Abstract
Obese Black women are at increased risk for development of gestational diabetes mellitus and have worse perinatal outcomes than do obese women of other ethnicities. Since hsp72 has been associated with the regulation of obesity-induced insulin resistance, we evaluated associations between glucose ingestion, hsp72 release and insulin production in Black pregnant women. Specifically, the effect of a 50-g glucose challenge test (GCT) on heat shock protein and insulin levels in the circulation 1 h later was evaluated. Hsp27 and hsp60 levels remained unchanged. In contrast, serum levels of hsp72 markedly increased after glucose ingestion (p = 0.0054). Further analysis revealed that this increase was limited to women who were not obese (body mass index <30). Insulin levels pre-GCT were positively correlated with body mass index (p = 0.0189). Median insulin concentrations also increased post GCT in non-obese women but remained almost unchanged in obese women. Post-GCT serum hsp72 concentrations were inversely correlated with post GCT insulin concentrations (p = 0.0111). These observations suggest that glucose intake during gestation in Black women rapidly leads to an elevation in circulating hsp72 only in non-obese Black women. The release of hsp72 may regulate the extent of insulin production in response to a glucose challenge and, thereby, protect the mother and/or fetus from development of hyperglycemia, hyperinsulinemia, and/or immune system alterations.
Keywords: Pregnancy, Glucose ingestion, hsp72, Black race, Body mass index, Insulin
Introduction
Glucose ingestion, either directly or as part of a meal, induces secretion of insulin. The release of insulin leads to a suppression of glucagon (the enzyme responsible for glucose production), gluconeogenesis (formation of glucose from lactate and amino acids), and glycogenolysis (the breakdown of glycogen to glucose)—all primarily in the liver (Aranoff et al. 2004). By this mechanism, basal blood glucose concentrations soon return to their normal range. In diabetic patients, induction of insulin after meals is insufficient to suppress glucagon secretion. As a consequence, the liver continues to produce endogenous glucose and circulating glucose levels become markedly elevated. This results in the appearance of hyperglycemia.
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance that has its first onset or recognition during pregnancy (American Diabetes Association 2002). It is believed to occur in 3–7 % of pregnancies in the USA and is associated with a range of adverse perinatal outcomes (Crowther et al. 2005). Pregnant women in the USA are now almost always screened for GDM, most commonly by an initial 50-g glucose challenge test (GCT) provided between 24 and 28 weeks gestation (Metzger et al. 2007). Women ingest a liquid containing 50 g glucose and their serum glucose level is monitored 1 h later. Individuals who exhibit elevated glucose readings (≥130–140 mg/dL) then typically undergo a diagnostic 3-h 100-g oral glucose tolerance test (OGTT) or a 2-h 75-g OGTT.
The prevalence of GDM and its consequences have been shown to vary between different racial and ethnic groups. The risk for development of GDM is higher in Black women as compared to Whites or Asians (Makgoba et al. 2012). Black women who are overweight or obese have a higher risk of developing GDM than do White, Hispanic, or Asian women of comparable body mass index (Shah et al. 2011; Kim et al. 2012). Black women with GDM have worse perinatal outcomes than do women of other races/ethnicities (Esakoff et al. 2011). The chance of developing diabetes after having been diagnosed with GDM is highest in Black women as compared with White women (Xiang et al. 2011).
It has been suggested that the induction of heat shock proteins may combat insulin resistance (McCarty 2006). Putative roles for hsp27, hsp60, and hsp72 in improving insulin signaling have been demonstrated (Chung et al. 2008; Simar et al. 2012; Marker et al. 2012). The mechanism appears to involve prevention of activation of c-Jun amino terminal kinase, an enzyme that inhibits insulin signal transduction and glucose tolerance. In this communication, we evaluated whether there are alterations in serum hsp27, hsp60, and hsp72 concentrations in the systemic circulation following a 1-h 50-g GCT in Black women. We hypothesized that a rapid increase in glucose-induced heat shock protein levels under these conditions would support a role for these proteins in the regulation of glucose metabolism during pregnancy.
Results and discussion
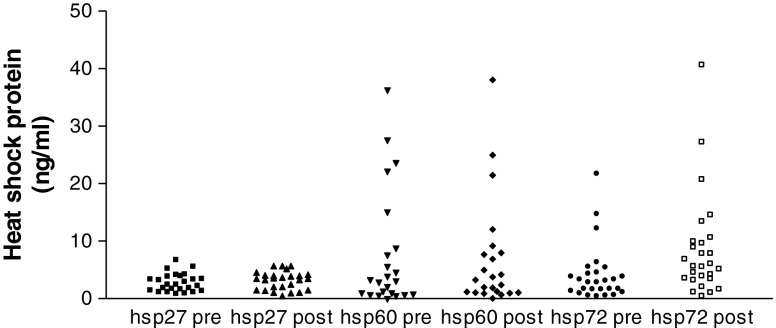
Twenty-three self-identified Black women who underwent a 1-h 50-g GCT at the outpatient obstetrics clinic at Weill Cornell Medical College comprised the study population. All signed a written informed consent form and the study was approved by the Institutional Review Board at Weill Cornell Medical College. Venous blood was obtained from a peripheral vein prior to and 1 h following the glucose ingestion. Serum was collected and stored in aliquots at −80 °C for subsequent testing. Paired samples were assayed for hsp27 (catalog #DYC 1580-S), hsp60 (catalog #DYC 1800-S) and hsp72 (catalog #DYC 1663-S) by commercial Enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, MN, USA). Insulin levels in the paired samples were measured by a commercial ELISA kit (Alpco Diagnositics, Salem, NH, USA; catalog # 80-INSHU-E01.1.6). Values were expressed as microliter international units per milliliter. The median serum concentrations of hsp27 and hsp60 did not change following glucose ingestion. The median (range) concentrations of hsp27 pre- and post-glucose were 2.4 (0.8–6.7) and 3.6 (0.6–5.8) ng/ml, respectively. The median (range) concentrations of hsp60 pre- and post-glucose were 3.1 (<0.6–36.3) and 3.4 (<0.6–38.2) ng/ml, respectively. In marked contrast, median (range) serum levels of hsp72 increased from 2.8 (0.4–21.7) ng/ml to 5.6 (0.4–40.6) ng/ml post-glucose ingestion (p = 0.0054). Results from the individual subjects are shown in Fig. 1. Median (range) insulin concentrations increased from 6.7 (0.4–79) μIU/ml preglucose to 19.0 (1.0–103) μIU/ml following glucose ingestion.
Fig. 1.
Circulating levels of hsp27, hsp60, and hsp 72 in pregnant Black women before and after a 1-h 50-g glucose challenge test. Serum was obtained prior to (pre) and 1 h following (post) ingestion of a 50-g glucose solution and tested for concentrations of hsp27, hsp60, and hsp70 by ELISA. Values were converted to nanograms per milliliter by reference to a standard curve that was generated in parallel to each assay. The lower limits of sensitivity were 31 pg/ml for hsp27, 625 pg/ml for hsp60, and 156 pg/ml for hsp72. The values were not normally distributed and so differences were evaluated by the nonparametric Mann–Whitney test
A 1-h time period is too short for de novo protein synthesis and the selective increase in hsp72 but not the other heat shock proteins argues against a massive cell necrosis and release of intracellular hsp72 as the mechanism for this observation. The increase in serum hsp72 concentration most likely is a consequence of the release of preformed hsp72 from a storage site into the circulation. There is a precedent for this observation. Exercise in man was shown to stimulate the release of hsp72 into the hepatic vein as early as 30 min (Febbraio et al. 2002). This strongly suggested that the hepatosplanchnic viscera (liver and spleen) were the source of the preformed hsp72 that was excreted into the bloodstream. This induced hsp72 release most likely performs unspecified maintenance functions in response to the physiological changes induced by physical exercise. A subsequent study by the same group demonstrated that hsp60 was also released into the hepatic vein during exercise and that the release of both hsp60 and hsp72 could be inhibited by simultaneous glucose ingestion (Febbraio et al. 2004). It was hypothesized that exercise placed a metabolic stress on the liver to synthesize more glucose and this was accompanied by the release of heat shock proteins into the circulation. Simultaneous ingestion of exogenous glucose during exercise alleviated a need for the liver to synthesize glucose and so metabolic stress was reduced and heat shock proteins were not released. Rapid exposure to a large amount of exogenous glucose to pregnant women in their midtrimester differs physiologically from providing glucose to men already engaged in physical exercise and so it is not unexpected that the release of hsp72 by the hepatosplanchnic viscera will differ in the two situations. Circulating hsp72 may be beneficial immediately following glucose ingestion in pregnant women to protect the fetus and/or mother from hyperglycemia, hyperinsulinemia, and/or immune system perturbations. Induction of GDM in rats has been shown to result in markedly elevated circulating levels of hsp72 (Saito et al. 2012), perhaps as a compensatory mechanism to minimize adverse consequences to the mother or fetus. Alternatively or concurrently, hp72 may also become elevated following a transient increase of endotoxin into the circulation as a consequence of glucose-induced intestinal osmotic stress (Dokladny et al. 2008).
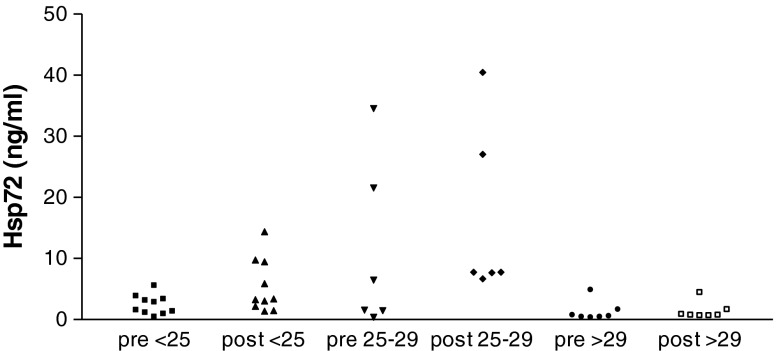
Several studies have demonstrated that obese or overweight Black women are at highest risk of developing GDM (Shah et al. 2011; Kim et al. 2012). We, therefore, evaluated the effect of body mass index (BMI, weight (in kilogram)/[height (in meter)]2) on hsp72 release and insulin production after glucose ingestion in our Black subjects. Although the number of women in each BMI group is small and, therefore, our results must be viewed as hypothesis testing and not definitive, the increase in circulating hsp72 was restricted to women who were not obese. In women who were of normal weight (BMI of <25) median (range) hsp72 levels increased from 2.2 (0.4–5.5) ng/ml prior to glucose to 3.5 (1.5–14.5) after glucose (p = 0.0355). Women who were overweight (BMI 25–29) had hsp72 levels that increased from 4.2 (0.5–34.7) ng/ml to 7.9 (6.8–40.6) ng/ml. This difference did not reach statistical significance (p = 0.1797). In obese women (BMI ≥ 30) hsp72 levels were 0.5 (0.3–4.8) ng/ml prior to glucose ingestion and 0.7 (0.6–4.4) ng/ml afterwards. Individual results are shown in Fig. 2. Postglucose hsp72 concentrations were significantly higher in women who were of normal weight (p = 0.0046) or overweight (p = 0.0012) as compared to levels in obese women. The difference in hsp72 concentrations after glucose ingestion between normal weight and overweight women did not reach statistical significance (p = 0.0559).
Fig. 2.
Circulating levels of hsp72 in pregnant Black women before and after a 1-h 50-g glucose challenge test as a function of BMI. Serum was obtained and assayed for hsp72 as described in Fig. 1. Subjects were classified as being of normal body weight (BMI of <25), overweight (BMI 25–29) or obese (BMI of ≥30)
Prior to glucose ingestion, insulin levels were positively correlated with BMI (Spearman r = 0.5327, p = 0.0189). This relationship was no longer present after glucose administration (r = −0.0543, p = 0.8250). Preglucose insulin levels were a median (range) of 3.3 (0.4–50) μIU/ml in non-obese women as opposed to 23.9 (4.2–79) μIU/ml in obese women (p = 0.0199). Following glucose ingestion median insulin levels increased to 17.6 (0.7–79) μIU/ml in non-obese women but, in marked contrast, remained similar to preglucose levels, 22.9 (6.1–102) μIU/ml, in the obese group. Thus, obese pregnant women have elevated insulin levels at 24–28 weeks gestation but glucose ingestion does not result in further induction. Perhaps insulin production may already be at maximum levels in this group.
Concentrations of hsp72 and insulin prior to glucose ingestion were not correlated (p = 0.0873). However, following glucose intake hsp72 and insulin levels in maternal sera were strongly negatively correlated (Spearman r = −0.5684, p = 0.0111). Thus, it appears that hsp72 is induced in pregnancy in response to excessive glucose ingestion, perhaps as a regulatory mechanism to prevent excessive insulin release and development of hyperinsulinemia. Previous investigations have demonstrated the role of hsp72 in prevention of hyperinsulinemia and insulin resistance (Kurucz et al. 2002; McCarty 2006; Chung et al. 2008; Kavanagh et al. 2011).
Obesity leads to a chronic inflammatory state and susceptibility to develop insulin resistance (Wellen and Hotamisligil 2005). A failure to release hsp72 into the circulation following glucose ingestion to regulate insulin levels, as demonstrated in our study, undoubtedly elevates the risk for development of hyperglycemia. Thus, susceptibility of obese pregnant Black women to develop GDM may be due, at least in part, to their inability to mobilize hsp72 release from the liver in response to a high caloric diet and the subsequent inability to effectively regulate insulin production. The precise mechanism of hsp72 exocytosis remains to be determined. The finding of obese women with hyperinsulinemia before glucose ingestion but who did not have gestational diabetes parallels previous observations made on obese pregnant women in a city in rural Brazil (Negrato et al. 2009). In that study, the obese women were shown to have other biochemical abnormalities consistent with a diagnosis of the metabolic syndrome. Whether obese pregnant Black women in New York manifest evidence of the metabolic syndrome and this contributes to an increased risk for adverse pregnancy outcome in this population deserves further study.
Six of the women in the study delivered preterm at 26.9–36.4 weeks gestation and only one woman, with a BMI of 34.1, was subsequently diagnosed as having GDM. There was no association between postglucose hsp72 levels or magnitude of the postglucose hsp72 increase and having a preterm vs. a term delivery. Similarly, postglucose hsp72 concentrations were unrelated to GCT results (data not shown).
Limitations of the present study include the relatively small number of women evaluated, the identification of only six obese subjects, and the absence, with one exception, of women who went on to develop GDM. It must also be acknowledged that the GCT, involving the ingestion of 50 g of glucose, is by itself a stress in many women and can be followed by nausea and other symptoms. It is thus possible that hsp72 induction may be in part a response to this digestive insult. The primary value of the investigation is its unique observation of hsp72 and insulin release into the circulation 1 h after glucose ingestion by pregnant women and the apparent negative effect of obesity on these occurrences. This finding will hopefully spark additional research to validate and expand on these initial observations and eventually lead to a more comprehensive understanding of the inter-relationship between diet, insulin resistance, and heat shock protein induction in Black women. The ultimate aim is to develop novel methods of prevention of GDM in individual susceptible women and to improve perinatal outcomes.
References
- Association AD. Gestational diabetes mellitus. Diabetes Care. 2002;25(Suppl 1):S94–S96. [Google Scholar]
- Aranoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 2004;17:183–190. doi: 10.2337/diaspect.17.3.183. [DOI] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther CA, Hillier JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occluding protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esakoff TF, Caughey AB, Block-Kurbisch I, Inturrisi M, Cheng YW. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J Maternal Fetal Neonatal Med. 2011;24:422–426. doi: 10.3109/14767058.2010.504287. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, et al. Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones. 2004;9:390–396. doi: 10.1379/CSC-24R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Streensberg A, Keller C, Krustrup P, et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544(3):957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring hsp70 deficiencies improves glucose tolerance in diabeteic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, England L, Sappenfield W, Wilson HG, Bish CL, Salihu HM, Sharma AJ. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Prev Chronic Dis. 2012;9:E88. doi: 10.5888/pcd9.110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG. 2012;119:276–282. doi: 10.1111/j.1471-0528.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- Marker T, Sell H, Zillessen P, Glode A, Kriebel J, Ouwens DM, et al. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:615–625. doi: 10.2337/db10-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66:527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- Negrato CA, Jovanovic L, Tambascia MA, Calderon IMP, Geloneze B, Dias A, Rudge MVC. Mild gestational hyperglycaemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab Res Rev. 2009;24:324–330. doi: 10.1002/dmrr.815. [DOI] [PubMed] [Google Scholar]
- Saito FH, Damasceno DC, Dallaqua B, Linhares IM, Rudge MV, De Mattos Paranhos Calderon I, Witkin SS. Heat shock protein production and immunity and altered fetal development in diabetic pregnant rats. Cell Stress Chaperones. 2012;18(1):25–33. doi: 10.1007/s12192-012-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Stotland NE, Cheng YW, Ramos GA, Caughey AB. The association between body mass index and gestational diabetes mellitus varies by race/ethnicity. Am J Perinatol. 2011;28:515–520. doi: 10.1055/s-0031-1272968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signaling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17:615–621. doi: 10.1007/s12192-012-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Li BH, Black MH, Sacks DA, Buchanan TA, Jacobsen SJ, Lawrence JM. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54(3016):3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]