Abstract
Introduction
The use of buccal fat pad as a grafting source in the closure of intraoral defects has gained popularity in the last quarter of this century. Because of the ease of access and rich blood supply, its use in oral defects is an attractive concept.
Methodology
The study comprised of 8 patients with oral submucous fibrosis, 1 patient with oroantral fistula, 1 patient with verrucous hyperplasia. The acquired oral defects following resection of pathology in the oral cavity, were reconstructed with pedicled buccal fat pad. The Post operative follow up at the intervals of 1st, 7th and 15th day, followed by 1st month, 2nd month and 3rd month was done.
Results
The procedure was successful in all the patients. Healing was satisfactory with no breakdown or liquefaction necrosis post operatively. All the patients had definitive colour change at the end of 1st post operative month owing to the epithelialisation. Residual defect was present in one patient diagnosed of verrucous hyperplasia on the 1st and the 7th post operative day which subsequently healed. In 8 patients with oral submucous fibrosis post operative mouth opening was measured in and was observed to be in the range of 12–26 mm on the 1st post operative day and 34–42 mm during 3rd month post operatively.
Conclusion
The results of this study support the view that the use of buccal fat pad is a simple, convenient and reliable method for the reconstruction of small to medium sized intra oral defects.
Keywords: Buccal pad of fat (BPF), Oral submucours fibrosis (OSMF), General anaesthesia (GA)
Introduction
Reconstruction in oral and maxillofacial area is a uniquely difficult task in its attempt to restore the individual`s facial expression, articulation of speech and deglutition. These are often based on a complex anatomy of soft tissues of the region and the underlying facial skeleton. Reconstruction in the maxillofacial area thus often has to deal with combined defects of both hard and soft tissues [1].
With large tissue deficits resulting from trauma, resection of malignancies or congenital malformations, transplantation of tissue may become necessary to accomplish complete and satisfactory tissue repair [1].
Many instances in surgery require of flaps to reconstruct intra oral defects like developmental abnormalities, post ablation defects following excision of fibrous bands in oral sub mucous fibrosis, Oro-antral and Oro-nasal fistulas created accidentally during extraction of teeth etc. There are basically 2 kinds of flaps used in reconstruction of intra oral defects namely local flaps and distant flaps. Local flaps have many advantages over the distant flaps like they are very near to defect, lesser morbidity and functional impairment.
Various surgical techniques have been suggested for the closure of oral defects such as primary closure, buccal mucosal graft, split thickness skin graft, allogenic graft, regional rotational flap, and the distant flap [2].
The type and size of the defect determines the technique to be used. The use of buccal fat pad as a grafting source in the closure of intra oral defects has gained popularity in the last quarter of this century [2].
Among the local flaps, buccal fat pad has no cosmetic hindrance (no extra oral scar) as it is nearer to intra oral defects, definite and rich in vasculature, pliable and can be adapted to defects, is also easy and less time consuming.
Materials and Methods
Ten patients with acquired oral defects following resection of pathology in the oral cavity were reconstructed with pedicled buccal pad of fat graft were included in the study.
All the patients were treated in the department of oral and maxillofacial surgery, oxford dental college, Bangalore. The group comprised of 6 males and 4 females aged between 9 and 80 years. The study group comprised of 8 patients with oral sub mucous fibrosis, 1 patient with oro-antral fistula, 1 patient with verrucous hyperplasia. Autogenous Buccal pad fat from the same side of the resection was used to reconstruct the defects arising due to excision of pathological lesion and traumatic injuries with tissue loss. The reconstruction using buccal fat pad was preferred for patients with low grade malignancies, benign tumors, and small to medium sized surgical defects. Large sized defects, diabetic patients and active infection in the recipient site was excluded from the study.
Mouth opening was recorded (Fig. 1), Routine blood investigations, biopsy and relevant radiographic evaluation were done, before the patients were included in the study. Area of the defect was measured in centimeters and prepared to accept the buccal fat pad (Fig. 2). The Buccal fat pad was exposed and mobilized either via the same incision which was used for excision of pathology or another incision was used to expose and mobilize the buccal fat pad (Fig. 3). The Buccal fat pad was transposed and sutured (Fig. 4) to the defect by tunneling the buccal fat pad into the defect. The Buccal fat pad was left uncovered to heal and epithelialise spontaneously.
Fig. 1.

Pre-operative mouth opening
Fig. 2.
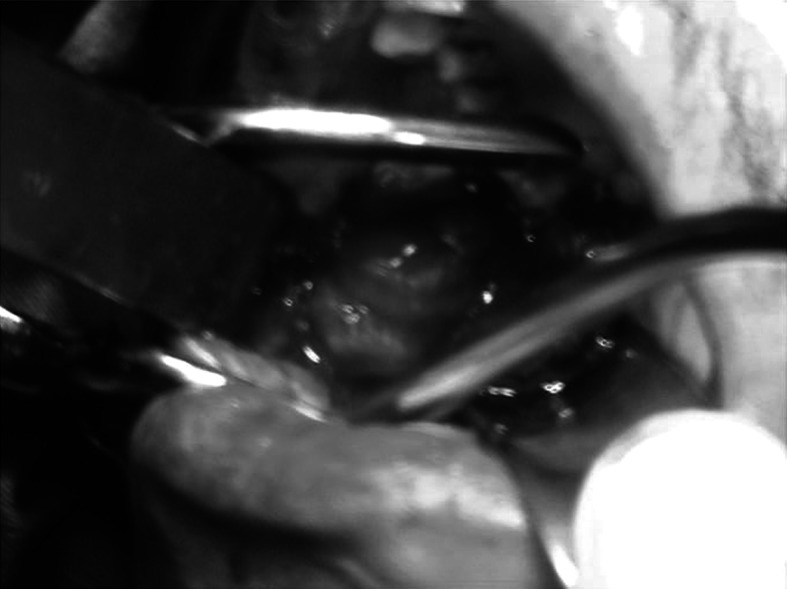
Surgical defect
Fig. 3.
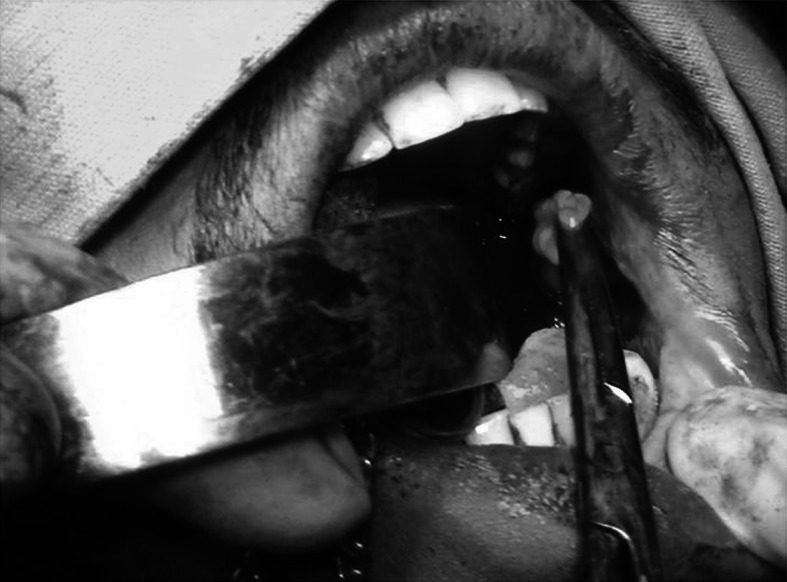
Buccal fat pad moblization
Fig. 4.
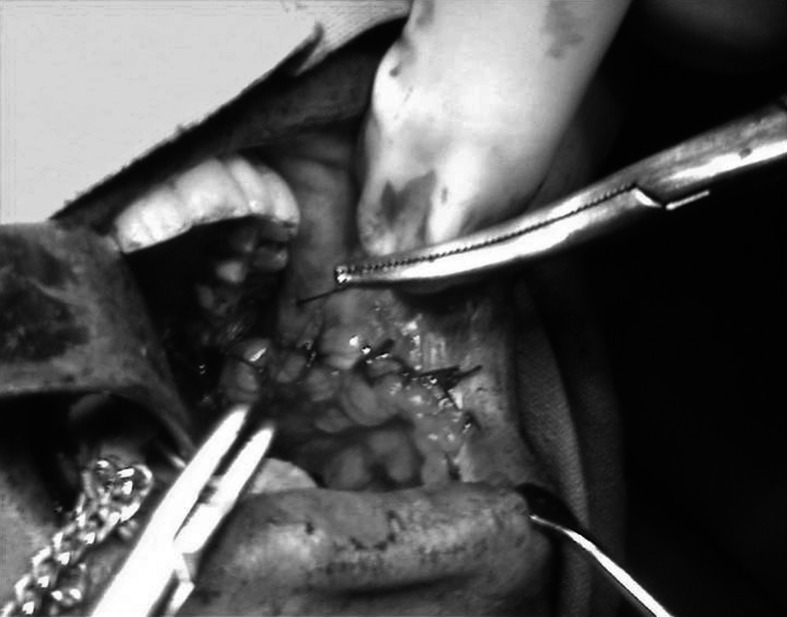
BFP sutured to the surgical defect
The surgical area and the buccal fat pad were evaluated post-operatively on 1st, 7th and 15th day, later at the intervals of 1st month (Fig. 5), 2nd month and 3rd month (Fig. 6).
Fig. 5.
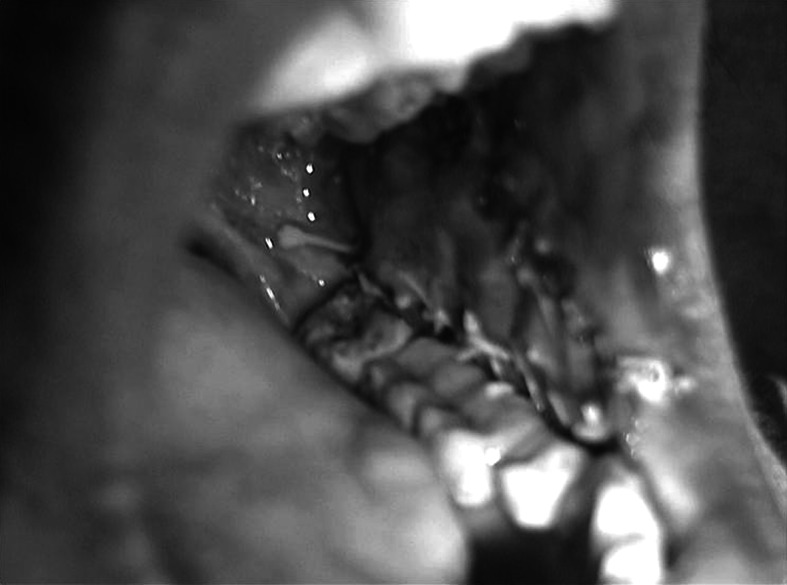
One month post-operatively
Fig. 6.

Post operative mouth opening
The wound was inspected for breakdown and presence or absence of infection. Assessment was made for the presence or absence of pain and foul smell. The buccal fat pad graft was assessed for liquefaction necrosis, graft epithelialisation, residual defect and colour. Mouth opening was assessed in millimeters on the post operative days of assessment. The presence or absence of infection was based on the Centre for disease control-Atlanta, USA, criteria for presence of post surgical infection. Healing was mainly assessed based upon the presence or absence of breakdown, infection, and liquefaction necrosis and graft epithelialisation
This study was conducted in the department of Oral and Maxillofacial surgery, The Oxford dental college, Bangalore with an aim in evaluating the versatility of buccal fat pad used in the reconstruction of oral defects.
Results
There were no signs of healing on the 1st post operative day in any of the patients. Healing was found to be satisfactory on 7th post operative day and was complete by the 3rd month post operatively (P = 0.00025).
There was no breakdown or liquefaction necrosis in any of the cases post operatively.
Infection was seen in 1 patient on the 1st post operative day and in 2 patients on the 7th post operative day which resolved before the 15th post operative day. The infection present in 2 patients was not statistically significant. (P = 0.4112)
Pain was present in all the patients on the 1st post operative day and in 8 patients on the 7th post operative day. Pain was not present in any of the patients during the follow up from 15th post operative day to 3rd month. Pain was statistically significant with a P value of 0.00025.
Foul smell was present in 5 patients on 1st post operative day and 2 patients on 7th post operative day. Foul smell was not seen in any of the patients on 15th post operative day. 1 patient had foul smell on the day of 1st month recall which subsided subsequently. Foul smell was statistically significant with the P value of 0.0952.
The colour change of the buccal fat pad graft from yellow to red was evaluated. There were no signs of colour change on the 1st post operative day in any of the patients. 3 patients showed colour changes on 7th post operative day. 9 patients had colour changes on the 15th post operative day. All the patients had a definitive colour change at the 1st post operative month. The improvements in the colour change were statistically significant. (P = 0.005)
Graft epithelialisation was seen in 3 patients on the 7th post operative day and was complete in all the patients at 15th post operative day. The changes in graft epithelialisation were statistically significant. (P = 0.00025)
Residual defect was present in 1 patient on the 1st and 7th post operative day. Residual defects were not seen in any of the patients after 15th post operative day. The presence of residual defects was not statistically significant. (P = 0.4113) (Table 1).
Table 1.
Evaluation Chart
| Parameters | Results | Study period | P values | |||||
|---|---|---|---|---|---|---|---|---|
| 1st day | 7th day | 15th day | 1st month | 2nd month | 3rd month | |||
| Healing | Absent | 10 (100.0) | 0 | 0 | 0 | 0 | 0 | 0.00025 |
| Present | 0 | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | ||
| Break down | Absent | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | – |
| Present | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Liquefaction necrosis | Absent | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | – |
| Present | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Infection | Absent | 9 (90.0) | 8 (80.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 0.4112 |
| Present | 1 (10.0) | 2 (20.0) | 0 | 0 | 0 | 0 | ||
| Pain | Absent | 0 | 2 (20.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 0.00025 |
| Present | 10 (100.0) | 8 (80.0) | 0 | 0 | 0 | 0 | ||
| Foul smell | Absent | 5 (50.0) | 8 (80.0) | 10 (100.0) | 9 (90.0) | 10 (100.0) | 10 (100.0) | 0.0952 |
| Present | 5 (50.0) | 2 (20.0) | 10 (100.0) | 1 (10.0) | 10 (100.0) | 10 (100.0) | ||
| Colour | Absent | 10 (90.0) | 7 (70.0) | 1 (10.0) | 0 | 0 | 0 | 0.005 |
| Present | 0 (10.0) | 3 (30.0) | 9 (90.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | ||
| Graft epitheliasation | Absent | 10 (100.0) | 3 (30.0) | 0 | 0 | 0 | 0 | 0.00025 |
| Present | 0 | 7 (70.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | ||
| Residual defects | Absent | 9 (90.0) | 9 (90.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 0.4113 |
| Present | 1 (10.0) | 1 (10.0) | 0 | 0 | 0 | 0 | ||
| Complications | Absent | 9 (90.0) | 9 (90.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 0.4113 |
| Present | 1 (10.0) | 1 (10.0) | 0 | 0 | 0 | 0 | ||
Complications
Partial loss of graft was seen in 1 patient on the 1st and 7th post operative day which resolved by the 15th post operative day and was not statistically significant (P = 0.4113).
Post operative mouth opening was measured in mm and observed to be in the range of 12–26 mm on the 1st post operative day with mean SD of 15.70 ± 4.06, 18–30 mm on 7th post operative day with mean SD of 24.60 ± 4.65, 22–37 mm on 15th post operative day with mean SD of 29.90 ± 4.99, 24–40 mm on the first month post operative day with the mean SD of 33.20 ± 5.12, 32–42 mm on 2nd month post operative day with mean SD of 36.80 ± 3.52, 34–42 mm on the 3rd month post operative day with mean SD of 38.90 ± 2.06.
Mouth opening was observed to be linearly and significantly increasing during the study period with P < 0.001.
Discussion
Intra oral defects may be obturated with local flaps such as a buccal advancement flap, a palatal pedicled flap, or a double-layered closure flaps using buccal and palatal tissues [3, 4]. However, the forementioned procedures produce large denuded areas, resulting in degrees of vestibular sulcus reduction and cannot be used to close large defects [4]. Distant flaps (tongue, temporalis muscle or naso-labial flaps etc.) have also been successfully used for intra oral reconstruction but, they are generally not preferred because of their invasiveness [2]. The buccal fat pad has gained popularity in the recent years. This mass of fatty tissue was apparently first mentioned by Heister.
The advantages of the buccal fat pad flap are simplicity and ease of technique and the high success rate [5]. To consider this as a success, the criteria that was taken into account was the complete epithelialisation of the graft [6]. The rich vascularity may explain the high success rate with this flap [7, 8]. The technique is simple. Nevertheless, some important points must be remembered. The most important is the careful manipulation of the flap in order to maintain its thin capsule. Mechanical suction must be avoided once the buccal fat pad is exposed. Further, blunt dissection, preferably with two vascular clamps, is mandatory. One to gently pull out the emergent part and the other to dissect the oral mucosa and other tissues surrounding the buccal fat pad [6].
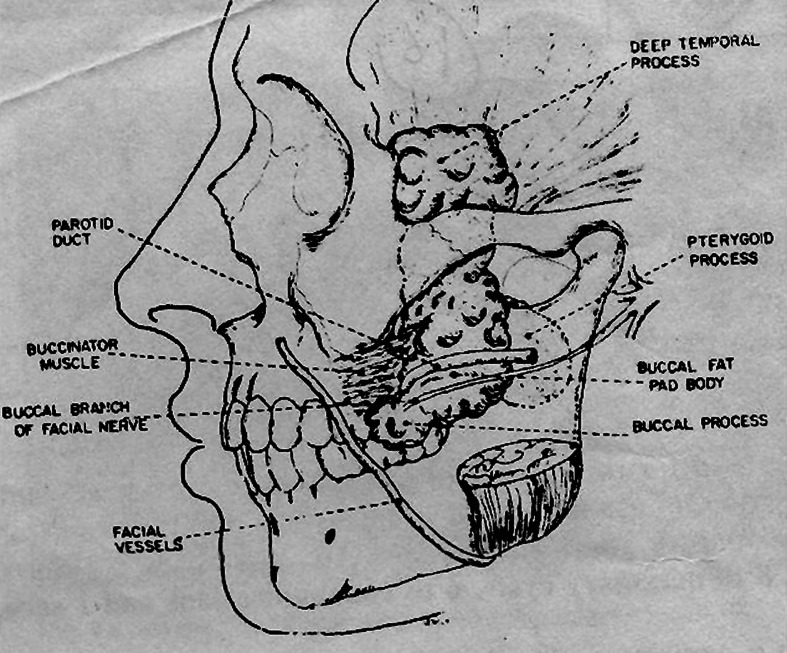
The buccal fat pad is a mass of specialized fatty tissue which is distinct from sub cutaneous fat. It was first mentioned by Heister. It consists of a main body and four extensions, buccal, pterygoid, superficial and deep temporal (Fig. 7). The body is centrally positioned and is located above the parotid duct and extends along the anterior border of masseter muscle. It courses medially to rest on the periosteum of the posterior maxilla and overlies the upper most fibres of buccinator muscle. Posteriorly it travels through the pterygo maxillary fissure in contact with the maxillary artery. The buccal extension lies superficially within the cheek. The pterygoid extension rests on the pterygoid muscle. The superficial and deep temporal extensions reside in the temporal region (Fig. 1). The average volume of the buccal fat pad is approximately 10 ml with a mean thickness of about 6 mm. The buccal fat is a specialized type of fat termed as syssarcosis, it enhances inter muscular motion. It is enclosed in a facial envelope which when open leads to herniation of the fat pad. The buccal fat pad has a good supply, efficient uptake at recipient site and spontaneous epithelialisation in oral cavity.
Fig. 7.
Anatomy of buccal fat pad
Egyedi first published the use of buccal fat pad as a pedicled graft for closure of oral defects, he lined it with split thickness skin graft [9]. Neder described the use of buccal fat pad as a free graft for intra oral defects [10]. Tideman showed that the pedicled fat pad graft when unlined would epithelialise in 1–4 weeks and therefore the use a split thickness graft was not necessary [11]. Yen first described application of buccal fat pad for oral sub mucous fibrosis, He found that a pedicled graft of buccal fat enables closure of oral defects up to 60 mm × 60 mm and 6 mm in thickness. He found no obliteration of the oral vestibule and very little morbidity at the donor site as compared to other local flaps [12]. Mehrotra et al. also used buccal fat pad in various maxillofacial surgeries like sub mucosal fibrosis, oro-antral fistulae and scar tissue adhesions in the cheek with good results [13]. Stajcic reported the use of pedicled buccal fat pad in the closure of oro-nasal and oro-antral communication following extractions in 56 patients with excellent results.
Hao reported that ideal defects to be reconstructed with the buccal fat pad are the maxillary defects due to their close anatomical locations. However, it can be applied in areas ranging from the angle of the mouth to the retromolar trigone and palate [14].
Size limitations of buccal fat pad must be known in order to provide successful outcome. Rapidis et al. have stated that in maxillary defects measuring more than 4 × 4 × 3 cm, the possibility of partial dehiscence of the flap is high due to impaired vascularity of the stretched ends of the flap [15]. Granizo et al. have stated that the closure of larger defects cannot be guaranteed without producing flap necrosis or creating a new fistula [6]. The buccal fat pad is an encapsulated, rounded, biconvex specialized fatty tissue, which is distinct from subcutaneous fat [16]. Buccal fat pad was considered a surgical nuisance for many years because of its accidental encounter during various operations in the pterygo mandibular area such as tumor, orthognathic, or trauma surgeries [15–17]. Successful closure of oro-antral fistula with buccal fat pad is widely reported in the literature. The use of buccal fat pad as a pedicled graft for closure of oral defects have been reported with good results [6, 8, 17].
Pedicled buccal fat pad has also been employed in the closure of surgical defects following tumor excision [7, 16], excision of leukoplakia and sub mucous fibrosis [16, 18], as well as closure of primary and secondary palatal clefts [5, 19].
When properly dissected and mobilized, the buccal fat pad provides a 7 × 4 × 3 cm pedicled graft. This graft with partly an axial pattern transfer from the transverse facial artery and partly with a mesenchymal random pattern transfer, owes its vascular inflow and outflow to small arterioles and venules in the flap. It therefore must be handled with great care and with a wide base preserved; otherwise a free fat graft will result [15].
The transferred buccal fat pad starts to epithelialise in a week and completes its epithelialisation within 6 weeks. At that time, the graft is covered with healthy looking oral mucosa. Although the histological process of epithelialisation has not been fully documented, it seems that the superficial layer of fat tissue is replaced by granulation tissue and is finally covered by stratified squamous epithelium migrating from the regions neighbouring the margins of the flap [15, 20]. Samman et al. examined histological samples from reconstructions and found that the surface of the healed graft was formed by Para keratotic stratified squamous epithelium with flattened rete ridges. No fat cells could be identified when paraffin embedded sections were stained with osmium tetra oxide. These finding support the view that the surface of the fat is replaced by fibrous tissue at least to the depth of 6–8 mm biopsy specimen [15, 17]. Epithelialisation in seven of the ten patients in our study on the seventh day and complete epithelialisation was observed after 4 weeks in all the patients. This is in agreement with the established facts in the literature.
The colour changes in the graft from yellowish white to red was attributed to the progression of epithelialisation which was seen after the seventh post operative day in three cases. The complete colour change of the graft was noticed from yellowish white to red by the end of 4 weeks owing to complete epithelialisation of the graft.
Healing of the graft usually occurred within 2–3 weeks, leaving a good mucosal surface [8, 21]. Histological nature of the healing process of the buccal fat pad was first reported by Samman et al. indicating partial fibrosis of the fatty tissue. In our study we observed that healing started from the first post operative day and was complete within 2–3 weeks post operatively.
Although infection is mentioned as a potential complication, analysis of the reported cases in our study proved that it is not true, because it has been reported in only one case on the first post operative day and two cases on the seventh post operative day which subsided subsequently.
The use of buccal fat pad in patients with prior local radiotherapy, malar hypoplasia, thin cheeks, Down’s syndrome is contraindicated [4, 15]. In our study we were not able to expose the buccal fat pad adequately in one of the patients with thin cheeks leading to a residual defect from insufficient graft material which was seen on the first and the seventh post operative day. It was left to epithelialise spontaneously but this did not seem to have any influence on the final result.
Foul smell was present in five of the ten cases in the first post operative day which was noticed in two of the patients on the seventh day owing to the infection which subsided subsequently. Foul smell was noticed in one of the patients at the end of first month post operatively due to the maintenance of bad oral hygiene.
According to Yeh and Malik the buccal fat pad, because of its anatomic position and the ease with which it can be approached and mobilized without causing any noticeable defects extra orally is best suited. It is highly vascularised graft and can cover the defect up to 3 × 5 cm in size without compromising its vascularity. Because of proximity of donor site to recipient site no other incision is required and other surgical procedure is not required to obtain the graft or its detachment. This pedicled graft is highly vascularised, so it is said to be resistant to infection. The procedure required to cover the buccal defect is not lengthy. It is simple and very much convenient to the patient. If it fails as a graft material, consequences are not serious, as other options are open. Because of all above advantages it was felt to use this pedicled buccal fat pad in the surgical management of oral sub mucous fibrosis [22, 23].
According to Yeh study in nine patients with oral sub mucous fibrosis, average increased mouth opening was 18.6 mm and according to Malik study in fifteen patients, average increased mouth opening was 24.3 mm. (Belgaum). In our study the mean of the pre operative mouth opening in patients with oral sub mucous fibrosis was 15.70 mm. The post operative mouth opening at the 3rd month was a mean of 38.90 mm. In addition to the advantage in mouth opening, it has the added advantage of the use of a local flap from the near proximity which results in a decreased morbidity, the reconstructive modality far more pliable than other modality. It also permits the use of other more complex modalities for reconstruction, should the need arise [22, 23].
Conclusion
The rich blood supply of the buccal fat pad and its easy mobilization and fewer complications make it an ideal flap. The use of buccal fat pad graft has been shown to be an easy, well tolerated and uncomplicated technique for reconstruction of intra oral defects. Because of these features of the buccal fat pad, it can be considered as a reliable modality for closure of defects that could not be repaired by other conventional procedures. The pliability of this fat pad permits it to be moulded to the shape of the defect or the area to be reconstructed, thus mimicking the physical characteristics of the recipient bed. If properly applied in selected cases, it results in complete success. In the light of these findings, we hope that the buccal fat pad will be used more often for various purposes in the future.
References
- 1.Booth PW (2006) Text book of oral & maxillofacial surgery
- 2.Alkan A, Dolanmaz D, Uzun E, Erdem E. The reconstruction of oral defects with buccal fat pad. Swiss Med Wkly. 2003;133:46–470. doi: 10.4414/smw.2003.10174. [DOI] [PubMed] [Google Scholar]
- 3.Guven O. A clinical study on oroantral fistulae. J Cranio Maxillofac Surg. 1998;26:267–271. doi: 10.1016/S1010-5182(98)80024-3. [DOI] [PubMed] [Google Scholar]
- 4.El-Hakim IE, El-Fakharany AM. The use of pedicled buccal fat pad and palatal rotating flaps in closure of oroantral communication and palatal defects. J Laryngol Otol. 1999;113:834–838. doi: 10.1017/S0022215100145335. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK. The use of a pedicled buccal fat pad graft for bone coverage in primary palatorrhapy: a case report. J Oral Maxillofac Surg. 2001;59(12):1499–1501. doi: 10.1053/joms.2001.28294. [DOI] [PubMed] [Google Scholar]
- 6.Martin Granizo R, Naval L, Costas A, Goizueta C, Rodriguez F, Monje F, Munoz M, Diaz F. Use of the buccal fat pad to repair intraoral defects: review of 30 cases. Br J Oral Maxillofac Surg. 1997;35:81–84. doi: 10.1016/S0266-4356(97)90680-X. [DOI] [PubMed] [Google Scholar]
- 7.Baumann A, Ewers R. Application of the buccal fat pad in oral reconstruction. J Oral Maxillofac Surg. 2000;58:389–392. doi: 10.1016/S0278-2391(00)90919-4. [DOI] [PubMed] [Google Scholar]
- 8.Abuabara A, Cortez ALV, Passeri LA, de Moraes M, Moreira RWF. Evaluation of different treatments for oroantral/oronasal communications: experience of 112 cases. Int J Oral Maxillofac Surg. 2006;35:155–158. doi: 10.1016/j.ijom.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Egyedi P. Utilisation of buccal fat pad for closure of oro-antral communication. J Maxillofac Surg. 1977;5:241–243. doi: 10.1016/S0301-0503(77)80117-3. [DOI] [PubMed] [Google Scholar]
- 10.Neder A. Use of buccal fat pad. Oral Surg Oral Med Oral Pathol. 1983;55:349–350. doi: 10.1016/0030-4220(83)90187-1. [DOI] [PubMed] [Google Scholar]
- 11.Lt Col AK Mehta, Col SS Panwar, Surg Cdr RK Verma, Lt Col AK Pal (2003) Buccal fat pad reconstruction in oral sub mucous fibrosis. MJAFI 59:340–341 [DOI] [PMC free article] [PubMed]
- 12.Yen DJ. Surgical treatment of sub mucous fibrosis. J Oral Surg. 1986;54:230–269. doi: 10.1016/0030-4220(82)90094-9. [DOI] [PubMed] [Google Scholar]
- 13.Mehrotra (2001) Buccal fat pad for reconstruction in maxillofacial surgery. Ind J Maxillofac Surg 16:7–11
- 14.Hao SP. Reconstruction of oral defects with the pedicled buccal fat pad flap. Otolaryngol Head Neck Surg. 2000;122:863–867. doi: 10.1016/S0194-5998(00)70015-5. [DOI] [PubMed] [Google Scholar]
- 15.Rapidis AD, Alexandridis CA, Eleftheriadis E, Angelopoulos AP. The use of the buccal fat pad reconstruction of oral defects; review of the literature and report of 15 cases. J Oral Maxillofac Surg. 2000;58:158–163. doi: 10.1016/S0278-2391(00)90330-6. [DOI] [PubMed] [Google Scholar]
- 16.Adeyemo WL, Ogunlewe MO, Ladeinde AL, James O. Closure of oro-antral fistula with pedicled buccal fat pad. A case report and review of literature. Afr J Oral Health. 2004;1(1):42–46. doi: 10.4314/ajoh.v1i1.31304. [DOI] [Google Scholar]
- 17.Samman N, Cheung LK, Tideman H. The buccal fat pad in oral reconstruction. Int J Oral Maxillofac Surg. 1993;22:2–6. doi: 10.1016/S0901-5027(05)80346-7. [DOI] [PubMed] [Google Scholar]
- 18.Ho KH. Excision of cheek leukoplakia and lining the defect with a pedicled buccal fat pad graft. Br Dent J. 1989;166:455–456. doi: 10.1038/sj.bdj.4806884. [DOI] [PubMed] [Google Scholar]
- 19.Hudson JW, Anderson JG, Russell RM, et al. Use of pedicled fat pad graft as an adjunct in the reconstruction of palatal cleft defects. Oral Surg. 1995;80:24. doi: 10.1016/s1079-2104(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 20.Hanazawa Y, Itoh K, Mabashi T, Sato K. Closure of oroantral communications using a pedicle buccal fat pad graft. J Oral Maxillofac Surg. 1995;53:1392–1396. doi: 10.1016/0278-2391(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 21.Tideman H, Bosanquet A, Scott J. Use of the buccal fat pad as a pedicled graft. J Oral Maxillofac Surg. 1986;44:435. doi: 10.1016/S0278-2391(86)80007-6. [DOI] [PubMed] [Google Scholar]
- 22.Malik NA, Deshpande MD, Pradhan VN. Use of the buccal fat pad in oral sub mucous fibrosis, Ind J Oral Maxillofac Surg XII(1):19–24
- 23.Yeh CY. Application of the buccal fat pad to the surgical treatment of oral sub mucous fibrosis. Int J Oral Maxillofac Surg. 1996;25:130. doi: 10.1016/S0901-5027(96)80058-0. [DOI] [PubMed] [Google Scholar]