Abstract
Background
This study sought to determine the efficacy of Botulinum toxin A (BTA) for the treatment of hyperkinetic lines of the face. Twenty three patients who were concerned for facial wrinkles and desiring correction are presented.
Methods
This clinical study evaluated the postoperative results of 23 patients who underwent treatment for facial wrinkles with BTA. Among the patients included in the study, 20 were males and remaining 3 were females. The age of the patients ranged from 27 to 46 years (mean 34.69 years) and the treatment was done in three different sessions and divided into 3 treatment subgroups of forehead, crow’s feet, and glabellar wrinkles.
Results
All the patients were followed up for a period of at least 6 months and graded for the response to treatment with BTA by the operator, observer and the patient independently using the facial wrinkle scale. The patient’s satisfaction to the treatment was also noted on all the follow-up visits on the satisfaction scale and the results were subjected to statistical analysis using Kappa analysis, Chi-square test and T test. The results showed that the treatment of facial hyperkinetic lines with BTA is associated with few adverse events like pain on injection, transient headache, and mild change in facial appearance in subjects with high hair line which are not serious and thus safe.
Conclusion
The findings of this study support the use of BTA for the treatment of hyperkinetic lines of the face although further studies with more sample size are required.
Keywords: Hyperkinetic lines, Face, Botulinum toxin A
Introduction
Hyper dynamic/long term facial animation seems to contribute to the cause of many undesired facial rhytides and furrows and to the development of soft tissue ptosis in many facial regions. Facial lines are caused by a number of different factors including intrinsic aging, sun exposure, gravity, and hyperactivity of underlying facial muscles [1]. This can occur naturally over time and is identified by certain biochemical, histological and physiologic changes that are enhanced by environmental exposure [1].
Numerous procedures designed to treat hyper functional facial lines namely, rhytidectomy, liposuction, brow lift, dermabrasion, chemical peel, collagen injections do not address the underlying problems and are associated with various complications. In contrast Botulinum toxin acts at the neuromuscular junction by irreversibly inhibiting the release of acetylcholine. Cosmetic denervation of the hyper functional musculature using Botulinum toxin has gained growing popularity over the years [2].
The arena of cosmetic surgery of the aging face has progressed from the knife to the needle. Botulinum toxin A (BTA) has been safely and effectively used for the treatment of various disorders, including cosmetic facial surgery for more than a decade in a safe and effective manner. Over the years there has been an increasing array of uses of Botulinum toxin in cosmetic facial surgery. Glabellar frown lines, forehead furrows, crow’s feet, and other muscle groups, which are a result of pull on skin by underlying facial mimetic musculature; have been treated with this toxin. Historically treatment has been aimed at repairing the cutaneous defect beneath the lines via soft tissue augmentation; these do not address the underlying musculature that causes wrinkles. This study is an attempt to treat the dynamic facial lines with BTA, thus addressing the underlying cause [2].
Materials and Methods
This clinical study was done on patients concerned for facial wrinkles and desiring correction. The patients were screened for their suitability for the study based on the inclusion and exclusion criteria. A detailed clinical format to note patients concern and expectations from the treatment, any condition which would interfere with the action of the drug and systemic health was utilized. The study design, the study purpose, and the potential risks of participation were discussed with each patient before enrollment, and written informed consent was obtained.
Inclusion Criteria
Patients presenting with wrinkles of the upper third of face (glabella, forehead, or crows’ feet) desiring treatment of the same.
Patients could have undergone prior Botulinum toxin treatment, provided they met the severity requirement.
There was no minimal severity requirement for resting appearance of these lines.
Patients were also required to complete the study and comply with study instructions.
Exclusion Criteria
History of neuromuscular disorder: Myasthenia gravis, Eaton–Lambert syndrome etc.
Reported allergy to components of the drug.
On medications like aminoglycosides, quinine, penicillamine and calcium channel blockers that would interfere with the neuromuscular function.
Pregnancy, lactation during the study period.
Patients with systemic disorders like hypertension, diabetes mellitus, heart diseases etc.
Any other condition or situation existed that would put the patient at significant risk.
Psychologically unstable patients or who have unrealistic goals and reasons.
Patients could be removed from the study at anytime because of inability to continue to follow up monitoring, or withdrawal of consent for a minimum period of 6 months.
All patients were to continue standard facial skin care regimen, throughout the study, but no other facial procedures were permitted during the study. The patients were photographed preoperatively, intraoperatively and at each follow up visit. Patients were photographed anteriorly at rest and maximal contraction of the concerned wrinkle group for the forehead and glabella group and bilaterally for the crows feet group.
Each vial of Botulinum toxin type A (Fig. 1) (Botox; Allergan, Inc., Irvine, CA) contains 100 U of Botulinum toxin, 0.5 mg of albumin, and 0.9 mg of sodium chloride, in a sterile vacuum dried form, without preservatives. All vials were reconstituted with 2.5 ml of 0.9 % sterile non preserved saline solution, yielding a final dilution of 40 U/ml of Botulinum toxin. 2.7 ml of non-preserved saline was used for reconstitution to account for the loss of saline (0.2 ml) in the hub of the needle. Care was taken to prevent frothing of the reconstituted drug to prevent any deactivation or denaturation. Once reconstituted the toxin was used as quickly as possible. The package insert suggests that the product should be used within 4 h.
Fig. 1.
Photograph showing Botulinum toxin type A used in the study
Patients were selected for the treatment procedure if they fulfilled the inclusion criteria. All patients signed a consent form, which allowed for the use of pictures to evaluate the treatment. On day 0 patients received intramuscular injections of BTA (Botox) for the correction of desired rhytide. The patients were evaluated based on the facial wrinkle scale as interpreted by the patient, operator and third person (observer) to prevent any bias. The skin of the areas to be treated is prepared with alcohol scrub and the patient is treated in the upright sitting position. In order to reduce the pain on injection ice can be applied to the operative site prior to the alcohol scrub and injection of the toxin and BD insulin syringe was used. Injections were administered at glabellar wrinkles, forehead wrinkles and bilaterally for the crows feet wrinkles as an outpatient procedure with no anesthesia.
Glabellar Wrinkles
The Glabellar marks are made 1 cm superior to the orbital rim for brow injections. The medial brow injection marks are made on an imaginary line from level of medial canthus superiorly for the corrugator and medial orbicularis injections, and the superolateral marks above the supraorbital notch not beyond the midpupillary line for the lateral corrugator and orbicularis. Finally, a mark is made in the midnasal area on the intersection of an imaginary “X” that connects the medial eyebrow to the contra lateral medial canthus. This is used for the procerus injection. The entire crux of the treatment is to place the BTA in proximity to the proper musculature without affecting the neighboring musculature, especially the levator palpebre and the extra ocular muscles. The muscles are then injected with 2 units of Botox using the predetermined injection marks and following technique. Asking the patient to scowl flexes the corrugator’s musculature and the resultant skin bulge can be palpated. First, a 0.5-in, 30-gauge needle is inserted at the medial brow landmark through the skin perpendicularly to approximately one half to three fourths of the needle depth on both sides. In theory, this should be in the corrugator supercili muscle and 0.05 cc is injected. The next point of injection is at the superolateral markings bilaterally. The final injection for the Glabellar region is given in the center of the procerus muscle by injecting on the “X” mark between the eyes. The muscle bulk to be injected can be pinched or the patient can be asked to animate the muscle to reduce the pain of injection. The needle is inserted into the nasal bridge to one half of its length and 0.05 cc is injected (Fig. 2).
Fig. 2.
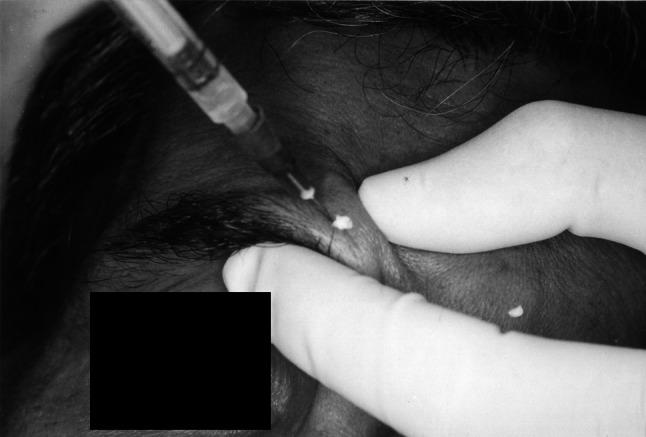
Injection of Botulinum toxin of markings placed on wrinkles formed in the glabellar region
Forehead Wrinkles
The patient is asked to raise eyebrows and the skin bulges are noted. The numbers of injections to be given depends on the extent of the wrinkle and severity and are marked. 0.05 cc per aliquot are injected equally spaced at 1.5–2 cm. (extent of diffusion of the toxin around the site of injection) from each other over the entire forehead not extending beyond the imaginary midpupillary line laterally. Only the most superior folds are treated, which allows the inferior portion of the frontalis to maintain function and enable raising the eyebrows. The lower most horizontal line or inferior most frontalis fibers are spared to prevent ptosis (Fig. 3).
Fig. 3.
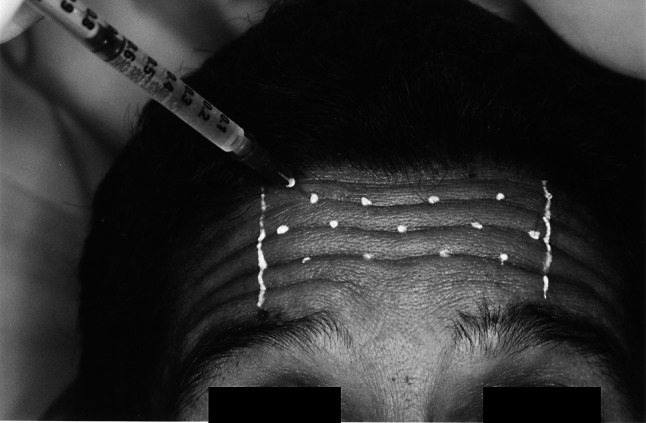
Injection of Botulinum toxin of markings placed on wrinkles formed in the forehead region
Crow’s Feet Wrinkles
Three injections were used in each lateral canthal area on each side of the face, at least 1 cm away from the bony orbital rim or 1.5 cm from the lateral canthus. Medial injection to this point has a risk of affection of the lateral rectus and thus causing diplopia. The superior site was at the lateral sub eyebrow (avoiding the inferior fibers of frontalis and brow ptosis) and the inferior injection was above the zygomaticus major (at the zygoma), which reduces the risk of potential lid droop. The middle injection was placed at the level of ocular canthus equidistant between the adjacent sites: 1 cm beneath the superior site and 1 cm above the inferior site. As the muscle is superficial and lies directly beneath the skin, without significant adiposity, superficial placement in sub dermal blebs ensures that the toxin is adequately exposed to the muscle and reduces the risk of intravascular injection and toxin hemodilution (Fig. 4).
Fig. 4.
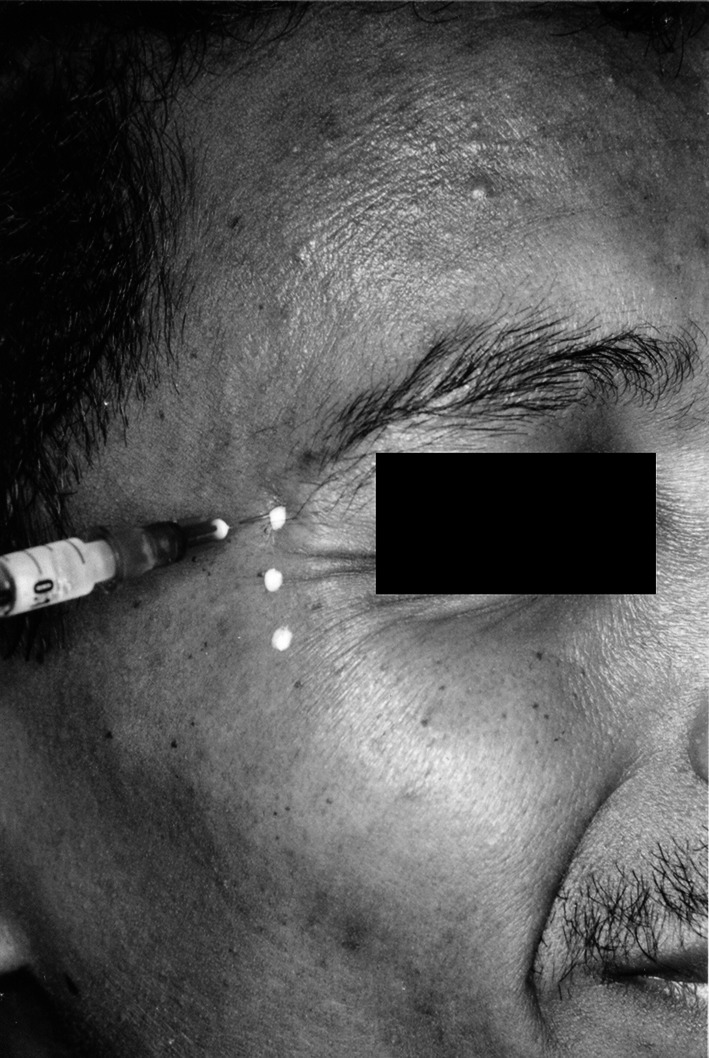
Injection of Botulinum toxin of markings placed on wrinkles formed in the region of crow’s feet
Patients were evaluated on pretreatment visit, onset, 7, 30, 60, 90, 120, 150 and 180 days for reduction in severity of the wrinkles using the facial wrinkle scale by the patient, the operator, and a trained observer (Figs. 5, 6, 7) Post treatment the patients were assessed for satisfaction of outcome following the treatment on a patient satisfaction scale at every follow up visit. The patients were monitored during, immediately after, and during the postoperative follow up for any adverse events. Adverse events were rated with respect to severity (mild, moderate, and severe), relation to treatment (none, possible, probable, or definite).
Fig. 5.
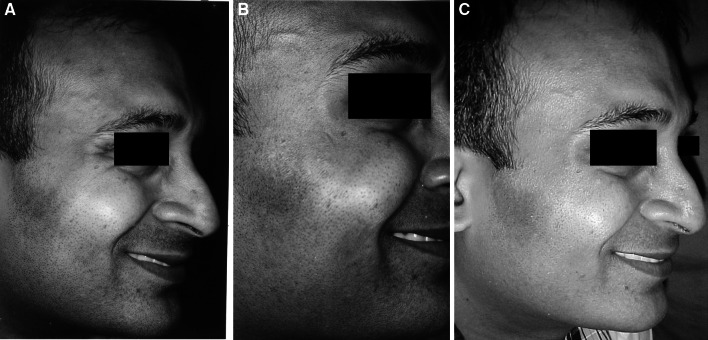
a Photograph showing crow’s feet wrinkles 1 week after injection of Botulinum toxin. b Photograph showing crow’s feet wrinkles 1 month after injection of Botulinum toxin. c Photograph showing crow’s feet wrinkles 6 months after injection of Botulinum toxin
Fig. 6.
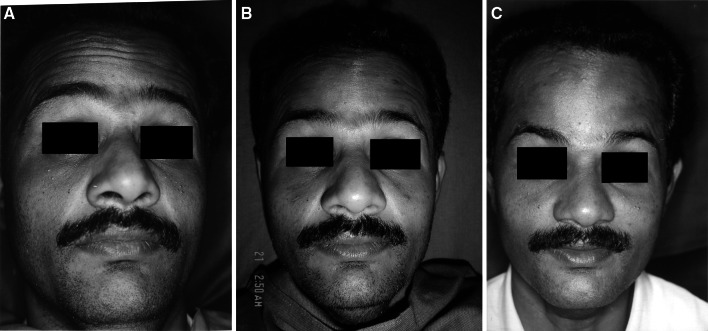
a Photograph showing forehead wrinkles 1 week after injection of Botulinum toxin. b Photograph showing forehead wrinkles 1 month after injection of Botulinum toxin. c Photograph showing forehead wrinkles 6 months after injection of Botulinum toxin
Fig. 7.
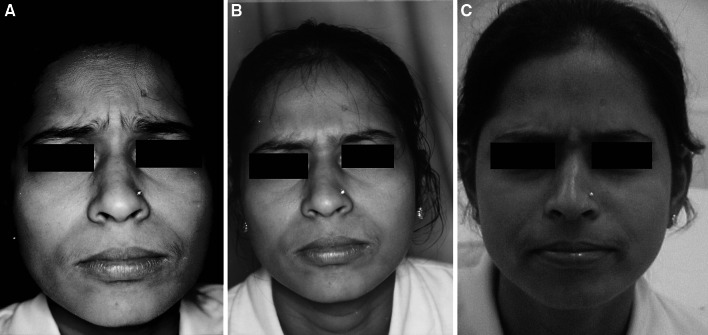
a Photograph showing glabellar wrinkles 1 week after injection of Botulinum toxin. b Photograph showing glabellar wrinkles 1 month after injection of Botulinum toxin. c Photograph showing glabellar wrinkles 6 months after injection of Botulinum toxin
Results
A total of 23 patients were enrolled and included in the study. Three patients (two in the glabella group and one in the forehead group) were lost to follow up during the study. The remaining 20 patients completed a minimum required follow up period of 6 months. Among the patients included in the study, 20 were males and remaining 3 were females. The age of the patients ranged from 27 to 46 years (average 34.69 years) and the treatment was done in three sessions.
The subjects were divided into 3 treatment subgroups of forehead, crow’s feet, and glabellar wrinkles. All the patients were followed up for a period of at least 6 months and graded for the response to treatment with BTA by the operator, observer and the patient independently using the facial wrinkle scale. The patient’s satisfaction to the treatment was also noted on all the follow-up visits on the satisfaction scale. Data was collected so as to record the efficacy and safety of the drug in each subgroup. The adverse effects to the treatment were also noted at every follow up visit.
The mean duration of onset of action was 1–6 days (mean 3 days). At the end of the study period of 6 months, the tabulated results were statistically analyzed to find out the significance between the operator, observer, and the patients self-assessment scores at relaxation and maximal contraction at each follow up visit. Kappa analysis and Chi-square test were used. According to the Chi square test, no significant difference between the three assessment scales was noted and thus all the scales were in agreement with the severity assessment of the subjects at every follow up visit. As per the Kappa analysis, all the values of comparison were less than two (0–0.15) thus showing good agreement between the assessment scores.
To define the degree of change in severity compared to the pretreatment baseline values at relaxation and contraction paired sample T test was employed to detect the change in mean differences. This revealed the change to be very highly significant with p values (P < 0.001) at all follow up visits in the relaxation scores. The paired sample test revealed very highly significant P values (P < 0.001) in the contraction scores except at 180 days scores (0.008, 0.009, 0.004) according to the patient, operator and observer assessment scores, which were highly significant. This infers that the difference in mean improvement of the severity compared to the baseline pretreatment values was very highly significant (P < 0.001) up to 180 days post treatment in the relaxation scores, and up to 150 days in the contraction scores. The mean difference in the severity scores at contraction at 180 days was highly significant thus showing slight decrease in the effect of the reduction of severity at 180 days. As, the scores at the end of 180 days were significant, it can be concluded that the toxin was successful in reducing the severity of wrinkles even at 180 days.
Efficacy Results of Individual Treatment Groups
Forehead Group
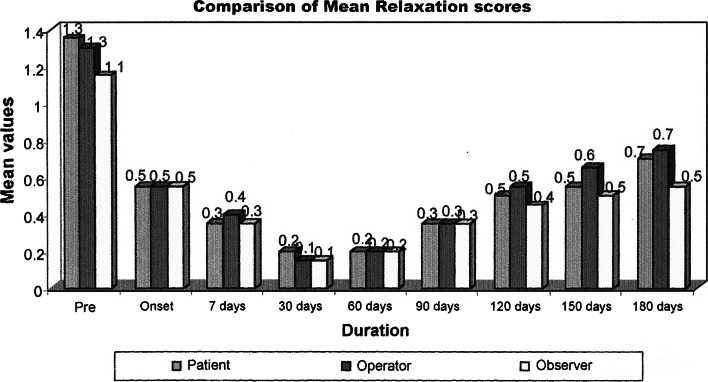
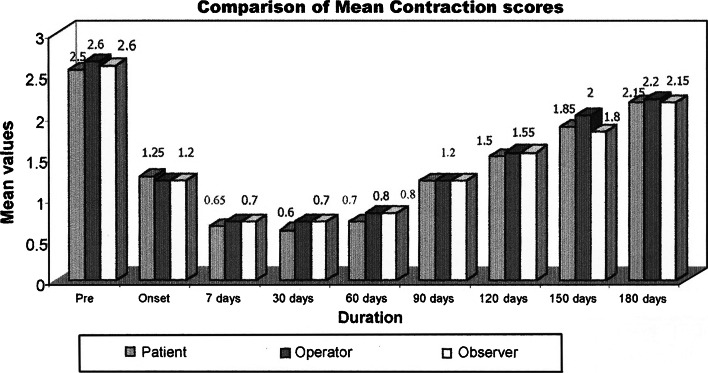
Seven patients were treated for their concern of the forehead wrinkles. All except one patient completed the study. Patients in the forehead group showed improvement in the mean wrinkle severity scores after treatment when compared to the pretreatment severity scores at rest, at maximal animation. The mean pretreatment scores in this group according to observers, operator and patient assessment scale were 1.33, 1.5, and 1.33 at relaxation 2.5, 2.66, and 2.5 at maximal contraction. The peak reduction in the mean severity scores was 0.33 seen at 7 days post treatment at relaxation and 30 days post treatment at maximal contraction. The same at the end of 6 months was 0.50, 0.50, and 0.33 as per the observer, operator and patient assessment at relaxation and 1.66 at maximal contraction. This showed a significant reduction in the severity of the wrinkles up to 6 months after treatment with BTA. (Graphs 1, 2, 3, 4).
Graph 1.
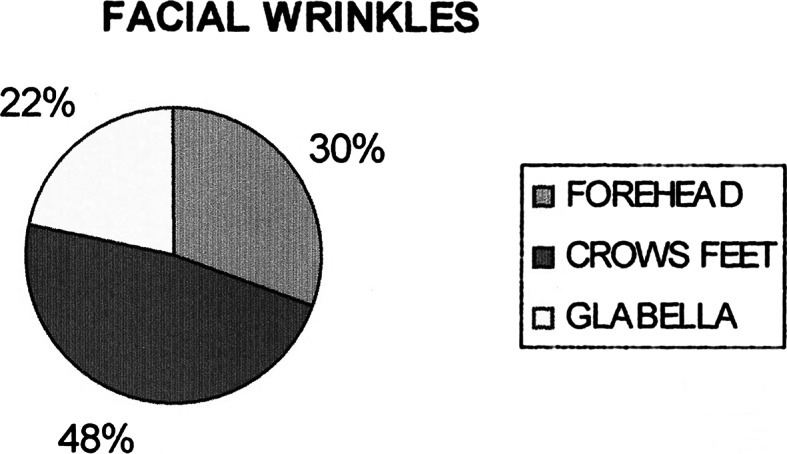
Distribution of facial wrinkles
Graph 2.
Comparison of mean relaxation scores
Graph 3.
Comparison of mean contraction scores
Graph 4.
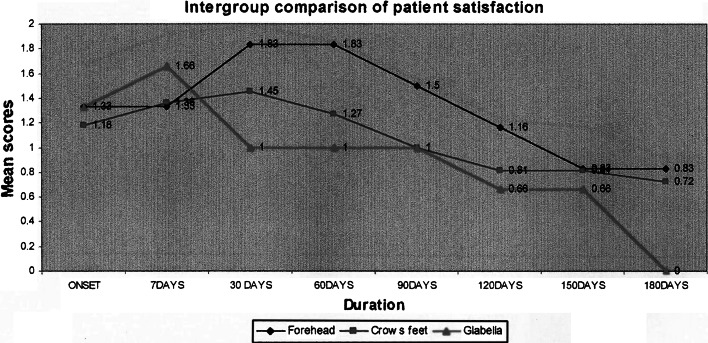
Intergroup comparison of patient satisfaction
Crow’s Feet Group
Eleven patients were treated for their concern of the crow’s feet wrinkles. All the patients in this group completed the study. The observer, operator and patient’s assessment mean pretreatment severity scores were 1.16, 1.27, and 1.45 at relaxation and 2.72, 2.72, and 2.63 at maximal contraction. The peak reduction in the observer and operator assessment mean severity scores was 0.09 at 30 days and at 60 days for patient assessment scores at relaxation and 0.72, 0.72, and 0.63 at 7 days post treatment at maximal contraction. The same at the end of 6 months was 0.63, 0.81, and 0.72 observer, operator and patient’s assessment at relaxation and 2.36, 2.36, and 2.27 at maximal contraction. This was lower than the pretreatment severity scores, thus indicating reduction in wrinkle severity.
Glabellar Group
Five patients were treated for their concern of the glabellar wrinkles. Two patients were lost to follow up and thus were excluded from the study. The mean pretreatment observer, operator assessment and patient assessment severity scores were 0.33, 1, 1.45 at relaxation and 2.33, 2.66, 2.63 at maximal contraction. The peak reduction in the observer, operator and patient assessment mean severity scores was 0, 0, 0.09 at 30, 30, 60 days at relaxation and 0.72, 0.72, and 0.63 at 7 days post injection at maximal contraction. The same at the end of 6 months period had returned to the baseline as noted at preinjection period. No further increase in severity of glabellar wrinkles was noted.
The overall response to the treatment of hyperkinetic lines of the upper third of the face was calculated by an overall assessment of mean scores by the patient, operator and the observer. The overall mean score pretreatment was 1.35, 1.30, and 1.15 at relaxation and 2.55, 2.65, and 2.60 at contraction. The peak reduction in mean severity scores was 0.20, 0.15, and 0.15 at 30 days at relaxation and 0.60, 0.70, and 0.70 at 30 days, 7 days, and 7 days post injection. The same peak effect was maintained up to 30 days post injection. The mean severity scores as per patient, operator and observer assessment at the end of 180 days were 0.70, 0.75, and 0.55 at relaxation and 2.15, 2.20 and 2.15 at maximal contraction The mean scores at the end of 180 days were lesser than that at the pretreatment period infers that the effect of treatment was persistent at the end of 180 days (6 months).
Patient Satisfaction Results
The patient satisfaction for the treatment was graded on a patient satisfaction scale at every follow up visit. The peak value for patient satisfaction was noted to be at 30 days for the forehead and crow’s feet group and at 7 days post treatment for the glabellar group. The overall peak satisfaction of the patients to this treatment was noted at 30 days. The mean values depicting the patient’s satisfaction to treatment at the end of 6 months did not touch ‘0’ except for the glabellar wrinkle group. This indicates the high satisfaction in relief of wrinkles following the treatment. Adverse events if any were noted at the follow up visit and classified as per the severity and their relation to the treatment procedure noted. Eight patients (32 %) reported no adverse events. The commonest complaint was local pain on injection. This was encountered in 11 patients (50 %). Two (9 %) patients reported with mild headache. Two patients (9 %) reported with a complaint of altered facial appearance of increased width of the forehead.
Botulinum toxin A treatment was well tolerated and no patient discontinued from either treatment groups because of adverse events. These results show that the treatment of facial hyperkinetic lines with BTA is associated with few adverse events like pain on injection, transient headache, and mild change in facial appearance in subjects with high hair line which are not serious and thus safe.
Discussion
Because of the differences in skin thickness and texture, Asians generally have faces with fewer fine rhytides and less laxity compared with Caucasians. Thicker dermis along with more collagen fiber explains lower incidence of finer wrinkles in Asians, whereas firmer attachment of the skin to the underlying tissues accounts for decreased laxity. For, these reasons, the chronic repetitive pulling by mimetic muscles on the skin is also expected to effect the facial wrinkles on the Asians differently.
The 100 U of the BTA in the vial used in this study was diluted with 2.5 ml of unpreserved saline solution, yielding a final dilution of 40 U/ml of Botulinum toxin as used by Klein, Carruthers et al. [1–3] and Somer et al. [4] 2.7 ml of non-preserved saline was used for reconstitution to account for the loss of saline (0.2 ml) in the hub of the needle as stated by Niamtu III [5].Various dilution ratios have been suggested in the previous studies ranging from 1 to 12 ml. The smaller volume, via capillary action, leaves a coating on the bottle that prevents the entire reconstituted drug from being drawn up in syringes, thus causing wastage of the drug and results in unworkable small amounts as reported by Niamtu III and Klien [1, 5]. Larger volumes of injections however are possibly associated with more discomfort and diffusion of the drug well beyond the area that is injected as reported by Niamtu III [5, 6], Hankins et al. [7] and Bendetto[8]. The drug once reconstituted can be drawn in a syringe with 30 gauge needle for injections. The Ultra fine II short needle was used due to its reported advantages like the unit dosing scale which is easy to interpret, causes minimal pain of injection, minimizes the dead space and thus the wastage of Botox as reported Carruthers et al. [9] and Flynn et al. [10].
The number of injection sites was decided based on the severity of the wrinkles. Due to anatomic variance, each patient presents a unique combination of dynamic rhytids on animation, thus the injections are tailored to that specific anatomy. Prior to the injection the site of injection was scrubbed with alcohol scrub, followed by chilling the site with ice. The sites of injection were marked with antiseptic ointment. The markings were not made with ink marker in order to prevent tattooing of the site of injection by the ink. The skin was penetrated vertically into the concerned muscle group. Some clinicians on the other hand utilize the tangential injection technique. Two units of the drug were injected per prick and the syringe changed after three to four injections to prevent pain of injection due to blunting of the needle tip.In the forehead, the drug was injected at least 1 cm above the eyebrow sparing the lower most wrinkle to prevent the chance of untoward diffusion into the upper eyelid causing ptosis and preserve the movement of the eyebrows as reported by Carruthers et al. [11], Niamtu III [6], Matarasso et al. [12] and Binder et al. [13]. Laterally, the drug was injected not beyond the imaginary midpupillary line to prevent lateral brow ptosis. The drug was distributed over the forehead by maintaining a distance of 1.5–2 cm between each injection site to allow for the diffusion of the drug. Following the injection upward pressure was applied in this region to prevent downward diffusion of the toxin.
The injections in the lateral orbital region for the crow’s feet were given at least 1 cm away from the lateral canthus or at least 1.5 cm lateral to the bony lateral orbital rim as described by Carruthers et al. [11], Matarasso [12] and Sposito [14]. Each lateral orbital region received 6 U at three sites on each side accounting to a total dose of 12 units. Following the procedure lateral pressure was applied to prevent untoward effects due to diffusion of the drug. None of the subjects were injected in the infraorbital region to prevent lower lid ptosis, and dryness of eyes as reported by Flynn et al. [15] and festooning as reported by Goldman et al. [16].
In the glabellar region the drug was injected at 5 sites as described by Hankins et al. [7] and Scott et al. [17]. Each muscle received a minimum effective dose of 2U accounting to a total of 10 U. Various doses have been employed to treat the glabellar lines ranging from 2 U to 7.5U per muscle group based on the severity of the wrinkles, but there have been reports that higher doses may not be necessary to treat majority of the patients with glabellar lines. The glabellar wrinkles have been reported to be the most difficult to treat and obtain consistent results. EMG guided injection in this complex muscle group has been advocated by Pribtkin et al. [18]. Following the procedure upward and outward pressure was applied. In order to reduce the pain on injection which, though minimal can be reduced by chilling the area with ice before injection. The ice was wrapped in a sterile glove to maintain the sterility of the field after the alcohol scrub. The use of ice balloons, topical EMLA creams, reconstitution with preserved saline and lignocaine, use of ultra fine insulin needles, and pinching the muscle bulk has also been recommended by various clinicians. Following injection of the toxin, manual pressure was applied at the injection site to prevent diffusion of the drug in unwanted muscle groups to prevent untoward paralytic effects and to prevent bruising. The subjects, to be included in the study should have completed the follow up for a minimum of 6 months as the duration of the effect has been reported to be ranging from 3 to 6 months as per previous studies. Patients were evaluated by the operator, a trained observer and the patient himself/herself based on the facial wrinkle scale at rest and at maximal contraction at the preoperative visit, and every day until the onset of action. The patient satisfaction was evaluated at all the follow up visits based on the patient satisfaction scale for a period of 6 months. At the end of 6 months the tabulated data was statistically analyzed to determine the significance between the operator, observer, and the patient’s self-assessment scores at relaxation and maximal contraction at each follow up visit utilizing the Kappa analysis and Chi-square test.
At every follow up visit, patients treated with BTX-A showed a significantly greater improvement in the severity of facial wrinkles. The safety profile was also excellent. These findings are consistent with previous reports in which BTX-A treatment consistently and safely reduced the severity. These findings are consistent with previous reports in which BTX-A treatment consistently, and safely, reduced the severity of facial lines. The drug was used on the same day of reconstitution to preserve the efficacy of the drug. Though, reports by Fulton et al. [19] say that the efficacy of the reconstituted toxin is unchanged until 30 days.
By day 7, post injection for every efficacy variable, the magnitude of improvement was only slightly less than the peak effect, which was seen at day 30. In previous studies, it has been reported that the effects of BTX-A treatment are typically seen within the first 24–72 h after injection [17, 19]. Thus, in order to detect the day of onset post injection the patients were recalled every day until the response was noted. It was, noted that the onset of action in the subjects was ranging from 1 to 6 days. (mean 3 days). This result is in consent with the studies done by Fulton et al. [19], Young et al. [20], Lew et al. [21] and Clay et al. [22] who reported that the toxin shows its effect within 3–5 days. Heckmann et al. [23] insisted that this longer duration can be due to the fact that the radial diffusion and uptake into relatively larger facial muscles continue over a much longer period than 5 days.
The effect in all variable groups peaked by 30 days and maintained the same until 60 days post treatment. This was similar with the study by Carruthers et al. [15]. However the effects of treatment were clinically apparent even 180 days post treatment. This was noted by the mean value being considerably lower than the pretreatment value. This suggests that at least some of the patients might have continued to benefit for more than 6 months after treatment as seen in previous studies reported by Hankins et al. [7], Lew et al. [21]. Another observation made was that the improvement in mean scores at relaxation at the end of 6 months was sustained too much lesser value than the pretreatment scores compared to that at maximal animation. This is in consistent with the results of the study by Carruthers et al. [15, 16] and Clay et al. [22].
Although the study was not designed to measure the duration of the effect of Botulinum toxin-A treatment of facial wrinkles, the results suggest that it is similar to the 3–6 months reported for other indications treated with BTX-A as reported by Bendetto [10] and Hankins et al. [24]. In the clinical use of BTX-A, across indications, the duration of effect is commonly defined as the time during which patients believe that their treatment goals are being met. At every post operative follow up the patients satisfaction scale was used to note patient’s response to treatment. The peak value for patient satisfaction was noted to be at 30 days for the forehead and crow’s feet group. The peak value for patient satisfaction scores in the glabella group was 7 days post treatment. This was consistent with the previous studies of Sadick et al. [24]. The overall peak satisfaction of the patients to this treatment was noted at 30 days. This result was consistent with previous studies conducted by Sadick et al. [25]. The patient satisfaction scores for the treatment subgroups except the glabellar group were above the “0” mark showing the satisfaction of the patients to the treatment was maintained beyond 180 days. Some patients were still satisfied with the treatment effect even when the severity assessment scores had reached the pretreatment baseline values. This shows that the effects of Botulinum toxin treatment seemed to be sustained longer than the effect observed at maximal attempted frown. Similar results were reported by Carruthers et al. who suggested that the cosmetic effects of Botulinum toxin treatment last longer than the direct muscle weakening action of the drug. They hypothesized the effect of dermal remodeling and a role of behaviour modification to explain the retention of the treatment benefits in resting appearance while preserving functionality and natural facial animation.
Adverse side effects include injection site pain, edema, and ecchymosis occur with varying frequencies in a large minority of patients; although their occurrence is, in part, technique-dependent, they should not be considered complications. Headaches, dry mouth sensation, and flu-like mild malaise can also occur after Botox injections. Patients who are on aspirin; anticoagulant medications (such as warfarin); nonsteroidal anti-inflammatory drugs; vitamin E; herbal remedies, like ginseng, ginkgo, and high doses of garlic; may experience higher rates of bruising. Botox injections can cause unintended side effects either from improper placement of injections or from localized diffusion of injected Botox into functionally important muscle fibers [24, 25].
Local complications like lid ptosis, ectropion, pseudoherniation of infraorbital fat pad, lagophthalmos, strabismus and brow malposition can occur when treating patients for facial rejuvenation [24, 25].
The safety profile of the drug in this study was excellent, which is consistent with the long history of the safe use of BTA for a variety of conditions. Most of the adverse events were mild and included pain on injection, head ache, and alteration in facial expression. Pain on injection was mild and immediate after the injection in all the patients and was not related to the drug but was associated to the insertion of the needle. This was considerably reduced by local cold application, and the use of ultra-fine BD syringes to inject the drug. Two (9 %) patients reported with mild headache. This was not attributed to the injected drug.
Two patients (9 %) reported with a complaint of altered facial appearance of increased width of the forehead. This was reported to be mild and was attributed to the relative inability of the patients to frown. Patients with this complaint had a high hair line (baldness), which could have been the possible cause of the condition, as this was not complained by any other subject in the forehead group. Patient 10 reported that his friends had found him appear more happy and satisfied after his treatment for crow’s feet. Further long-term studies of patients’ responses to BTX-A treatment are necessary to fully define the duration of effect for this indication.
Conflict of Interest
None.
References
- 1.Klien AW. Dilution and storage of botulinum toxin. Dermtol Surg. 1998;24:1179–1180. doi: 10.1016/S1076-0512(98)00178-2. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers JA, Lowe NJ, Menter A, Gibson J, Nordquist M, Mordaunt J, Wexler P, Eadie N. Double blind, placebo: controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112:1089–1098. doi: 10.1097/01.PRS.0000076504.79727.62. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, Wexler P, Eadie N. A multicenter, double blind, randomized placebo: controlled study of the efficacy and safety of botulinum toxin type A in the treatment glabellar lines. J Am Acad Dermatol. 2002;46(6):840–849. doi: 10.1067/mjd.2002.121356. [DOI] [PubMed] [Google Scholar]
- 4.Sommer B, Zschoke I, Bergfeld D, Sattler G, Augustin M. Satisfaction of patients after treatment with botulinum toxin for Dynamic facial lines. Dermatol Surg. 2003;29:456–460. doi: 10.1046/j.1524-4725.2003.29113.x. [DOI] [PubMed] [Google Scholar]
- 5.Niamtu Joseph., III Botulinum toxin A: a review of 1,085 oral and maxillofacial patients treatments. J Oral Maxillofac Surg. 2003;61:317–324. doi: 10.1053/joms.2003.50069. [DOI] [PubMed] [Google Scholar]
- 6.Niamtu J., III The use of botulinum toxin in cosmetic facial surgery. Oral Maxillofac Surg Clin North America. 2000;12(4):595–612. [Google Scholar]
- 7.Hankins CL, Strimling R, Rogers G. Botulinum A toxin for glabellar wrinkles: dose and response. Dermatol Surg. 1998;24:1181–1183. doi: 10.1016/S1076-0512(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 8.Benedtto AV. The cosmetic uses of botulinum toxin type A. Int J Dermatol. 1999;38:641–655. doi: 10.1046/j.1365-4362.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 9.Caruthers J, Carruthers JA. Aesthetic botulinum toxin A in the mid and lower face and neck. Dermatol Surg. 2003;29:468–476. doi: 10.1046/j.1524-4725.2003.29115.x. [DOI] [PubMed] [Google Scholar]
- 10.Flynn TC, Carruthers JA, Caruthers J. Surgical pearl: the use of the Ultra-Fine II short needle 0.3 cc insulin syringe for botulinum toxin injections. J Am Acad Dermatol. 2002;46(6):931–933. doi: 10.1067/mjd.2002.119202. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers JA, Caruthers J. Clinical indications and injection technique for the cosmetic use of Botulinum A exotoxin. Dermatol Surg. 1998;24:1189–1194. doi: 10.1016/S1076-0512(98)00189-7. [DOI] [PubMed] [Google Scholar]
- 12.Matarosso SL. Complications of Botulinum A exotoxin for hyper functional lines. Dermatol Surg. 1998;24:1249–1254. doi: 10.1016/S1076-0512(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 13.Binder WJ, Blitzer A, Brinn MF. Treatment of hyper functional lines of the face with botulinum toxin A. Dermatol Surg. 1998;24:1198–1205. doi: 10.1016/S1076-0512(98)00181-2. [DOI] [PubMed] [Google Scholar]
- 14.Matilde MS. New indications for botulinum toxin type-A in cosmetic: mouth and neck. Plast Reconstr Surg. 2002;110:601–613. doi: 10.1097/00006534-200208000-00037. [DOI] [PubMed] [Google Scholar]
- 15.Flynn TC, Carruthers JA, Carruthers JA. Botulinum A toxin treatment of the lower eyelid improves infraorbital rhytides and widens the eye. Dermatol Surg. 2001;27:703–708. doi: 10.1046/j.1524-4725.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldman MP. Festoon formation after infraorbital botulinum toxin A: a case report. Dermatol Surg. 2003;29:560–561. doi: 10.1046/j.1524-4725.2003.29130.x. [DOI] [PubMed] [Google Scholar]
- 17.Connor MS, Karlis V, Ghali GE. Management of the aging forehead: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:642–648. doi: 10.1067/moe.2003.240. [DOI] [PubMed] [Google Scholar]
- 18.Pribtkin EA, Greco TM, Goode RI, Keane WM. Patient selection in the treatment of glabellar wrinkles with botulinum toxin type A injection. Arch Otolaryngol Head Neck Surg. 1997;123:321–326. doi: 10.1001/archotol.1997.01900030103013. [DOI] [PubMed] [Google Scholar]
- 19.Fulton JE. Botulinum toxin: the Newport Beach experience. Dermatol Surg. 1998;24:1219–1224. doi: 10.1016/S1076-0512(98)00184-8. [DOI] [PubMed] [Google Scholar]
- 20.Ahn K-Y, Park M-Y, Park D-H, Han D-G. Botulinum toxin A for the treatment of facial Hyperkinetic wrinkles in Koreans. Plast Reconstr Surg. 2000;105:778–784. doi: 10.1097/00006534-200002000-00050. [DOI] [PubMed] [Google Scholar]
- 21.Lew H, Yun YS, Lee SY, Kim SJ. Effect of botulinum toxin A on facial wrinkle lines in Koreans. Ophthalmologica. 2002;216:50–54. doi: 10.1159/000048297. [DOI] [PubMed] [Google Scholar]
- 22.Cather JC, Cather JC, Menter A. Update on botulinum toxin for facial esthetics. Dermatol Clin. 2002;20:749–761. doi: 10.1016/S0733-8635(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 23.Heckman M, Teichmann B, Schroeder U, Sprengelmeyer R, Ceballos-Baumann AO. Pharmacologic denervation of frown muscles enhances baseline expression of happiness and decreases baseline expression of anger, sadness, and fear. J Am Acad Dermatol. 2003;49(2):213–216. doi: 10.1067/S0190-9622(03)00909-5. [DOI] [PubMed] [Google Scholar]
- 24.Sadick N. Botulinum toxin type B for glabellar wrinkles: a prospective open label response study. Dermatol Surg. 2002;28(9):817–821. doi: 10.1046/j.1524-4725.2002.02037.x. [DOI] [PubMed] [Google Scholar]
- 25.Sadick N, Herman AR. Comparison of botulinum toxins A and B in the esthetic treatment of facial rhytides. Dermatol Surg. 2003;29:340–347. doi: 10.1046/j.1524-4725.2003.29082.x. [DOI] [PubMed] [Google Scholar]