Abstract
The signature symptom of alcohol-induced persisting amnestic disorder, more commonly referred to as alcoholic Korsakoff’s syndrome (KS), is anterograde amnesia, or memory loss for recent events, and until the mid 20th Century, the putative brain damage was considered to be in diencephalic and medial temporal lobe structures. Overall intelligence, as measured by standardized IQ tests, usually remains intact. Preservation of IQ occurs because memories formed before the onset of prolonged heavy drinking — the types of information and abilities tapped by intelligence tests — remain relatively well preserved compared with memories recently acquired. However, clinical and experimental evidence has shown that neurobehavioral dysfunction in alcoholic patients with KS does include nonmnemonic abilities, and further brain damage involves extensive frontal and limbic circuitries. Among the abnormalities are confabulation, disruption of elements of executive functioning and cognitive control, and emotional impairments. Here, we discuss the relationship between neurobehavioral impairments in KS and alcoholism-related brain damage. More specifically, we examine the role of damage to prefrontal brain systems in the neuropsychological profile of alcoholic KS.
Keywords: Alcoholism, Korsakoff’s syndrome, frontal brain circuitry, executive functions, emotion
Introduction
In vivo neuroimaging studies and postmortem analyses of the brains of alcoholics have revealed abnormalities associated with prolonged and extensive alcohol consumption. Likewise, results of neuropsychological tests sensitive to impaired mental processes after damage to particular brain systems have disclosed abnormalities in cognitive abilities and emotional functioning in chronic alcoholics (Oscar-Berman and Marinkovic 2007; Chanraud et al. 2010). One of the striking and tragic legacies of decades of chronic alcoholism, often accompanied by poor nutrition, is the severe neuropsychiatric condition, alcohol-induced persisting amnestic disorder (American Psychiatric Association 2000), commonly known as alcoholic Korsakoff’s syndrome (KS). Although the condition most often is associated with protracted alcohol abuse and concomitant thiamine (vitamin B1) deficiency, other ailments such as prolonged gastrointestinal disturbances can deplete thiamine and lead to KS (Charness 2010; Oscar-Berman and Evert 1997).. Additional rare causes of KS have been reported in the absence of thiamine deficiency: intraventricular hemorrhage; thalamic infarction; T-cell lymphoma; Creutzfeldt-Jakob disease; and multiple sclerosis (see Oscar-Berman and Evert 1997). The focus of the present review, however, is alcoholic KS.
Alcoholic KS usually is preceded by Wernicke’s encephalopathy, an acute, transient stage of neurological symptoms that include confusion, impairments of consciousness, difficulties moving eye muscles, and problems with gross muscle control. With thiamine treatment, good nutrition, and alcohol abstinence, Wernicke’s encephalopathy can resolve; it tends to develop into the more stable and chronic condition of alcohol-induced persisting amnestic disorder, i.e., alcoholic KS (Sechi and Serra 2007; Victor, 1994; Zahr et al. 2011). However, many authors continue to refer to alcoholic KS as Wernicke-Korsakoff syndrome. Caine et al. (1997) suggested an operational definition of Wernicke-Korsakoff syndrome. This definition includes unremitting anterograde amnesia and “disorientation in the absence of an acute confusional state,” and two of the following: dietary deficiency, occulomotor abnormalities, cerebellar dysfunction and “either an altered mental state or mild memory impairment” (page 54). Note that this terminology describes a broad combination of symptoms of the acute clinical presentation of Wernicke’s encephalopathy together with the subsequent chronic and stable disorder of alcohol-induced persisting amnestic disorder. In the present review, our primary focus is on the permanent KS condition, unless otherwise indicated.
Alcoholic KS is characterized most dramatically by amnesia, but the amnesia does not encompass all memories equally. Instead, memory loss for recent events (anterograde amnesia) is considerably more severe than loss of memories for information learned prior to the onset of alcoholism (retrograde amnesia). Thus, many early memories are intact, and general intelligence scores remain within the normal range (Oscar-Berman et al. 1993). For decades subsequent to Korsakoff’s initial description of the disorder named after him (Victor and Yakovlev 1955), anterograde amnesia was the dominant focus of clinical assessment and research inquiry, and damage within Papez (1937) circuit, especially diencephalic damage, was thought to be the source of the amnesia (Brion 1969; Talland 1965; Victor et al. 1971). To account for additional cognitive and emotional symptoms observed in KS patients, several writers included cortical damage as well (Barbizet 1963; Talland 1965), although there was no consensus on the locus. Investigators stressed the idea that the diencephalic damage in KS patients was caused by thiamine deficiency, and patients with acute, alcoholic Wernicke’s encephalopathy who do not receive thiamine treatment, have shown evidence of hemorrhagic lesions within the region around the diencephalon (Kashi et al. 2009). However, other researchers suggested that the cortical abnormalities were caused by alcohol neurotoxicity or by other conditions associated with alcoholism (e.g., liver disease or head trauma) (Charness 2010).
There still are conflicting opinions about the pathogenesis of KS, with much of the debate revolving around the relative contributions of nutritional deficiency and alcohol neurotoxicity (Ribeiro and Pereira 2010). In any case, it should be noted that Korsakoff’s own account of the neuropathology of the syndrome also included damage to large regions of the brain, including cerebral cortex and structures deep within the brain; however precise cortical and subcortical loci were not detailed (Victor and Yakovlev 1955). Rather, Korsakoff described mainly the neuropsychological characteristics that he observed in association with various etiologies. In fact, he reported virtually all of the important cognitive and emotional impairments that might accompany or contribute to the anterograde amnesia (Banks 1996), some of which remain understudied to this day. The diencephalic brain damage that has come to be implicated in the amnesia of KS was detailed much later by investigators such as Brion (1969) and Victor et al. (1971, 1989) who examined postmortem pathology but used differing neuropsychological criteria to classify their patients. To date, controversy remains regarding the critical lesion sites (Harper 2009; Visser et al. 1999). Therefore, the idea that the syndrome can be confirmed with postmortem neuropathological evidence alone without precise knowledge of ante mortem neuropsychological symptomatology and a clinical picture, may be misleading when attempting to reconcile differing accounts of the neuropathology. A possible solution was proposed by Caine et al. (1997), who suggested an operational definition of KS, that is, unremitting anterograde amnesia, a clear sensorium, and two of the following: dietary deficiency, occulomotor abnormalities, and cerebellar dysfunction.
Nevertheless, with increased sensitivity of neurobehavioral assessment tools and the availability of functional and structural brain imaging techniques, the true complexity of the disorder is being revealed. In addition to anterograde amnesia, other domains of cognitive impairment have been described. These include deficits in attention, abstract thinking, cognitive flexibility, and behavior regulation (Oscar-Berman and Evert 1997). Additionally, KS patients often appear to have diminished volition, they seem emotionally apathetic, and they give an impression of being unaware of their disabilities (Talland 1965; Victor and Yakovlev 1955; Warner 1934). Thus, the combined picture clearly began to point to dysfunctional frontal brain circuitry. Consequently, systematic study ensued, and now frontal-system dysfunction is recognized as a primary contributor to the behavioral characteristics of the disorder. In this review, we first describe the prefrontal neuroanatomical networks that are vulnerable in alcoholic KS. We then summarize key domains of associated neurobehavioral impairment.
Neuroanatomical Circuitry of Prefrontal Brain Systems
A complex and interconnected circuitry involving overlapping brain regions defines the neural substrate associated with the various mnemonic and nonmnemonic deficits observed in alcoholism (see Figs. 1 and 2) (Barrett et al. 2007; Bowirrat and Oscar-Berman 2005; Harper and Matsumoto 2005; Makris et al. 2008; Oscar-Berman and Bowirrat 2005; Oscar-Berman and Marinkovic 2007; Schulte et al. 2010; Zahr et al. 2010). The key cortical and subcortical components of this network (the Extended Reward and Oversight System; Figure 1) are frontal areas (dorsolateral prefrontal, ventral/orbitofrontal, and anterior cingulate) and their underlying white matter, the insula, and limbic regions (hippocampus, amygdala, nucleus accumbens, and the ventral diencephalon: the hypothalamus, basal forebrain, sublenticular extended amygdala, and a large portion of the ventral tegmentum) (Barbas 2007; Barrett et al. 2007; Makris et al. 2008; Pfefferbaum and Sullivan 2005; Pitel et al. 2009; Yeterian et al. 2012). Results of in vivo magnetic resonance imaging (MRI) and postmortem neuropathological studies of non-Korsakoff (i.e., uncomplicated) alcoholics indicate that the most apparent cortical abnormalities occur in the frontal lobes, with concurrent thinning of the corpus callosum (Pfefferbaum et al. 1996), and concomitant compromise of pontocerebellar and cerebellothalamocortical systems (Sullivan 2003). Compromise of components in the circuitry of this extensive reward and oversight system may adversely influence remote regions within that circuit, resulting in characteristic alcoholism-related cognitive and motor deficits. However, even when one component may be compromised, another component may be invoked as a compensatory processing adjunct in situations where alcoholics are faced with difficult cognitive challenges (Oscar-Berman and Marinkovic 2007; Zahr et al. 2010).
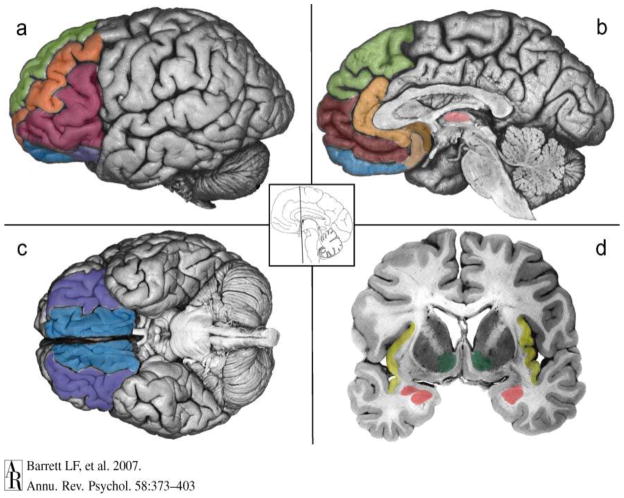
Figures 1 and 2. Prefrontal brain circuitry.
Figure 1 shows the Extended Reward and Oversight System, and Figure 2 highlights its cortical components. The ventral system includes two closely connected circuits that are anchored in the orbitofrontal cortex (OFC; Figure 2c). The more sensory system involves the lateral sector of the OFC (a, c, purple). It is closely connected to the anterior insula (d, yellow) and the basolateral complex in the amygdala (2d, rose, ventral aspect). The visceromotor circuitry includes the ventral portion of the ventromedial prefrontal cortex, which lies in the medial sector of the OFC (2a, b, c, blue) where the medial and lateral aspects of OFC connect; ventromedial prefrontal cortex is closely connected to the amygdala (including the central nucleus, d, rose, dorsal aspect) and the subgenual parts of the anterior cingulate cortex on the medial wall of the brain (2b, copper and peach). The dorsal system is associated with mental state attributions including the dorsal aspect of the ventromedial prefrontal cortex corresponding to the frontal pole (b, maroon), the anterior cingulate (2b, peach), and the dorsomedial prefrontal cortex (2a, b, green). Ventrolateral prefrontal cortex is shown in red (2a). Structures in the reward circuitry include the OFC, dorsolateral prefrontal (2a, orange) and cingulate cortex (2b, copper and tan), the thalamus (b, light pink), the ventral striatum (2d, green), the amygdala (2d, rose) and hippocampus (2d, gray), and the limbic brainstem. (Adapted from Makris et al. 2008 and Barrett et al. 2007.)
Pathological brain aberrations in patients with alcoholic KS likely involve this same extended circuitry, and other brain regions as well. That is, although damage to limbic structures and atrophy of portions of prefrontal cortex have been clearly and consistently associated with KS (Moselhy et al. 2001; Oscar-Berman et al. 2004; Pitel et al. 2009), involvement of specific diencephalic regions remains emphasized: the mammillary bodies and the region enclosed by the anterior and mediodorsal thalamic nuclei, including the parataenial nucleus (Caulo et al. 2005; Harding et al. 2000; Reed et al. 2003; Vetreno et al. 2012). Other affected brain areas are periaquaeductal and periventricular gray matter, the basal forebrain, hippocampal regions, middle cingulate cortex, and the cerebellum (Pitel et al. 2009; Sullivan et al. 2000; Sullivan and Marsh 2003; Vetreno et al. 2012; Zahr et al. 2010; Zahr et al. 2011).
High-order association areas such as prefrontal cortex (Figure 2) allow us to attend to and maintain relevant information in mind and resist interference, which in turn, is necessary for understanding the significance of perceptions and actions. Traditionally, primate prefrontal cortex has been classified into at least three primary subdivisions — lateral, orbital (or ventral), and medial — each having different cytoarchitectonic characteristics and afferent/efferent connections (Fuster 2008; Yeterian et al. 2012). Recent neuroimaging studies of the human frontal lobes have suggested that they are organized hierarchically in a rostro-caudal direction. As such, cognitive control is supported in progressively caudal regions during concrete action decisions, whereas rostral regions control more abstract decisions and actions (Badre and D’Esposito 2009). This neuroanatomical schema is important to keep in mind when considering clinical deficits of decision-making and impulse control, which in turn increase the propensity of the affected individual to make poor and risky decisions, including continued abuse of alcohol or other substances.
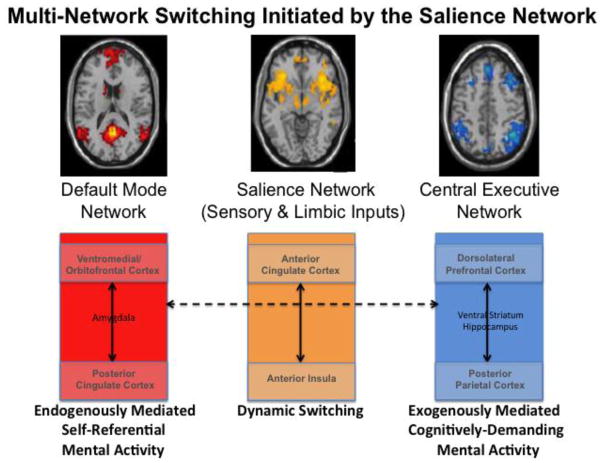
Through the multiple innervations of its different centers, preserved prefrontal brain circuitry is essential for such functions as memory consolidation and recall, spatial and contextual sensory processing, integration of stimulus-reward associations, decision-making, and determination of mood (Bressler and Menon 2010; Barrett et al. 2007; Rissman and Wagner 2012; Rushworth et al. 2011; Wallis 2007). Executive functions and appreciation of non-emotional environmental stimuli are thought to be under control of the lateral prefrontal brain system (Tanji and Hoshi 2008), with connections that include parietal cortex, the ventral striatum, and the hippocampus (Oscar-Berman and Bowirrat 2005; Santarelli et al. 2003), and their interconnected fiber pathways (Schmahmann and Pandya 2006). Response inhibition, emotional expression, and memory for social cues are controlled by a ventral/orbital frontal circuit, with strong amygdala influence (Barbas et al. 2003; LoPresti et al. 2008). The medial prefrontal cortex, most notably the anterior cingulate region, essential for inhibitory control and error-monitoring, has a role in emotions as well, specializing in the expression of emotions through pathways to autonomic structures (Barbas et al. 2003; Jackson et al. 2006; Schulte et al. 2010). Figure 3 represents an example of one such model of a large-scale network involving prefrontal brain circuitry and multiple connections (Bressler and Menon 2010).
Figure 3. An example of a model of a large-scale network involving prefrontal brain circuitry with multiple connections.
This model is based upon a review of findings from structural and functional neuroimaging studies (Bressler and Menon 2010). It hypothesizes that the salience network (middle) initiates dynamic switching between the central-executive (right) and default-mode (left) networks, and mediates between attention to endogenous and exogenous events. Sensory and limbic inputs are processed by the anterior insula, which detects salient events and initiates appropriate control signals to regulate behavior via the anterior cingulate cortex (and homeostatic states via the mid and posterior insular cortex). Key nodes of the salience network include the anterior cingulate cortex and the anterior insula; the default-mode network includes the ventromedial prefrontal cortex and posterior cingulate cortex, with input from the amygdala; the central-executive network includes the dorsolateral prefrontal cortex and posterior parietal cortex, with input from the ventral striatum and hippocampus. (Adapted from Bressler and Menon 2010.)
Together, these cortical-subcortical networks control high-level cognitive and emotional processes important for learning reward-values and affective-properties of stimuli, and they allow us to modulate our responses (Rushworth et al. 2011; Wood and Grafman 2003). Thus, this system is strongly involved in many bio-behavioral functions impaired in alcoholics, and its breakdown and dysfunction are responsible for a variety of abnormalities, e.g., impaired maintenance and monitoring of spatial and object information (Müller et al. 2002); disruption of decision-making (Bechara 2003; Bolla et al. 2005; Brand et al. 2005; LeDoux 2000; Pandya and Yeterian 2002; Patterson et al. 2002; Poldrack et al. 1999); insensitivity to feedback or rewards (Brand et al. 2009; Wrase et al. 2007); impairments in emotional control and behavioral inhibition (Ochsner and Gross 2007); and initiating drug use or relapse after protracted abstinence (Oscar-Berman and Bowirrat 2005; Goldstein and Volkow 2011). Indeed, Goldstein and Volkow (2002) proposed that disrupted function of the system — with emphasis on prefrontal cortex — leads to a syndrome of Impaired Response Inhibition and Salience Attribution in all addictions. This syndrome is characterized by “attributing excessive salience to the drug and drug-related cues, decreased sensitivity to non-drug reinforcers, and decreased ability to inhibit maladaptive or disadvantageous behaviors” (Goldstein and Volkow 2011, p. 652). The authors provided an informative schematic figure, based upon neuroimaging findings, that shows differences in prefrontal brain activity between addicted and healthy individuals involving functional domains such as attention and memory, decision-making and inhibitory control, and emotion and motivation. These same functional domains are the predominant dysfunctional characteristics of alcoholic KS patients, and here we review literature related to them. However, we caution that our use of the terminology representing the domains is generic, general, and atheoretical. We acknowledge that the definitions of the major constructs may differ across subfields within the cognitive neurosciences and addictions (Alvarez and Emory 2006; Goldstein and Volkow 2011; Knudsen 2007; Posner 2011; Rolls 2008); this is reflected in large part in the complex and widespread underlying neurocircuitry.
Functional Domains of Prefrontal System Abnormalities in Alcoholic Korsakoff’s Syndrome
Before the advent of sophisticated neuroimaging techniques, damage within the diencephalon was believed to be responsible for the amnesia characteristically associated with KS. However, a few investigators, including Korsakoff himself, considered the syndrome to be accompanied by a combination of cortical and diencephalic lesions (Barbizet 1963; Talland 1965; Victor and Yakovlev 1955; Warner 1934) but neuroanatomical specificity was absent. Specific emphasis on frontal involvement was apparent, however, when Victor et al. (1971) reported mild cortical atrophy primarily involving the frontal regions in 27% of cases with Wernicke-Korsakoff syndrome; the frontal atrophy was concurrent with histopathological evidence showing 88% of the cases having damage in the medial dorsal thalamic nucleus, and 100% of cases involving the medial mammillary nucleus. Over the next two decades, there were additional reports of frontal brain abnormalities in conjunction with diencephalic pathology associated with alcoholic KS (Adams et al. 1993; Cala et al. 1978; Cave and Squire 1992; Harper and Kril 1993; Hunter et al. 1989; Jacobson and Lishman 1990; Jernigan et al. 1991a; Jernigan et al. 1991b; Oscar-Berman and Evert 1997; Paller et al. 1997; Pfefferbaum et al. 1992; Shimamura et al. 1988; Volkow et al. 1992). More recently, combined neuropsychological and neuroimaging evidence has suggested that alcoholic KS likely is linked to an interruption of complex cerebellothalamocortical and limbic circuitry rather than to damage mainly of the diencephalon (Fama et al. 2004; Sullivan and Marsh 2003; Zahr et al. 2010; Zahr et al. 2011). Further, this circuitry is densely interconnected with other cortical and subcortical brain regions and networks, including the default mode network, which is implicated in executive control processes such as attention and inhibition, and in addictions (Buckner et al. 2011; Goldstein and Volkow 2011; Raichle and Snyder 2007).
Although the issue is far from settled regarding whether or not the brain abnormalities of alcoholics with and without KS differ along a continuum (Brokate et al. 2003; Pitel et al. 2008; Zahr et al. 2011), there is considerable evidence that both groups do have gray and white matter pathology, most especially damage in prefrontal cortex (Moselhy et al. 2001; Pitel et al. 2008; Pitel et al. 2009; Pitel et al. in press), although the degree of damage generally is greater in KS patients. In uncomplicated alcoholics, for example, structural imaging studies have clearly documented gray matter abnormalities in prefrontal cortex, most notably in the dorsolateral prefrontal region. These anomalies have been associated with longer lifetime alcohol use (Fein et al. 2002; Chanraud et al. 2007) and poorer neuropsychological functioning (Chanraud et al. 2007), and deficits have persisted from six months to six years or more of abstinence (Chanraud et al. 2010; Makris et al. 2008; Wobrock et al. 2009). Additionally, in short-term and long-term abstinent alcoholics, functional MRI (fMRI) studies have reported enhanced BOLD responses in bilateral dorsolateral prefrontal and anterior cingulate regions to alcohol-related cues relative to control cues (Grüsser et al. 2004; Heinz et al. 2007). In general, then, the primary distinction between the neuropathologies associated with uncomplicated alcoholism and KS is the extensive involvement of limbic and diencephalic brain regions in the latter group (Buhler and Mann 2011; Pitel et al. 2009; Pitel et al. in press; Sullivan and Pfefferbaum 2009).
Attention and Memory
In addition to brain-based evidence of frontal system abnormalities in KS patients, neurobehavioral evidence also has implicated frontal involvement. Indeed, initial neuropsychological evidence of frontal system damage in KS patients raised the possibility that it may play a role — as yet undefined — in their anterograde amnesia. For example, Korsakoff noted that a weakness in attentional control might be partly responsible for the recall failure, or conversely, that the memory disorder might prevent the execution of attentional strategies, or both (Banks 1996; Victor and Yakovlev 1955). In any case, because attentional deficits in KS were assumed to reflect fragile endogenous control processes, frontal deficits were implied. Moreover, if KS patients had impaired attentional abilities, their ability to learn at least some stimulus characteristics would be limited. Therefore, we conducted experimental tests of this notion. First we assessed the cognitive strategies employed by KS patients as they learned visual discrimination problems, and we found inefficient use of relevant visual information in favor of perseverative choices of preferred but irrelevant stimuli (Oscar-Berman 1973). Subsequently, we tested KS patients with a procedure that permitted an analysis of the specific stimulus features to which the patients attended (Oscar-Berman and Samuels 1977). The patients showed a selective responsivity to preferred stimulus attributes and a disregard for the relevance of other attributes. Moreover, even when the patients were provided with memory aids so that the information required for problem solution was fully available, they continued to choose their preferred stimuli. In another study (Parkinson 1979), KS patients were presented with lists of dichotic-listening stimuli, consisting of word-number pairs, with instructions to (a) divide their attention between ears and report by stimulus category, and (b) focus their attention and report by ear. The KS patients were impaired not only on the number of category reports but also on the ear reported second in the selective attention condition. The authors attributed poor accuracy with category reports to an impairment of semantic feature processing, and the deficit on the ear reported second to the patient’s sensitivity to proactive interference. While the deficits likely are tied to prefrontal abnormalities, we still do not know the extent to which they result from abnormalities in separate, independent functions that happen to be located in the same damaged brain areas. In short, the relationship between impairments in specific components of attention and prefrontal damage in KS has not been studied systematically.
By contrast, as is summarized extensively by others, including contributors to this issue of Neuropsychology Review, there have been innumerable studies exploring the relationship of prefrontal damage to memory impairments in KS (Bardenhagen et al. 2007; Fama et al. 2004; Oscar-Berman and Bardenhagen 1998). In brief, the findings have revealed a disproportionate impairment in recall memory relative to recognition memory, an impairment in identifying the temporal context or temporal sequence of memories, and repetitive spontaneous confabulations (Gilboa and Verfaellie 2010; Kopelman 2008; Shimamura et al. 1991).
Prospective memory, which relies more on the frontal lobes than retrospective memory (Umeda et al. 2011), also is impaired in KS patients. Prospective memory differs from other types of memory in that it involves the processes and strategies by which one plans and organizes memory, which are important, in turn, for monitoring and then performing future actions. In one study, Shimamura et al. (1991) described a series of experiments comparing KS patients, non-KS amnesic patients, and frontal lobe patients on a variety of mnemonic and nonmnemonic neuropsychological tests. They found that KS patients were as impaired as frontal patients on tasks of prospective memory. However, Brunfaut et al. (2000) later reported that aspects of prospective memory were preserved in KS patients. Conflicting results often reflect differences in methodologies employed by different laboratories. For example, Groot et al. (2002) evaluated prospective memory in patients with memory disorders (only one of whom had KS) and found performance to be correlated with scores on conventional memory and executive function tests. Importantly, the investigators noted that time-based tasks are more difficult than event-based tasks because the former place higher demands on inhibitory control mechanisms, and compensatory strategies improve prospective memory functioning. Clearly, further work is needed to understand the characteristics and scope of prospective memory impairments in KS, including strategic versus automatic monitoring of cueing, cue salience, and planning.
Confabulations are memory distortions that occur in patients with alcoholic KS, and Borsutzky et al. (2008) investigated the nature of these types of false memories in KS patients. The researchers used a standardized confabulation interview to investigate memory domains most affected by confabulations in KS, and they found that the patients confabulated most within the episodic/autobiographical memory domain, i.e., mainly with respect to their personal past and present. In another study of confabulation in KS patients, Kessels and colleagues (2008) distinguished between spontaneous and provoked confabulations, each having different underlying cognitive mechanisms and possible neuroanatomical underpinnings. Provoked confabulations — related to intrusions on memory tests — were quantified by third-party ratings; spontaneous confabulations, which may be due to executive dysfunction or a source memory deficit, were assessed with a confabulation battery. The investigators found deficits by the KS patients in source memory, in which the patients incorrectly assigned previously learned words to the wrong word list. Also, the KS patients had extensive executive deficits, but no relationship between the severity of these deficits and the severity of confabulation or intrusions on a memory task was found. Thus, the findings provided evidence for dissociation between spontaneous confabulation, provoked confabulation, and false memories. It should be noted that basic questions remain about how confabulation should be defined, how many types of confabulation there are, their underlying neurocognitive mechanisms, and their neural bases (Gilboa and Verfaellie 2010). In any case, because confabulations are characteristic of KS, Borsutzky et al. (2008) recommended that the screening of confabulation tendencies be used as a supplementary clinical tool for a detailed description of the memory profile of KS patients.
Although memory researchers continue to differentiate among aspects of the memory impairments related to damage in diencephalic, medial temporal, and prefrontal areas, results have remained inconsistent (Brokate et al. 2003). Nonetheless, studies by other investigators, including contributors to the present issue of Neuropsychology Review, have described memory impairments in KS patients that likely reflect the consequences of frontal system damage to the signature amnesia (Kopelman et al. 2009; Modirrousta and Fellows 2008; Spiegel and Lim 2011; van Geldorp et al. 2012).
Executive Functions
There is no definitive one-to-one relationship between intact executive functions and frontal lobe integrity (Alvarez and Emory 2006; Elliott 2003; Fuster 2008). However, executive functions have been linked to frontal brain systems, and there is abundant neurobehavioral evidence that alcoholic KS patients have impairments in executive functioning. As defined by Lezak (1982), “Executive functions comprise those mental capacities necessary for formulating goals, planning how to achieve them, and carrying out the plans effectively. They are at the heart of all socially useful, personally enhancing, constructive, and creative activities. With the executive functions intact, a person can suffer many different kinds and combinations of sensory, motor, and cognitive deficits and still maintain the direction of his own life and be productive as well. Impairment or loss of these functions compromises a person’s capacity to maintain an independent, constructively self-serving, and socially productive life no matter how well he can see and hear, walk and talk, and perform tests” (p. 281).
Theorists differ with regard to which of many diverse cognitive functions are to be considered as executive functions (Alvarez and Emory 2006; Stuss and Knight 2002), but at the very least, the term encompasses a host of processes that act in harmony and are responsible for the high-level action of monitoring and controlling behaviors necessary for maintaining focus and achieving outcomes in possibly adverse circumstances (Williams et al. 2009). Thus, executive skills include an array of complex mental abilities such as connecting past experiences with present actions; planning future behavior when faced with novel tasks; making judgments; changing behaviors and strategies; paying attention; and remembering details to guide decision-making. Executive skills also are involved in evaluating risks, recognizing future consequences of our actions, choosing between good and bad actions, suppressing unacceptable social responses, determining similarities and differences among objects or events, initiating or postponing responses, and prioritizing or switching tasks. These complex sets of behaviors are achievable only if the neurological structures and functions are intact, and frontal brain circuitry is associated with orchestration and control of these processes (Fuster 2008; Stuss and Knight 2002). The deficits observed in KS patients, which include disinhibition, restricted attention, increased susceptibility to interference, poor judgment and planning abilities, difficulty solving problems (irrespective of the type of material or the sensory modality), abnormal response perseveration, and blunted affect, are clear indications that the syndrome includes executive dysfunction (Krabbendam et al. 2000; Oscar-Berman and Evert 1997). Here, we review exemplary categories of executive impairments observed in KS: (a) difficulties the patients might have in the course of daily living; (b) deficits revealed with formal neurobehavioral tests; and (c) deficiencies in the ability to make adaptive reward-guided decisions. Our coverage does not include deficits in working memory, which are the bases of discussions in other papers in this issue (also see Oscar-Berman and Bardenhagen 1998).
Skills for daily living
Van Oort and Kessels (2009) used ecologically valid tests of executive dysfunction in KS patients to examine various subtypes of the deficits that the authors considered to be relevant to clinical practice, and that the patients would encounter in daily life, such as planning a map route, switching between tasks (response inhibition), and self monitoring. Sixteen of their 20 KS patients were impaired on at least one of the subtests, most notably on a subtest developed to assess planning of unstructured tasks and shifting attention between them (i.e., telling a story, completing arithmetic problems, and writing down the names of pictures of objects printed on a series of cards).
The ability to estimate the duration of time is another practical skill in which frontal-executive functions play a crucial role (Berlin 2004; Fuster 2008), and an abnormal internal clock will have significance for recognizing future consequences resulting from current actions. Korsakoff even suggested that patients who confabulate might have a disturbed sense of time, which results in confusion about events that occurred at different time periods (Victor and Yakovlev 1955). A negative correlation between magnitude of time estimation and performance on a test of cognitive estimation thought to assess frontal functions in KS patients has been viewed as evidence for the role of frontal systems in time estimation (Shaw and Aggleton 1994). In a study by Mimura and colleagues (2000), the researchers examined the relationships between memory, frontal-executive functions, and temporal estimation by comparing KS patients and patients with frontal damage on several measures of time judgment. They reasoned that if the internal clocks of the patients were abnormally accelerated, decelerated, or variable, time judgments would be correspondingly faulty but still would conform to a constant proportion of clock time. However, neither of the groups of patients showed such constancy. Compared to control subjects, the atypical temporal estimates of the KS and frontal patients varied in relation to clock time, depending on the amount of clock time involved: The KS patients’ impairments were apparent on the long temporal durations (more than 30 seconds), whereas the frontal patients’ estimations were tied to shorter intervals. Therefore, the internal clocks of both groups likely were not functioning normally and may have reflected memory-related difficulties as well. In any case, an abnormal internal clock will influence the ability to relate one’s current actions to future consequences.
Performance on formal neuropsychological tests
Other components of executive functions have been measured with standard neuropsychological assessment methods. In one study that compared performance of abstinent alcoholics with and without KS on documented measures of prefrontal integrity (Oscar-Berman et al. 2004), results showed that the KS patients were impaired on tests of memory, fluency, cognitive flexibility, and perseveration. Although the uncomplicated alcoholics showed some frontal system deficits, the impairments were mild compared to KS patients’ and were related to duration of abstinence (less than five years), duration of abuse (more than 10 years), and amount of alcohol consumed. Thus, impairments in executive functions were severe in KS patients, and the mild deficits observed in the non-KS alcoholics depended upon their drinking history.
In another study, Krabbendam et al. (2000) compared patients with KS to uncomplicated alcoholics and healthy control subjects on a neuropsychological battery that included three tests of frontal-executive functions, and on MRI brain structure measures. The uncomplicated alcoholics had normal brain structure volumes and cognitive performance profiles. The patients with KS, in addition to showing memory and visuoperceptual deficits, also had impairments on two of the three tests of executive functions (Word Fluency and Stroop Interference), and their brain structure volumes were reduced (most apparent as increased volume of the third ventricle).
Reward-guided decision-making
Studies have shown that KS patients are deficient in adaptive decision-making skills, which are presumed to reflect executive dysfunction. For example, Brand et al. (2005) employed a Game of Dice Task to assess decision-making abilities, risk-taking behavior, and insensitivity to future consequences in gambling situations. The task imposed explicit and stable rules for reinforcement and punishment. When the KS patients were compared to healthy controls on that task, and on other neuropsychological tests as well, results showed that the patients were highly impaired. Moreover, deficits on the Game of Dice Task were correlated with specific subcomponents of executive functions (categorization, monitoring, and using feedback), as measured by a modified Wisconsin Card Sorting Test. Because the investigators wanted to ensure that the decision-making deficits in the KS patients in risky situations were caused by executive dysfunctions rather than by impairments in processing feedback, they later used the same gambling test and a modified version of it, in which the feedback components were removed (Brand et al. 2009). The researchers found that KS patients again showed a preference for the risky alternatives linked to reductions in executive functioning, irrespective of whether or not feedback was provided.
A key aspect of decision-making involves updating the value of behavioral options, which rely on the executive controlled by prefrontal cortex. In an early study (Oscar-Berman et al. 1976), we compared the performance of alcoholic KS patients to that of uncomplicated alcoholics and healthy control subjects on each of three different schedules of spatial probability learning (50:50, 70:30, and 30:70) using monetary reinforcement. Instructions were minimal, and to earn money, the subjects simply had to choose between two left-right alternatives that were differentially rewarded according to the three schedules. Although on all three schedules, choice ratios by the nonalcoholic subjects approximated the reinforcement ratios, the choice ratios of KS patients on the second and third schedules remained close to the reinforcement ratio acquired with the first schedule. In addition, the KS patients made an abnormal number of perseverative errors. On most measures, performance by the uncomplicated alcoholics fell between that of the other two groups. A more recent study has used fMRI in conjunction with differential-reinforcement learning tasks to investigate the integrity of neural mechanisms underlying reward-guided decision-making in uncomplicated alcohol-dependent patients (Park et al. 2010). The results showed that functional connectivity between striatum and dorsolateral prefrontal cortex predicted impairments in behavior and the magnitude of alcohol craving. Although KS patients have yet to be included in brain imaging studies of decision-making, results in the uncomplicated alcoholics are in line with other findings of abnormalities in prefrontal circuitry in addictions and the pivotal role that frontal brain systems have in adaptive updating of action values and behavioral regulation (Goldstein and Volkow 2011; Koob and Volkow 2010; Wrase et al. 2007).
A somatic marker hypothesis has been proposed, which suggests that somatic or internal bodily signals guide adaptive decision-making processes when we are faced with complex or conflicting cognitive choices such as those often studied in gambling tasks (Bechara et al. 2005; Damasio 1996). Processing these internal “emotional” signals can influence our decisions. Further, the relationship between decision-making and emotion (see next section) is emphasized by results showing that different negative feelings can influence judgments and lead to dissimilar choices (Bechara 2004; Lerner and Keltner 2001). Studies using gambling tasks in substance abusers have shown decision-making impairments in these patients (Fein et al. 2006; Verdejo et al. 2004). Alcoholism also can lead to deficits in sensitivity to reward (Oscar-Berman et al. 1976), an important determinant of effective decision-making (Bechara et al. 2003; Fein et al. 2006). Studies have suggested that changes in the reward system (Makris et al. 2008) lead to the creation of an allostatic state, which represents a chronic deviation from reward set point, and one hypothesis holds that, in an allostatic state, loss of reward leads to disregulation of brain neurotransmitters (Koob and Le Moal 2008a, 2008b; Koob and Volkow 2010). Several studies have provided support for this hypothesis; they point to lower brain activity in alcoholics in regions such as the frontal lobes, ventral striatum, and limbic system (Bowirrat and Oscar-Berman 2005; Marinkovic et al. 2009; Phan et al. 2004). However, the architecture supporting intact executive functions and top-down control of behavior is complex and likely involves interconnected functional networks that include — in addition to prefrontal cortex, ventral striatum, and limbic system — numerous other regions such as the thalamus and cerebellum (Dosenbach et al. 2008; Wijnia and Goossensen 2010), all reported to be compromised in KS (Zahr and Sullivan 2008).
In sum, executive dysfunction is an indisputable characteristic of alcoholic KS. Because of the ubiquity of executive impairments associated with this disorder, Kessels and colleagues (2008) recommended revision of the DSM classification of “alcohol-induced persisting amnestic disorder” in order to include executive dysfunction. They proposed that, in so doing, the rehabilitation process would be facilitated and might even be helpful in reducing memory impairments and confabulatory behavior.
Emotion
Executive functioning and emotional functioning can be closely linked, such that different emotions will influence decisions and actions differently (Verdejo et al. 2004). Emotions engage strong mental and physical states and rely on a seamless coordination among multiple neurophysiological systems spanning different levels of the neuraxis (Davidson et al. 2003; Panksepp et al. 2002). Lesions in distinctly different areas of the brain will disrupt emotional processing at different levels or stages, e.g., during perception or evaluation of emotional stimuli, and at stages whereby emotional responses are expressed. Therefore, a common feature shared by theories of emotional dysfunction is that multiple brain structures, encompassing widespread brain systems, are involved, including prefrontal cortices (which are particularly involved in the final processing of emotionally relevant stimuli), the anterior cingulate, insula, amygdala, and basal ganglia (Davidson et al. 2003; Goldstein and Volkow 2011; Koob and Le Moal 2008a; Panksepp et al. 2002). Not surprisingly, therefore, emotional abnormalities accompanying long-term chronic alcoholism cover a broad spectrum. Moreover, Talland (1965) speculated that KS patients’ failure to sustain emotional involvement in ongoing events might be an important mechanism in the maintenance of their severe anterograde amnesia. However, that the emotional abnormalities in KS patients may be separable from their memory loss, was demonstrated in a study by Feinstein et al. (2010) showing that emotional feelings in patients with amnesia endured well beyond the conscious recollection for the events that initially triggered the emotion.
Korsakoff noted that KS patients showed little concern about their condition and about events that typically would cause elation or grief in others. In general, the emotional lives of KS patients were bland, monotonous, and devoid of passion or curiosity, and they seemed to lack intentions, plans, or goals (Banks 1996; Victor and Yakovlev 1955). The apathy and emotional flatness in KS patients also are characteristic of patients with bilateral frontal-lobe damage unrelated to alcoholism (Lezak et al. 2004; Moselhy et al. 2001). Like individuals with frontal brain damage, alcoholics have impairments in cognitive processing of emotional signals (Jackson et al. 2006; Marinkovic et al. 2009; Monnot et al. 2001; Oscar-Berman and Marinkovic 2007; Schulte et al. 2010; Tsuchida and Fellows 2012), and they are impaired in social skills, which in turn, impacts their ability to implement their preferred strategies for interpersonal interactions (Gaffney et al. 1998; Verdejo et al. 2004). One interpersonal skill is perspective taking, the ability to make inferences about the knowledge, thoughts, and feelings of others. This aspect of social cognition is known to be disrupted both with severe alcoholism (Uekermann and Daum 2008) and following prefrontal cortical damage (Bramham et al. 2009; Tranel et al. 2002). To explore perspective-taking ability in KS patients, Oosterman et al. (2011) used a story comprehension task in which inferences had to be made that either relied on perspective-taking or not, and other aspects of executive function were assessed using an extensive neuropsychological test battery. The performance of KS patients declined with increasing story complexity, but the pattern of decline for perspective-taking and non-perspective-taking stories was similar compared to that of a control group of nonalcoholic subjects. Furthermore, the performance decline with increasing task complexity was directly related to the overall decline in executive functioning measured with the neuropsychological battery. The authors concluded that aspects of executive function unrelated to perspective-taking per se, appeared to underlie the KS patients’ difficulties with story comprehension.
Systematic studies evaluating emotional dysfunction in KS patients have revealed deficits in labeling and recognition of anger, fear, and surprise. KS patients also are deficient in processing emotional prosody and in judging the intensity of emotions (Clark et al. 2007; Montagne et al. 2006; Oscar-Berman et al. 1990; Snitz et al. 2002). We examined the ability of KS patients to identify different affective states using facial expressions and voice intonations (affective prosody) as stimuli (Oscar-Berman et al. 1990). We found that KS patients showed deficits in emotional perception in both modalities, but since we did not separate the different task demands of prosody and semantic content, Snitz et al. (2002) explored those aspects of the task. They examined affective prosody discrimination and identification in KS patients, and observed impairments when the semantic content was either neutral or incongruent with prosody. The results suggested that KS patients were impaired in interpreting the meaning of affective prosody in the absence of semantic cues as to the emotional content of sentences. Montagne et al. (2006) investigated the perception and recognition of emotional expressions in KS patients by showing them video clips in which a neutral facial expression gradually changed into an emotional expression. The investigators gauged the amount of expression on the face that was needed for correct identification. They found that the KS patients showed impaired recognition of facial expressions of anger, fear and surprise, and they interpreted the pattern as evidence for a general expression-recognition deficit, which likely reflected dysfunction of frontal brain circuitry, including connections with the amygdala.
In a study that sought to differentiate KS patients’ deficits in judgments of emotional stimuli from those of uncomplicated alcoholics and healthy control subjects, we asked participants to rate stimuli according to emotional valence and intensity (Clark et al. 2007). The primary difference among the groups was that the KS patients attributed the most positive valence to neutral stimuli. Overall, the pattern of behavioral results implicated bi-hemispheric frontal and subcortical involvement in the abnormalities of emotion identification, but it was not clear whether the findings were due to a deficiency in emotional processing itself, or whether deteriorated basic processes underlying all kinds of judgment tasks resulted in affective judgment errors. Therefore, Brand et al. (2003) analyzed possible underlying cognitive processes of emotional and nonemotional judgments in KS patients using a cognitive estimation test consisting of four dimensions (size, weight, quantity, and time) and an affective judgment task comprising negative, neutral, and positive words. In a later study in the same laboratory (Labudda et al. 2010), the researchers also assessed valence classification performance for emotional pictures. In both studies, the KS patients’ results showed marked deficits concerning both cognitive estimation and affective judgments. The deficits were highly intercorrelated, and performance was related to processing speed, executive functions, and memory, suggesting a common basis for cognitive estimation and affective judgments.
The relationship between emotional impairments and abnormalities of frontal brain circuitry in KS has been based primarily on inferences made in the absence of neuroimaging data with KS patients. However, fMRI studies of emotional functioning in patients with focal lesions of different subregions within prefrontal areas have disclosed that damage to ventromedial prefrontal cortex impaired the detection of subtle facial expressions of emotion (Tsuchida and Fellows 2012). Such patients had difficulty distinguishing emotional from neutral expressions. In contrast, patients with left ventrolateral prefrontal lesions were able to detect the presence of emotional signals but had difficulty discriminating between specific emotions. These effects were regionally specific: Dorsomedial prefrontal damage had no effect on either aspect of emotion recognition. The findings suggested that separable processes relying critically on distinct regions within prefrontal cortex are responsible, on the one hand, for detecting emotional signals from facial expressions and, on the other, for correctly classifying such signals.
Other studies have used fMRI scans to document numerous instances of emotional abnormalities in non-KS alcoholics (Marinkovic et al. 2009; Oscar-Berman and Marinkovic 2007; Urban et al. 2007), and Schulte et al. (2010) described contributions of cortico-limbic fiber degradation to emotional dysfunction and impaired cognitive control of emotionally motivated actions in alcoholism. Thus, fiber tractography, together with functional neuroimaging, represent promising technologies to explore the role of abnormalities of regional fronto-cortico-limbic-striatal-cerebellar connectivity in dysregulation of emotion in KS.
Summary, Conclusions, and Some Remaining Questions
Alcoholic KS occurs more often with prolonged alcoholism than from other etiologies, and it is characterized by severe anterograde amnesia that is out of proportion to other symptoms. Additional neuropsychological changes occur as well, including a variety of cognitive impairments and emotional abnormalities. The amnesia in alcoholic KS patients is thought to be caused mainly by damage to diencephalic and limbic structures, but cortical and cerebellar gray- and white-matter damage contribute to aspects of memory loss. Other neuropsychological impairments such as poor attention, difficulty with executive functions, and emotional flatness and apathy likely reflect dysfunction of a large and highly integrated fronto-cortico-striatal-cerebellar circuitry. Our understanding of KS has come a long way since Korsakoff’s initial descriptions more than a century ago. Neuropsychological and neuroimaging techniques have revolutionized the ways we can explore brain and behavioral abnormalities. By skillfully applying these techniques, scientists now recognize that anterograde amnesia in KS is only a piece of a much more complex condition. Here we focused principally on the nonmnemonic neuropsychological dysfunctions associated with brain damage to prefrontal brain systems.
While advances have been made in understanding the contribution of dysfunction of prefrontal circuitry to neurobehavioral deficits in KS, many questions remain unanswered. We do not yet know, for example, the full nature of the attentional weaknesses in KS nor the extent to which they contribute to the memory impairments (or conversely, whether the impaired memory interferes with the execution of attentional strategies) (Banks 1996). Additionally, we do not know precisely how emotional changes in KS are related to the memory defect, nor to what extent, and in what ways emotional involvement is essential for encoding or retrieving memories (or conversely, whether memory is essential to emotional processes) (Schulte et al. 2010).
The anterograde amnesia of KS is unremitting. Although there is mounting evidence from research on uncomplicated alcoholism that damage to one component of the affected brain circuitry can lead to compensatory functionality from another component (Oscar-Berman and Marinkovic 2007; Zahr et al. 2010), this remains an open question regarding alcoholic KS. For example, might the extensive and combined frontal and cerebellar damage in KS reduce the brain’s ability to establish functional compensation, thereby contributing to the permanence of the disorder?
Another unexplored question concerns whether or not frontal system damage in alcoholic KS is manifested differently in men and women. This is likely, because of the plethora of gender differences in brain and behavioral abnormalities in alcohol use disorders generally (Medina et al. 2008; Pfefferbaum and Sullivan 2002; Urban et al. 2007).
The results of recent neurobehavioral and neuroimaging studies have revealed frontal system dysfunctions in alcoholic KS that parallel those reported for most addictions, including widespread prefrontal hypoactivity during exposure to cognitive and emotional challenges (Goldstein and Volkow 2011). Moreover, the roles ascribed to disruption of prefrontal circuitry common to KS and the addictions are emotion disregulation and interference with executive control. Although frontal system circuitry is a highly integrated system, its subcomponents (dorsolateral prefrontal, ventral/orbitofrontal, and medial frontal), including their underlying connections, are involved in diverse neurobehavioral functions. What remains to be determined is the relative contribution of the subcomponents to the various domains of impairment, and whether the functions of the subcomponents are affected differently in KS, uncomplicated alcoholism, and other addictions. As results of continued research begin to answer these many questions, treatment and management options will expand for the afflicted individuals (Sechi and Serra 2007).
Acknowledgments
Support for the writing of this review came from the US Department of Health and Human Services NIAAA R01-AA07112 and K05-AA00219, and from the Medical Research Service of the US Department of Veterans Affairs.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcoholism: Clinical and Experimental Research. 1993;17(2):205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neuroscience. 2009;10(9):659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WP. Korsakoff and amnesia. Consciousness and Cognition. 1996;5(1–2):22–26. doi: 10.1006/ccog.1996.0003. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. Journal of Anatomy. 2007;211(2):237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;4(1):25–37. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbizet J. Defect of memorizing of hippocampal-mammillary origin: a review. Journal of Neurology, Neurosurgery & Psychiatry. 1963;26:127–135. doi: 10.1136/jnnp.26.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardenhagen FJ, Oscar-Berman M, Bowden SC. Rule knowledge aids performance on spatial and object alternation tasks by alcoholic patients with and without Korsakoff’s amnesia. Neuropsychiatric Disease and Treatment. 2007;3(6):907–918. doi: 10.2147/ndt.s1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Risky business: Emotion, decision-making, and addiction. Journal of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the NY Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends in Cognitive Science. 2005;9(4):159–164. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Berlin HA. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(5):1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Borsutzky S, Fujiwara E, Brand M, Markowitsch HJ. Confabulations in alcoholic Korsakoff patients. Neuropsychologia. 2008;46(13):3133–3143. doi: 10.1016/j.neuropsychologia.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency Syndrome. American Journal of Genetics Part B: Neuropsychiatric Genetics. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Bramham J, Morris RG, Hornak J, Bullock P, Polkey CE. Social and emotional functioning following bilateral and unilateral neurosurgical prefrontal cortex lesions. Journal of Neuropsychology. 2009;3(1):125–143. doi: 10.1348/174866408X293994. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of Korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology. 2005;19(3):267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Kalbe E, Steingass HP, Kessler J, Markowitsch HJ. Cognitive estimation and affective judgments in alcoholic Korsakoff patients. Journal of Clinical and Experimental Neuropsychology. 2003;25(3):324–334. doi: 10.1076/jcen.25.3.324.13802. [DOI] [PubMed] [Google Scholar]
- Brand M, Pawlikowski M, Labudda K, Laier C, von Rothkirch N, Markowitsch HJ. Do amnesic patients with Korsakoff’s syndrome use feedback when making decisions under risky conditions? An experimental investigation with the Game of Dice Task with and without feedback. Brain and Cognition. 2009;69(2):279–290. doi: 10.1016/j.bandc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brion S. Korsakoff’s syndrome: clinico-anatomical and physiopathological considerations. In: Talland GA, Waugh NC, editors. The pathology of memory. New York, NY: Academic Press; 1969. pp. 22–29. [Google Scholar]
- Brokate B, Hildebrandt H, Eling P, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff’s syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–428. doi: 10.1037/0894-4105.17.3.420. [DOI] [PubMed] [Google Scholar]
- Brunfaut E, Vanoverberghe V, d’Ydewalle G. Prospective remembering of Korsakoffs and alcoholics as a function of the prospective-memory and on-going tasks. Neuropsychologia. 2000;38(7):975–984. doi: 10.1016/s0028-3932(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcoholism: Clinical and Experimental Research. 2011;35(10):1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. Journal of Neurology, Neurosurgery & Psychiatry. 1997;62(1):51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala LA, Jones B, Mastaglia FL, Wiley B. Brain atrophy and intellectual impairment in heavy drinkers--a clinical, psychometric and computerized tomography study. Australian and New Zealand Journal of Medicine. 1978;8(2):147–153. doi: 10.1111/j.1445-5994.1978.tb04502.x. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, et al. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128(7):1584–1594. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2(2):151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Sullivan EV. Structural imaging of alcoholic abuse. In: Shenton ME, Turetsky BI, editors. Understanding neuropsychiatric disorders. New York, NY: Cambridge University Press; 2010. [Google Scholar]
- Charness ME. Overview of the chronic neurologic complications of alcohol. uptodate.com. 2010:1–12. [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21(3):346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences (Series in affective science) New York, NY: Oxford University Press; 2003. [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders. British Medical Bulletin. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. Journal of the International Neuropsychological Society. 2004;10:427–441. doi: 10.1017/S135561770410310X. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2002;26(4):558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, et al. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. NeuroImage. 2006;32(3):1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Duff MC, Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proceedings of the National Academy of Sciences. 2010;107(17):7674–7679. doi: 10.1073/pnas.0914054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4. London: Academic Press; 2008. [Google Scholar]
- Gaffney LR, Thorpe K, Young R, Collett R, Occhipinti S. Facial skills, expectancies and drinking in adolescents. Addictive Behaviors. 1998;23(5):587–599. doi: 10.1016/s0306-4603(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Verfaellie M. Introduction—telling it like it isn’t: The cognitive neuroscience of confabulation. Journal of the International Neuropsychological Society. 2010;16(06):961–966. doi: 10.1017/S135561771000113X. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8(5):645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berlin) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril JJ. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol and Alcoholism. 2009;44(2):136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Alcohol-induced brain damage. Rockville, MD: NIH Publications; 1993. Neuropathological changes in alcoholics; pp. 39–69. [Google Scholar]
- Harper CG, Matsumoto I. Ethanol and brain damage. Current Opinion in Pharmacology. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, et al. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcoholism: Clinical and Experimental Research. 2007;31(7):1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hunter R, McLuskie R, Wyper D, Patterson J, Christie JE, Brooks DN, et al. The pattern of function-related regional cerebral blood flow investigated by single photon emission tomography with 99mTc-HMPAO in patients with presenile Alzheimer’s disease and Korsakoff’s psychosis. Psychological Medicine. 1989;19(4):847–855. doi: 10.1017/s0033291700005560. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jacobson RR, Lishman WA. Cortical and diencephalic lesions in Korsakoff’s syndrome: a clinical and CT scan study. Psychological Medicine. 1990;20(1):63–75. doi: 10.1017/s0033291700013234. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991a;15(3):418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Schafer K, Butters N, Cermak LS. Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology. 1991b;4(3):175–186. [PubMed] [Google Scholar]
- Kashi MR, Henderson GI, Schenker S. Wernicke’s Encephalopathy. In: McCandless DW, editor. Metabolic encephalopathy. New York, NY: Springer; 2009. pp. 281–301. [Google Scholar]
- Kessels RPC, Kortrijk HE, Wester AJ, Nys GMS. Confabulation behavior and false memories in Korsakoff’s syndrome: role of source memory and executive functioning. Psychiatry and Clinical Neurosciences. 2008;62(2):220–225. doi: 10.1111/j.1440-1819.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annual Review of Neuroscience. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008b;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. Alcohol and frontal lobe impairment: fascinating findings. Addiction. 2008;103:736–737. doi: 10.1111/j.1360-0443.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol and Alcoholism. 2009;44(2):148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Visser PJ, Derix MM, Verhey F, Hofman P, Verhoeven W, et al. Normal cognitive performance in patients with chronic alcoholism in contrast to patients with Korsakoff’s syndrome. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12(1):44–50. doi: 10.1176/jnp.12.1.44. [DOI] [PubMed] [Google Scholar]
- Labudda K, von Rothkirch N, Pawlikowski M, Laier C, Brand M. Categorization abilities for emotional and nonemotional stimuli in patients with alcohol-related Korsakoff syndrome. Cognitive and Behavioral Neurology. 2010;23(2):89–97. doi: 10.1097/WNN.0b013e3181d83aa4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Fear, anger, and risk. Journal of Personality and Social Psychology. 2001;81(1):146–159. doi: 10.1037//0022-3514.81.1.146. [DOI] [PubMed] [Google Scholar]
- Lezak MD. The problem of assessing executive functions. International Journal of Psychology. 1982;17(1–4):281–297. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. Journal of Neuroscience. 2008;28(14):3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism: Clinical and Experimental Research. 2009;33(11):1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M, Kinsbourne M, O’Connor M. Time estimation by patients with frontal lesions and by Korsakoff amnesics. Journal of the International Neuropsychological Society. 2000;6(5):517–528. doi: 10.1017/s1355617700655017. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia. 2008;46(12):2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon SJ, Lovallo WR, Ross E. Altered emotional perception in alcoholics: deficits in affective prosody comprehension. Alcoholism: Clinical and Experimental Research. 2001;25(3):362–369. [PubMed] [Google Scholar]
- Montagne B, Kessels RPC, Wester AJ, de Haan EHF. Processing of emotional facial expressions in Korsakoff’s syndrome. Cortex. 2006;42(5):705–710. doi: 10.1016/s0010-9452(08)70408-8. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol and Alcoholism. 2001;36(5):357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Müller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. Journal of Cognitive Neuroscience. 2002;14(5):673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Handbook of emotion regulation. Vol. 5. New York, NY: Guilford Press; 2007. The neural architecture of emotional regulation; pp. 87–109. [Google Scholar]
- Oosterman JM, de Goede M, Wester AJ, van Zandvoort MJE, Kessels RPC. Perspective taking in Korsakoff’s syndrome: the role of executive functioning and task complexity. Acta Neuropsychiatrica. 2011;23(6):302–308. doi: 10.1111/j.1601-5215.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Hypothesis testing and focusing behavior during concept formation by amnesic Korsakoff patients. Neuropsychologia. 1973;11(2):191–198. doi: 10.1016/0028-3932(73)90007-9. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Bardenhagen F. Nonhuman primate models of memory dysfunction in neurodegenerative disease: contributions from Comparative Neuropsychology. In: Tröster AI, editor. Memory in neurodegenerative disease. Cambridge: Cambridge University Press; 1998. pp. 3–20. [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Disease and Treatment. 2005;1(3):211–229. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Clancy JP, Weber DA. Discrepancies between IQ and memory scores in alcoholism and aging. Clinical Neuropsychologist. 1993;7(3):281–296. [Google Scholar]
- Oscar-Berman M, Evert D. Alcoholic Korsakoff’s syndrome. In: Nussbaum PD, editor. Handbook of neuropsychology and aging (Critical Issues in Neuropsychology) New York: Plenum Press; 1997. [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N. Emotional perception and memory in alcoholism and aging. Alcoholism: Clinical and Experimental Research. 1990;14(3):383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004;28(4):667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Sahakian BJ, Wikmark G. Spatial probability learning by alcoholic Korsakoff patients. Journal of Experimental Psychology: Human Learning and Memory. 1976;2:215–222. [PubMed] [Google Scholar]
- Oscar-Berman M, Samuels I. Stimulus-preference and memory factors in Korsakoff’s syndrome. Neuropsychologia. 1977;15(1):99–106. doi: 10.1016/0028-3932(77)90119-1. [DOI] [PubMed] [Google Scholar]
- Paller KA, Acharya A, Richardson BC, Plaisant O, Shimamura AP, Reed BR, et al. Functional neuroimaging of cortical dysfunction in alcoholic Korsakoff’s syndrome. Journal of Cognitive Neuroscience. 1997;9(2):277–293. doi: 10.1162/jocn.1997.9.2.277. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. The anatomical substrates of emotional behavior: the role of the cerebral cortex. In: Grafman J, Boller F, editors. The frontal lobes. 2. Vol. 7. New York: Elsevier; 2002. [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97(4):459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;38(4):725. [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. The Journal of Neuroscience. 2010;30(22):7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson SR. The amnesic Korsakoff syndrome: a study of selective and divided attention. Neuropsychologia. 1979;17(1):67–75. doi: 10.1016/0028-3932(79)90023-x. [DOI] [PubMed] [Google Scholar]
- Patterson JC, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. NeuroImage. 2002;17(4):1797–1806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20(4):752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002;15(3):708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30(2):423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectroscopy. 2004;9(4):258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Pitel A-L, Aupée A-M, Chételat G, Mézenge F, Beaunieux H, de la Sayette V, et al. Morphological and glucose metabolism abnormalities in alcoholic Korsakoff’s syndrome: group comparisons and individual analyses. PLoS ONE. 2009;4(11):e7748. doi: 10.1371/journal.pone.0007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, de la Sayette V, Viader F, et al. Episodic and working memory deficits in alcoholic Korsakoff patients: the continuity theory revisited. Alcoholism: Clinical and Experimental Research. 2008;32(7):1229–1241. doi: 10.1111/j.1530-0277.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chételat G, Le Berre AP, Desgranges B, Eustache F, Beaunieux H. Neurology. Macrostructural abnormalities in Korsakoff’s syndrome compared to uncomplicated alcoholism. in press. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Posner MI. Imaging attention networks. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Reed L, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, et al. FDG-PET Findings in the Wernicke-Korsakoff Syndrome. Cortex. 2003;39(4–5):1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro AM, Pereira SRC. Animal Models of Alcohol-Induced Dementia. In: De Deyn PP, Van Dam D, editors. Animal models of dementia. New York, NY: Springer; 2010. pp. 665–683. [Google Scholar]
- Rissman J, Wagner AD. Distributed representations in memory: insights from functional brain imaging. Annual Review of Psychology. 2012;63:101–128. doi: 10.1146/annurev-psych-120710-100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Memory, attention, and decision-making. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York, NY: Oxford University Press, USA; 2006. [Google Scholar]
- Schulte T, Mũller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues in Clinical Neuroscience. 2010;12(4):554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurology. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Shaw C, Aggleton JP. The ability of amnesic subjects to estimate time intervals. Neuropsychologia. 1994;32(7):857–873. doi: 10.1016/0028-3932(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LS. What is the role of frontal lobe damage in memory disorders? In: Levin HS, Eisenber HM, Benton AL, editors. Frontal lobe function and dysfunction. New York, NY: Oxford University Press; 1991. [Google Scholar]
- Shimamura AP, Jernigan TL, Squire LR. Korsakoff’s syndrome: radiological (CT) findings and neuropsychological correlates. Journal of Neuroscience. 1988;8(11):4400–4410. doi: 10.1523/JNEUROSCI.08-11-04400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Hellinger A, Daum I. Impaired processing of affective prosody in korsakoff’s syndrome. Cortex. 2002;38(5):797–803. doi: 10.1016/s0010-9452(08)70046-7. [DOI] [PubMed] [Google Scholar]
- Spiegel DR, Lim KJ. A case of probable Korsakoff’s syndrome: a syndrome of frontal lobe and diencephalic structural pathogenesis and a comparison with medial temporal lobe dementias. Innovations in Clinical Neuroscience. 2011;8(6):15–19. [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. 1. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff s syndrome: Relation to ataxia. Neuropsychology. 2000;14(3):341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27(2):301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology. 2003;61(12):1716–1719. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff Syndrome. Alcohol and Alcoholism. 2009;44(2):155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talland GA. Deranged memory: a psychonomic study of the amnesic syndrome. New York, NY: Academic Press; 1965. [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiological Reviews. 2008;88(1):37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cerebral Cortex. 2012;22 doi: 10.1093/cercor/bhr370. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103(5):726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Umeda S, Kurosaki Y, Terasawa Y, Kato M, Miyahara Y. Deficits in prospective memory following damage to the prefrontal cortex. Neuropsychologia. 2011;49(8):2178–2184. doi: 10.1016/j.neuropsychologia.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Urban T, Oscar-Berman M, Marinkovic K, Sawyer K, Harris G. Neural correlates of gender differences in the processing of emotional stimuli. Paper presented at the Society for Neuroscience; San Diego, CA. November.2007. [Google Scholar]
- van Geldorp B, Bergmann HC, Robertson J, Wester AJ, Kessels RPC. The interaction of working memory performance and episodic memory formation in patients with Korsakoff’s amnesia. Brain Research. 2012;1433(C):98–103. doi: 10.1016/j.brainres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Van Oort R, Kessels RPC. Executive dysfunction in Korsakoff’s syndrome: time to revise the DSM criteria for alcohol-induced persisting amnestic disorder? International Journal of Psychiatry in Clinical Practice. 2009;13(1):78–81. doi: 10.1080/13651500802308290. [DOI] [PubMed] [Google Scholar]
- Verdejo A, Aguilar de Arcos F, Perez Garcia M. Alterations in the decision making processes linked to the ventromedial prefrontal cortex in drug-abusing patients. Review of Neurology. 2004;38(7):601–606. [PubMed] [Google Scholar]
- Vetreno RP, Ramos RL, Anzalone S, Savage LM. Brain and behavioral pathology in an animal model of Wernicke’s encephalopathy and Wernicke-Korsakoff syndrome. Brain Research. 2012;1436:178–192. doi: 10.1016/j.brainres.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M. Alcoholic dementia. Canadian Journal of Neurological Science. 1994;21:88–99. doi: 10.1017/s031716710004899x. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome: a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Philadelphia, PA: Davis, F. A; 1971. [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2. Philadelphia, PA: F.A. Davis; 1989. [Google Scholar]
- Victor M, Yakovlev PI. S.S. Korsakoff’s psychic disorder in conjunction with peripheral neuritis. Neurology. 1955;5:394–406. doi: 10.1212/wnl.5.6.394. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Krabbendam L, Verhey FRJ, Hofman PAM, Verhoeven WMA, Tuinier S, et al. Brain correlates of memory dysfunction in alcoholic Korsakoff’s syndrome. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(6):774–778. doi: 10.1136/jnnp.67.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. American Journal of Psychiatry. 1992;149(8):1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. The Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Warner FJ. The brain changes in chronic alcoholism and Korsakoff’s psychosis. The Journal of Nervous and Mental Disease. 1934;80(6):629. [Google Scholar]
- Wijnia JW, Goossensen A. Cerebellar neurocognition and Korsakoff’s syndrome: An hypothesis. Medical Hypotheses. 2010;75(2):266–268. doi: 10.1016/j.mehy.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Annals of Behavioral Medicine. 2009;37(2):126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wölwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism. European Archives of Psychiatry and Clinical Neuroscience. 2009;259(3):143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012;48(1):58–81. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nature Reviews Neurology. 2011;7(5):284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychology Review. 2010;20(3):280–289. doi: 10.1007/s11065-010-9141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Sullivan EV. Translational studies of alcoholism: bridging the gap. Alcohol Research & Health. 2008;31(3):215–230. [PMC free article] [PubMed] [Google Scholar]