
D1 and D2 cell types in NAc are not synonymous with direct/indirect pathways.
NAc neurons show cocaine-induced changes in synaptic plasticity.
New technologies examine involvement of D1 and/or D2 projection neurons.
Acknowledgments
This work was funded by National Institutes of Health grants R01 DA03906, R01 DA12513, and P50 DA015369 (PWK), and F32 DA031519 (RJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 2.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 5.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- 10.Augood SJ, Westmore K, Emson PC. Phenotypic characterization of neurotensin messenger RNA-expressing cells in the neuroleptic-treated rat striatum: a detailed cellular co-expression study. Neuroscience. 1997;76:763–774. doi: 10.1016/s0306-4522(96)00449-6. [DOI] [PubMed] [Google Scholar]
- 11.Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 13.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 14.Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 15.Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- 16.Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364:340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats--I. forebrain gabaergic innervation. Neuroscience. 1996;70:93–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell P, Lavin A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997;17:2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109:12764–12769. doi: 10.1073/pnas.1210797109. [The authors used reversible neurotransmission blockade (as in Hikida et al., 2010) to examine the contribution of striatal subpopulations to reward-based learning and behavioral flexibility. Neurotransmission blockade in D1-MSNs results in impaired learning while blockade in D2-MSNs is associated with loss of behavioral flexibility when the task calls for a switch in learning strategies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience. 2012 doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci U S A. 2011;108:4206–4211. doi: 10.1073/pnas.1101424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Novak M, Halbout B, O'Connor EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, et al. Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci. 2010;30:11973–11982. doi: 10.1523/JNEUROSCI.2550-10.2010. [This study makes the important discovery that disruption of mGluR5 sigaling in D1-MSNs alone is sufficient to attenuate reinstatement of cocaine seeking.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [The authors used transgenic mice expressing tetanus toxin under the control of a tetracycline-responsive element and microinjected an AAV-tTA virus into the striatum driven by a SP or Enk promoter to induce the toxin and block neurotransmission in a cell type-specific manner. Importantly, this blockade and its behavioral effects could be reversed by doxycycline. While coordinated neurotransmission of both cell types appeared to be required for the acute locomotor activating effects of psychostimulants, each pathway distinctively contributed to rewarding or aversive learning tasks.] [DOI] [PubMed] [Google Scholar]
- 27*.Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d'Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [The authors used a clever strategy to specifically ablate striatal D2-MSNs combining CRE-mediated expression of diphtheria toxin receptor in D2-expressing neurons under the control of the A2a promoter coupled with i.c.v. delivery of diphtheria toxin to the whole striatum or selectively in NAc. The authors demonstrate that loss of D2-MSNs in the full striatum results in locomotor hyperactivity while loss of D2 cells in the NAc does not impact locomotor activity but results in an increased amphetamine CPP.] [DOI] [PubMed] [Google Scholar]
- 28*.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [The authors show that deletion of Trk-B from NAc MSNs has cell type-specific effects on cocaine CPP, with loss from D1-MSNs increasing and loss from D2-MSNs decreasing preference scores. Because the loss of TrkB in D2-MSNs is associated with an increase in neuronal activity, they similarly used optogenetic activation of D1- or D2-MSNs and recapitulated these effects on cocaine CPP.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [This study found that D1-MSNs and D2-MSNs in dorsal striatum contribute to drug-related learning, by using DREADDs to transiently reduce excitability of striatopallidal or striatonigral neurons. This is one of the first studies to use DREADD-mediated inhibition of neuronal populations to affect behavioral responding in rats.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacAskill AF, Little JP, Cassel JM, Carter AG. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat Neurosci. 2012;15:1624–1626. doi: 10.1038/nn.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [This is an excellent review on the role that dopamine plays in synaptic plasticity of striatal projection neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 34.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [This study used spike-timing-dependent plasticity protocols to assess the contribution of dopamine to synaptic plasticity at corticostriatal synapses in isolated D1-MSNs or D2-MSNs. The authors found that under the right circumstances both cell types were capable of expressing LTP and LTD, but in situations where dopamine levels were perturbed as shown in a Parkinson's model the ability to produce this plasticity was disrupted. This study indicates that dopamine receptor signaling is importantly involved in bidirectional plasticity in medium spiny neurons, and shows that LTP and LTD are observed in both cell types, contradicting previous findings.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- 38.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [This study shows that extinction training but not abstinence after self-administration produced changes in PSD proteins associated with glutamatergic transmission specifically in NAc core, including increased Homer1b/c and decreased surface mGluR5. Loss of mGluR5-dependent LTD in NAc core after self-administration was dependent upon extinction training. These changes may represent an adaptive response designed to suppress cocaine seeking.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 42*.Brown AL, Flynn JR, Smith DW, Dayas CV. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol. 2011;14:1099–1110. doi: 10.1017/S1461145710001367. [Researchers used behavioral phenotyping to classify rats as relapse-vulnerable or relapse-resilient and identified changes in expression of synaptic plasticity-related transcripts in striatal regions that were independent of cocaine consumption.] [DOI] [PubMed] [Google Scholar]
- 43.Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience. 2010;168:48–60. doi: 10.1016/j.neuroscience.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K-W, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, An S, Zhang L, Zhang L. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neuroscience Letters. 2012;517:118–122. doi: 10.1016/j.neulet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Dietz DM, Sun Haosheng, Lobo Mary Kay, Cahill Michael E., Chadwick Benjamin, Gao Virginia, Koo Ja Wook, Mazei-Robison Michelle S., Dias Caroline, Maze Ian, Damez-Werno Diane, Dietz Karen C., Scobie Kimberly N., Ferguson Deveroux, Christopher Daniel, Ohnishi Yoko, Hodes Georgia E., Zheng Yi, Neve Rachael L., Hahn Klaus M., Russo Scott J., Nestler Eric J. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nature Neuroscience. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma X-M, Huang J-p, Xin X, Yan Y, Mains RE, Eipper BA. A Role for Kalirin in the Response of Rat Medium Spiny Neurons to Cocaine. Molecular Pharmacology. 2012;82:738–745. doi: 10.1124/mol.112.080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toda S, Shen H, Kalivas P. Inhibition of Actin Polymerization Prevents Cocaine-induced Changes in Spine Morphology in the Nucleus Accumbens. Neurotoxicity Research. 2010;18:410–415. doi: 10.1007/s12640-010-9193-z. [DOI] [PubMed] [Google Scholar]
- 51.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology. 2011;61:1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. In Vivo Cocaine Experience Generates Silent Synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. A Silent Synapse-Based Mechanism for Cocaine-Induced Locomotor Sensitization. The Journal of Neuroscience. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. [In this elegant study, the authors used transgenic Fos-GFP mice to electrophysiologically characterize NAc MSNs strongly activated by cocaine. Cocaine sensitization produced higher levels and longer lasting expression of silent synapses in GFP+ compared to GFP- MSNs. Moreover, these silent synapses differed phenotypically from those that had been previously characterized as they were not associated with increased NR2B.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koya E, Golden SA, Harvey BK, Guez DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of sparsely distributed cocaine-activated accumbens neurons prevents context-specific psychomotor sensitization. Nat Neurosci. 2009 doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]