Abstract
Executive functions are processes that act in harmony to control behaviors necessary for maintaining focus and achieving outcomes. Executive dysfunction in neuropsychiatric disorders is attributed to structural or functional pathology of brain networks involving prefrontal cortex (PFC) and its connections with other brain regions. The PFC receives innervations from different neurons associated with a number of neurotransmitters, especially dopamine (DA). Here we review findings on the contribution of PFC DA to higher-order cognitive and emotional behaviors. We suggest examination of multifactorial interactions of an individual’s genetic history, along with environmental risk factors, can assist in the characterization of executive functioning for that individual. Based upon the results of genetic studies we also propose genetic mapping as a probable diagnostic tool serving as a therapeutic adjunct for augmenting executive functioning capabilities. We conclude that preservation of the neurological underpinnings of executive functions requires the integrity of complex neural systems including the influence of specific genes and associated polymorphisms to provide adequate neurotransmission.
Keywords: Executive functions, dopamine, prefrontal cortex, genetics, Reward Deficiency Syndrome (RDS)
Introduction
“Executive functions comprise those mental capacities necessary for formulating goals, planning how to achieve them, and carrying out the plans effectively. They are at the heart of all socially useful, personally enhancing, constructive, and creative activities. With the executive functions intact, a person can suffer many different kinds and combinations of sensory, motor, and cognitive deficits and still maintain the direction of his own life and be productive as well. Impairment or loss of these functions compromises a person’s capacity to maintain an independent, constructively self-serving, and socially productive life no matter how well he can see and hear, walk and talk, and perform tests” (Lezak, 1982).
Defining Executive Functions
Executive functions are complex cognitive abilities requiring the synchronization of several subprocesses to achieve a particular goal (Cohen et al. 2009). They control and regulate other abilities and behaviors and involve cognitive control processes that regulate thought and action on representations stored in the PFC (Smith and Jonides, 1999). Executive functions are localized in neural networks and, when activated, enable access to the stored actions. Indeed, executive functions can be viewed as computational procedures or algorithms that are localized in neural networks (Laird et al., 2009). Theorists differ with regard to whether executive functions are unified with respect to process, nor whether they include specific cognitive abilities (Baddeley, 1996; Rolls, 1996; Shallice & Burgess, 1991; Stuss & Benson, 1984, 1986; Stuss & Alexander, 2000). Consensus is lacking with regard to which of many diverse cognitive functions are considered relevant to executive functioning. At the very least, the term encompasses a host of processes that act in harmony and are responsible for the higher-level action of monitoring and controlling behaviors necessary for maintaining focus and achieving outcomes in possibly adverse circumstances. The efficacy measure of any successful high mental skill is a function of our intellectual integrity and mental capabilities. The outcome of our behavior depends on the ability of our brain to exert control over its processing of reflexive reactions to the environment and directing those behaviors toward conscious as well as non-conscious goals (Williams et al., 2009a).
Executive skills
A recent hypothetical mechanism for executive function postulates several sub-components. In a frequently cited classification, Smith and Jonides (1999) distinguished between mechanisms relating to (a) attention and inhibition, (b) task management, (c) planning, (d) monitoring and (e) coding. There is, however, no consensus on the number and the precise nature of functional subcomponents. Recent research has concentrated on those sub-processes that are relatively well defined in both theoretical and empirical terms.
Executive skills include an array of conscious and complex mental abilities that help us to: connect past experiences with present actions; plan future behavior when faced with novel tasks; judge; organize; change behavior and strategies; pay attention and remember details for our decision making. Executive skills give us the ability to: evaluate risks, recognize future consequences resulting from current actions, choose between good and bad actions, override and suppress unacceptable social responses, determine similarities and differences among objects or events, initiate or postpone, negate actions and to prioritize and switch among tasks. These very complex sets of decisions are achievable only if the neurological structures and functions are intact. The need for an executive control mechanism has been postulated for non-routine situations requiring a supervisory system, for example, for selecting an appropriate action from variety of options, inhibition of inappropriate actions and keeping in working memory the plan of action as well as the results of the plan. Flexible goal-directed behavior requires an executive control for optimizing behavior. Deciding which action to take is biased by the anticipation of the action’s outcome. Mismatch between anticipated and actual outcome can be used to optimize behavior. If an anticipated reward is not delivered, the error can be used for changing the previously learned behavioral pattern. All of these features distinguish the healthy humans from other animals and primates, because humans have a highly developed brain capable of abstract reasoning, language, introspection, and problem solving.
Neuropsychological evidence suggests that executive processing is intimately connected with the intact function of the frontal cortices and their underlying connections. Hence, the brain has an executive system that is hierarchical and yet distributed rather than strictly localized (Vaidya and Stollstorff, 2008).
The PFC and Executive Functioning
The English scientist Grey Walter in 1964 was one of first to confirm the involvement of the PFC in human executive functions by using electrophysiological evidence. He discovered the contingent negative variation, a slow negative potential recorded from the anterior part of the head during preparation of subjects to receive a stimulus or to make a movement. It is known that the PFC is a major brain structure with considerable functional heterogeneity in humans (Robbins and Arnsten, 2009). The PFC lies anterior to the motor and premotor areas and consists of multimodal association cortex (dorsolateral convexity and anteromedial surface) as well as limbic cortex (anterior cingulate and posterior orbitofrontal areas). The PFC is divided into three regions: lateral PFC, orbitofrontal (ventral) cortex, and medial frontal cortex (which include the anterior cingulate cortex). All PFC areas connect reciprocally with the dorsomedial thalamic nucleus.
A recent PUBMED search (1-22-12) resulted in 1,146 articles concerned with the relationship of the PFC and executive functions. It is well-known that the PFC is critical to many cognitive abilities that are considered particularly human, and forms a large part of a neural system crucial for normal socio-emotional and executive functioning in humans and other primates. PFC matures later in development than more caudal regions, and some of its neuronal subpopulations exhibit more complex dendritic arborizations. Comparative work as reviewed by Teffer and Semendeferi (2012) suggests that that the human prefrontal cortex differs from that of closely related primate species less in relative size than it does in organization. In fact a phylogenetically recent reorganization of frontal cortical circuitry may have been critical to the emergence of human-specific executive and social-emotional functions and developmental pathology in these same systems underlies many psychiatric and neurological disorders, including autism and schizophrenia.
The neural architecture of human PFC is probably more sophisticated or organized differently compared to cortical areas in other species. In spite of the fact that the major executive functions of the frontal lobes have been identified as: higher-level reasoning, analytical thinking, multi-tasking, decision-making, and problem solving, as well as creative thinking, important tasks of the PFC in executive function still need clarification. The exact role of other PFC structures involved in the neural circuits needs to be identified and their relationship to the subcortical structures elucidated. We argue that while the brain has networks that act in harmony and an executive system that is hierarchical and yet diffuse rather than strictly localized, the various sub-regions of the P3FC perform a unified set of interrelated roles. These interrelations can to be studied through a neural network model incorporating the frontal lobes and other regions including: the basal ganglia, dorsomedial, lateral, and anterior nucleus of the thalamus, amygdala, hippocampus, cingulate cortex, cerebellum, temporal cortex, and parietal cortex Computational neural network modelling may unravel clues that will fuel our understanding of how executive functioning works as a cohesive network of connectivity rather than an unrelated diffuse set of anatomical loci (Bishop, 1995). Another tool used to learn how executive function actually works by evaluation of impairments in this precise and important complex system.
Impairments of Executive Functions
Executive dysfunction has been associated with a range of disorders generally attributed to structural or functional frontal lobe pathology. Neuroimaging, with PET and fMRI, have confirmed this relationship (Mirsky et al., 2011); however, attempts to link specific aspects of executive functioning to discrete prefrontal foci have been inconclusive. Instead, the emerging view suggests that executive function is mediated by dynamic and flexible networks that can be characterized using functional integration and effective connectivity analyses. This vision is compatible with the clinical presentation of executive dysfunction associated with a range of pathologies. For example, both healthy adults and schizophrenic patients activate a qualitatively similar neural network during executive task performance, consistent with the engagement of a general-purpose cognitive control network, with critical nodes in the dorsolateral PFC and anterior cingulate cortex (ACC). However, patients with schizophrenia show altered activity with deficits in the dorsolateral PFC, ACC, and mediodorsal nucleus of the thalamus. Increases in activity are evident in other PFC areas, which could be compensatory in nature (Minzenberg et al., 2009).
An interruption of cognitive/executive function often results in a various pattern of deficits including distractibility, social unreliability, untrustworthiness, lack of initiative, impulsivity and profound disinhibition. Cognitive symptoms may occur in many neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, psychiatric illness such as schizophrenia, depression, obsessive compulsive disorder and Reward Deficiency Syndrome (RDS) [Blum et al, 1996], as well as pervasive developmental disorders such as attention-deficit hyperactivity disorder. In fact, executive dysfunction syndromes are commonly encountered in psychosomatic medicine (Andersson et al., 2008).
Executive dysfunction describes the inability to delay reward, modify behavior, match context and a lack of capacity for self control, an underestimation of harm and a lack of regard for consequences. While executive function is associated with both the initiation and the modulation of behavior lack of initiation and lack of control of behavior that might be concurrent features of executive dysfunction (Hall et al., 1994). It has been argued that reward processing and error signals after reward non-delivery are not generally considered executive functions, as such functions operate in a bottom-up, unsupervised fashion. However, Taylor et al (2004) found an interaction between reward and retrieval from working memory in the right dorsolateral prefrontal cortex. Main effects of load and reward occurred in adjacent regions of the ventrolateral PFC during retrieval. The data demonstrate that when subjects perform a simple working memory task, financial incentives motivate performance and interact with some of the same neural networks that process various stages of working memory. Areas of overlap and interaction may integrate information about value, or they may represent a general effect of motivation increasing neural effort.
Clearly, any loss of synchrony or timing in neurotransmission will disrupt mental processes dependent upon the integration of signal transmission for function. Primary factors disrupting brain integration are transitory mental, emotional, physical or biochemical stress (Duncko, 2003).
Stressors could be described as extremely powerful environmental and psychological incentives. According to Richard S Lazarus, stress is a feeling experienced when “the demands exceed the personal and social resources the individual is able to mobilize” (Lazarus et al., 1993). Fatigue resulting from lack of sleep is one of the most common forms of physical stress affecting brain integration (McEwen, 2007; Williams et al., 2009b). In general, and for most people these factors causing loss of brain integration are transitory. Physiological stress, a major factor, which could occur from psychological trauma, injury, or even from memory loss, is biochemical in nature and a direct result of our emotional states, particularly activation of our survival emotions, and is thus subconscious in origin (Khan et al., 2000).
Patients with traumatic brain injury, particularly when mild, may appear normal, claim to be normal, and show no impairment on standard cognitive related testing. Nevertheless, they may suffer a potentially devastating syndrome involving a constellation of disabilities, including executive functioning deficits. Interestingly, complete recovery of executive function in some patients can occur, perhaps due to functional reorganization within executive networks (Macqueen et al., 2003). The destructive consequence of closed head injuries are potentially catastrophic neurobehavioral symptoms, following major or minor head trauma (Lux, 2007). A number of studies suggest that the use of low pressure hyperbaric oxygen may have positive outcomes in patients presenting brain injury whereby executive functioning is improved and other associated symptoms like Post-Traumatic Stress Disorder (Harch and McCullough, 2007; Harch et al. 2009; Bowirrat et al. 2010).
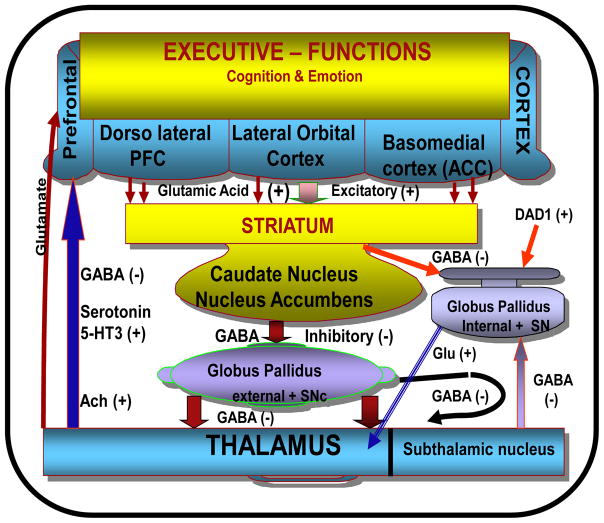
Converging neuropsychological and neuroimaging evidence suggests that abnormalities in practical real-life decision-making form a prominent part of the profile of the cognitive deficit associated with patients that have frontal damage restricted to the ventromedial PFC that includes the orbitofrontal region (Damasio, 1994; Bechara et al., 1998; Bechara et al., 1999; Rahman et al., 1999 Rogers et al., 1999a; Manes et al., 2002; Robbins and Arnsten, 2009). Such cognitive deficits are considered in the context of risk-taking or impulsivity because patients tend to pursue inappropriate actions, often without foresight, that are immediately rewarding, but are likely to have undesirable consequences for the patient’s well being in the long-term. Damage or abnormalities in other structural regions such as the anterior cingulum circuit may cause malfunction to the motivation of behavior (Flint and Eastwood, 1988; Shue and Douglas, 1992; Semkovska et al., 2001). Damage to the dorsolateral prefrontal circuit causes instability in the organizational aspects of executive functioning and integrating information, focusing attention, and deciding on response. Also, injury to the lateral orbitofrontal circuit produces critical damage to the integration of limbic and emotional information into contextually appropriate behavioral responses (Mega and Cummings, 1994; Houk, 2001; Tekin and Cummings, 2002) (see Figure 1).
Fig 1.
The figure shows the division of the prefrontal cortex (PFC): The dorsolateral PFC, lateral orbital cortex and basomedial cortex that originates in the anterior cingulated cortex (ACC), and their connection with the subcortical structures (striatum, globus pallidus, substantia nigra and the thalamus). Neurotransmitters involved in modulations of frontosubcortical circuit are also shown. Damage or alteration of these structures or the neurochemistry will cause different emotional and executive dysfunction.
- Damage to the dorsolateral PFC circuit will cause inflexibility of thought, problems solutions deficit and deficit in regulating adaptive and goal –directed behavior.
- Damage to the lateral orbital cortex and its connections will cause significant affective behaviors such as aggressiveness, hyperactivity, and labile emotions ranging from euphoria to dysphoria.
- Damage to the basomedial cortex will induce cognitive impairment and mutism.
Neurochemical pathology of the modulatory catecholaminergic (dopamine, serotonin and noradrenalin) and cholinergic neurotransmitters is also known to play an important role in emotional and cognitive functions.
Recent research by Chen et al. (2009) studying the relationship between executive dysfunction and frontal and non-frontal white matter using diffusion tensor imaging measurements on 13 subjects with amnesic mild cognitive impairment (aMCI), 11 subjects with early Alzheimer’s disease (AD), and 16 control subjects provided important insights. The aMCI and early AD patients showed executive function impairments with differential performance in frontal-related behaviors. Both groups also showed increased mean diffusion in the genu of the corpus callosum and left frontal periventricular white matter (PVWM). The early AD group showed an additional decrease in fractional anisotropy of bilateral frontal PVWM and in the genu of the corpus callosum. The frontal PVWM was associated with performance on the Verbal Fluency Test, the Wisconsin Card Sorting Test, and Part B of the Trail Making Test (psychomotor performance/attention). Executive function was impaired in subjects with aMCI and early AD and was associated with frontal and parietal PVWM changes. These changes may be due to early AD degeneration of the lateral cholinergic projections or to early change of the superior longitudinal fasciculus.
Neurochemicals and Neuromodulators in the PFC
Concurrent with the elucidation of the connections between aspects of executive function and damaged neurophysiology, there is evidence that disorders and pathology of brain and behavior are partially a result of neurochemical imbalance of the modulatory catecholaminergic and the cholinergic neurotransmitters. This interest, in part, stems from the longstanding consensus that these neural systems play a critical role in many neuropsychiatric disorders, as well as in normal and pathologic aging (Blum et al., 1977; Seamens and Yang, 2004, Bäckman et al., 2010). In this review we discuss separately the role of the important neurotransmitters involved directly in executive functions.
The Role of Dopamine (DA) in Executive Functions
The dopaminergic system of the brain is heterogeneous and multi-functional. Most DA-containing cells develop from a single embryological cell group that originates at the mesencephalic–diencephalic junction. The cell group is divided into three systems with different projections. The best known is the nigrostriatal system, which originates in the zonal compact of the substantia nigra. Two others are associated with the ventral tegmental area. The boundaries between these ‘systems’ are not well defined (Ikemoto, 2010). At the pharmacological level, DA receptors are broadly classified into two families: the D1 like (comprising D1 and D5 subtypes) and D2 like (comprising D2, D3, and D4 subtypes) (Missale, 1998). While DA receptors are found throughout the nervous system (Blum, 1969), they demonstrate high concentrations within the brain’s limbic regions, basal ganglia, and frontal cortical areas, all of which are involved importantly in emotional and motivational regulation (Laviolette,, 2007). DA exerts its effects on PFC neural activity via multiple receptor subtypes. The DA receptors (D1, D2, D3 and D4) are localized within the PFC in different levels and distribution, although the subcellular localization of these receptors differs. Expression of D1-like receptors on principal pyramidal neurons in the PFC appears to be substantially greater than D2-like (D2 and D4) receptors (Gaspar et al. 1995), whereas both types of DA receptors were localized on GABAergic interneurons, and may also reside on presynaptic excitatory glutamate terminals (Sesack et al. 1995; Mrzijak et al. 1996; Muly et al. 1998; Wedzony et al. 2001). Expression of D3 receptors in the PFC is very low (Levesque et al. 1992). However, numerous studies showed that activation of D1, D2, or D4 receptors exert dissociable electrophysiological actions on the activity of different classes of PFC neurons (Seammans and Yang, 2004). Yet, despite these anatomical and neurophysiological findings, the majority of studies have focused on the role of PFC DA in functions such as working memory and on the role of D1-like receptors. Nevertheless, D1 and D2 receptors act cooperatively to mediate behavioral flexibility and a consensus of evidence highlights the position that DA acting via D1-like and D2 receptors is crucial. Because DA D1 receptors in the PFC are several times more abundant than D2 receptors (Hall et al., 1994), the relationship between D1 receptors and PFC functions have been widely investigated.
Wise (2004) hypothesized that DA and PFC systems are critical for the control of thought and behavior. Additionally, the PFC is of central importance to higher cognition and plays a critical role in working memory and attentional control (Hopfinger et al., 2000; O’Reilly et al., 2002), and the DA system is integrally involved with both motor control and reward/motivation (Cohen and Frank, 2009; Cohen et al., 2002; Blum et al. 2006). The interaction of DA within the PFC also likely serves a specialized computational function. DA is thought to enable behavioral flexibility in these pathways by facilitating the learning and execution of adaptive behavioral responses (Goto, 2005). The nonlinear interactions in the differing trajectories of these systems during development result in changing patterns of cognitive functions over time; they may also lead to paradoxical outcomes, for which enhancement of one function through dietary intervention (epigenetics) may be at the expense of another.
DA is one of a number of neuromodulators present in the PFC. The other neurotransmitters, noradrenaline and serotonin (5-HT, 5-hydroxytryptamine), and acetylcholine, are also known to play an important role in cognitive functions, are also widely distributed and participate in neurotransmission in the PFC (Goldman-Rakic et al., 1990; Oscar-Berman et al., 1991; Williams and Goldman-Rakic, 1993; Mrzijak et al., 1996; Muly et al., 1998). The marked influence of these neurochemical systems on prefrontal working memory processes has been widely described.
Although the mechanisms by which DA modulates cognitive performance are still mysterious, experimental studies in both humans and animals have demonstrated a fascinating and multivariate role for DA transmission. Central DA systems have a role in processing and encoding of emotionally salient information, at the anatomical signal transduction levels of analysis (Smith et al., 2011). In addition a growing body of evidence also implicates central DA systems in cognitive processes dependent on integral frontostriatal connections (Bolton et al., 2010). Therefore, it appears that executive deficits in spatial working memory observed in frontal patients has a considerable degree of selectivity, but it is not simply produced by damage to the PFC. It seems likely that performance on this task is a product of interactions between cortical and subcortical structures, and their neurochemical innervations, especially DA which serves to regulate performance depending on the precise task requirement and baseline levels of DA (Sambataro et al. 2011).
Muller et al. (1998) reported that systemic administration of a mixed D1/D2 agonist facilitated working memory, whereas the selective D2 agonist had no effect, indicating that the dopaminergic modulation of working memory processes is mediated principally via D1 receptors. Also, D1 receptors are believed to have an inverted U-shaped dose/response curve for working memory whereby either too much or too little DA will result in sub-optimal performance that impairs prefrontal functions (Vijayraghavan et al., 2007). A seminal study by Brozoski et al. (1979) showed a relatively selective role for prefrontal DA as distinct from other prefrontal monoamines (that is, noradrenaline and serotonin) in spatial working memory. Further work has elucidated this specific contribution of DA to working memory functions at the psychological (Floresco and Phillips, 2001; Floresco et al. 2001, 2006, Chudasama and Robbins, 2004), anatomical (Goldman-Rakic et al., 1989; Smiley et al., 1992; Smiley & Goldman-Rakic, 1993), cellular (Sawaguchi et al., 1990; Sawaguchi and Goldman-Rakic, 1991; Williams and Goldman-Rakic, 1995), and molecular (i.e., receptor) levels of analysis (Sawaguchi and Goldman-Rakic, 1991). Thus, there is accumulation and considerable evidence for a special role for DA D1, but not D2 receptors in spatial working memory, based on evidence using iontophoresis or intracerebral drug infusion (Floresco and Magyar, 2006). Overall there is consensus that spatial working memory function depends upon an optimal level of DA function within the PFC (Williams and Goldman-Rakic, 1995; Arnsten, 1997; Zahrt et al., 1997; Floresco and Phillips, 2001). Thus whereas D4 receptor activity may act to antagonize the effects that D1 and D2 receptors exert over behavioral flexibility the inverse correlations between D1 and D2 receptors or abnormal increases in D2 receptor activity also cause a more general impairment in behavioral flexibility. These findings suggest that deficits in these forms of executive functioning observed in disorders linked to dysfunction of the DA system may be attributable in part to aberrant increases or decreases in mesoaccumbens DA activity.
Less is known of the role of D2 receptors in cognition, but previous studies have shown that D2 receptors in the hippocampus might play some roles via hippocampal-PFC interactions (Fujishiro et al., 2005). Hippocampal D2 receptor binding shows positive linear correlations not only with memory function but also with frontal lobe functions, while hippocampal D1 receptor binding had no association with any memory and prefrontal functions. Hippocampal D2 receptors seem to contribute to local hippocampal functions (long-term memory) and to modulation of brain functions outside the hippocampus (frontal lobe functions), which are mainly subserved by PFC, via the hippocampal-PFC pathway. Takahashi et al. (2007) suggested that orchestration of prefrontal D1 receptors and hippocampal D2 receptors might be necessary for human executive function including working memory. Indeed, Kemppainen et al. (2003) reported that a reduction of D2 receptors in the hippocampus (HPC) in Alzheimer’s disease patients was correlated with memory impairments. In addition, hippocampal D2 receptors appear to be involved in synaptic plasticity. It has been reported that D2 antagonist inhibited long-term potentiation in the hippocampus (Frey et al., 1990; Manahan-Vaughan and Kulla, 2003), the key mechanism underlying memory consolidation (Jay, 2003; Lynch, 2004). While this may be true we must be clear in that long-term hippocampal-mediated memory is not the same as with working memory, a main component in executive function.
In addition, it is becoming increasingly apparent that mesocortical DA transmission contributes to other forms of executive functions regulated by the frontal lobes that are distinct from working memory processes. Furthermore, the specific DA receptor pharmacology that underlies these effects appears to be substantially different from that which mediates working memory. It seems that the collective work of the PFC, make it apparent that dopaminergic input to the frontal lobes forms an essential network of the neural circuits that mediate a variety of cognitive and executive functions, including working memory, behavioral flexibility, and decision-making. Each of these executive functions engages distinct types of cognitive operations and functional neural circuits.
The anatomical distribution of dopaminergic projections strongly implicates DA in higher-level cognitive abilities, specifically in PFC functions (Arnsten et al., 1997; Nieoullon, 2002). There is an anterior-posterior gradient in brain DA concentration, which is highest in the PFC. (Williams and Goldman-Rakic, 1993). Thus, the distribution of the mesocortical dopaminergic fibers suggests a greater influence on anterior brain structures.
Several lines of pharmacological evidence confirm the role of DA in PFC function. First, in monkeys, depletion of DA in PFC or pharmacological blockade of DA receptors impaired working memory tasks. This working memory impairment was as severe as the deficit in monkeys with PFC lesions and was not observed in monkeys when serotonin or norepinephrine was depleted (Sawaguchi et al, 1991).
Serotonin
The serotonin (5-HT) 2 family of receptors has three receptors (5-HT2A, 5-HT2B, and 5-HT2C), which are similar in terms of their molecular structure, pharmacology, and signal transduction effects (Roth et al, 1998; Barnes and Sharp, 1999; Celada et al, 2004). However 5-HT2B and 5-HT2C receptors are less important and not as widely expressed as the 5-HT2A receptors. Serotonin (5-HT) 2A receptors are widely distributed, in different regions in cortical and subcortical areas, with high levels in the frontal cortex, suggesting a particularly important role in PFC function (Roth, 1998; Celada et al,, 2004).
Postsynaptic activation may increase activity in pyramidal glutamatergic neurons and mediate various executive functions. More specifically, reciprocal cortical-raphe pathways may allow the ventral PFC to inhibit stress-induced neural activity in the brainstem when stressors are perceived as controllable. However, early adversity and negative attitudes may be associated with higher frontal 5-HT2A receptor levels and greater risk for stress-induced psychopathology, and certain 5-HT2A gene variants have been associated with increased risk for impulsive behavior.
5-HT2A receptors are ideally located to play a role in reciprocal cortical-raphe pathways, (Bhagwagar et al, 2006) which, in turn, help regulate the stress response based on assessments about stressor controllability (Amat et al, 2006). Particular gene variants, early adversity, and negative attitudes may be associated with higher frontal 5-HT2A receptor levels and greater risk for stress-induced psychopathology.
Manipulations of Cholinergic, Serotonergic, and Noradrenergic Systems
Other neurotransmitters also play a role in the PFC and executive functioning (Oscar-Berman et al., 1991). One of the important direct strategies that may explain the role of cholinergic, serotonergic and noradrenergic (NA) systems on fronto-executive processing is by observations following manipulations of these systems. Indeed, manipulating the cholinergic system demonstrates its strength in predicting patterns of attentional dysfunction, and direct manipulations of 5-HT levels has only been explored using dietary tryptophan depletion which has little effect on tasks that require the integrity of the dorsolateral PFC (Park et al., 1994) but does impair visual discrimination reversal (Park et al., 1994; Rogers et al., 1999b; Clarke et al., 2006). There is increasing evidence suggesting that abnormalities in serotonergic innervations of the frontal cortex may contribute to decision-making deficit (Park et al., 1994; Rogers et al., 1999c). This further supports the general hypothesis that decreased 5-HT function increases impulsivity (Soubrié, 1986).
These effects of neurochemical manipulations in the PFC provide some insight into the nature of the functional interactions between these neurochemical systems. It now seems likely that the central 5-HT system and its interactions with acetylcholine probably mediate reward-related information processing and this has implications for a number of neuropsychiatric disorders (Insel and Winslow, 1992; Lucey et al., 1997; Hermesh et al. 2003).
There is burgeoning evidence that the NA system, specifically the coeruleus--cortical NA projections to diverse forebrain sites, including the neocortical mantle and the hippocampus, are implicated in attentional set-shifting. There is already substantial evidence that manipulations of the NA system effect working memory functions in nonhuman primates in a way perhaps similar to the effects of DA neuromodulation (Arnsten and Robbins 2002, Amsten et al 1984). NA has been shown to be a key neurotransmitter for working memory in human in study carried out by Chamberlain et al. (2006). Similarly to DA, an optimal level of NA appears critical for working memory.
Parallel investigations have also suggested a role for central, particularly prefrontal, NA in attentional functioning based on electrophysiological studies in monkeys (Aston-Jones and Cohen, 2005), and studies in rats of the effects of profound cortical depletion of NA (Carli et al., 1983; Cole and Robbins, 1992; Milstein et al., 2007).
Additional Neural Circuitry involved in Executive and Cognitive Functions
Aspects of cognition are integral components of executive functions. They require neural processes necessary to support the flexible use of information in the execution of adaptive and goal-directed behavior. Of the many different processes involved, learning and memory are two essential components. However, in addition to learning and memory, the ability to enable adaptive behavioral responses also depends on the capacity to focus and sustain attention on the relevant stimuli and to hold this information in working memory while choosing and executing the appropriate motor response. It also involves more complex information processing functions, such as the ability to abstract information and to establish a system of rules for responding appropriately in different contexts and to suppress other competing behavioral responses.
Cognition is not a solitary function but rather contingent on the efficacy of multiple and divisible central nervous system networks. The neural circuits that have been implicated most prominently in mediating executive functions, the information processing and decision-making processes of the human brain comprise reciprocal cortical-subcortical connections. These circuits originate in the PFC and project to various subcortical structures such as the ventral and dorsal striatum (nucleus accumbens and basal ganglia, respectively). They then go back by way of the thalamus to the region of the frontal cortex from which they originated (Alexander et al., 1986). The subcortical centers can have an important impact on behavior, but their effect will depend on command processes at higher centers, governed by both feedback (phasic arousal/reinforcement) and synaptic action at higher levels. Synaptic action depends on cortical receptor levels, which degrades when it is either over-aroused or under-aroused (Levy, 2006). Therefore, a reciprocal relationship exists between the higher cortical centers and the lower subcortical centers. In fact, cortical activities regulate subcortical activities through executive modulation of prepotent assessments and emotional responses. Subcortical systems alternatively regulate the cortex by tuning its activities to the demands or opportunities provided by the environment. Cortical controls buy us time, as needed for planning and intelligent action. Subcortical controls provide energy, focus, and direction, as needed for relevant emotion-guided behavior (Lewis and Todd, 2007; Simpson et al. 2007).
It is becoming increasingly apparent that cortical-subcortical pathways operate with dexterity and in harmony. They do not operate autonomously and randomly and they are subject to the influence of input from many other areas of the brain. For example, in addition to the PFC, vital sources of input to the nucleus accumbens are the hippocampus and amygdala (Chambers et al., 2003). The functional relationship between these structures is fundamental to both behavioral and physiologic regulation, and this understanding has served to challenge the traditional distinction made between the cognitive and affective contributions to decision making (Everitt and Robbins, 2005). The hippocampus is necessary for the formation of long-term memories and contributes to higher-level decision-making processes by representing the relations between discrete stimuli, thereby providing information relating to the overall context (O’Reilly et al., 2001). The amygdala is involved in processing the emotion (i.e., affective) valence of sensory stimuli and relaying this information to the PFC as well as the instigation of the synchronized pattern of physiologic and behavioral changes that constitute the stress response to real or perceived dangers (Phelps and LeDoux, 2005). This includes the release of glucocorticoid hormones, where appropriate concentrations of glucocorticoids are necessary for mnemonic functions to be accomplished effectively (McGaugh, 2004). The hippocampus in turn, together with the PFC, is involved in the feedback regulation of these glucocorticoid concentrations (de Kloet, 2005). These processes are part of the executive functions that are engaged during the activity of decision making - which is just the ability to choose between the available options. It is also through our executive functions that we can appreciate cause and effect, and thus anticipate the possible outcomes of our actions. The PFC generates possible behavioral alternatives in response to the specific nature and emotional valence of sensory stimuli. This information is then relayed to the nucleus accumbens, where input from the hippocampus is thought to “gate” neural activity in such a way that the motor response chosen is that most appropriate to the overall context (O’Donnell, 1995). In this way, the circuitry involving the hippocampus, PFC, and nucleus accumbens plays a key role in enabling behavioral flexibility (Atallah, 2004). This is in contrast to the trajectory that includes the basal ganglia (dorsal striatum), which has been associated with the learning and implementation of the sequences of motor output that constitute habitual learned behavioral response patterns (Packard and Knowlton, 2002; Atallah, 2004). It is thought that the system involved to support behavior and which of these systems is engaged depends on the nature of the circumstances; that is, it depends on the integration of these systems with other systems that can access the organism’s goal in the context of the task at hand as well as the organism’s past victories and failures in reaching such goals (Packard and Wingard, 2004). Under routine and familiar conditions, customary responses may be the most effective, but any unexpected change will necessitate a switch either from one set of learned responses to another or to an entirely new set of behaviors based on a novel combination of internal representations gained from previous experience.
The modulation of states that allow the organism to be receptive to stimulation (e.g., alertness or arousal) is likely mediated by several ascending pathways, which are generally identified by their neurotransmitters: the dopaminergic pathways that originate in the midbrain; the cholinergic, serotonergic, and adrenergic pathways that originate in the brainstem; and the cholinergic pathways that originate in the basal forebrain (Everitt and Robins, 1997; Arnsten and Li, 2005; Chamberlain, 2006). Although the specific functions in which these subsystems are involved are not yet fully understood, these pathways have widespread cortical targets, and substantial evidence suggests that their input can also be modulated by feedback from cortical sites (see figure 2).
Fig 2.
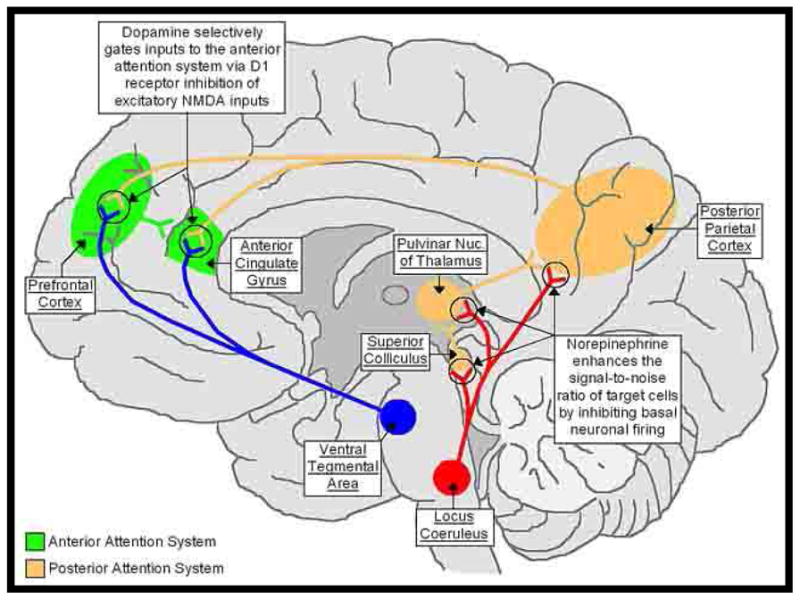
This schematic illustrates the neuroanatomy of the meso-limbic system highlighting specific neurotransmitters such as dopamine and norepinephrine and receptor interactions.
Can We Modulate Executive Functions through Epigentics?
Neuronal plasticity (e.g., neurogenesis, synaptogenesis, cortical re-organization) refers to neuron-level changes that can be stimulated by experience. Cognitive plasticity (e.g., increased dependence on executive function) refers to adaptive changes in patterns of cognition related to brain activity. Mechanisms of neural plasticity underpin cognitive plasticity and in turn, neural plasticity is stimulated by cognitive plasticity. Greenwood and Parasuraman (2010) have suggested that neural plasticity is stimulated by learning and novelty, and the plasticity is enhanced by dietary manipulations (low-fat, dietary restriction) and aerobic exercise. Moreover, studies supporting the use of environmental manipulations, compensatory strategy training, and techniques to improve underlying skills, including attention and prospective memory, have been reviewed by Mateer (1999).
The availability of certain neurotransmitters can be influenced by dietary supply of their amino acid precursor. For example, tryptophan is the dietary precursor of serotonin and tyrosine is that of DA and norepinephrine (Fernstrom 1990). Many of these neurotransmitters serve a dual role, functioning also as growth factors that influence the intricate choreography of growth of neural systems in the developing brain (Lauder 1993; Levitt et al. 1997). Thus, one of the mechanisms whereby changes in the availability of nutrient supply may result in disturbances of specific brain and behavioral functions during development is through their selective effect on some of these systems and not others. This emerging understanding has important implications for the design and interpretation of studies on the cognitive effects of specific nutrients during development (Chen et al. 2011).
Reward Related Genes and Executive Functions
Bertolino et al. (2010) demonstrated that a functional SNP (rs1076560) within the Dopamine D2 Receptor Gene (DRD2) predicts striatal binding of the two radiotracers to DA transporters and D2 receptors as well as the correlation between striatal D2 signaling with PFC activity during performance of a working memory task. These data are consistent with the possibility that the balance of excitatory/inhibitory modulation of striatal neurons may also affect striatal outputs in relationship with prefrontal activity during working memory performance within the cortico-striatal-thalamic-cortical pathway. Furthermore, Markett et al. (2010) found a significant interaction between nicotinic acetylcholine receptor (rs#1044396) and a haplotype block covering all three dopaminergic polymorphisms (rs#1800497, rs#6277, rs#2283265) on working memory capacity. This effect only became apparent on higher levels of working memory load. This is the first evidence from a molecular genetics perspective that these two neurotransmitter systems interact on cognitive functioning. Frank and Hutchinson (2009) found effects of the commonly studied Taq1A polymorphism on reinforcement-based decisions were due to indirect association with C957T of the dopamine receptor gene.
It is noteworthy that overexpression of D2 under pathological conditions such as schizophrenia and Parkinson’s disease could give rise to motivational and timing deficits (Drew et al., 2007). The increase in DA D2 receptors has been shown by others to prevent storage of lasting memory traces in PFC networks and impair executive functions (Xu et al., 2009).
Interestingly, Stelzel et al. (2009) showed that catechol-O-methyltransferase (COMT)Val (158)Met polymorphism effects on working memory performance are modulated by the DRD2/ANKK1-TAQ-Ia polymorphism. Val- participants -- characterized by increased prefrontal DA concentrations--outperformed Val+ participants in the manipulation of working memory contents, but only when D2 receptor density could be considered to be high. Stlzel et al. (2009) suggested that this beneficial effect of a balance between prefrontal DA availability and D2 receptor density reveals the importance of considering epistasis effects and different working memory subprocesses in genetic association studies. However, these genetic effects may not be present or are too subtle to detect in healthy subjects (Blanchard et al., 2011). Moreover, it has also recently been found that participants with Val/Val of the COMT gene involved in a 17 week running exercise program improved cognitive performance to a greater extent compared to individuals carrying a Met allele. From the present results it is concluded that an increase in physical fitness provides a means to improve cognitive functioning via dopaminergic modulation (Stroth et al., 2010). In a related study of HIV and methamphetamine dependence, dopaminergic overactivity in PFC conferred by the Met/Met genotype appeared to result in a liability for executive dysfunction and potentially associated risky sexual behavior due to poor judgment (Bousman et al., 2010).
To reiterate, the dopaminergic system of the brain is heterogeneous and multi-functional. It is a system with many important neurochemical functions and has been credited with resultant behavioral effects such as “pleasure,” “stress reduction” and “wanting” (Blum et al., 1997, 2000; Bowirrat and Oscar-Berman, 2005; Blum et al., 2008). In addition to its contributory role in addressing higher-order cognition and executive functions, it is very important in relapse of substance seeking behavior (Blum et al., 2009). It is well-known that acute alcohol intoxication significantly impairs executive functioning and judgment. Most recently, Lyvers and Tobias- Webb (2010) found a dose-dependent selective disruption of PFC functioning by alcohol. They suggested that alcohol-associated perseveration on the Wisconsin Card Sorting Test may reflect the inhibitory effect of alcohol preventing DA release in the PFC.
Finally, being somewhat complex, abnormal increases in D(2) receptor activity cause a more general impairment in behavioral flexibility especially in patients with attention deficit hyperactivity disorder (Barnes et al., 2011). These findings suggest that deficits in these forms of executive functioning observed in disorders linked to dysfunction of the DA system may be attributable in part to aberrant increases or decreases in mesoaccumbens DA activity (Zang et al., 2007; Arnsten, 2009; Dickinson and Elvevåg, 2009; Haluk and Floresco, 2009; MacDonald et al. 2009; Braet et al., 2011). The control of DA release in PFC and other brain regions is regulated by many neurotransmitters and second messenger genes and constitute a genetic map that could provide important information relating to a predisposition to poor judgment.
Development of a Genetic Map to Identify Individuals at Risk for Impaired Judgment
Executive dysfunctions are linked to flawed DA metabolism, and especially to low D2 receptor density, as well as other neurotransmitter deficits due to specific gene polymorphisms. Moreover, executive dysfunctions result from abnormalities in the mesolimbic system of the brain, which directly links abnormal craving behavior with a defect in the DRD2 gene, as well as other dopaminergic genes (D1, D3, D4, and D5, DATA1, MAO, COMT), including many genes associated with the brain reward function (Pinto & Ansseau, 2009) as listed in Table 1 below.
Table 1.
Reward Pathways and candidate genes involved in executive function physiology
| Signal Transduction | Serotonin | GABA | Dopamine |
|---|---|---|---|
| ADCY7 | HTRIA | GABRA2 | COMT |
| AVPR1A | HTRIB | GABRA3 | DDC |
| AVPR1B | HTR2A | GABRA4 | DRD1 |
| CDK5R1 | HTR2C | GABRA6 | DRD2 |
| CREB1 | HTR3A | GABRB1 | DRD3 |
| CSNKLE | HTR3B | GABRB2 | DRD4 |
| FEV | MAOA | GABRB3 | DRD5 |
| FDS | MAOB | GABRD | SLC18A2 |
| FOSL1 | SLC64A | GABRE | SLC6A3 |
| FOSL2 | TPH1 | GABRG2 | TH |
| GSK3B | TPH2 | GABRG3 | |
| JUN | GABRQ | ||
| MAPK1 | SLC6A7 | ||
| MAPK3 | SL6A11 | ||
| MAPK14 | SLC32A1 | ||
| MPD2 | GAD1 | ||
| MGFB | GAD2 | ||
| NTRK2 | DB1 | ||
| NTSR1 | |||
| NTSR2 | |||
| PPP1R1B | |||
| PRKCE |
| Cannabinoid | Glycine | Cholinergic | Opioid | NDMA |
|---|---|---|---|---|
| CNR1 | GLRA1 | CHRMI | OPRMI | GR1K1 |
| FAAH | GLRA2 | CHRM2 | OPRKI | GRINI |
| GLRB | CHRM3 | PDYN | GRIN2A | |
| GPHN | CHRM5 | PMOC | GRIN2B | |
| CHRNA4 | PRD1 | GRIN2C | ||
| CHRNB2 | OPRL1 | GRM1 | ||
| PENK | ||||
| PNOC |
| Adrenergic | Stress | Drug Metabolizing | Others |
|---|---|---|---|
| ADRA1A | CRH | ALDH1 | BDNF |
| ADRA2B | CRHEP | ALDH2 | CART |
| ADRB2 | CRHR1 | CAT | CCK |
| SLC6A2 | CRHR2 | CYPZE1 | CCKAR |
| DRA2A | GAL | ADH1A | CLOCK |
| DRA2C | NPY | ADH1B | HCRT |
| ARRB2 | NPY1R | ADH1C | LEP |
| DBH | NPY2R | ADH4 | NR3C1 |
| NPY5R | ADH5 | SLC29A1 | |
| ADH6 | TAC | ||
| ADH6 | |||
| ADH7 |
The genesis of all behavior, be it “normal” (socially acceptable) or “abnormal” (socially unacceptable), derives from an individual’s genetic makeup at birth. This genetic predisposition, due to multiple gene combinations and polymorphisms, is expressed differently based on numerous environmental factors including family, friends, educational and socioeconomic status, environmental contaminant exposure, and the availability of psychoactive drugs and unhealthy foods. The core of predisposition to these behaviors is a set of genes interacting with the environment, which promote a feeling of wellbeing via neurotransmitter interaction at the “reward center” of the brain (located in the meso-limbic system) leading to normal DA release (Kendler et al 2011).
Subjects afflicted with executive decision making dysfunction carry polymorphic genes in dopaminergic pathways that result in hypo-dopominergic function caused by a reduced number of dopamine D2 receptors, reduced synthesis of DA (by DA beta –hydroxylase), reduced net release of pre-synaptic DA (from e.g., the DA D1 receptor), increased synaptic clearance due to a high number of DA transporter sites (DA transporter), and low D2 receptor densities (DA D2 receptor), making such people more vulnerable to addictive behaviors and relapse because of poor judgment (Comings and Blum, 2000).
The inability to make good decisions involves shared genes and their mRNA expressions and behavioral tendencies, including dependence on alcohol, psycho-stimulants, marijuana, nicotine (smoking), and opiates, altered opiate receptor function, carbohydrate issues (e.g., sugar-binging), obesity, pathological gambling, premeditated aggression, stress, pathological aggression, and certain personality disorders, including novelty-seeking, and sex addiction. The common theme across all of these substances and behaviors is that they induce pre-synaptic DA release (Dreyer, 2010). Spectrum disorders such as ADHD, Tourettes syndrome, and autism also are included due to DA dysregulation. Other rare mutations (Sundaram et al., 2010) have been associated with Tourettes and autism. One example includes the association with Neuroligin 4 (NLGN4), a member of a cell adhesion protein family that appears to play a role in the maturation and function of neuronal synapses (Lawson-Yuen et al., 2008) that could have an impact on executive functions. The roles of many of these reward genes and associated polymorphisms have been reviewed in a recent paper by Blum et al. (2012).
Limitations and Future Directions
We have suggested that examination of multifactorial interactions of an individual’s genetic history, along with environmental risk factors, can assist in the characterization of executive functioning for that individual. We also have proposed that genetic studies may provide genetic mapping as a probable diagnostic tool serving as a therapeutic adjunct for augmenting executive functioning capabilities. However, considerable additional research is required prior to any definitive interpretation. Thus, we encourage other investigators to extend this work by analyzing families with histories of executive dysfunction for determining genotypes. This especially should include examination of a variety of reward genes. Certainly studies involving larger populations even in a few families over many generations (if possible) would strengthen this potentially important concept.
Conclusions
The differential modulation of fronto-executive function by discrete neurochemical systems highlights a degree of specificity for these “nonspecific” neuromodulatory pathways, which hitherto have been underestimated. These systems interact not only within the PFC at the level of single pyramidal neurons, but also at the level of functional modules in order to optimize overall executive control. Preservation of the neurological underpinnings of executive functions requires integrity of entire neural systems as well as specific genes and associated polymorphisms. Genetic mapping may serve as a probable diagnostic tool and a therapeutic target for eventual augmentation of executive functioning capabilities.
Highlights.
Review of PFC DA in higher-order cognitive and emotional behaviors.
Role of important neurotransmitters involved directly in executive functions (EF).
Influence of genetic history and environmental risk factors on EF.
Genetic mapping as a therapeutic target for augmenting EF capabilities.
Acknowledgments
Marlene Oscar Berman is the recipient of grants from NIAAA (R01 AA07112, K05 AA 00219) and from the Medical Research Service of the US Department of Veterans Affairs.
Footnotes
Disclosures:
The authors claim no conflict of interest.
Contributor Information
Abdalla Bowirrat, Email: bowirrat@netvision.net.il.
Thomas JH Chen, Email: tjhchen@yahoo.com.tw.
Marlene Oscar-Berman, Email: oscar@bu.edu.
Amanda LH Chen, Email: ac8858@gmail.com.
Mallory Kerner, Email: pathmedical@aol.com.
John Giordano, Email: michg@hotmail.com.
Frank Fornari, Email: ffornari@me.com.
References
- Alexander G, DeLong M, Strick PL. Parallel organization of functionally relevant segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Barder HE, Hellvin T, Løvdahl H, Malt UF. Neuropsychological and electrophysiological indices of neurocognitive dysfunction in bipolar II disorder. Bipolar Disorders. 2008;10(8):888–99. doi: 10.1111/j.1399-5618.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatr. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Robbins TW. Neurochemical modulation of prefrontal cortical functions in humans and animals. In: Stuss D, Knight R, editors. The prefrontal cortex. New York: Oxford University Press; 2002. pp. 51–84. [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus- norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Frank MJ, O’Reilly RC. Hippocampus, cortex, and basal ganglia: insights from computational models of complementary learning systems. Neurobiol Learn Mem. 2004;82:253–67. doi: 10.1016/j.nlm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Della Sala S. “Working memory and executive control” (PDF) Philos Trans R Soc Lond, B, Biol Sci. 1996;351 (1346):1397–403. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Barnes JJ, Dean AJ, Nandam LS, O’Connell RG, Bellgrove MA. The molecular genetics of executive function: role of monoamine system genes. [accessed Mar 11 2011];Biol Psychiatry [Epub] 2011 15;69(12):e127–43. doi: 10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacol. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18:428–37. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. 2010;34:670–7. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Taurisano P, Pisciotta NM, Blasi G, Fazio L, Romano R, Gelao B, Lo Bianco L, Lozupone M, Di Giorgio A, Caforio G, Sambataro F, Niccoli-Asabella A, Papp A, Ursini G, Sinibaldi L, Popolizio T, Sadee W, Rubini G. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS One. 2010;22(5)(2):e9348. doi: 10.1371/journal.pone.0009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby PJ. Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [11C]MDL 100,907. Am J Psychiat. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- Bishop CM. Neural Networks for Pattern Recognition. Oxford: Oxford University Press; 1995. [Google Scholar]
- Blanchard MM, Chamberlain SR, Roiser J, Robbins TW, Müller U. Effects of two dopamine-modulating genes (DAT1 9/10 and COMT Val/Met) on n-back working memory performance in healthy volunteers. Psychol Med. 2011;41:611–8. doi: 10.1017/S003329171000098X. [DOI] [PubMed] [Google Scholar]
- Blum K. The effect of dopamine and other catecholamines on neuromuscular transmission. Arch Int Pharmacodyn Ther. 1969;181(2):297–306. [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward Deficiency Syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen ALC, Chen TJ, Braverman ER, Reinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Theoretical Biology and Medical Modelling. 2008;5(24):1–16. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Downs BW, Bowirrat A, Waite RL, Braverman ER, Madigan M, Oscar-Berman M, DiNubile N, Stice E, Giordano J, Morse S, Gold M. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing “deprivation-amplification relapse therapy” (DART) Postgrad Med. 2009;121(6):176–96. doi: 10.3810/pgm.2009.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen TJH, Meshkin B, Downs BW, Gordon CA, Blum S, Mengucci JF, Braverman ER, Arcuri V, Varshavskiy M, Deutsch R, Martinez-Pons M. Reward deficiency syndrome in obesity: A preliminary cross-sectional trial with a genotrim variant. Advances in Therapy. 2006;23(6):1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- Blum K, Fornari F, Downs BW, Waite RL, Giordano J, Smolen A, Lui Y, Tain J, Majmundr N, Braverman ER. Genetic Addiction Risk Score (GARS): Testing for Polygentic Predisposition and Risk for Reward Deficiency Syndrome (RDS) In: King C, editor. Chapter 19: in Gene Therapy Applications. 2011. pp. 327–362. [Google Scholar]
- Blum K, Sheridan PJ, Chen THJ, Wood RC, Braverman ER, Cull JG, Comings DE. The Dopamine D2 receptor gene locus in Reward Deficiency Syndrome: Meta-analyses. In: Blum K, Noble EP, editors. Handbook of Psychiatric Genetics. Vol. 236. Boca Raton, FL: CRC Press; 1997. pp. 407–34. [Google Scholar]
- Blum K, Wallace JE, Schwertner HA, Meyer E, Morgan WW. Central supersensitivity to norepinephrine and amphetamine following brain chemical sympathectomy by 6-hydroxydopamine. Life Sci. 1977;20(10):1705–13. doi: 10.1016/0024-3205(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Marioni RE, Deary IJ, Harris SE, Stewart MC, Murray GD, Fowkes FG, Price JF. Association between polymorphisms of the dopamine receptor D2 and catechol-o-methyl transferase genes and cognitive function. Behav Genet. 2010;40(5):630–8. doi: 10.1007/s10519-010-9372-y. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Atkinson JH, Heaton RK, Grant I, Everall IP. Hnrc Group T. COMT Val158Met polymorphism, executive dysfunction, and sexual Risk behavior in the context of HIV infection and methamphetamine dependence. Interdiscip Perspect Infect Dis. 2010;2010:678648. doi: 10.1155/2010/678648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency Syndrome. Am J Med Genet Part B. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Chen TJH, Blum K, Madigan M, Bailey JA, Chen LHC, Downs BW, Braverman ER, Radi S, Waite RL, Kerner M, Giordano J, Morse S, Oscar-Berman M, Gold M. Neuro-psychopharmacogenetics and neurological antecedents of Posttraumatic Stress Disorder: Unlocking the mysteries of resilience and vulnerability. Curr Neuropharmacol. 2010;8:335–358. doi: 10.2174/157015910793358123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet W, Johnson KA, Tobin CT, Acheson R, McDonnell C, Hawi Z, Barry E, Mulligan A, Gill M, Bellgrove MA, Robertson IH, Garavan H. fMRI activation during response inhibition and error processing: The role of the DAT1 gene in typically developing adolescents and those diagnosed with ADHD. Neuropsychologia. 2011;49(7):1641–1650. doi: 10.1016/j.neuropsychologia.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–80. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5- HT1A and 5-HT2A receptors in depression. J Psychiat Neurosci. 2004;29:252–65. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–3. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiat. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, Madigan MA, Waite RL, Bailey JA, Kerner M, Yeldandi S, Majmundar N, Giordano J, Morse S, Miller D, Fornari F, Braverman ER. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: proposing an addiction candidate gene panel map. J Psychoactive Drugs. 2011;43:108–27. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, But Not Serotonin, Regulates Reversal Learning in the Marmoset Caudate Nucleus. The Journal of Neuroscience, 16 March 2011. 2006;31(11):4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behavioural Brain Res. 2009;199(1):141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cohen M, Ylvisaker M, Hamilton J, Kemp L, Claiman B. Errorless learning of functional life skills in an individual with three aetiologies of severe memory and executive function impairment. Neuropsychol Rehabil. 2009;26:1–22. doi: 10.1080/09602010903309401. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacol. 1992;7:129–42. [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacol. 2004;29:1628–36. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes’ Error: Emotion, Reason and the Human Brain. New York: G.P. Putnam; 1994. [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dickinson DC, Elvevåg B. Genes, cognition and brain through a COMT lens. Neurosci. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010 Dec;2(12):92. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncko R, Schwendt M, Jezova D. Altered glutamate receptor and corticoliberin gene expression in brain regions related to hedonic behavior in rats. Pharmacol Biochem Behav. 2003;76(1):9–16. doi: 10.1016/s0091-3057(03)00164-3. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(1):81–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. Aromatic amino acids and monoamine synthesis in the CNS: influence of the diet. J Nutr Biochem. 1990;1:508–17. doi: 10.1016/0955-2863(90)90033-h. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Eastwood MR. Frontal lobe syndrome and depression in old age. J Geriatr Psychiatry Neurol. 1988;1:53–55. doi: 10.1177/089198878800100110. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set-shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience. 2009;24;164:131–40. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Umegaki H, Suzuki Y, Oohara-Kurotani S, Yamaguchi Y, Iguchi A. Dopamine D2 receptor plays a role in memory function: implications of dopamine-acetylcholine interaction in the ventral hippocampus. Psychopharmacology (Berl) 2005;182(2):253–61. doi: 10.1007/s00213-005-0072-x. [Epub 2005 Oct 19] [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, LeMoine C. D1 and D2 receptor gene expression in rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–12. doi: 10.1038/nn1471. [Epub 2005 May 22] [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Neuronal and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Front Aging Neurosci. 2010;2:150. doi: 10.3389/fnagi.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacol. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacol. 2009;34:2041–52. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Harch PG, Fogarty EF, Staab PK, Van Meter K. Low pressure hyperbaric oxygen therapy and SPECT brain imaging in the treatment of blast-induced chronic traumatic brain injury (post-concussion syndrome) and post traumatic stress disorder: a case report. Cases J. 2009;2:6538. doi: 10.1186/1757-1626-0002-0000006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harch PG, Mccullough V. The Oxygen Revolution. Hatherleigh Press; New York and London: 2007. [Google Scholar]
- Hermesh H, Weizman A, Gur S, Zalsman G, Shiloh R, Zohar J, Gross-Isseroff R. Alternation learning in OCD/schizophrenia patients. European Neuropsychopharmacology. 2003;13:87–91. doi: 10.1016/s0924-977x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neurosci. 2000;3:284– 291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Houk JC. Neurophysiology of frontal-subcortical loops, frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Psychiatry and Neurology. New York: Guilford Publications; 2001. pp. 92–113. [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35(2):129–50. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Neurobiology of obsessive compulsive disorder. The Psychiatric Clinics of North America. 1992;15:813–824. [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry. 2011;168(1):29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen N, Laine M, Laakso MP, Kaasinen V, Någren K, Vahlberg T, Kurki T, Rinne JO. Hippocampal dopamine D2 receptors correlate with memory functions in Alzheimer’s disease. Eur J Neurosci. 2003;18:149–154. doi: 10.1046/j.1460-9568.2003.02716.x. [DOI] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48(2):148–55. [PubMed] [Google Scholar]
- Kravariti E, Morgan K, Fearon P, Zanelli JW, Lappin JM, Dazzan P, Morgan C, Doody GA, Harrison G, Jones PB, Murray RM, Reichenberg A. Neuropsychological functioning in first-episode schizophrenia. Br J Psychiatry. 2009;195(4):336–45. doi: 10.1192/bjp.bp.108.055590. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: Evidence for a final common pathway in Schizophrenia? Schizophr Bull. 2007;33(4):971–81. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals role of receptors and second messengers. Trends Neurosci. 1993;16:233–40. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008 May;16(5):614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu Rev Psychol. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–59. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–74. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Levy F, Krebs PR. Cortical-subcortical re-entrant circuits and recurrent behaviour. Aust NZJ Psychiatry. 2006;40(9):752–58. doi: 10.1080/j.1440-1614.2006.01879.x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Todd RM. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Develop. 2007;22(4):406–30. [Google Scholar]
- Lezak MD. The problem of assessing executive functions. Internat J Psychol. 1982;17:281–97. [Google Scholar]
- Lucey JV, Burness CE, Costa DC, Gacinovic S, Pilowsky LS, Ell PJ, Marks IM, Kerwin RW. Wisconsin Card Sorting Task (WCST) errors and cerebral blood flow in obsessive-compulsive disorder (OCD) Br J Med Psychol. 1997;70:403–411. doi: 10.1111/j.2044-8341.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Tobias-Webb J. Effects of acute alcohol consumption on executive cognitive functioning in naturalistic settings. Addict Behav. 2010;35:1021–1028. doi: 10.1016/j.addbeh.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Lux WE. A neuropsychiatric perspective on traumatic brain injury. J Rehabil Res Dev. 2007;44(7):951–62. doi: 10.1682/jrrd.2007.01.0009. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Cervenka S, Farde L, Nyberg L, Bäckman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia. 2009;47:2299–304. doi: 10.1016/j.neuropsychologia.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Macqueen BD, Pachalska M, Sniegocki M, Lukowicz M, Pufal A. An evaluation of executive functions in sportsmen after traumatic brain injury. Ortop Traumatol Rehabil. 2003;5(6):767–80. [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Markett SA, Montag C, Reuter M. The Association between dopamine DRD2 polymorphisms and working memory capacity Is modulated by a functional polymorphism on the nicotinic receptor gene CHRNA4. J Cogn Neurosci. 2010;22:1944–54. doi: 10.1162/jocn.2009.21354. [DOI] [PubMed] [Google Scholar]
- Mateer CA. Executive function disorders: rehabilitation challenges and strategies. Semin Clin Neuropsychiatry. 1999;4:50–9. doi: 10.1053/SCNP00400050. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6(4):358–70. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DE, Dalley JW, Robbins TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. In: Psychopharmacology (Berl) 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, Miller BL, Boxer AL. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol. 2011;24:50–8. doi: 10.1097/WNN.0b013e318223f6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzijak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Muly EC, 3rd, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, von Cramon DY, Pollmann S. D1- versus D2- receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, McNamara P, Freedman M. Delayed-response tasks: Parallels between experimental ablation studies and findings in patients with frontal lesions. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Injury. New York: Oxford University Press; 1991. pp. 231–255. [Google Scholar]
- O’Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: Representational organization and neuromodulatory control. Cerebral Cortex. 2002;12(3):246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psych Rev. 2001;108:311–45. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. Amygdala and emotional modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 2004;82:243–52. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacol. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pinto E, Ansseau M. Genetic factors of alcohol dependence. Encephale. 2009 Oct;35(5):461–469. doi: 10.1016/j.encep.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- Robbins TW. From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, Hopwood A, Wallace C, Deakin JF, Sahakian BJ, Robbins TW. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacol (Berl) 1999b;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacol. 1999c;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between Small, Likely Rewards and Large, Unlikely Rewards Activates Inferior and Orbital Prefrontal Cortex. J Neurosci. 1999a;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Fazio L, Taurisano P, Gelao B, Porcelli A, Mancini M, Sinibaldi L, Ursini G, Masellis R, Caforio G, Di Giorgio A, Niccoli-Asabella A, Popolizio T, Blasi G, Bertolino A. DRD2 Genotype-Based Variation of Default Mode Network Activity and of Its Relationship with Striatal DAT Binding. Schizophr Bull. 2011 Oct 5; doi: 10.1093/schbul/sbr128. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Effects of dopamine antagonists on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, King SW, Bressler CN, Watson SJ, Lewis DA. Electron microscopic visualization of dopamine D2 receptors in the forebrain: cellular, regional, and species comparisons. Soc Neurosci Abstr. 1995;21:365. [Google Scholar]
- Semkovska M, Bedard MA, Stip E. Hypofrontality and negative symptoms in schizophrenia: synthesis of anatomic and neuropsychological knowledge and ecological perspectives. Encephale. 2001;27:405–415. [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shue KL, Douglas VI. Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain Cogn. 1992;20:104–124. doi: 10.1016/0278-2626(92)90064-s. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Ince PG, Higham CE, Gelsthorpe CH, Fernando MS, Matthews F, Forster G, O’Brien JT, Barber R, Kalaria RN, Brayne C, Shaw PJ, Stoeber K, Williams GH, Lewis CE, Wharton SB. MRC Cognitive Function and Ageing Neuropathology Study Group. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol Appl Neurobiol. 2007;33(6):670–83. doi: 10.1111/j.1365-2990.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex. 1993;3:223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, Dickerson BC, Viswanathan A, Albert MS, Blacker D, Greenberg SM. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492–9. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23:S28–33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Soubrié P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci. 1986;9:319–364. [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Effects of dopamine-related gene-gene interactions on working memory component processes. Eur J Neurosci. 2009;29:1056–63. doi: 10.1111/j.1460-9568.2009.06647.x. [DOI] [PubMed] [Google Scholar]
- Stroth S, Reinhardt RK, Thöne J, Hille K, Schneider M, Härtel S, Weidemann W, Bös K, Spitzer M. Impact of aerobic exercise training on cognitive functions and affect associated to the COMT polymorphism in young adults. Neurobiol Learn Mem. 2010;94:364–72. doi: 10.1016/j.nlm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychological Res. 2000;63(3–4):289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- Sundaram SK, Huq AM, Wilson BJ, Chugani HT. Tourette syndrome is associated with recurrent exonic copy number variants. Neurology. 2010 May;74(20):1583–1590. doi: 10.1212/WNL.0b013e3181e0f147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Hayashi M, Okubo Y, Takano A, Ito H, Suhara T. Memory and frontal lobe functions; possible relations with dopamine D2 receptors in the hippocampus. Neuroimage. 2007;34:1643–1649. doi: 10.1016/j.neuroimage.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21:1045–54. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Teffer K, Semendeferi K. Human prefrontal cortex Evolution, development, and pathology. Prog Brain Res. 2012;195:191–218. doi: 10.1016/B978-0-444-53860-4.00009-X. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Dev Disabil Res Rev. 2008;14(4):261–7. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brainimmunocytochemical study. J Physiol Pharmacol. 2001;51:205–221. [PubMed] [Google Scholar]
- Williams LE, Bargh JA, Nocera CC, Gray JR. The unconscious regulation of emotion: nonconscious reappraisal goals modulate emotional reactivity. Emotion. 2009a;9(6):847–5. doi: 10.1037/a0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual Differences in Executive Functioning: Implications for Stress Regulation. Annals of Behavioral Medicine. 2009b;37(2):126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xu TX, Sotnikova TD, Liang C, Zhang J, Jung JU, Spealman RD, Gainetdinov RR, Yao WD. Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J Neurosci. 2009;29:14086–99. doi: 10.1523/JNEUROSCI.0974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–7. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]