Abstract
Expression of intracellular heat shock protein 27 (Hsp27) rises in the brain of animal models of cerebral ischemia and stroke. Hsp27 is also released into the circulation and the aim of the present study was to investigated if serum Hsp27 (sHsp27) levels are altered in patients with acute ischemic stroke. sHsp27 was measured in 15 patients with acute ischemic stroke and in 14 control subjects comparable for age, sex, and cardiovascular risk factors. In patients, measurements were performed at admission and 1, 2, and 30 days thereafter. At admission, mean sHsp27 values were threefold higher in patients than in controls. In patients, sHsp27 values dropped after 24 h, rose again at 48 h, and markedly declined at 30 days, indicating the presence of a temporal trend of sHsp27 values following acute ischemic stroke.
Keywords: Acute ischemic stroke, Heat shock protein 27, Serum biomarker, Stress response
Acute ischemic stroke (AIS) is a leading cause of morbidity and mortality worldwide (van der Worp and van Gijn 2007). Heat shock protein-27 (Hsp27), an intracellular polypeptide with cytoprotective activity, is potently induced by both ischemic and oxidative stress (Brownell et al. 2012). In experimental models of cerebral ischemia/stroke, there is a rapid induction of Hsp27 into the brain (Brownell et al. 2012; Kato et al. 1995; Imura et al. 1999). In addition, in vivo and in vitro studies have shown that Hsp27 overexpression confers neuroprotection against ischemic cerebral injury by inhibiting activation of the apoptosis signal-regulating kinase 1 and, subsequently, the mitochondrial prodeath cascade (Stetler et al. 2008). Hsp27 is also released into the circulation in response to either cell damage or cellular stresses and extracellular Hsp27 is biologically active as it can modulate both inflammatory and immune processes (Banerjee et al. 2011; Salari et al. 2013). We have previously reported that serum Hsp27 (sHsp27) levels are a marker of distal symmetrical polyneuropathy in diabetic patients (Gruden et al. 2008). However, there are no data on circulating Hsp27 in AIS. In this case–control study, we investigated if sHsp27 levels are altered in patients with AIS and studied their temporal trend after an acute ischemic event.
Methods
All consecutive patients admitted (September 2009–January 2010) to the Emergency Department of the San Giovanni Battista Hospital-Turin with computer tomography (CT)- or magnetic resonance imaging (MRI)-proven cerebral infarction were recruited. Subjects admitted 24 h after the onset of symptoms or with hemorrhagic stroke, transient ischemic attack, neoplasms, inflammatory conditions, and serum creatinine of >2 mg/dl were excluded. Patients who died in the month after admission and those who underwent thrombolytic therapy were withdrawn from the study. In the remaining 15 patients, clinical data were recorded and neurological impairment assessed by the NIHSS scores. Stroke volume (n = 11) was evaluated on 24–48 h follow-up brain CT/MRI scans. Fourteen comparable blood donors serve as a control group. Ethics committee approval was obtained and all subjects provided written informed consent. Body mass index (BMI) was calculated as weight × height−2. Hypertension was defined as systolic blood pressure of >140 mmHg and/or diastolic blood pressure of >90 mmHg or treatment with antihypertensive drugs. Triglycerides, total cholesterol, high-density lipoprotein cholesterol, and serum creatinine levels were measured by standard laboratory techniques. Serum levels were measured by ELISA (Calbiochem San Diego, CA, USA). Serum Hsp27 was measurable in all subject with right skewed distribution of values, thus values were log-transformed. Variables are presented either as mean ± SD or as median (25th–75th percentile) as appropriate. Student’s t test was used for comparisons between groups.
Results and discussion
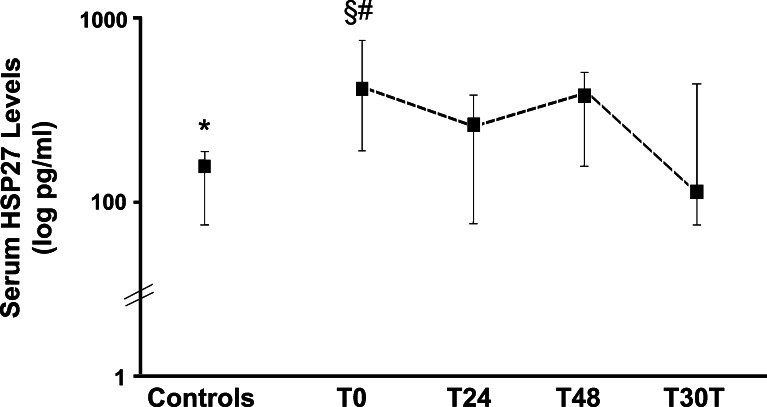
In this study, we have provided the first evidence that sHsp27 levels are increased in patients with AIS, a leading cause of morbidity and mortality in the Western World. Indeed, sHsp27 levels were threefold higher in patients than in control subjects (Fig. 1) though the two groups were comparable for both demographic parameters and cardiovascular risk factors (Table 1).
Fig. 1.
Serum Hsp27 levels in control subjects and in patients with acute ischemic stroke at admission (T0) and 24 h (T24), 48 days (T48), and 30 days (T30d) thereafter. Logarithmic scale. Boxes represent median values and upper and lower horizontal lines show 25th and 75th percentiles, respectively. Student t test: *p = 0.001 controls vs. T0, p = 0.691 controls vs. T30d. Repeated measurements ANOVA p = 0.002 for T0-T24-T48-T30d; §p = 0.016 T0 vs. T24, p = 0.100 T0 vs T48, #p = 0.006 T0 vs T30d
Table 1.
Characteristics of patients with acute ischemic stroke and controls
| Patients with acute ischemic stroke | Control subjects | p | |
|---|---|---|---|
| N | 15 | 14 | |
| Age (years) | 74.4 ± 18.5 | 74.7 ± 4.46 | 0.95 |
| Males (%) | 46.7 % | 57.15 % | 0.42 |
| Smokers no/ex/yes | 10/2/3 | 7/2/5 | 0.37 |
| BMI (kg/m2) | 26.0 ± 3.6 | 26.2 ± 3.4 | 0.78 |
| Hypertension (%) | 80.0 % | 78.6 % | 0.46 |
| Total cholesterol (mmol/l) | 4.90 ± 1.12 | 5.15 ± 0.88 | 0.61 |
| LDL-cholesterol (mmol/l) | 3.02 ± 0.91 | 2.89 ± 0.78 | 0.94 |
| HDL-cholesterol (mmol/l) | 1.22 ± 0.27 | 1.47 ± 0.28 | 0.01 |
| Triglycerides (mmol/l) | 1.37 (0.75–1.72) | 1.10 (0.94–1.58) | 0.71 |
| Creatinine (mg/dl) | 1.0 ± 0.3 | 0.88 ± 0.2 | 0.25 |
| Pre-hospital delay (hours) | 9.4 ± 5.0 | ||
| Neurological impairment | |||
| Mild (%) | 73.3 | – | |
| Moderate (%) | 13.3 | – | |
| Severe (%) | 13.3 | – | |
| Mean stroke volume (cm3) | 27.4 ± 46.6 | ||
This increase in circulating Hsp27 levels may mirror an increased Hsp27 expression into the brain. In keeping with this hypothesis, Hsp27 is upregulated in animal models of cerebral ischemia and stroke. Furthermore, both cell injury and necrosis are known inducers of Hsp release (Brownell et al. 2012; Kato et al. 1994, 1995; Imura et al. 1999).
sHsp27 levels were not measured prior to the ischemic event; therefore, we cannot exclude the possibility that a rise in sHsp27 precedes and possibly predicts the occurrence of AIS. However, a 6-year follow-up period study of healthy women has recently shown that sHsp27 levels are not predictive of AIS (Kardys et al. 2008). Furthermore, a Hsp27 release prior to stroke would not have logical explanation as circulating HSP are released by pathologically changed or damaged cells. Finally, in our study, sHsp27 levels dropped 30 days after the event and were no longer significantly different from those measured in control subjects (Fig. 1) and this pattern in time is consistent with the hypothesis that AIS induces an acute rise in sHsp27 levels with a return to baseline within a month.
We found no correlation between sHsp27 levels and any clinical/laboratory parameter, including prehospital delay, National Institutes of Health Stroke Scale (NIHSS) score, and stroke volume. Consistently, a recent study has shown that levels of anti-Hsp27 antibodies, which are produced in response to extracellular Hsp27 release, are increased in patients with acute ischemic stroke, but do not correlate with either severity or prognosis (Azarpazhooh et al. 2010). This is not surprising because in experimental models Hsp27 overexpression is observed not only in ischemic perilesional areas, but also in non-ischemic remote ipsi- or even contralateral regions, likely because of spreading depression (Kato et al. 1995, Popp et al. 2009). In patients with AIS, the study of sHsp27 temporal trend showed that values significantly changed over time (p = 0.002 repeated measurements ANOVA). Specifically, there was a significant fall at 24 h and a new rise at 48 h (Fig. 1). The underlying mechanism is unknown; however, studies in experimental animals have shown that middle cerebral artery occlusion followed by reperfusion induces Hsp27 expression in both microglia and neurons of the perilesional area as well as in reactive astrocytes distributed widely in both perilesional and remote areas (Kato et al. 1995; Popp et al. 2009). Expression in perilesional neurons is rapid, but transient, possibly explaining the drop in sHsp27 levels herein observed. By contrast, Hsp27 expression in reactive astrocytes greatly increases after 1 day reperfusion. This raises the possibility that sHsp27 derived from astrocytes may account for the delayed sHsp27 rise observed in our patients and reflect the ongoing repairing process.
Of interest, the three patients who died during hospital stay and the two patients who were discharged with severe neurological impairment did not show this characteristic biphasic temporal trend in sHsp27, suggesting a potential prognostic relevance of sHsp27 measurements. However, the sample size was too small to further explore this possibility. The topic is, however, of relevance as a recent study in an experimental model of stroke has shown that post-ischemic viral delivery of Hsp27 causes a significant reduction in brain lesion size (van der Weerd et al. 2010; Badin et al. 2009), underlying the importance of Hsp27 induction in neuroprotection and paving the way for future therapeutic applications.
In conclusion, this study shows that sHsp27 levels are increased in patients with AIS and have a characteristic pattern of change over time. Further studies are required to assess the potential clinical relevance of these preliminary findings.
Acknowledgments
This study is supported by grants from the Piedmont Region and the University of Turin.
References
- Azarpazhooh MR, Mobarra N, Parizadeh SM, Tavallaie S, Bagheri M, Rahsepar AA, Ghayour-Mobarhan M, Sahebkar A, Ferns GA. Serum high-sensitivity C-reactive protein and heat shock protein 27 antibody titers in patients with stroke and 6-month prognosis. Angiology. 2010;61:607–612. doi: 10.1177/0003319709360524. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Lin CF, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, Redmond EM, Morrow D, Huston A, Shayne M, Langstein HN, Miller-Graziano CL, Strickland J, O'Donoghue L, De AK. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011;71:318–327. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- Badin RA, Modo M, Cheetham M, Thomas DL, Gadian DG, Latchman DS, Lythgoe MF. Protective effect of post-ischaemic viral delivery of heat shock proteins in vivo. J Cereb Blood Flow Metab. 2009;29:254–263. doi: 10.1038/jcbfm.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell SE, Becker RA, Steinman L. The protective and therapeutic function of small heat shock proteins in neurological diseases. Front Immunol. 2012;3:1–10. doi: 10.3389/fimmu.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Schalkwijk C, Pinach S, Stehouwer CD, Witte DR, Fuller JH, Perin PC, EURODIAB Prospective Complications Study Group Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Shimohama S, Sato M, Nishikawa H, Madono K, Akaike A, Kimura J. Differential expression of small heat shock proteins in reactive astrocytes after focal ischemia: possible role of beta-adrenergic receptor. J Neurosci. 1999;19:9768–9779. doi: 10.1523/JNEUROSCI.19-22-09768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardys I, Rifai N, Meilhac O, Michel JB, Martin-Ventura JL, Buring JE, Libby P, Ridker PM. Plasma concentration of heat shock protein 27 and risk of cardiovascular disease: a prospective, nested case–control study. Clin Chem. 2008;54:139–146. doi: 10.1373/clinchem.2007.094961. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Liu XH, Araki T, Kato K, Itoyama Y. Immunohistochemical localization of the low molecular weight stress protein HSP27 following focal cerebral ischemia in the rat. Brain Res. 1995;679:1–7. doi: 10.1016/0006-8993(95)00198-Y. [DOI] [PubMed] [Google Scholar]
- Kato H, Liu Y, Kogure K, Kato K. Induction of 27-kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res. 1994;634:235–244. doi: 10.1016/0006-8993(94)91926-7. [DOI] [PubMed] [Google Scholar]
- Popp A, Jaenisch N, Witte OW, Frahm C (2009) Identification of ischemic regions in a rat model of stroke. Plose One 4:(3):e4764. doi:10.1371/journal.pone.0004764 [DOI] [PMC free article] [PubMed]
- Salari S, Seibert T, Chen YX, Hu T, Shi C, Zhao X, Cuerrier CM, Raizman JE, O'Brien ER. Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages. Cell Stress Chaperones. 2013;18:53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weerd L, Tariq Akbar M, Aron Badin R, Valentim LM, Thomas DL, Wells DJ, Latchman DS, Gadian DG, Lythgoe MF, de Belleroche JS. Overexpression of heat shock protein 27 reduces cortical damage after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:849–856. doi: 10.1038/jcbfm.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, van Gijn J. Acute ischemic stroke. N Engl J Med. 2007;357:572–579. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]