Abstract
This retrospective study was conducted to evaluate the role of platelet-rich plasma (PRP) in the reconstruction of segmental mandibular defects using iliac bone grafts. Thirteen patients underwent reconstruction of post-resection segmental defects of the mandible using titanium reconstruction plates, cortico cancellous iliac bone graft. The patients were randomly separated into two groups. One group of the patients received a PRP graft in addition to the iliac bone graft. Post-operative dimensions of the graft were measured and compared to assess the efficacy of PRP in reconstruction of segmental defects. The post-operative follow-up radiographs confirmed consolidation of the graft in all cases and the segmental defect was obliterated. Thereby mandibular continuity was successfully achieved in all cases. Two patients in the non-PRP group developed an infection and were administered additional antibiotics. The infection was contained and the grafts survived. The use of PRP along with autogenous bone graft may be advantageous since it appeared to enhance the quantity of bone formed. Further long-term follow-up and studies are required to effectively establish the efficacy of PRP and autogenous free bone grafts in the reconstruction of bony defects.
Keywords: PRP, Mandibular reconstruction, Free graft
Introduction
Secondary reconstruction of continuity defect of the mandible for the restoration of form and function is a challenge. Microvascular composite or free flaps are advocated when the defect is composite as in case of malignant tumour resection [1–9]. Being vascular, they are considered more reliable. However, they fall short of the quantity of bone required to reconstruct the mandibular height and the donor site morbidity is higher [10–12]. In India, due to the limitations in armamentarium and the cost factor that is higher due to the skill and time involved in microvascular surgeries, patients often choose a free graft for reconstruction prior to attempting a microvascular surgery. Commercially available bone morphogenetic proteins are also way above the reach of common man.
In comparison, though immediate or primary reconstruction of post-resection continuity defects of the mandible with microvascular transfer has increased in usage but, cancellous free bone grafting is still performed in Indian teaching institutions and hospitals. This graft has proved to be advantageous over all other forms of reconstruction in terms of reliability and decreased donor site morbidity. Moreover, when composite reconstruction is not needed as in non-malignant cases, micro-vascular surgery is not sought as first choice. Free grafts can also manage the volume required for reconstructing the height, width and curvature of the mandible especially in secondary reconstructions [6, 13–16]. This technique has been improvised and modified by several surgeons over the years to increase its efficacy, survival and decrease the rate of resorption.
Cancellous particulate bone grafts were compressed in tray form to mimic the mandibular contour in the 1960s and 1970s. Gaining popularity, custom made trays were fabricated to match mandibular form [17, 18]. Several additives have been tried to enhance the ossification of the graft.
Of them, platelet-rich plasma (PRP) has been found to be effective by many authors. PRP is made from the patient’s own blood by centrifugation. By sequestering and concentrating the platelets to 338 %, the platelet-derived growth factor and transforming growth factor beta can be concentrated to levels that are clinically usable. Platelets adhere, aggregate and generate a pro-coagulant surface at the site of vascular injury which promotes thrombin generation and fibrin formation. Theoretically, platelets are known to release growth factors (PDGF, TGF-beta?), cytokines (PF4, CD40L), chemokines and active metabolites responsible for neoangiogenesis, tissue repair and regeneration. Concentrating platelets in a fibrin clot or a glue provides a higher dose of these healing proteins to the site of application [19]. The cell membrane receptors of cancellous bone grafts are capable to responding to these growth factors as evidenced by monoclonal antibody assessment. Marx et al. [20] reported their evidence of 1.62 to 2.16 times greater radiographic maturation rate and increased histomorphometric bone density in grafts with PRP in comparison to grafts without PRP. Earlier, Tayaponsak et al. [21] had published an early radiographic healing in 33 patients who underwent mandibular reconstruction on addition of autogenous fibrin adhesive to the cancellous bone graft.
However, review of clinical as well as in vitro studies shows a great variation in the results obtained, possibly due to the site and method of implantation of the grafts. In spite of these varied results obtained, we have continued to use PRP in various clinical cases ranging from secondary alveolar bone grafting to fracture healing and mandibular segmental defect reconstruction since the consolidation achieved has been evidently superior to that obtained while using the bone graft alone.
In this prospective study, we have comparatively analysed the quantum of bone regenerated to assess the efficacy of PRP in the secondary reconstruction of continuity defects of the mandible after segmental resection for odontogenic tumours. All 13 patients were reconstructed using iliac cortico-cancellous block bone graft and of them randomly chosen 8 received PRP in addition to the bone graft.
Materials and Methods
All patients who reported for secondary reconstruction of the mandible 6 to 12 months after their primary surgery were screened for inclusion in the study. The criteria of selection was based on the following points:
The resected lesion should be non-malignant.
The defect size should be greater than 4 cm and lesser than 6 cm not involving the condyle or the ramus.
The patient should be below 50 years of age without any systemic diseases like diabetes, hypertension or hormonal imbalance.
The patient should not have any other infectious diseases and should test negative for hepatitis B virus, hepatitis C virus, human immunodeficiency viruses 1 and 2, human T-lymphotropic viruses 1 and 2, and Treponema pallidum.
All patients were given extensive information about the procedure and all consented to the type of reconstruction. Thirteen patients fulfilled the inclusion and exclusion criteria during the study period. There were 7 (53.8 %) males and 6 (46.2 %) females in the study group. Of these 7 (53.8 %) underwent reconstruction using autogenous iliac crest graft (AICG) for the defect while the rest 6 (46.2 %) underwent reconstruction with AICG with PRP. Of these 9 (69.2 %) cases were resected for ameloblastoma while rest were resected to treat aggressive odontogenic keratocysts. The mean age of the study group was 29 ± 7.64 years (19–48 years). Every alternate case was selected for each group.
Preparation of the PRP Gel
40 ml of autologous blood was withdrawn prior to surgery. The blood was drawn into four 10 ml test tubes containing 0.5 ml of sodium citrate solution (anticoagulant). The tubes were centrifuged at 1,500 rpm for 10–15 min to obtain 3 components. The middle layer of PRP (platelet-rich plasma) was separated from the PPP (platelet-poor plasma) and RBC (red blood corpuscles). The separated PRP was again centrifuged at 1,500 rpm for 10–15 min and the middle layer was collected in a beaker along with 10 % calcium chloride. 5,000 units of topical bovine thrombin was added and kept in water bath for 30–40 min to obtain the PRP in gel form.
Surgical Technique (Figs. 1, 2)
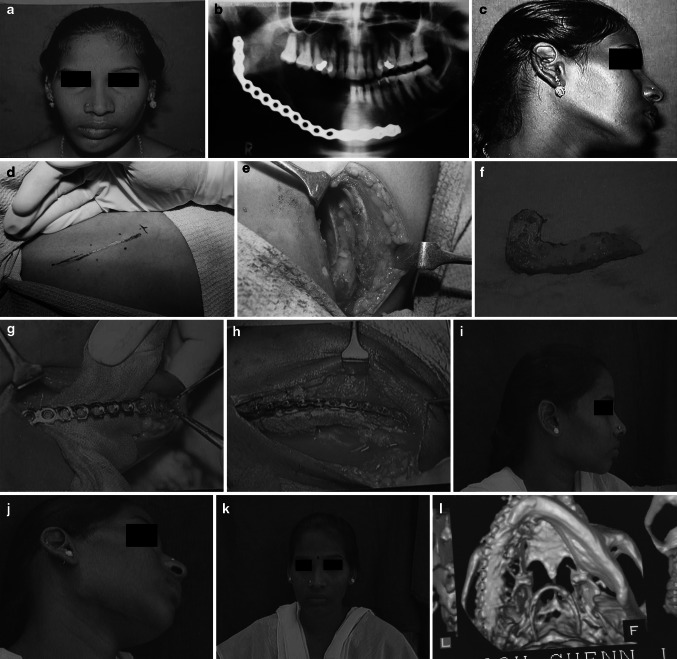
Fig. 1.
a Pre operative view, b Preoperative x-ray, c Preoperative view, d Iliac incision line, e Iliac graft harvest site, f Harvested Graft, g Mandibular recipient site prepared, h Harvested Graft implanted in the mandible, i Post operative view, j Post operative view, k Post operative view, l Post operative 3DCT
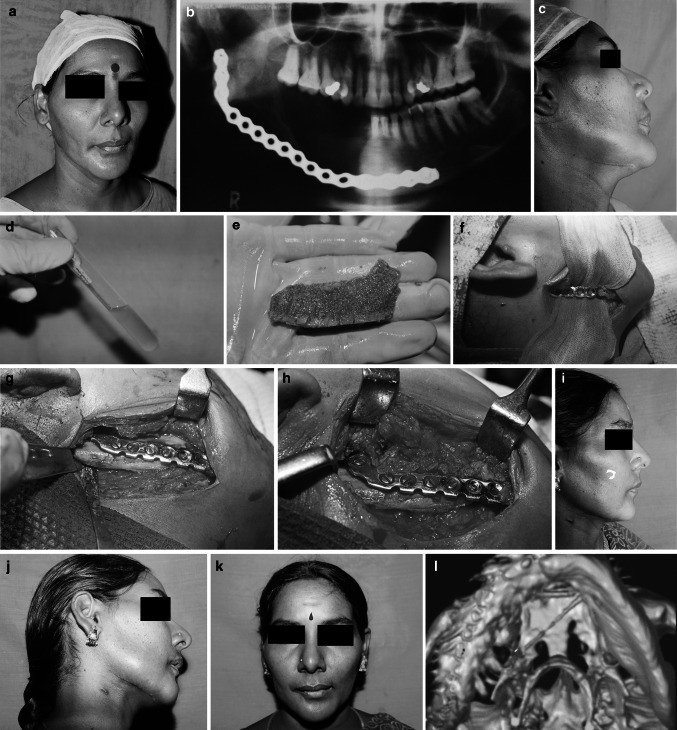
Fig. 2.
a Pre operative view, b Preoperative x-ray, c Preoperative view, d Prepared -prp, e Harvested Iliac Graft, f Mandibular recipient site prepared, g Iliac Graft Fixed to reconstruction plate to bridge the defect, h PRP packed in and around the defect in the mandible and the free graft, i Post operative view, j Post operative view, k Post operative view, l Post operative 3DCT
Under GA and naso-endotracheal intubation, the patient was adequately prepared and draped. Approach to the defect site was gained through pre-existing scars in the submandibular region. The bony stumps were exposed on both the lingual and buccal sides and freshened. The occlusion was temporarily fixed by maxillo-mandibular fixation to re-create the actual size of the defect. The length, breadth and height of the graft required was assessed. If the reconstruction plate was misshapen or not made of titanium, it was replaced with an appropriate one.
The cortico-cancellous block graft was harvested from either the anterior or posterior iliac crest depending on the volume of bone required. Additional cancellous bone was harvested using bone scoops. The harvested graft was remodelled and contoured according to the segmental continuity defect. Cancellous bone graft was applied between the cortical plates.
In the cases in which PRP was used additionally, the PRP along with the cancellous graft particles were applied between the cortical plate and the titanium plates. Wound was closed in layers without a drain and antibiotics administered.
As soon as the patient was ambulant, an orthopantomogram (OPG) was taken with extreme precaution to maintain the midline, occlusion and uniform magnification. All patients were regularly followed-up. OPG and CT were repeated on the same machine by the same radiologist to standardize the length, breadth and Hounsfield units. Unbroken continuity of the graft was taken as success of the graft.
OPG measurements taken The mid-point of the graft, the space between the proximal and distal margins of the pre-existing defect was marked. A line was drawn at this point parallel to the posterior border of the ramus. The height of the graft was then measured as the length of this line from the lower border to the crest. Actual length was arrived at by assessing the magnification.
CT measurements CT measurements were done by the same radiologist who employed radiographic markers to ascertain the standardisation.
Statistics
All data were entered and analyzed using Statistical Package for Social Service (SPSS version 16.0, IBM, Chicago, IL, USA). Descriptive statistics were presented for the variables. Independent t test was employed to identify the difference between the two types of materials used. Paired t test was employed to find the difference between the immediate and 6 month post-operative parameters. P value of <0.05 was taken as statistical significance.
Results
Follow-up was done for a minimum of 6 months. The post-operative follow up radiographs confirmed consolidation of the graft in all cases and the segmental defect was obliterated. Mandibular continuity was successfully achieved in all cases. Two patients in the non-PRP group developed an infection and wound dehiscence. They were administered antibiotics and wound dressed regularly till it healed by secondary intension. The infection was contained and the grafts survived. Prosthetic rehabilitation using removable dentures was done in all the cases.
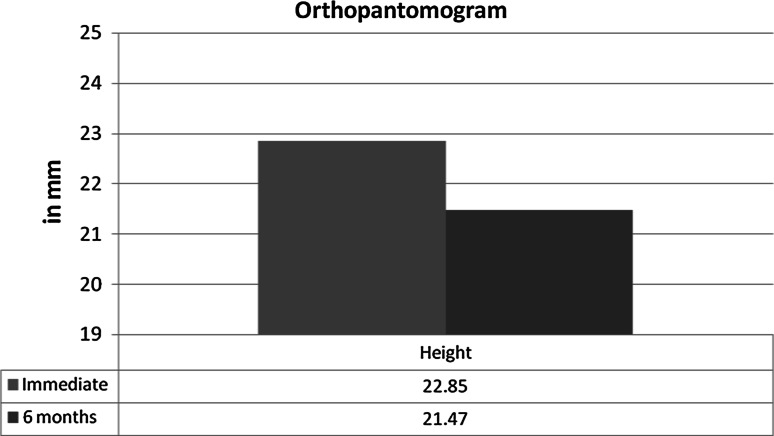
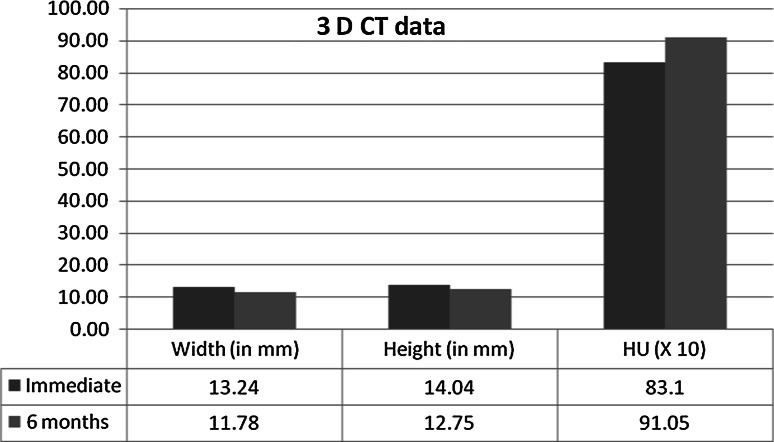
Table 1 depicts the descriptive statistics of the outcomes. It is observed that there is shrinkage of the graft in the study population. Graph 1 indicates the difference in terms of height measurement between the immediate and 6 months post-operative data measurements obtained using OPG. The value is significantly different (P ≤ 0.005). Graph 2 indicates the 3D CT data difference in terms of linear measurement between the immediate and 3 months post-operative data measurements. The value is significantly different (P ≤ 0.05) for all the measurements.
Table 1.
Descriptive statistics of the outcome measures in the study population
| Minimum | Maximum | Mean | Std. deviation | |
|---|---|---|---|---|
| OPG | ||||
| Immediate | ||||
| Height in mm | 18.00 | 28.00 | 22.85 | 3.05 |
| 6 months | ||||
| Height in mm | 17.28 | 25.80 | 21.47 | 2.83 |
| CT data | ||||
| Immediate | ||||
| Width in mm | 11.56 | 16.80 | 13.24 | 1.63 |
| Immediate | ||||
| Height in mm | 12.11 | 16.91 | 14.04 | 1.38 |
| Immediate | ||||
| Bone quality In HU | 701.00 | 911.00 | 831.38 | 55.16 |
| 6 months | ||||
| Width in mm | 10.88 | 14.44 | 11.78 | 0.93 |
| 6 months | ||||
| Height in mm | 10.28 | 15.80 | 12.75 | 1.48 |
| 6 months | ||||
| Bone quality in HU | 827.14 | 1018.54 | 910.49 | 67.74 |
Graph 1.
Comparison of the OPG data between immediate and 6 months post-operative in terms of height in millimeters
Graph 2.
Comparison of the 3D CT data between immediate and 6 months post-operative in terms of linear measurement and quality of bone formed
Table 2 depicts the comparison of the two treatment modalities with the various parameters. Significant difference exists in relation to the immediate length in OPG data. In CT data, width and height in immediate as well as the 6 months period varied significantly.
Table 2.
Comparison of the treatment modalities with parameters
| Iliac graft | Iliac graft + PRP | P value | |
|---|---|---|---|
| OPG data | |||
| Immediate | |||
| Height in mm | 23.71 ± 2.56 | 21.83 ± 3.49 | 0.290 |
| 6 months | |||
| Height in mm | 22.03 ± 2.15 | 20.81 ± 3.57 | 0.460 |
| CT data | |||
| Immediate | |||
| Width in mm | 14.12 ± 1.77 | 12.21 ± 0.49 | 0.027* |
| Immediate | |||
| Height in mm | 13.71 ± 1.11 | 14.41 ± 1.67 | 0.383 |
| Immediate | |||
| Bone quality in HU | 838.14 ± 28.79 | 823.50 ± 78.53 | 0.654 |
| 6 months | |||
| Width in mm | 11.98 ± 1.19 | 11.56 ± 0.48 | 0.434 |
| 6 months | |||
| Height in mm | 12.14 ± 1.18 | 13.47 ± 1.55 | 0.106 |
| 6 months | |||
| Bone quality in HU | 876.52 ± 37.40 | 950.12 ± 76.38 | 0.045* |
* P <. 01
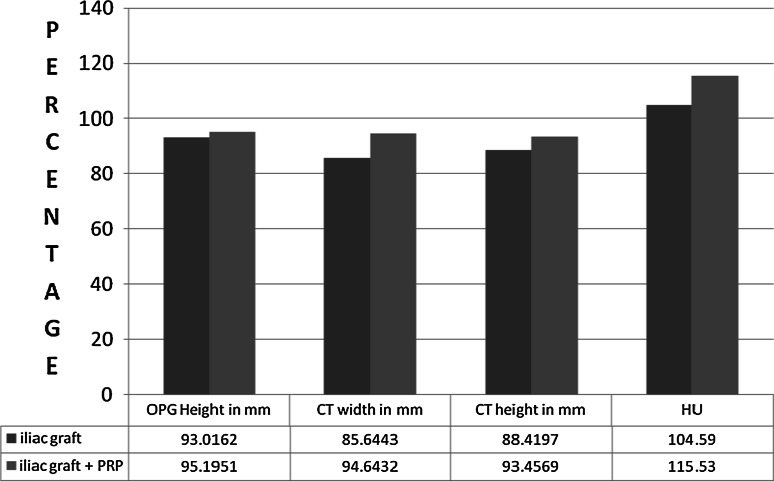
The efficacy of the material was calculated using the 6 month post operative measurement divided by the immediate measurement and expressed as percentage. Graph 3 indicates the measurements of the value. It is observed that the height in OPG was not significant (P ≥ 0.05). The width (P ≤ 0.05), height and Hounsfield unit difference was significantly different on comparison (P ≤ 0.001). It is observed that the graft shrinkage is higher when AICG is used alone whereas the shrinkage is less when the AICG is mixed with autogenous PRP. Additionally the quality of the bone formed is better with AICG and PRP combination as indicated by higher Hounsfield units.
Graph 3.
Comparison of mean of the efficacy as percentage of the outcome parameters in the type of graft used
Discussion
Several authors have successfully applied PRP for various bone defects including reconstruction of mandible [22–25].
Merks et al. [23] successfully reconstructed segmental defects of the mandibles that were resected for malignancy. Of the 8 cases, they evidenced good bone remodelling in the biopsy taken after 6 months during implant insertion. They successfully achieved three dimensional reconstruction with successful prosthetic rehabilitation. In our cases too all patients were provided removable dentures. However fixed prosthetic rehabilitation using implants was not attempted due to economic reasons.
Thorn et al. [25] reported good healing on the usage of fibrin glue, a two-component glue, where one component is a concentrated fibrinogen solution with platelet growth factors and the other is a thrombin solution derived from PRP. This glue when added to particulate bone graft has been successfully used in the reconstruction of continuity defects of 8 mandibles. In our series the cortical plates were used for bulk reconstruction and the cancellous iliac bone graft was applied between the cortical plates. Mandibular continuity was achieved successfully.
Simon et al. [24] reports a novel experience in the immediate reconstruction of the mandibles in 11 patients with ameloblastoma in Tanzania. The concentration of platelets in the PRP used varied from 1,000 × 109/L to 2,000 × 109/L. In 6 of the patients, the tumour was excised from the cortical scaffold, irradiated, perforated, and re-fixed to fit the defect filled with particulate bone mixed PRP. In 5 of the patients, a mixture of bone and PRP was packed into the defect under the reconstruction plates without using the cortical scaffold. Four of the 6 scaffold patients suffered complications. None from the group reconstructed without scaffolds suffered any complications.
The concentration of the PRP that was used was not uniform but since the failure rate reported was low, no correlation could be drawn between the concentration and graft take.
Animal studies on PRP and its mechanism of action has yielded varied results [26, 27].
Systematic review of published literature to assess the benefits of use of PRP in extraction sockets concludes that the use of platelet concentrates may be beneficial for reducing post-operative pain and inflammation, thereby improving quality of life in the early period after extraction. No systematic acceleration of osseous healing at the post-extraction site could be demonstrated, suggesting that platelet concentrates per se probably exert a negligible effect on bone regeneration [28].
Mooren et al. [29] reporting their 8 year experience with 20 patients who underwent a reconstruction of the mandible, by use of pre-shaped 2.3-mm titanium plates, autogenous cortical bone plates and autogenous particulate bone with PRP conclude that the fact that all gaps in this study were far larger than 5 cm and in all cases continuity was achieved attests to the validity of the method reported. The increased antimicrobial effect and the increased proliferation of osteoblasts are likely responsible for the results achieved using PRP. Two patients in the non-PRP group developed an infection and wound dehiscence. The absence of post surgical infection rate in the PRP cases in our series also may have been due to the antimicrobial effect of the platelet concentrate.
Study of the relationship of resorptive and depository surfaces to the maintenance of bone graft volume in the growing rabbit facial skeleton [30] reveals that the kind of bone used may also play a role. It was observed that particulate cortical bone did not heal in a predictable fashion but a cortical block as a solitary implant yielded better results as in our series of cases [31]. We employed a mixture of both cortical and cancellous graft and achieved the desired continuity and bulk.
Since Pogrel et al. [7] described failure of mandibular reconstruction as loss of continuity, requiring further grafting, all 13 cases had been successfully grafted with an evidence of better ossification in the PRP group. Though anatomic shape could not be achieved perfectly, due to the presence of pre-existing scars, it was acceptable to the patient and prosthetic rehabilitation using removable dentures was done, comparable to other clinical studies in mandibular reconstruction using PRP [20, 23–25].
The evidence of an increased number of wound dehiscence due to infection in the non-PRP cases may support the antimicrobial effect of PRP [32–35].
The rigid cortical block graft serves as a scaffold for wound healing and maintains the shape of the mandible and obliterates the cosmetic defect as desired by the patient.
Conclusion
The use of PRP along with autogenous bone graft may be advantageous since it appeared to enhance the quantity of bone formed. Further long-term follow-up and studies are required to effectively establish the efficacy of PRP and autogenous free bone grafts in the reconstruction of bony defects.
References
- 1.Chiapasco M, Biglioli F, Autelitano L, et al. Clinical outcome of dental implants placed in fibula-free flaps used for the reconstruction of maxillo-mandibular defects following ablation for tumors or osteoradionecrosis. Clin Oral Implants Res. 2006;17:220. doi: 10.1111/j.1600-0501.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- 2.González-García R, Naval-Gías L, Rodríguez-Campo FJ, et al. Vascularized free fibular flap for the reconstruction of mandibular defects: clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:191. doi: 10.1016/j.tripleo.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo D. Fibular free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71. doi: 10.1097/00006534-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SN, Lowe D, Fisher SE, et al. Health related quality of life and clinical function after primary surgery for oral cancer. Br J Oral Maxillofac Surg. 2002;40:11. doi: 10.1054/bjom.2001.0706. [DOI] [PubMed] [Google Scholar]
- 5.Urken ML, Buchbinder D, Weinberg H, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. Laryngoscope. 1991;101:935. doi: 10.1288/00005537-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Goh BT, Lee S, Tideman H, et al. Mandibular reconstruction in adults: a review. Int J Oral Maxillofac Surg. 2008;37:597. doi: 10.1016/j.ijom.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Pogrel MA, Podlesh S, Anthony JP, et al. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200. doi: 10.1016/S0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 8.Urken ML, Weinberg H, Vickery C, et al. Oromandibular reconstruction using microvascular composite free flaps (Report of 71 cases and a new classification scheme for bony, soft-tissue, and neurologic defects) Arch Otolaryngol Head Neck Surg. 1991;117:733. doi: 10.1001/archotol.1991.01870190045010. [DOI] [PubMed] [Google Scholar]
- 9.Urken ML, Buchbinder D, Costantino PD, et al. Arch oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Otolaryngol Head Neck Surg. 1998;124:46. doi: 10.1001/archotol.124.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Brown JS, Magennis P, Rogers SN, et al. Trends in head and neck microvascular reconstructive surgery in Liverpool (1992–2001) Br J Oral Maxillofac Surg. 2006;44:364. doi: 10.1016/j.bjoms.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Cawood JI, Stoelinga PJ, Blackburn TK. The evolution of preimplant surgery from preprosthetic surgery. Int J Oral Maxillofac Surg. 2007;36:377. doi: 10.1016/j.ijom.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Hayter JP, Cawood JI. Oral rehabilitation with endosteal implants and free flaps. Int J Oral Maxillofac Surg. 1996;25:3. doi: 10.1016/S0901-5027(96)80004-X. [DOI] [PubMed] [Google Scholar]
- 13.Schimmele SR. Delayed reconstruction of continuity defects of the mandible after tumor surgery. J Oral Maxillofac Surg. 2001;59(11):1340–1344. doi: 10.1053/joms.2001.27826. [DOI] [PubMed] [Google Scholar]
- 14.Canter HI, Vargel I, Mavili MEJ. Reconstruction of mandibular defects using autografts combined with demineralized bone matrix and cancellous allograft. J Craniofac Surg. 2007;18:95. doi: 10.1097/01.scs.0000244921.07383.cc. [DOI] [PubMed] [Google Scholar]
- 15.Foster RD, Anthony JP, Sharma A, et al. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66. doi: 10.1002/(SICI)1097-0347(199901)21:1<66::AID-HED9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Schliephake H, Schmelzeisen R, Husstedt H, et al. Comparison of the late results of mandibular reconstruction using nonvascularized or vascularized grafts and dental implants. J Oral Maxillofac Surg. 1999;57:944. doi: 10.1016/S0278-2391(99)90015-0. [DOI] [PubMed] [Google Scholar]
- 17.Cheung LK, Samman N, Tong AC, et al. Mandibular reconstruction with the Dacron urethane tray: a radiologic assessment of bone remodeling. J Oral Maxillofac Surg. 1994;52:373. doi: 10.1016/0278-2391(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 18.Samman N, Luk WK, Chow TW, et al. Custom-made titanium mandibular reconstruction tray. Aust Dent J. 1999;44:195. doi: 10.1111/j.1834-7819.1999.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 20.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Radiol Endod. 1998;85:638. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Tayaponsak PJ, O’Brien DA, Monteiro CB, et al. Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J Oral Maxillofac Surg. 1994;52:161. doi: 10.1016/0278-2391(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 22.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294. doi: 10.1016/S0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 23.Merkx MAW, Fennis JPM, Verhagen CM, et al. Reconstruction of the mandible using preshaped 2.3 mm titanium plates, autogenous particulate cortico-cancellous bone grafts and platelet-rich plasma: a report on eight patients. Int J Oral Maxillofac Surg. 2004;33:733. doi: 10.1016/j.ijom.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Simon EN, Merkx MAW, Shubi FM, et al. Reconstruction of the mandible after ablative surgery for the treatment of aggressive, benign odontogenic tumours in Tanzania: a preliminary study. Int J Oral Maxillofac Surg. 2006;35:421. doi: 10.1016/j.ijom.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Thorn JJ, Sørensen H, Weis-Fogh U, et al. Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:95. doi: 10.1054/ijom.2003.0461. [DOI] [PubMed] [Google Scholar]
- 26.Mooren RE, Dankers AC, Merkx MA, Bronkhorst EM, Jansen JA, Stoelinga PJ. The effect of platelet-rich plasma on early and late bone healing using a mixture of particulate autogenous cancellous bone and Bio-oss: an experimental study in goats. Int J Oral Maxillofac Surg. 2010;39(4):371–378. doi: 10.1016/j.ijom.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Del Fabbro M, Bortolin M, Taschieri S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int J Oral Maxillofac Surg. 2011;40:891–900. doi: 10.1016/j.ijom.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Plachokova AS, van den Dolder J, Stoelinga PJ, et al. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2007;18:244. doi: 10.1111/j.1600-0501.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 29.Mooren RE, Merkx MA, Kessler PA, Jansen JA, Stoelinga PJ. Reconstruction of the mandible using preshaped 2.3-mm titanium plates, autogenous cortical bone plates, particulate cancellous bone, and platelet-rich plasma: a retrospective analysis of 20 patients. J Oral Maxillofac Surg. 2010;68(10):2459–2467. doi: 10.1016/j.joms.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Zins JE, Kusiak JF, Whitaker LA, et al. The influence of the recipient site on bone grafts to the face. Plast Reconstr Surg. 1984;73:371. doi: 10.1097/00006534-198403000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Merkx MAW, Maltha JC, Freihofer HPM, et al. Incorporation of particulate bone grafts in the facial skeleton. Biomaterials. 1999;20:2029. doi: 10.1016/S0142-9612(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 32.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89:417. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 34.Krijgsveld J, Zaat SA, Meeldijk J, et al. Microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem. 2000;275:20374. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 35.Yeaman MR. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]