Abstract
Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a binding receptor for hepatitis C virus (HCV). Binding of HCV envelope protein E2 to target cells is a prerequisite to DC-SIGN-mediated signaling. Using cell lines with stable or transient expression of DC-SIGN, we investigated effects of soluble HCV E2 protein on ERK pathway. MEK and ERK are activated by the E2 in NIH3T3 cells stably expressing DC-SIGN. Treatment of the cells with antibody to DC-SIGN results in inhibition of the E2 binding as well as the E2-induced MEK and ERK activation. In HEK293T cells transiently expressing DC-SIGN, activation of MEK and ERK is also induced by the E2. Activation of ERK pathway by HCV E2 through DC-SIGN provides useful information for understanding cellular receptor-mediated signaling.
Keywords: DC-SIGN, HCV, Envelope protein E2, ERK
Introduction
Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is expressed on dendritic cells and some subsets of macrophages (Geijtenbeek et al. 2000; Soilleux et al. 2002). A variety of viruses, such as human immunodeficiency virus (HIV), Ebola virus, cytomegalovirus, dengue virus, hepatitis C virus (HCV), human herpesvirus 8, measles virus, West Nile virus, severe acute respiratory syndrome coronavirus, avian H5N1 influenza virus, and herpes simplex virus, bind to susceptible cells by targeting DC-SIGN, establishing chronic infection (Pöhlmann et al. 2001; Alvarez et al. 2002; Halary et al. 2002; Tassaneetrithep et al. 2003; Gardner et al. 2003; Rappocciolo et al. 2006; de Witte et al. 2006; Davis et al. 2006; Chen and Subbarao 2007; Wang et al. 2008; de Jong et al. 2008). DC-SIGN enhances viral entry into target cells through interaction with the viral envelope glycoproteins (Lozach et al. 2007). As HCV envelope binding receptor, DC-SIGN captures and delivers HCV particles to the liver through interaction with HCV envelope protein E2 (Lozach et al. 2003; Pöhlmann et al. 2003).
Modulation of some signaling pathways by DC-SIGN leads to the induction of immune responses against numerous pathogens and especially Raf-1 plays a central role in the processes (den Dunnen et al. 2009; Gringhuis and Geijtenbeek 2010; Geijtenbeek et al. 2009; Svajger et al. 2010). For instance, modulation of Toll-like receptor signaling by DC-SIGN via Raf-1-dependent acetylation of NF-kappaB is involved in regulation of adaptive immunity by dendritic cells to bacterial, fungal, and viral pathogens (Gringhuis et al. 2007). Binding of the HIV-1 envelope glycoprotein gp120 to DC-SIGN induces Raf-1-dependent phosphorylation of the NF-kappaB subunit p65, which is required for the generation of full-length HIV transcripts (Gringhuis et al. 2010). Increased Rho-GTPase activity triggered by HIV-stimulated DC-SIGN signaling is required for HIV replication (Hodges et al. 2007). The phagocytosis of Escherichia coli by HEK293/DC-SIGN stable transfectants could be inhibited by the inhibitors specific for Raf and NF-kappaB (Iyori et al. 2008). Thus, signaling events triggered by pathogens through DC-SIGN may provide a molecular basis for the mechanisms underlying infection and immunity.
Binding of HCV envelope protein E2 to target cells is a prerequisite to cellular receptor-mediated signaling. Given the involvement of early signaling events in the viral pathogenesis, we focused on regulation of mitogen-activated protein kinase (MAPK) pathways by HCV E2 through the relevant receptors (Zhao et al. 2005, 2006, 2007). It has been shown that ERK, a key MAPK pathway, is affected by HCV proteins or infectious virus and plays an impotent role in HCV pathogenicity. Activation of ERK signaling is detectable in stable cell lines expressing HCV core protein, and that HCV core protein neurotoxicity may be mediated by sustained activation of ERK (Giambartolomei et al. 2001; Paulino et al. 2011). HCV E2 protein activates ERK in human hepatic stellate cells (Mazzocca et al. 2005). Blocking ERK pathway by kinase inhibitor U0126 reduces HCV replication in human hepatoma cells (Pei et al. 2011). Recent report shows that HCV activates ERK signaling cascades involved in activation of matrix metalloproteinase-2 and B cell lymphoma 2 (Li et al. 2012). We have reported that p38 MAPK pathway is upregulated by HCV E2 in HEK293T cells transiently expressing DC-SIGN (Chen et al. 2010). Based on our previous work, using cell lines with stable or transient expression of DC-SIGN, we further investigated effects of HCV E2 protein on ERK pathway.
Materials and methods
Materials
DC-SIGN expression plasmid pcDNA3-DC-SIGN was obtained through the AIDS Research and Reference Reagent Program (NIAID, NIH). The DC-SIGN cDNA was amplified from human dendritic cells and cloned into pcDNA3 vector (Pöhlmann et al. 2001). Soluble HCV subtype 1a E2 protein expressed in Chinese hamster ovary cells and mouse anti-E2 mAb were kind gifts of Michael Houghton (Chiron Corporation, USA) (Mazzocca et al. 2005). Goat anti-HCV E2 antibody was purchased from Biodesign International (Saco, Maine). Mouse anti-human DC-SIGN mAb (clone DCN46) was purchased from BD PharMingen (San Diego, CA). Rabbit antibodies against MEK, ERK, phospho-MEK (Ser217/221), or phospho-ERK (Thr202/Tyr204) were from Cell Signaling Technology (Beverly, MA). Fluorescein isothiocyanate-conjugated rabbit anti-goat IgG was from Jackson ImmunoResearch (West Grove, PA). Alkaline phosphatase-conjugated goat anti-rabbit IgG was from Vector Lab (Burlingame, CA). Mannan, nitro blue tetrazolium, and 5-bromo-4-chloro-3-indolyl-phosphate were from Sigma (St. Louis, MO). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA).
Cell lines
NIH3T3 cells stably expressing DC-SIGN (NIH3T3/DC-SIGN) was obtained through the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). The NIH3T3/DC-SIGN was generated by stable transduction of NIH3T3 with MLV vector MX-DC-SIGN encoding human DC-SIGN (Wu et al. 2002). NIH3T3/DC-SIGN, NIH3T3, and HEK293T cells were grown in Dulbecco’s modified Eagle’s medium containing 10 % fetal bovine serum.
Flow cytometry
NIH3T3/DC-SIGN and NIH3T3 cells were incubated for 1 h at 37 °C with 6 μg/ml HCV E2 protein, washed twice with phosphate-buffered saline (PBS) to remove unbound protein, followed by incubation with 6 μg/ml goat anti-E2 Ab for another 1 h. After two washes with PBS, cells were stained with Fluorescein isothiocyanate-conjugated rabbit anti-goat IgG for 30 min at 4 °C, washed with PBS, fixed in 1 % paraformaldehyde, and subjected to flow cytometry on a FACSCalibur (BD, San Jose, CA) using CellQuest software for data acquisition and analysis.
For receptor competition assay, NIH3T3/DC-SIGN cells were pre-incubated for 1 h with 10 μg/ml anti-DC-SIGN mAb, washed twice with PBS, and incubated with 6 μg/ml E2 protein for another 1 h. After two washes with PBS, cells were incubated with goat anti-E2 Ab and the E2 binding was detected as above.
Cell treatment
NIH3T3/DC-SIGN cells were cultured in the serum-free medium containing 2 μg/ml HCV E2 protein for 5 min, 15 min, 30 min, 1 h, and 12 h, respectively. Cells were washed with PBS and lysed as described (Zhao et al. 2005). Two micrograms per milliliter of E2 was mixed with 4 μg/ml mouse anti-E2 mAb at 37 °C for 1 h. Cells were serum starved for 48 h prior to treatment for 30 min at 37 °C with 2 μg/ml E2, the mixture of E2-E2 mAb, or mannan at various concentrations (0, 10, 200, and 400 μg/ml). In addition, cells serum-starved were pre-incubated for 1 h with anti-DC-SIGN mAb at different concentrations (0, 5, 10, and 20 μg/ml) prior to treatment with 2 μg/ml E2. After the above treatment, cell lysates were prepared and subjected to Western blot analysis.
Transient transfection
Transient transfection was performed using Lipofectamine 2000 according to the manufacturer’s instructions. To evaluate response to HCV E2 in transfectants, DC-SIGN expression plasmid pcDNA3-DC-SIGN or pcDNA3.1 vector was transfected into HEK293T cells. At 48 h after transfection, cells were serum starved for an additional 7 h before treatment with 2 μg/ml E2 or the E2-E2 mAb for 30 min at 37 °C. Cells were harvested and lysed for measurement of DC-SIGN and kinases.
Western blotting
Proteins in cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and blocked with nonfat milk. Membranes were incubated overnight at 4 °C with antibodies against total and phosphorylated kinases or DC-SIGN, followed by incubation with alkaline phosphatase-conjugated secondary antibody. Immune complexes were visualized with 5-bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium substrates. In some experiments, protein band intensity was quantified using GeneTools software from SynGene (Cambridge, UK).
Results
HCV E2 binds to NIH3T3/DC-SIGN cells
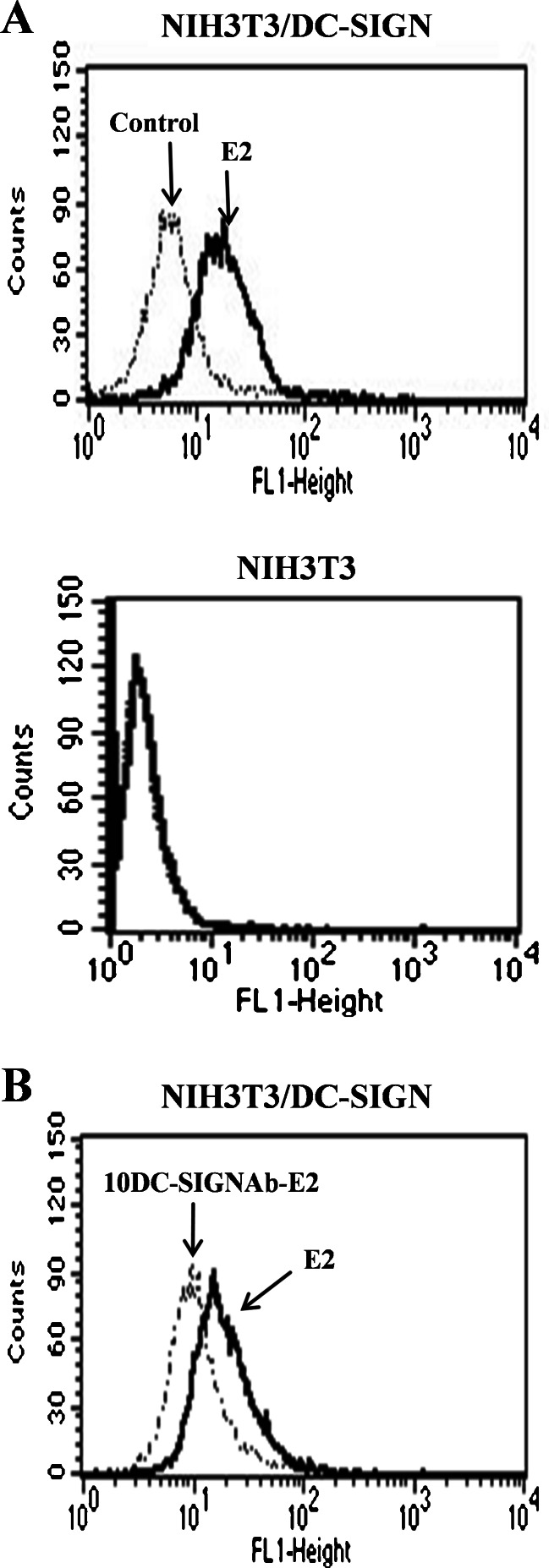
Binding of HCV E2 protein to target cells via relevant receptors is required for subsequent signaling. The soluble HCV E2 protein was used as a biological signal to determine whether the E2-DC-SIGN interaction is associated with ERK pathway. NIH3T3/DC-SIGN with stable expression of DC-SIGN was chosen to be a cell model in response to the HCV E2. HCV E2 binding to NIH3T3/DC-SIGN was analyzed by flow cytometry. Figure 1a showed that the E2 bound to NIH3T3/DC-SIGN cells at a high level, which is consistent with the notion that DC-SIGN is a high affinity binding receptor for HCV E2 (Lozach et al. 2003). Control was omission of the E2 incubation and staining with the goat anti-E2 Ab and the secondary antibody to establish background level of binding. In contrast, the E2 did not bind to DC-SIGN-deficient parental NIH3T3 cells. Next, the E2 binding was evaluated in the cells pre-incubated with anti-DC-SIGN mAb prior to the E2 incubation. Figure 1b showed that the anti-DC-SIGN mAb pre-incubation led to inhibition of the E2 binding. Thus, DC-SIGN mediates HCV E2 protein binding to target cells.
Fig. 1.
HCV E2 binding to NIH3T3/DC-SIGN via DC-SIGN. a NIH3T3/DC-SIGN and NIH3T3 cells were incubated with the E2 and the E2 binding was detected by flow cytometry using goat anti-E2 Ab and Fluorescein isothiocyanate-conjugated rabbit anti-goat IgG. Cells were stained with the goat anti-E2 Ab and the secondary antibody as control. b NIH3T3/DC-SIGN cells were pre-incubated with anti-DC-SIGN mAb prior to the E2 incubation. The E2 binding was analyzed as above. Similar results were obtained in three independent experiments, and one representative experiment is shown
ERK pathway is activated by HCV E2
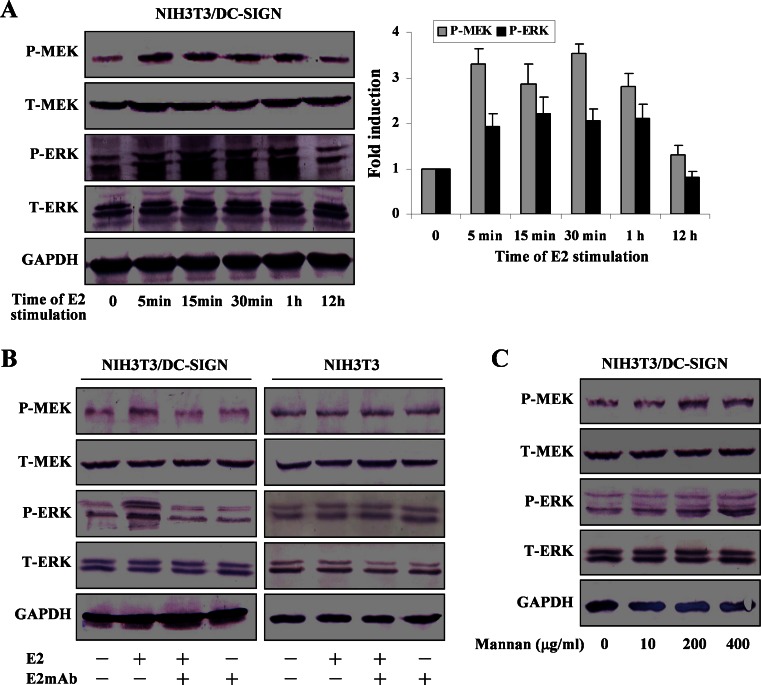
MEK-ERK cascades were analyzed in NIH3T3/DC-SIGN cells cultured with HCV E2 protein for the different time periods. Western blot analysis showed that phosphorylation of MEK and ERK was markedly enhanced after the E2 incubation (Fig. 2a). The kinase phosphorylation was rapidly increased by the E2 at 5 min, maintaining at higher levels by 1 h, and declining at 12 h. Total MEK and ERK were constant in samples. Glyceraldehyde-3-phosphate dehydrogenase levels were determined as a control for protein loading. Moreover, levels of MEK and ERK phosphorylation in NIH3T3/DC-SIGN cells stimulated with the E2-E2 mAb were lower than those with the E2 stimulation (Fig. 2b). In case of NIH3T3 cells, the E2 had no effect on phosphorylation of the kinases. The data indicated activation of the kinases by the E2. Mannan is a natural ligand for DC-SIGN. It is thus interesting to examine whether ERK pathway would be affected by mannan. As shown in Fig. 2c, levels of MEK and ERK phosphorylation were slightly increased in NIH3T3/DC-SIGN cells exposed to the various concentrations of mannan, suggesting that ligation of DC-SIGN with mannan also results in activation of ERK pathway.
Fig. 2.
Activation of ERK pathway by HCV E2. a NIH3T3/DC-SIGN cells were cultured with the E2 for the indicated time periods. Cell lysates were prepared for assessment of total (T-) and phosphorylated (P-) MEK and ERK by Western blotting using antibodies against total or phosphorylated kinases. Quantification was made using GeneTools software, and results correspond to the ratio between the amount of phosphorylated MEK or ERK and the amount of total MEK or ERK normalized to the control without E2 stimulation. Results represent mean and SD of three experiments. b NIH3T3/DC-SIGN and NIH3T3 cells were treated with the E2 or the E2-E2 mAb. c NIH3T3/DC-SIGN cells were treated with mannan at the indicated concentrations. Total and phosphorylated MEK and ERK were analyzed by Western blotting. Representative results out of four experiments are shown
ERK signaling is triggered by HCV E2 in NIH3T3/DC-SIGN cells
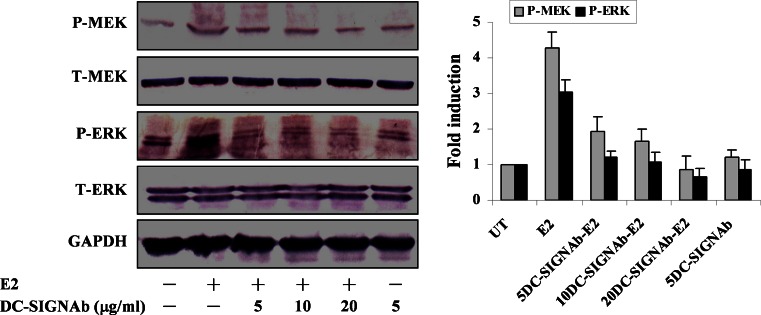
Since DC-SIGN mediated the HCV E2 binding (Fig. 1), we wondered whether the increased phosphorylation of MEK and ERK was induced by the E2 through interaction with DC-SIGN expressed on the cells. The kinases were thus evaluated in NIH3T3/DC-SIGN cells pretreated with anti-DC-SIGN mAb at the indicated concentrations prior to the E2 treatment. We found that pretreatment with the anti-DC-SIGN mAb reduced the E2-induced phosphorylation of MEK and ERK, and 20 μg/ml anti-DC-SIGN mAb almost abolished the increased phosphorylation of kinases (Fig. 3). The inhibition of MEK and ERK phosphorylation was in parallel with the inhibition of the E2 binding by the anti-DC-SIGN mAb (Fig. 1b). As a control, kinase phosphorylation was also evaluated in the cells treated with the anti-DC-SIGN mAb alone. These results indicated that interaction of HCV E2 with DC-SIGN accounts for MEK and ERK activation.
Fig. 3.
Effect of DC-SIGN mAb on MEK and ERK phosphorylation induced by HCV E2. NIH3T3/DC-SIGN cells were treated with anti-DC-SIGN mAb at the indicated concentrations before treatment with the E2. Total (T-) and phosphorylated (P-) MEK and ERK were analyzed by Western blotting. Quantification was made using GeneTools software, and results correspond to the ratio between the amount of phosphorylated MEK or ERK and the amount of total MEK or ERK normalized to the untreated control (UT). Results represent mean and SD of three experiments
ERK signaling is triggered by HCV E2 in HEK293T cells transiently expressing DC-SIGN
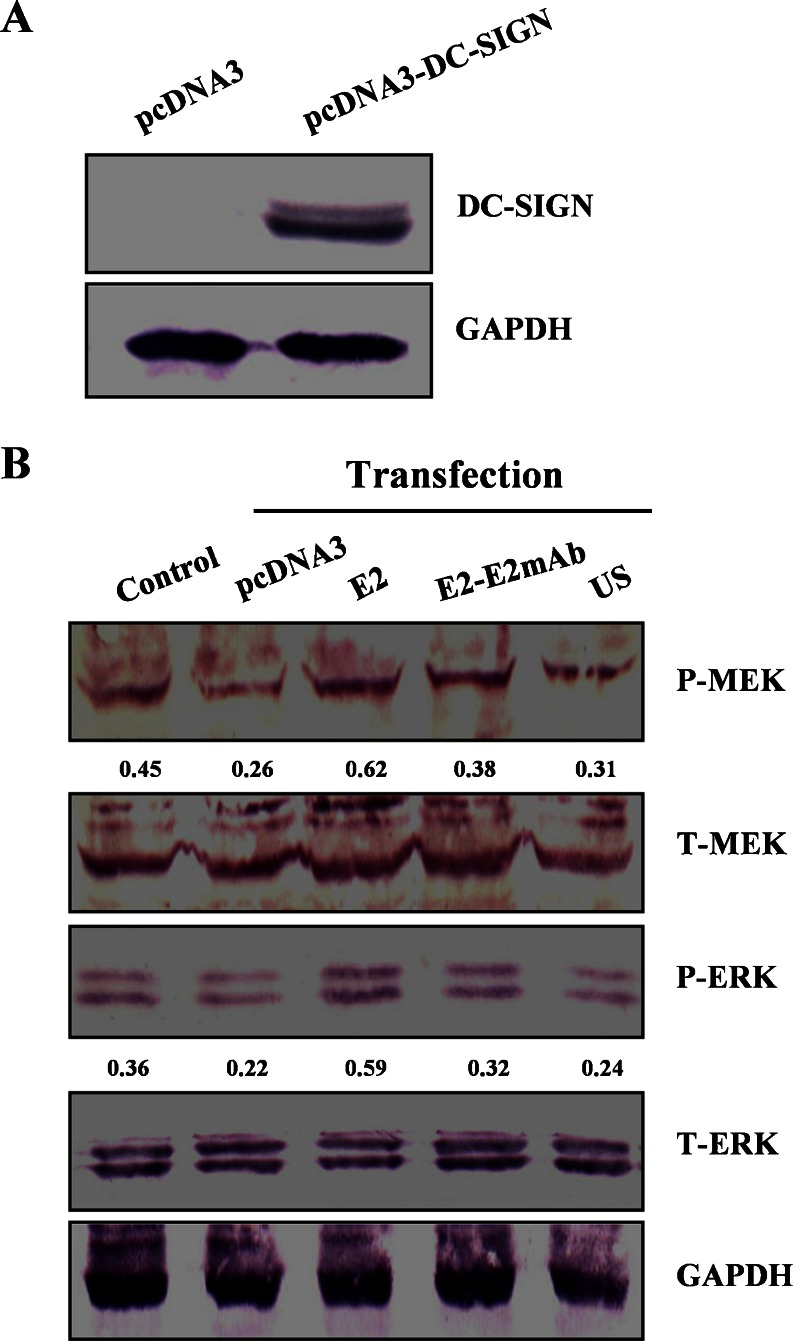
We further investigated regulation of ERK pathway by the HCV E2 in DC-SIGN transient transfectants beyond NIH3T3/DC-SIGN cells. HEK293T is a recommended cell line to express the pcDNA3-DC-SIGN plasmid (Pöhlmann et al. 2001). The signaling ability of DC-SIGN was evaluated in HEK293T with DC-SIGN transfection. At 48 h post-transfection, DC-SIGN was efficiently expressed in HEK293T cells transfected with the pcDNA3-DC-SIGN (Fig. 4a). In the presence of E2, levels of MEK and ERK phosphorylation were increased in the DC-SIGN transfected cells when compared as the un-transfected cells (control) as well as the transfected cells without the E2 stimulation (US), and the levels of MEK and ERK phosphorylation were reduced following the E2-E2 mAb stimulation (Fig. 4b), implying that HCV E2-DC-SIGN interaction is responsible for MEK and ERK phosphorylation.
Fig. 4.
Phosphorylation of MEK and ERK in HEK293T transiently expressing DC-SIGN under HCV E2 stimulation. a HEK293T cells were transfected with pcDNA3-DC-SIGN expression plasmid or pcDNA3 vector, and DC-SIGN expression was detected by Western blotting. b The transfected cells were stimulated with the E2 or the E2-E2 mAb. Total (T-) and phosphorylated (P-) kinases were analyzed by Western blotting. Changes in the amount of phosphorylated MEK or ERK over the amount of total MEK or ERK are shown below each blot. Control untransfected and unstimulated, US pcDNA3-DC-SIGN transfection without the E2 stimulation. Representative results out of three experiments are shown
Discussion
Pathogens are known to trigger a set of pattern recognition receptors including DC-SIGN, leading to activation of intracellular signaling processes that shapes the adaptive immunity (Gringhuis and Geijtenbeek 2010). DC-SIGN ligation results in ERK activation implicated in maturation of dendritic cells (Caparrós et al. 2006). We wondered whether the signaling of DC-SIGN is restricted to dendritic cells with natural expression of DC-SIGN. Using cell lines with stable or transient expression of DC-SIGN, we addressed effects of HCV E2 protein on ERK pathway.
Studies suggest that interference of HCV E2 protein with certain signaling pathways is involved in the molecular mechanisms underlying HCV-associated disorders (Balasubramanian et al. 2003, 2005; Munshi et al. 2003). At an early stage of HCV infection, E2 binds to susceptible cells through interaction with the relevant receptors. We thus assumed that interaction of HCV E2 with the cellular receptors may trigger signaling events responsible for HCV pathogenesis. Many reports have demonstrated the importance of DC-SIGN engagement with toll-like receptor signaling in the maturation process of dendritic cells. However, signaling events triggered by HCV E2 through DC-SIGN are poorly defined. HCV receptors including CD81, low-density lipoprotein receptor, and scavenger receptor class B type 1 have been identified using the truncated soluble HCV envelope glycoprotein E2 (Flint et al. 1999; Wünschmann et al. 2000; Scarselli et al. 2002). In this study, influence of the soluble HCV subtype 1a E2 protein on ERK signaling was evaluated. In accordance with previous reports that the different forms of HCV E2 protein interacted with some primary cells and cell lines stably or naturally expressing DC-SIGN (Gardner et al. 2003; Lozach et al. 2003; Pöhlmann et al. 2003; Cormier et al. 2004), our data showed that the HCV E2 protein bound to target cells via DC-SIGN. We next investigated response of ERK pathway to the E2 stimulation. The specific phosphorylation and activation of MEK and ERK was induced by the E2 in NIH3T3/DC-SIGN but not NIH3T3 cells, implying that DC-SIGN mediates E2-triggered ERK signaling. Mannan inhibits HCV E2 protein binding to DC-SIGN as well as HCV pseudovirus entry into immature dendritic cells (Gardner et al. 2003; Lozach et al. 2003; Cormier et al. 2004; Kaimori et al. 2004). In our experiments, mannan was assessed for its capacity to affect ERK pathway. The ligation of DC-SIGN with mannan resulted in the slight activation of MEK and ERK. Similar to the viral ligand HCV E2, mannan also initiates ERK pathway by targeting DC-SIGN.
CD81 is a central regulator of cellular events required for HCV infection. Binding of HCV E2 to CD81 induces ERK phosphorylation (Mazzocca et al. 2005). The CD81 engagement activates Raf/MEK/ERK signaling cascades, resulting in affecting postentry events of HCV life cycle (Brazzoli et al. 2008). We reported the regulation of MAPK pathways by the HCV E2 through CD81 and low-density lipoprotein receptor (Zhao et al. 2005, 2006, 2007). Respiratory syncytial virus glycoprotein G interaction with DC-SIGN activates ERK, allowing immunomodulation and diminution of dendritic cell activation (Johnson et al. 2012). Consistent with this finding, our data showed that the phosphorylation of MEK and ERK was increased by the E2 in NIH3T3/DC-SIGN and HEK293T cells stably or transiently expressing DC-SIGN. The competitive experiment was carried out to validate the specificity of E2-DC-SIGN interaction responsible for such increase. The inhibition of E2-induced kinase phosphorylation by the antibody against DC-SIGN was consistent with the inhibitory effect on the E2 binding, indicating that the interaction of DC-SIGN with HCV E2 accounts for the activation of ERK pathway. Further studies are still necessary to establish the linkage between DC-SIGN ligation and ERK activation. Our data suggest that the interaction of DC-SIGN with HCV E2 triggers ERK signaling, which may deliver a survival signal to susceptible cells required for HCV entry and replication. Activation of Ras/Raf/MEK pathway facilitates HCV replication (Zhang et al. 2012). Although biological functions for the activation of ERK pathway in relation to HCV infection remain to be elucidated, the ERK signaling triggered by E2 through DC-SIGN represents an early signaling event following HCV infection.
Acknowledgments
We acknowledge T.D. Martin and V.N. KewalRamani (the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) for providing the NIH3T3/DC-SIGN cells; S. Pöhlmann, F. Baribaud, F. Kirchhoff, and R.W. Doms (the AIDS Research and Reference Reagent Program, NIAID, NIH) for providing the pcDNA3-DC-SIGN plasmid; and Chiron Corporation (Emeryville, USA) for providing the HCV E2 protein and the E2 mAb. This work was supported by grants from the National Natural Science Foundation of China (30771928) and the Shanghai Leading Academic Discipline Project (B901).
Footnotes
Lan-Juan Zhao and Wen Wang contributed equally to this work.
References
- Alvarez CP, Lasala F, Carrillo J, Muñiz O, Corbí AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- Balasubramanian A, Koziel M, Groopman JE, Ganju RK. Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clin Infect Dis. 2005;41(Suppl 1):S32–37. doi: 10.1086/429493. [DOI] [PubMed] [Google Scholar]
- Brazzoli M, Bianchi A, Filippini S, Weiner A, Zhu Q, Pizza M, Crotta S. CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes. J Virol. 2008;82:8316–8329. doi: 10.1128/JVI.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparrós E, Munoz P, Sierra-Filardi E, Serrano-Gómez D, Puig-Kröger A, Rodríguez-Fernández JL, Mellado M, Sancho J, Zubiaur M, Corbí AL. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- Chen J, Subbarao K. The immunobiology of SARS. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Chen QL, Zhu SY, Bian ZQ, Zhao LJ, Cao J, Pan W, Qi ZT. Activation of p38 MAPK pathway by hepatitis C virus E2 in cells transiently expressing DC-SIGN. Cell Biochem Biophys. 2010;56:49–58. doi: 10.1007/s12013-009-9069-0. [DOI] [PubMed] [Google Scholar]
- Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci U S A. 2004;101:14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CW, Nguyen HY, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MA, de Witte L, Bolmstedt A, van Kooyk Y, Geijtenbeek TB. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. J Gen Virol. 2008;89:2398–2409. doi: 10.1099/vir.0.2008/003129-0. [DOI] [PubMed] [Google Scholar]
- de Witte L, Abt M, Schneider-Schaulies S, van Kooyk Y, Geijtenbeek TB. Measles virus targets DC-SIGN to enhance dendritic cell infection. J Virol. 2006;80:3477–3486. doi: 10.1128/JVI.80.7.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol Immunother. 2009;58:1149–1157. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint M, Maidens C, Loomis-Price LD, Shotton C, Dubuisson J, Monk P, Higginbottom A, Levy S, McKeating JA. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73:6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JP, Durso RJ, Arrigale RR, Donovan GP, Maddon PJ, Dragic T, Olson WC (2003) L-SIGN (CD 209 L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci U S A 100:4498–4503 [DOI] [PMC free article] [PubMed]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, den Dunnen J, Gringhuis SI. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009;4:879–890. doi: 10.2217/fmb.09.51. [DOI] [PubMed] [Google Scholar]
- Giambartolomei S, Covone F, Levrero M, Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene. 2001;20:2606–2610. doi: 10.1038/sj.onc.1204372. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Geijtenbeek TB. Carbohydrate signaling by C-type lectin DC-SIGN affects NF-kappaB activity. Methods Enzymol. 2010;480:151–164. doi: 10.1016/S0076-6879(10)80008-4. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- Halary F, Amara A, Lortat-Jacob H, Messerle M, Delaunay T, Houlès C, Fieschi F, Arenzana-Seisdedos F, Moreau JF, Déchanet-Merville J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/S1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, Simmons A. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- Iyori M, Ohtani M, Hasebe A, Totsuka Y, Shibata K. A role of the Ca2+ binding site of DC-SIGN in the phagocytosis of E. coli. Biochem Biophys Res Commun. 2008;377:367–372. doi: 10.1016/j.bbrc.2008.09.142. [DOI] [PubMed] [Google Scholar]
- Johnson TR, McLellan JS, Graham BS. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J Virol. 2012;86:1339–1347. doi: 10.1128/JVI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimori A, Kanto T, Kwang Limn C, Komoda Y, Oki C, Inoue M, Miyatake H, Itose I, Sakakibara M, Yakushijin T, Takehara T, Matsuura Y, Hayashi N. Pseudotype hepatitis C virus enters immature myeloid dendritic cells through the interaction with lectin. Virology. 2004;324:74–83. doi: 10.1016/j.virol.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Liu Y, Luo Z, Kang L, Qu J, Liu W, Xia X, Liu Y, Wu K, Wu J. Hepatitis C virus activates Bcl-2 and MMP-2 expression through multiple cellular signaling pathways. J Virol. 2012;86:12531–12543. doi: 10.1128/JVI.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, Staropoli I, Foung S, Amara A, Houles C, Fieschi F, Schwartz O, Virelizier JL, Arenzana-Seisdedos F, Altmeyer R (2003) DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem 278:20358–20366 [DOI] [PubMed]
- Lozach PY, Burleigh L, Staropoli I, Amara A. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol Biol. 2007;379:51–68. doi: 10.1007/978-1-59745-393-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocca A, Sciammetta SC, Carloni V, Cosmi L, Annunziato F, Harada T, Abrignani S, Pinzani M. Binding of hepatitis C virus envelope protein E2 to CD81 up-regulates matrix metalloproteinase-2 in human hepatic stellate cells. J Biol Chem. 2005;280:11329–11339. doi: 10.1074/jbc.M410161200. [DOI] [PubMed] [Google Scholar]
- Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- Paulino AD, Ubhi K, Rockenstein E, Adame A, Crews L, Letendre S, Ellis R, Everall IP, Grant I, Masliah E. Neurotoxic effects of the HCV core protein are mediated by sustained activation of ERK via TLR2 signaling. J Neurovirol. 2011;17:327–340. doi: 10.1007/s13365-011-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R, Chen H, Lu L, Zhu W, Beckebaum S, Cicinnati V, Lu M, Chen X. Hepatitis C virus infection induces the expression of amphiregulin, a factor related to the activation of cellular survival pathways and required for efficient viral assembly. J Gen Virol. 2011;92:2237–2248. doi: 10.1099/vir.0.032581-0. [DOI] [PubMed] [Google Scholar]
- Pöhlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Munch J, Kirchhoff F, Doms RW. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J Virol. 2001;75:4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhlmann S, Zhang J, Baribaud F, Chen Z, Leslie GJ, Lin G, Granelli-Piperno A, Doms RW, Rice CM, McKeating JA. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J Virol. 2003;77:4070–4080. doi: 10.1128/JVI.77.7.4070-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR Jr (2006) DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol 176:1741–1749 [DOI] [PubMed]
- Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. J Pathol. 2002;98:511–516. doi: 10.1002/path.1205. [DOI] [PubMed] [Google Scholar]
- Svajger U, Anderluh M, Jeras M, Obermajer N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell Signal. 2010;22:1397–1405. doi: 10.1016/j.cellsig.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SF, Huang JC, Lee YM, Liu SJ, Chan YJ, Chau YP, Chong P, Chen YM. DC-SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem Biophys Res Commun. 2008;373:561–566. doi: 10.1016/j.bbrc.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Martin TD, Vazeux R, Unutmaz D, KewalRamani VN. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wünschmann S, Medh JD, Klinzmann D, Schmidt WN, Stapleton JT. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol. 2000;74:10055–10062. doi: 10.1128/JVI.74.21.10055-10062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Gong R, Qu J, Zhou Y, Liu W, Chen M, Liu Y, Zhu Y, Wu J. Activation of the Ras/Raf/MEK pathway facilitates hepatitis C virus replication via attenuation of the interferon-JAK-STAT pathway. J Virol. 2012;86:1544–1554. doi: 10.1128/JVI.00688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LJ, Wang L, Ren H, Cao J, Li L, Ke JS, Qi ZT. Hepatitis C virus E2 protein promotes human hepatoma cell proliferation through the MAPK/ERK signaling pathway via cellular receptors. Exp Cell Res. 2005;305:23–32. doi: 10.1016/j.yexcr.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Zhang XL, Zhao P, Cao J, Cao MM, Zhu SY, Liu HQ, Qi ZT. Up-regulation of ERK and p38 MAPK signaling pathways by hepatitis C virus E2 envelope protein in human T lymphoma cell line. J Leukoc Biol. 2006;80:424–432. doi: 10.1189/jlb.0106014. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Zhao P, Chen QL, Ren H, Pan W, Qi ZT. Mitogen-activated protein kinase signaling pathways triggered by the hepatitis C virus envelope protein E2: implications for the prevention of infection. Cell Proliferat. 2007;40:508–521. doi: 10.1111/j.1365-2184.2007.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]