Abstract
Glutathione S-transferases (GSTs) are members of a multifunctional antioxidant enzyme superfamily that play pivotal roles in both detoxification and protection against oxidative damage caused by reactive oxygen species. In this study, a complementary DNA (cDNA) encoding a sigma class GST was identified in the Chinese honey bee, Apis cerana cerana (AccGSTS1). AccGSTS1 was constitutively expressed in all tissues of adult worker bees, including the brain, fat body, epidermis, muscle, and midgut, with particularly robust transcription in the fat body. Relative messenger RNA expression levels of AccGSTS1 at different developmental stages varied, with the highest levels of expression observed in adults. The potential function of AccGSTS1 in cellular defenses against abiotic stresses (cold, heat, UV, H2O2, HgCl2, and insecticides) was investigated. AccGSTS1 was significantly upregulated in response to all of the treatment conditions examined, although the induction levels were varied. Recombinant AccGSTS1 protein showed characteristic glutathione-conjugating catalytic activity toward 1-chloro-2,4-dinitrobenzene. Functional assays revealed that AccGSTS1 could remove H2O2, thereby protecting DNA from oxidative damage. Escherichia coli overexpressing AccGSTS1 showed long-term resistance under conditions of oxidative stress. Together, these results suggest that AccGSTS1 is a crucial antioxidant enzyme involved in cellular antioxidant defenses and honey bee survival.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0394-7) contains supplementary material, which is available to authorized users.
Keywords: Glutathione-S-transferase, Oxidative stress, Antioxidant defense, Apis cerana cerana
Introduction
Glutathione S-transferases (GSTs) belong to a large and diverse gene family of dimeric enzymes that are involved in the cellular detoxification of both natural and artificial molecules (Lumjuan et al. 2005; Ding et al. 2003; Rogers et al. 1999; Tang and Tu 1994). These enzymes are widely expressed in both prokaryotic and eukaryotic cells. Based on sequence identity, the mammalian GSTs are grouped into the following seven classes: alpha, mu, pi, theta, sigma, omega, and zeta (Flanagan and Smythe 2011). In insects, this highly diverse gene family falls into six major subclasses, including sigma, omega, theta, zeta, and insect-specific delta and epsilon (Hayes et al. 2005; Tu and Akgul 2005). GST enzymes exhibit remarkably broad and overlapping substrate specificities. They can catalyze the conjugation of the reduced glutathione (GSH) to electrophilic centers of a wide range of exogenous or endogenous toxic compounds, including cancer chemotherapeutic agents, chemical carcinogens, insecticides, herbicides, and oxidative stress products (Hayes et al. 2005). Some members also manifest glutathione peroxidase activity and promote the metabolism of organic hydroperoxides generated in cells (Ayyadevara et al. 2005; Burmeister et al. 2008; Singh et al. 2001; Tang and Tu 1994). As modulated expression levels of GST under oxidative stress are thought to represent an adaptive response (Hayes and Pulford 1995), GST expression and activity are recognized as biomarkers of exposure to oxidative stressors (Durou et al. 2007; Nair et al. 2011).
The majority of reports on insect GSTs have concentrated on their roles in insecticide resistance via the detoxification of insecticides (Enayati et al. 2005; Lumjuan et al. 2011; Yang et al. 2009; Che-Mendoza et al. 2009). GSTs confer resistance by detoxifying lipid peroxidation products induced by pyrethroids, thereby protecting tissues from oxidative damage (Vontas et al. 2001), as well as by binding pyrethroid molecules in a sequestration mechanism, thereby offering passive protection (Kostaropoulos et al. 2001). Recently, studies on their roles in mediating oxidative stress responses have been extensively reported (Burmeister et al. 2008; Kampkotter et al. 2003; Li et al. 2008; Meng et al. 2009; Nair and Choi 2011; Umasuthan et al. 2012; Yang et al. 2012), and these studies have shown that GSTs reduce lipid hydroperoxides through a selenium-independent pathway. In Chironomus riparius fourth instar larvae, the upregulation of GSTs upon exposure to the pro-oxidative stress inducer paraquat indicates their involvement in oxidative stress defense mechanisms, while their induction upon exposure to cadmium and silver nanoparticles suggests a protective role (Nair and Choi 2011). In addition, Umasuthan et al. (2012) provided evidence that a Ruditapes philippinarum sigma-like GST could play a significant role in cellular defenses against oxidative stress caused by bacteria.
Because GST genes play important roles in detoxification pathways and the oxidative stress response, studies on GST resistance have been widely reported in insects, including Drosophila melanogaster (Li et al. 2008; Low et al. 2007; Sawicki et al. 2003; Tang and Tu 1994; Tu and Akgul 2005), Anopheles gambiae (Chen et al. 2003; Ding et al. 2003, 2005; Ranson et al. 2000, 2001), and Bombyx mori (Yamamoto et al. 2006; Yu et al. 2008), but information about other important species is rather limited. Honey bees, which serve as an excellent model for social behavior research, are a major plant pollinator and thus play essential roles in agriculture and ecological balance. Moreover, the Chinese honey bee, Apis cerana cerana, is suffering from decreasing populations size due to various environmental stressors including temperature, heavy metals, pesticides, and ultraviolet (UV) radiation (Xu et al. 2009), which are all known inducers of oxidative stress (Lushchak 2011; Kottuparambil et al. 2012). In this study, we report on the molecular cloning and characterization of a sigma class GST from A. cerana cerana. The expression profiles of AccGSTS1 in response to various oxidative stresses were evaluated to establish the cellular defense function of this GST member against oxidative exposure. The potential roles of an AccGSTS1 fusion protein in antioxidant defense were also investigated. Our results provide a better perspective on the mechanisms of resistance to oxidative stress in the honey bee.
Materials and methods
Experimental insects and treatments
Chinese honeybees (A. cerana cerana) used in the study were obtained from Shandong Agricultural University (Taian, China). Larvae and pupae worker bees were classified by the age, eye color, and shape. The larvae, pupae, and 1-day-old adult workers were taken from the hive, and the 2-week-old adult workers were collected at the entrance of the hive when they returned to the colony after foraging. Tissues from adults (15 days), including the brain, fat body, epidermis, muscle, and midgut, were dissected on ice. The midgut was separated under a steremicroscope. Two-week-old adults were caged into groups of 30 and maintained in an incubator at a constant temperature (34 °C) and humidity (70 %) under a 24-h dark regimen. They were fed on a pollen-and-sucrose mixture before treatments. For cold and heat shock treatment, the temperature of the incubator was maintained at 4 and 43 °C, respectively. The UV light (30 mJ/cm2) was introduced to the incubator for UV treatment. For H2O2 treatment, honey bees were injected with 20 μl of H2O2 (50 μM of H2O2/worker) between the first and second abdominal segments using a sterile microscale needle. For metal treatment, groups of 30 adults were equally fed a mixture containing pollen and HgCl2 (3 μg) with a micropipette. For generating pesticide resistance, three types of pesticides (phoxim, cyhalothrin, and acaricide) were diluted to a final concentration of 20 mg/l and fed to the worker bees. For the control, groups were fed only a pollen–sucrose solution. All honey bees were frozen in liquid nitrogen and stored at −70 °C until use.
RNA extraction, cDNA synthesis, and DNA preparation
Total RNA was extracted from the honeybees using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. To eliminate genomic DNA contamination, each 2 μg sample of RNA was treated with 2 U of DNase I at 37 °C for 1 h. Then, complementary DNA (cDNA) was synthesized using the EasyScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s protocol. Genomic DNA was isolated from the whole bodies of adult bees using the EasyPure Genomic DNA Extraction Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions.
Isolation of the full-length cDNA sequence of AccGSTS1
Primers S1 and S2 were designed and synthesized (Shanghai Sangon Biotechnological Company, China) based on the conserved regions of the sigma class GSTs from other insects. These primers were then used to obtain the internal region of AccGSTS1. Based on the sequence of the amplified fragment, two pairs of specific primers, 5R1/2 and 3R1/2, were subsequently designed and used for 5′ and 3′ rapid amplification of cDNA ends (RACE), respectively. The full-length cDNA sequence was determined by aligning the 5′ and 3′ UTR fragments with the internally conserved region. Another two primers, QC1 and QC2, were then synthesized to amplify the complete cDNA sequence of AccGSTS1. All primers and PCR amplification conditions used in this study are listed in Tables 1 and 2, respectively. All PCR products were separated by 1 % agarose gel electrophoresis, purified using a gel extraction kit (Solarbio, Beijing, China), ligated into pEASY-T3 vectors (TransGen Biotech, China), and transformed into Escherichia coli strain DH5α competent cells. The positive clones were selected for sequencing.
Table 1.
Primer sequences used in this study
| Name | Primer sequence (5′-3′) | Description |
|---|---|---|
| S1 | TATTTTAATATTCCTGGTCTTG | cDNA sequence primer, forward |
| S2 | GCATGTCAGTCAAAGATTCCTC | cDNA sequence primer, reverse |
| 5R1 | GCGATCAAGCGAGAAATAGCC | 5′ RACE forward primer, outer |
| 5R2 | GATTTAATCTTGGGCCATTCTTC | 5′ RACE forward primer, inner |
| AAP | GGCCACGCGTCGACTAGTAC(G)14 | Abridged anchor primer |
| AUAP | GGCCACGCGTCGACTAGTAC | Abridged amplification primer |
| 3R1 | CCAAAGTTCCCTTGCTTCTC | 3′ RACE reverse primer, outer |
| 3R2 | GGAAAGTTGACGTGGGCAG | 3′ RACE reverse primer, inner |
| B26 | GACTCTAGACGACATCGA(T)18 | 3′ RACE universal primer, outer |
| B25 | GACTCTAGACGACATCGA | 3′ RACE universal primer, inner |
| QC1 | AGTCTGTGGAAGACCGTCTC | Full-length cDNA primer, forward |
| QC2 | GTATCAAACGCGATTTAATG | Full-length cDNA primer, reverse |
| SN1 | CAAAGAGGAGAGGTATGGTGG | Genomic sequence primer, forward |
| SN2 | GTTGAGAAGCAAGGGAAC | Genomic sequence primer, reverse |
| SN3 | GGCTATTTCTCGCTTGATCGC | Genomic sequence primer, forward |
| SN4 | CCATAGATTTGGGTCGATTCTTC | Genomic sequence primer, reverse |
| GS-1 | CCAAAGTTCCCTTGCTTCTCAAC | qPCR (copy number) primer, forward |
| GS-2 | GCATGTCAGTCAAAGATTCCTC | qPCR (copy number) primer, reverse |
| GZ-1 | CGAATAAAAGGGGAGGGAGGAG | Standard control primer, forward |
| GZ-2 | CAGGCTTTGATGGACGGATTC | Standard control primer, reverse |
| SD-s | GGCTATTTCTCGCTTGATCGC | Real-time PCR primer, forward |
| SD-x | CGTAATCCACCACCTCTATCG | Real-time PCR primer, reverse |
| β-actin-s | GTTTTCCCATCTATCGTCGG | Standard control primer, forward |
| β-actin-x | TTTTCTCCATATCATCCCAG | Standard control primer, reverse |
| YH-F | GGATCCATGTCCACGTATAAATTGATT | Protein expression primer, forward |
| YH-R | GAGCTCAGATATGACCATAGATTTGGG | Protein expression primer, reverse |
Table 2.
PCR amplification conditions in this study
| S1/S2 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 49 °C, and 50 s at 72 °C for 35 cycles; 5 min at 72 °C |
| 5R1/AAP | 10 min at 94 °C; 40 s at 94 °C, 40 s at 51 °C, and 1 min at 72 °C for 28 cycles; 5 min at 72 °C |
| 5R2/AUAP | 10 min at 94 °C; 40 s at 94 °C, 40 s at 48 °C, and 40 s at 72 °C for 35 cycles; 5 min at 72 °C |
| 3R1/B26 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 49 °C, and 1 min at 72 °C for 28 cycles; 5 min at 72 °C |
| 3R2/B25 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 52 °C, and 40 s at 72 °C for 35 cycles; 5 min at 72 °C |
| QC1/QC2 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 45 °C, and 1 min at 72 °C for 35 cycles; 5 min at 72 °C |
| SN1/SN2 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 43 °C, and 1 min 30 s at 72 °C for 35 cycles; 5 min at 72 °C |
| SN3/SN4 | 10 min at 94 °C; 40 s at 94 °C, 40 s at 51 °C, and 1 min 30 s at 72 °C for 35 cycles; 5 min at 72 °C |
| YH-F/YH-R | 10 min at 94 °C; 40 s at 94 °C, 40 s at 50 °C, and 40 s at 72 °C for 35 cycles; 5 min at 72 °C |
Bioinformation analyses and phylogenetic characterization of AccGSTS1
Homologous AccGSTS1 protein sequences were retrieved using the basic local alignment search tool (BLAST) program from the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and aligned using DNAman version 5.2.2 (Lynnon Biosoft, Quebec, Canada). Conserved domains (CDs) were identified using the PROSITE profile analysis (Bairoch et al. 1997) and CD database from NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The molecular mass and isoelectric point of AccGSTS1 was predicted using PeptideMass (http://web.expasy.org/compute_pi/). The tertiary structure was predicted using the online protein structure prediction tool SWISS-MODEL (http://swissmodel.expasy.org/). Phylogenetic analysis was performed using Molecular Evolutionary Genetic Analysis (MEGA) version 4.1 with the neighbor-joining (NJ) method.
Amplification of genomic sequence and copy number analysis
To obtain the genomic DNA sequence of AccGSTS1, two pairs of specific primers (SN1/SN2 and SN3/SN4) were designed and synthesized according to the full-length cDNA of AccGSTS1. PCR amplification was performed using the genomic DNA of A. cerana cerana as the template. The products were purified, cloned into pMD18-T (TaKaRa, Dalian, China), and transformed into competent E. coli DH5α cells for sequencing. Two overlapping fragments obtained were spliced to form the AccGSTS1 gene. The primers and reaction conditions are provided in Tables 1 and 2, respectively. The copy number of AccGSTS1 in the honey bee genome was determined by real-time quantitative PCR, as described by Mason et al. (2002).
Real-time quantitative PCR
AccGSTS1 transcription was analyzed by quantitative PCR (qPCR) using the SYBR Premix Ex Taq (TaKaRa, Dalian, China) and the CFX96TM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The PCR mix was composed of 12.5 μl SYBR Premix Ex Taq, 2.0 μl of 1:10 diluted cDNA, 0.5 μl of each primer (10 mM), and 9.5 μl PCR grade water in a final volume of 25 μl. The PCR reaction was carried out as follows: 1 cycle of 95 °C for 30 s; 40 cycles at 95 °C for 5 s, 56 °C for 15 s, and 72 °C for 15 s; and then a single melt cycle from 65 to 95 °C. The β-actin gene (GenBank ID XM640276), which is stably expressed (Yang et al. 2012; Umasuthan et al. 2012), was used as an internal control. Specific primers for AccGSTS1 (SD-s, SD-x) and the reference gene (β-actin-s, β-actin-x) are listed in Table 1. Each sample was run in triplicate. The relative expression level of AccGSTS1 was normalized to that of actin messenger RNA (mRNA) and was analyzed using CFX Manager software version 1.1 and the 2−ΔΔCT method (Livak and Schmittgen 2001).
Overexpression and purification of recombinant AccGSTS1
The full-length cDNA of AccGSTS1 was amplified using the primers YH-F and YH-R, in which a BamHI site and a SacI site were introduced, respectively. The PCR product was cloned into the prokaryotic expression vector pET-30a (+) and transformed into E. coli strain BL21 (DE3). The cells were grown in Luria–Bertani (LB) broth with kanamycin at 37 °C, and 1 mM final concentration isopropyl-1-thio-β-galactopyranoside (IPTG) was added to the cultures when the cell density reached 0.4–0.6 OD600. After expression, recombinant AccGSTS1 was purified by Ni2+-nitrilotriacetate (Ni-NTA) resin (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The homogeneity of the enzyme preparation was analyzed by 15 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were assayed by Coomassie Blue staining.
Determination of enzyme activity
Recombinantly expressed and Ni-NTA purified AccGSTS1 was used for enzyme assays. GST activity was measured as previously described (Habig et al. 1974), with some modifications. Specifically, the reaction was performed in a 1-ml reaction system that contained 0.1 M sodium phosphate buffer (pH 6.5), 5 mM 1-chloro-2,4-dinitrobenzene (CDNB), 5 mM GSH, and an appropriate amount of purified recombinant protein. The change in absorbance at 340 nm for 1 min was converted into moles CDNB conjugated with GSH per minute per milligram of protein. The molar extinction coefficient (9.6 mM−1 cm−1) for CDNB was used to convert absorbance into moles.
In vitro peroxidase activity
AccGSTS1 at different concentrations (0, 10, 20, 50, and 100 μg/ml) was incubated in medium containing 100 mM Hepes buffer (pH 7.0) and 10 mM DTT at 37 °C for 10 min. The reaction (a total volume of 500 μl) was initiated by the addition of 11.6 μl of 30 % H2O2. After incubation for 0, 2, 5, and 10 min, 100 μl of 100 % (w/v) trichloroacetic acid was added to stop the reaction. Then, 200 μl of 10 mM Fe(NH4)2(SO4)2 and 100 μl of 2.5 M KSCN were added, resulting in the red-colored ferrithiocyanate complex. The remaining peroxide content was determined by measuring the absorbance decrease at 475 nm.
DNA cleavage assay
The DNA cleavage assay was performed using the mixed-function oxidation (MFO) system that can cause DNA damage. The reaction mixture (25 μl) containing 2 μg of supercoiled pUC19 plasmid DNA (TaKaRa, Dalian, China), 3 μM FeCl3 and 10 mM DTT in 100 mM Hepes buffer (pH 7.0) and increasing concentrations of AccGSTS1 was incubated at 37 °C for 3 h and was then subjected to 1 % agarose gel electrophoresis for the determination of DNA cleavage.
Disc diffusion assay
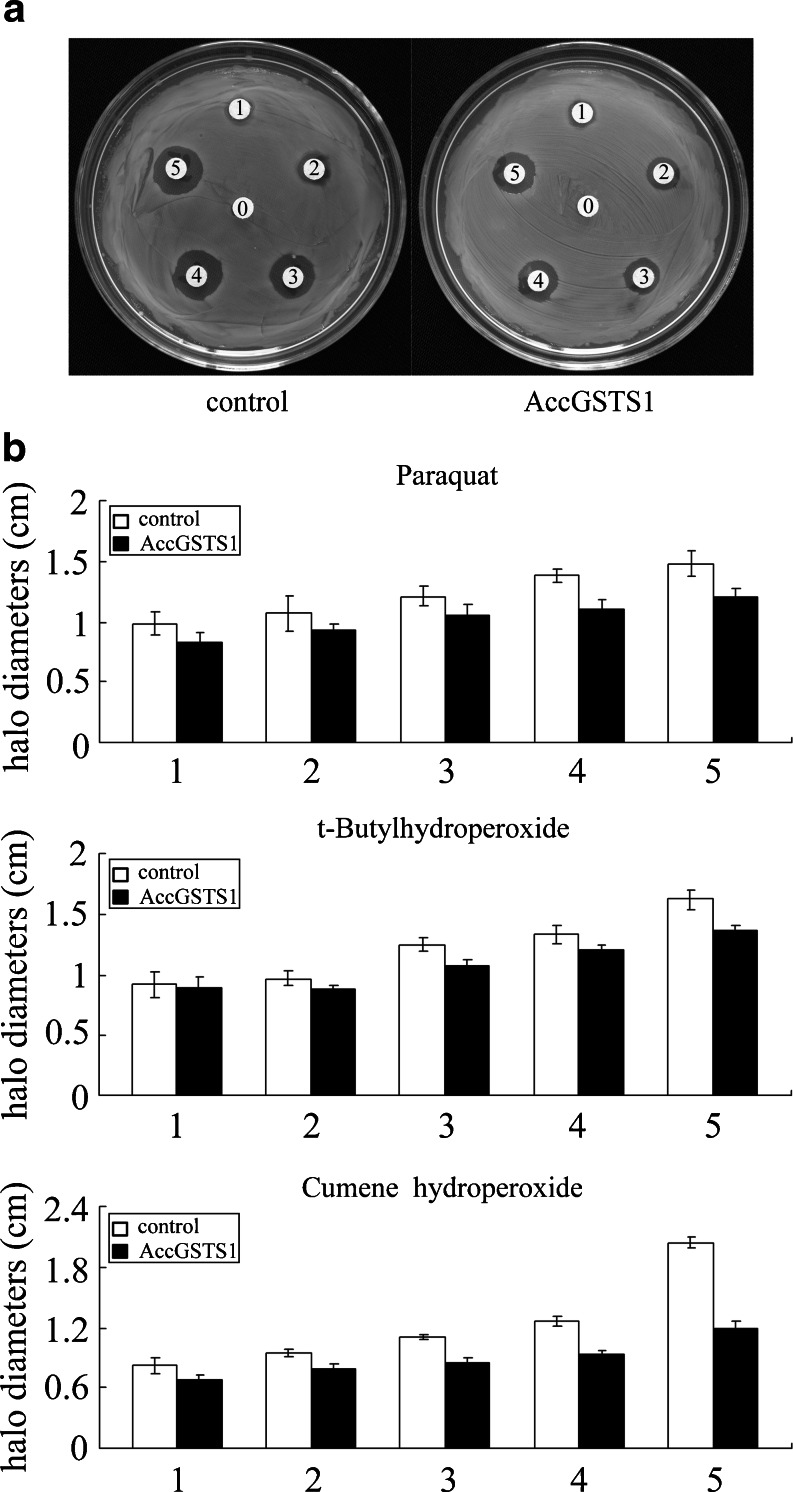
E. coli BL21 (DE3) cells were cultured in LB broth with kanamycin at 37 °C until the cell density reached 0.4–0.6 OD600, then IPTG was introduced to the medium at a final concentration of 1 mM. After being induced for 6 h, approximately 5 × 108 cells overexpressing recombinant AccGSTS1 were overlaid onto LB–kanamycin agar and incubated at 37 °C for 1 h. Cells with the pET-30a (+) vector only were used as the control. Filter discs (6 mm in diameter) soaked in different concentrations of paraquat, cumene hydroperoxide, and t-butylhydroperoxide were placed on the surface of the top agar and incubated at 37 °C for 24 h, and then the killing zones around the discs were measured (Burmeister et al. 2008).
Statistical analysis
The gene expression pattern and in vitro experimental data were subjected to Duncan’s multiple range test with an analysis of variance (ANOVA) using Statistical Analysis System (SAS) version 9.1 software. Comparisons showing significant differences are shown.
Results and discussion
Isolation and molecular characterization of AccGSTS1
The full-length cDNA sequence of a Sigma class GST (AccGSTS1) from A. cerana cerana was obtained using a combination of reverse transcription PCR (RT-PCR) and RACE and was deposited in GenBank with the accession number HQ828076. This sequence encompasses a 615 bp open reading frame (ORF) that encodes a 204-amino acid residue protein, in addition to a 5′-untranslated region (UTR) of 60 bp and a 3′-UTR of 163 bp. No trans-splicing was observed at the 5′ end of the transcripts obtained by RACE. The AccGSTS1 protein has a predicted molecular mass of 23.737 kDa and a theoretical isoelectric point of 8.75. The predicted molecular mass is consistent with other sigma class GSTs, which have an average molecular mass of 23 kDa (Blanchette et al. 2007).
Distribution of sigma class GSTs in the honey bee differs substantially from that in the fruit fly and mosquito; there are four sigma class GSTs in Apis mellifera, indicating gene expansion (Corona and Robinson 2006). Our previous study isolated one sigma class GST gene from the Chinese honey bee, AccGSTS4 (Yu et al. 2012). In contrast, only one sigma class GST gene is found in the D. melanogaster genome (Tu and Akgul 2005) as well as the A. gambiae mosquito, with two distinct transcripts arising from alternative splicing (Ding et al. 2003).
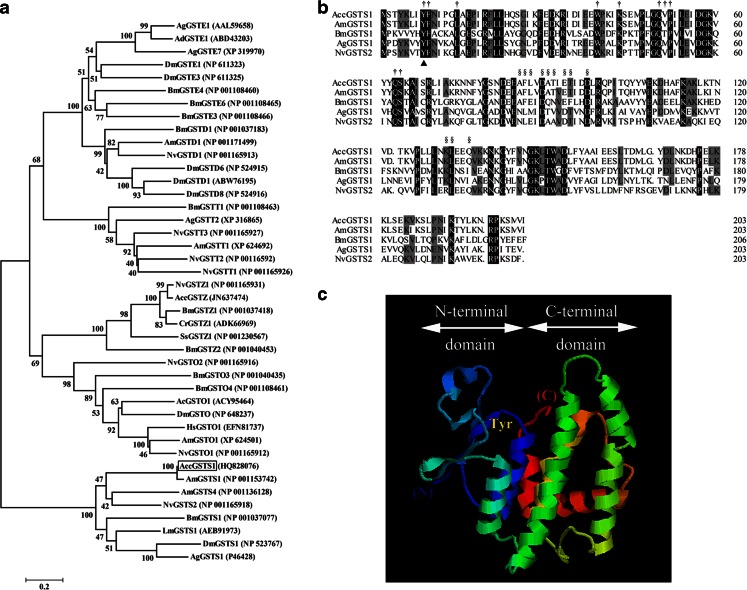
Phylogenetic analysis was also performed to investigate the evolutionary position of AccGSTS1 and its homologues in insects (Fig. 1a). As expected, the tree classified AccGSTS1 alongside other sigma class GSTs. Alignment of AccGSTS1 with its homologues in A. mellifera, B. mori, D. melanogaster, A. gambiae, Locusta migratoria, and Nasonia vitripennis, demonstrated 72.86–98 % similarity. Residues at the N-terminal domains of these GSTs, which contribute to the binding of GSH, were shown to be conserved (Fig. 1b), including a Tyr8 that is critical for stabilization of GSH (Blanchette et al. 2007). On the contrary, residues at the C-terminal domain, where the substrate binding site (H-site) is located, were highly diverse. The 3D molecular structure of AccGSTS1 was predicted by SWISS-MODEL using 1m0uA (1.75 Å) as the template (Fig. 1c). These two proteins share 37.811 % sequence identity, and the model showed that AccGSTS1 possesses the typical cytosolic GST structure, including a conserved N-terminal domain with a βαβαββα motif and a C-terminal domain composed entirely of α-helices.
Fig. 1.
Molecular characterization of AccGSTS1. a Phylogenetic relationship between AccGSTS1 and GSTs from A. cerana cerana, A. mellifera, N. vitripennis, B. mori, A. gambiae, and D. melanogaster, in addition to GSTs from other insects. Species represented: Cr, Chironomus riparius; Ss, Sus scrofa; Hs, Harpegnathos saltator; Ac, Anopheles cracens; Ad, Anopheles dirus; and Lm, Locusta migratoria. The tree was constructed using NJ-based bootstrapped phylogeny in MEGA 4 with 1,000 bootstrap replicates; values are indicated at each node. AccGSTS1 is boxed. The GenBank accession numbers of protein sequences are indicated in parentheses. b Sequence alignment of AccGSTS1 with known homologs. GSTs from A. mellifera (NP_001153742), N. vitripennis (NP_001165918), B. mori (NP_001037077), A. gambiae (XP_311546), L. migratoria (AEB91973), and D. melanogaster (NP_725653) were used. Identical residues are shaded black, while 75 % conserved residues are shown in gray. The catalytic residue Tyr is marked with triangle. The GSH-binding sites and substrate-binding sites are highlighted with dagger and section symbols, respectively. c The tertiary structure of AccGSTS1. The N-terminal domain (βαβαββα motif) containing the G-site and the C-terminal domain (all α-helical motifs) harboring the H-site are shown. The conserved Tyr at position 8 responsible for the stabilization of GSH is indicated
Genomic structure and copy number of AccGSTS1
To elucidate the genomic DNA structure of AccGSTS1, the genomic sequence was amplified using genomic DNA as the template for PCR amplification. The full-length AccGSTS1 genomic sequence (GenBank accession number HQ828082) is 1,865 bp. The positions and sizes of the introns were noted by aligning the cDNA sequences of AccGSTS1 with the genomic DNA sequence. There are a total of three introns, each of which is within the ORF. The nucleotide sequences at the splice junctions are consistent with the canonical GT–AG rule. Next, the genomic sequence of AccGSTS1 was aligned with other GSTS1 sequences; interestingly, although their intron numbers and lengths are different, AccGSTS1, BmGSTS1, and AgGSTS1 share a conserved intron positioned at the 44th codon (Fig. 2). Likewise, previous reports have demonstrated that almost half of the GSTs in the silkworm (Yu et al. 2008) and mosquito (Ding et al. 2003) share a conserved position at approximately the 50th codon from the 5′ end of the gene. Intron positions have been successfully used to delineate the deep phylogenetic relationships (Rogozin et al. 2005). Thus, although the putative AccGSTS1 shows a greater identity to AmGSTS1 than BmGSTS1 or AgGSTS1, AccGSTS1 may have a closer relationship with BmGSTS1 and AgGSTS1. The elucidation of the genomic sequence of AccGSTS1 in this study establishes a platform for deepening our understanding of the organization of the A. cerana cerana GST superfamily.
Fig. 2.
Schematic representation of intron positions of sigma class GST genes. Lengths of genomic DNA sequences from A. cerana cerana (HQ828082), A. mellifera (NW_001254614.1), B. mori (DQ862466), A. gambiae (NT_078267.5), and D. melanogaster (NT_033778.3) are indicated according to the scale below. Light gray and gray are used to highlight the exons and introns, respectively. The initiation and termination codons are indicated by inverted triangles and asterisks, respectively
We next performed qPCR to investigate the copy number of AccGSTS1. AccGSTZ1, which we previously demonstrated to be a single-copy gene by Southern blot (Yan et al. 2012), was used as an internal standard. The correlation coefficients of AccGSTZ1 and AccGSTS1 were rather good, i.e., 0.999 (Supplementary Figure 1), and these results demonstrate that AccGSTS1 is present as a single copy in the A. cerana cerana genome (Table 3).
Table 3.
Estimation of copy number of AccGSTS1 gene in Apis cerana cerana
| Experiments | AccGSTS1 | AccGSTZ1 | AccGSTS1/AccGSTZ1 | Copy number | ||
|---|---|---|---|---|---|---|
| Ct values | Calculation results | Ct values | Calculation results | |||
| 1 | 25.27 | −3.97 | 25.01 | −4.19 | 0.95 | 1 |
| 2 | 27.09 | −4.56 | 26.52 | −4.68 | 0.97 | 1 |
| 3 | 29 | −5.18 | 28.5 | −5.34 | 0.97 | 1 |
Tissue and stage-specific expression profiles of AccGSTS1
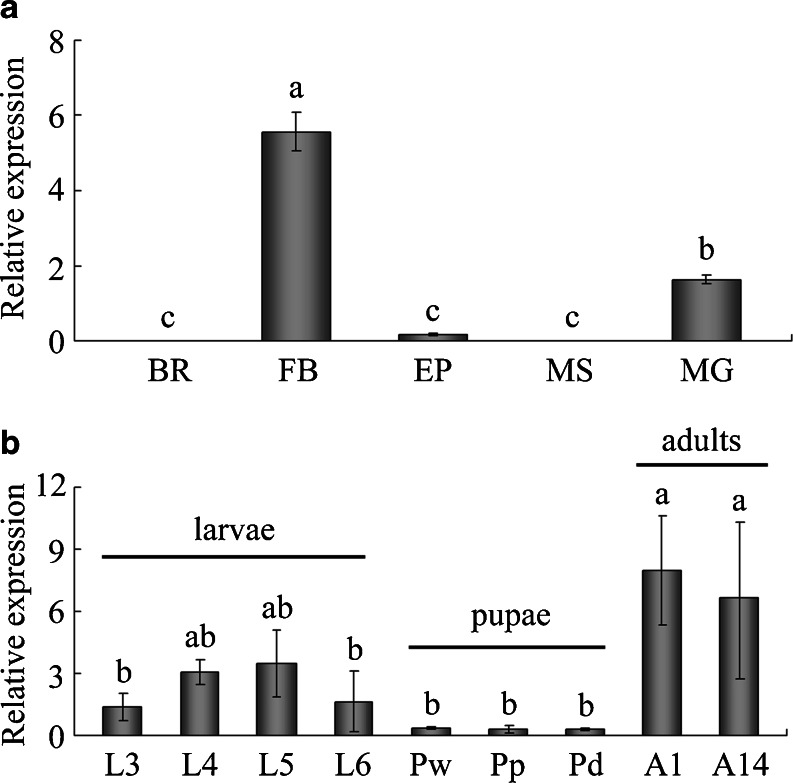
The distribution of AccGSTS1 was analyzed by qPCR using multiple tissues from adult worker bees, including the brain, fat body, epidermis, muscle, and midgut. As shown in Fig. 3a, the mRNA level of AccGSTS1 was most robust in the fat body, and the expression was appreciable in the midgut, but the transcripts in the brain were virtually undetectable, suggesting tissue-specific expression patterns. In insects, the fat body and midgut are the central metabolic organs that play important roles in detoxification/degradation of xenobiotics and protection from oxidative stress (Arrese and Soulages 2010; Enayati et al. 2005; Sawicki et al. 2003). In addition, there is evidence that insect GSTs can detoxify many plant allelochemicals and synthetic insecticides (Li et al. 2007). Thus, the higher mRNA levels of AccGSTS1 in these two tissues suggest a potential functional role in protection against the toxicity of xenobiotics present in the diet of honey bees. GST genes have also been reported to be widely expressed in the fat body and midgut of other insect species (Chintapalli et al. 2007; Krishnan and Kodrík 2006). In D. melanogaster, 28 out of 38 (73.7 %) GST genes, belonging to a total of 5 classes, are expressed in the midgut of third instar larvae (Li et al. 2008), and in B. mori, the percentage is 73.9 % (Yu et al. 2008). GSTs have also been found in large concentrations in other tissues. For example, DmGSTS1-1 was thought to be anchored to the flight muscle of D. melanogaster by the hydrophobic/acidic N-terminal extension and was demonstrated to be instrumental in protecting this highly aerobic tissue from by-products of oxidative stress (Singh et al. 2001). Although this N-terminal extension on the sigma class GST also occurs in some other Muscamorphan insects, such as Musca domestica, Glossina morsitans, and Mayetola destructor, anchoring of this GST to the flight muscle is not universal. Absence of such GST proteins in other insects including A. cerana cerana suggests that there may be quite substantial differences in the biochemistry of flight muscle between different insect groups (Flanagan and Smythe 2011).
Fig. 3.
Transcriptional patterns of AccGSTS1 in different tissues and during different stages of development. a Distribution of AccGSTS1 in the brain (BR), fat body (FB), epidermis (EP), muscle (MS), and midgut (MG). b The mRNA transcripts of AccGSTS1 at the following developmental stages: larvae (L3–L6 instars), pupae (Pw white eyes, Pp pink eyes, and Pd dark eyes) and adults (A1 1-day postemergence and A14 14-day post-emergence adults). The mRNA expression of AccGSTS1 was quantified and normalized to that of the A. cerana cerana β-actin gene. Vertical bars represent the mean ± SD (n = 3). Different letters above the bar indicate significant differences, according to P < 0.01 in SAS
To determine the regulation of AccGSTS1 at different developmental stages, the expression of AccGSTS1 in larvae, pupae, and adults was measured by qPCR. Larvae and pupae worker honey bees were generally classified according to age, eye color, and shape. As for the adults, we chose the 1-day-old bees and 2-week-old foragers mainly considering the role of AccGSTS1 in oxidative stress. Only foragers have the chance to go outside of the hive and exposed to external environment and therefore challenged by excessive ROS and oxidative stress. Both of 1-day-old bees and 2-week-old hive bees are alive in the comb and suffered less oxidative damage than foragers. Using bees at the stage of 1-day-old bees and 2-week-old foragers can not only determined the expression of AccGSTS1 at different development stages but also considered the factors of oxidative stress. Moreover, behavior may affect the expression of AccGSTS1 in 2-week-old foragers and hive bees; further studies could be done to verify this possibility. The results suggested the developmental regulation of AccGSTS1 with the highest mRNA levels in adults (Fig. 3b), the stage at which the bees have the chance to leave the hive and forage for nectar and pollen for the remainder of their lives (Ament et al. 2008). The transcripts remained unchanged from the last-instar larvae to dark eye pupae, indicating that AccGSTS1 may be constitutively expressed in the pupae. Maximum expression of AccGSTS1 was shown to occur during a period in the lifecycle of the bees when they are exposed to the external environment. It is during this time that they encounter various stresses and are therefore more susceptible to toxic chemicals and oxidative damage. In D. melanogaster, the sole sigma class GST plays a dominant protective role against oxidative damage (Singh et al. 2001; Alias and Clark 2007). Taken together, the high expression of AccGSTS1 at this stage indicates that it may play important roles in detoxification as well as antioxidant defense.
Induction of AccGSTS1 after exposure to various environmental stresses
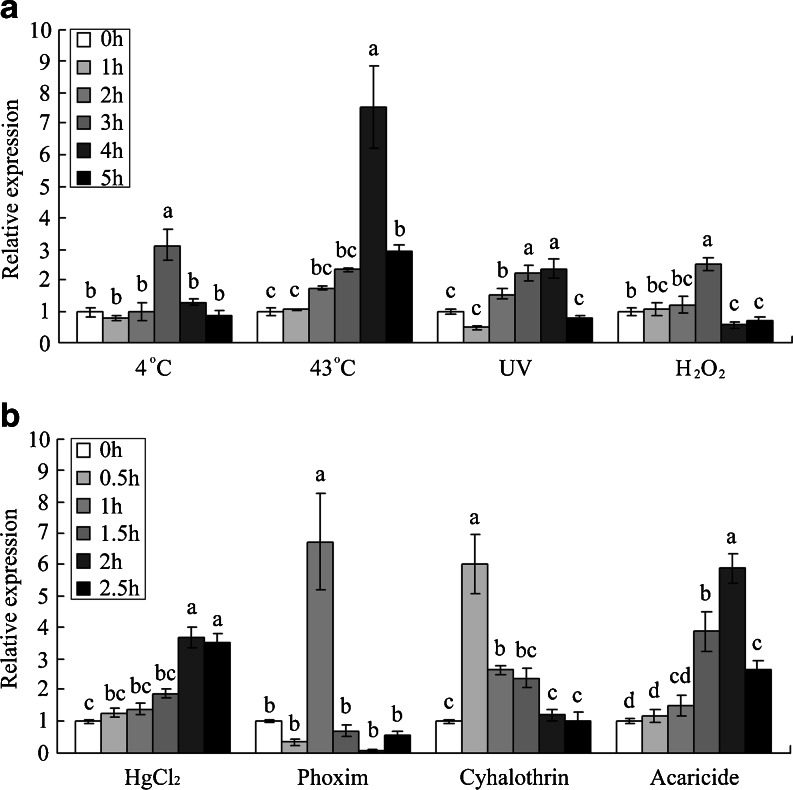
GSTs are known to be an important component of the oxidative stress response. Previous studies have indicated that environmental conditions such as temperature, heavy metals, salinity, pesticides, and UV radiation can induce oxidative stress (Lushchak 2011; Kottuparambila et al. 2012). To elucidate the potential involvement of AccGSTS1 in antioxidant defense, transcripts of AccGSTS1 under cold (4 °C), heat (43 °C), H2O2, HgCl2, and insecticide (phoxim, cyhalothrin, and acaricide) exposure were examined by qPCR (Fig. 4). AccGSTS1 expression was upregulated under all treatments examined, although the mRNA levels were increased by different degrees. Alterations to GST expression induced by various factors have been extensively reported (Meng et al. 2009; Nair and Choi 2011; Umasuthan et al. 2012; Yang et al. 2012), and GSTs are known to act as detoxification agents against the chemotoxicity of xenobiotics. Elevated levels of GST are also correlated with resistance to insecticides (Ranson et al. 2001; Vontas et al. 2001). Moreover, in Nilaparvata lugens, GST levels are elevated primarily to protect tissues by conferring resistance against oxidative damage (Vontas et al. 2001). Thus, the high expression of AccGSTS1 may present a defense mechanism against increased levels of oxidative stress caused by different environmental challenges. The induction of GST has been proposed to represent an evolutionarily conserved cellular response to oxidative stress (Hayes et al. 2005), and individual GSTs present in different insect species or different subclasses within the same species are regulated independently in response to various stressors. Among the six classes of GSTs, sigma, as well as delta and epsilon GSTs, has been shown to play key roles in antioxidant defense in various organisms (Singh et al. 2001; Hayes et al. 2005; Oakley 2005; Frova 2006; Blanchette et al. 2007). Taken together, we hypothesize that upregulated AccGSTS1 expression may be associated with antioxidant tolerance in honey bees.
Fig. 4.
Expression patterns of AccGSTS1 under various abiotic stresses. a The mRNA levels of AccGSTS1 upon exposure to cold (4 °C), heat (43 °C), UV radiation, and H2O2. Samples were collected at 0, 1, 2, 3, and 4 h after different treatments. b The expression level of AccGSTS1 after bees were fed with a heavy metal (HgCl2) and insecticides (phoxim, cyhalothrin, and acaricide) for 0, 0.5, 1, 1.5, and 2 h. The mRNA expression of AccGSTS1 was quantified and normalized to that of the A. cerana cerana β-actin gene. Vertical bars represent the mean ± SD (n = 3). Different letters above the bar indicate significant differences, according to P < 0.01 in SAS
Transgenic worms expressing a Caenorhabditis elegans GSTO-1::GFP fusion protein under the control of the GSTO-1 promoter experienced transcriptional upregulation of GSTO-1 under pro-oxidant stress, which subsequently led to an increase in stress resistance in the worms (Burmeister et al. 2008). In particular, the overexpression of C. elegans GSTP2-2 in transgenic worms was found not only to lead to resistance to paraquat, heat shock, UV radiation, and hydrogen peroxide but also to an increased median lifespan (Ayyadevara et al. 2005). Therefore, an increase in AccGSTS1 expression under various abiotic stresses may protect honey bees from ROS damage to ensure survival and may represent an adaptive response against oxidative stress.
Bacterial expression and purification of AccGSTS1
AccGSTS1 was overexpressed as a fusion protein with a cleavable N-terminal His-tag in E. coli BL21 (DE3). SDS-PAGE analysis revealed that this AccGSTS1 fusion protein was expressed in a soluble form after induction with IPTG (Fig. 5). The recombinant protein had a molecular size of approximately 23 kDa, which is in agreement with its predicted value. This recombinant protein was further purified using an Ni-nitrilotriacetic acid spin column. Finally, 4.8 mg of highly purified AccGSTS1 from 250 mL of LB medium was obtained. In previous studies, recombinant Sigma class GSTs from the fall webworm (Yamamoto et al. 2007), silkworm (Yamamoto et al. 2006), and earthworm (LaCourse et al. 2009) exhibited specific activities for CDNB ranging between 1.9 and 4.4 μmol min−1 mg−1. In the present study, the specific activity of the final product toward CDNB was 2.1 μmol min−1 mg−1, revealing that active AccGSTS1 was successfully expressed in E. coli cells.
Fig. 5.
Overexpression and purification of recombinant AccGSTS1 fusion protein in E. coli BL21 (DE3). Lanes M protein marker; U and I un-induced and IPTG-induced cellular extract, respectively; P purified AccGSTS1 protein
Biochemical characterization of AccGSTS1
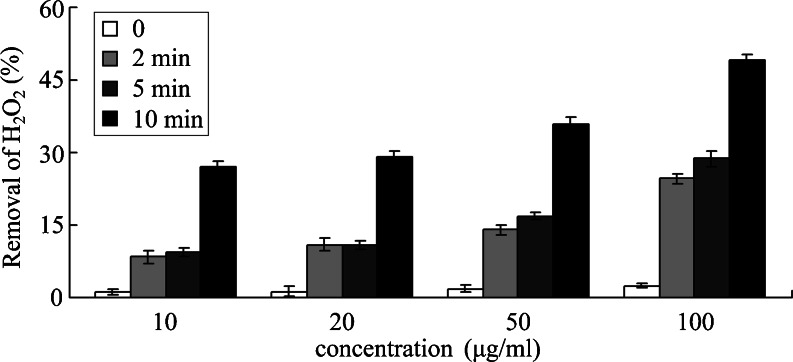
As sigma class proteins have been shown to be involved in lipid peroxidation in Drosophila (Agianian et al. 2003), we next determined the antioxidant properties of AccGSTS1 by measuring the removal of H2O2 in a reaction mixture with DTT as an electron donor (Suttiprapa et al. 2008). As shown in Fig. 6, the rate of H2O2 destruction was gradually increased with increasing concentrations of recombinant protein and incubation time, suggesting that AccGSTS1 can catalyze the removal of H2O2 in a concentration- and time-dependent manner. To further evaluate the capacity of AccGSTS1 to protect DNA from oxidative damage, we employed a thiol-dependent MFO system, in which hydroxyl radicals are produced and convert supercoiled plasmid DNA into its nicked form (lane 3) (Fig. 7). The purified AccGSTS1 was found to prevent supercoiled pUC19 from degrading in a dose-dependent manner (lanes 4–9). Protection against DNA cleavage in the thiol-MFO system was due to the removal of activated oxygen species (Lim et al. 1993), suggesting that AccGSTS1 may be involved in the protection mechanism under oxidative stress. In addition, DNA repair capacity has been shown to correlate with increased stress resistance and thus may lead to a longer lifespan (Hyun et al. 2008). Therefore, AccGSTS1 may also be associated with the lifespan of the honey bee. GST peroxidase activity is of particular importance to insects because they do not possess any selenium-dependent glutathione peroxidase activity and therefore depend on GSTs for the reduction of organic hydroperoxides (Singh et al. 2001). The observation that AccGSTS1 could remove H2O2 and protect DNA indicates that AccGSTS1 may play an important role in the survival of insects under oxidative stress.
Fig. 6.
Effect of AccGSTS1 on the removal of H2O2. Peroxidase activity of AccGSTS1 in different concentrations and incubation times. Abscissa indicates different concentration of recombinant protein (10, 20, 50, and 100 μg/ml) added in the reaction mixture. White, light gray, dark gray, and black represent incubation times (0, 2, 5, and 10 min, respectively). Values shown represent the mean (SD) of three experiments
Fig. 7.
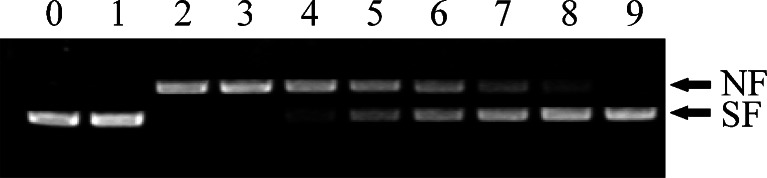
Ability of AccGSTS1 to protect against DNA damage in the MFO system. Lane 0 pUC19 plasmid DNA only; lane 1 pUC19 plasmid DNA + FeCl3; lane 2 pUC19 plasmid DNA + FeCl3 + dithiothreitol (DTT); lane 3 pUC19 plasmid DNA + FeCl3 + BSA; lanes 4–9 pUC19 plasmid DNA + FeCl3 + DTT + purified AccGSTS1 (5, 10, 50, 100, 150, and 200 mg/ml, respectively). SF supercoiled form, NF nicked form
To provide direct evidence that AccGSTS1 is responsible for antioxidant defense, a disc diffusion assay was performed. E. coli cells overexpressing AccGSTS1 were exposed to paraquat, t-butylhydroperoxide and cumene hydroperoxide, which are known oxidative stress inducers (Burmeister et al. 2008). Following overnight exposure, the inhibition zones of the bacteria expressing AccGSTS1 were found to be much smaller than those of cells transfected with the vector only (Fig. 8a). The halo size reductions were 18 % for paraquat, 19 % for t-butylhydroperoxide, and 41 % for cumene hydroperoxide (Fig. 8b). Earlier studies have demonstrated that paraquat treatment results in oxidative stress and induces the expression of several classes of GST genes in C. riparius (Nair and Choi 2011). Similarly, cumene hydroperoxide and t-butylhydroperoxide are also widely used as model substances for studying the mechanism of cell injury resulting from oxidative stress (Sawicki et al. 2003; Tang and Tu 1994). In the present study, paraquat, t-butylhydroperoxide and cumene hydroperoxide adsorbed to the filter disc may have induced oxidative stress in E. coli cells. The decrease in the size of the killing zones for cells over-expressing AccGSTS1 provides further evidence that AccGSTS1 can serve as an effective antioxidant enzyme that protects cells from oxidative stress.
Fig. 8.
Antioxidant activity of AccGSTS1 according to a disc diffusion assay. a Approximately 5 × 108E. coli cells overexpressing AccGSTS1 were flooded on the LB agar plates. Paper discs, which adsorbed paraquat, cumene hydroperoxide, and t-butylhydroperoxide in concentration gradients, were placed on the agar plates seeded with bacteria. The plates were incubated for 24 h at 37 °C. The labels 0, 1, 2, 3, 4, and 5 on filter discs represent the different concentrations of paraquat (0, 50, 100, 150, 200, and 250 μM, respectively), cumene hydroperoxide (0, 50, 100, 150, 200, and 250 mM, respectively), and t-butylhydroperoxide (0, 25, 50, 100, 200, and 300 mM, respectively). Bacteria transfected with pET-30a (+) (vector only) were used as controls. The halo diameter of the inhibition zones is shown in b
Conclusions
In the present study, we identified and characterized a sigma class GST from A. cerana cerana. This enzyme (AccGSTS1) is represented by a single locus in the genome and possesses the conserved functional domains of the GST superfamily. The temporal expression levels of AccGSTS1 in response to various environmental stresses indicate that AccGSTS1 may play a biological role in oxidative stress-related defense mechanisms. Its induction under HgCl2, phoxim, cyhalothrin, and acaricide exposure may result in the enhanced tolerance of A. cerana cerana to other insecticides and xenobiotics. Recombinant AccGSTS1 was shown to remove H2O2 and protect DNA from oxidative damage, and a disc diffusion assay demonstrated a protective role upon exposure to oxidative stress. Taking into account these observations, our results suggest that AccGSTS1 may be involved in detoxification mechanisms and may be an important antioxidant enzyme against oxidative stress. In conclusion, these findings may be useful for studying the comparative molecular functions of different GST classes across various insect lineages.
Electronic supplementary material
(JPEG 18 kb)
Acknowledgments
This work was funded by the China Agriculture Research System (no. CARS-45), Agro-scientific Research in the Public Interest (number 200903006) and the National Natural Science Foundation (number 31172275) in China.
Contributor Information
Xingqi Guo, Phone: +86-538-8245679, FAX: +86-538-8226399, Email: xqguo@sdau.edu.cn.
Baohua Xu, Phone: +86-538-8245679, FAX: +86-538-8226399, Email: bhxu@sdau.edu.cn.
References
- Agianian B, Tucker PA, Schouten A, Leonard K, Bullard B, Gros P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J Mol Biol. 2003;326:151–165. doi: 10.1016/S0022-2836(02)01327-X. [DOI] [PubMed] [Google Scholar]
- Alias Z, Clark AG. Studies on the glutathione S-transferase proteome of adult Drosophila melanogaster: responsiveness to chemical challenge. Proteomics. 2007;7:3618–3628. doi: 10.1002/pmic.200700070. [DOI] [PubMed] [Google Scholar]
- Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA. 2008;105:4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Ann Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Benes H, Shmookler Reis RJ, Liebau E, Zimniak P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette B, Feng X, Singh BR. Marine glutathione S-transferases. Mar Biotechnol. 2007;9:513–542. doi: 10.1007/s10126-007-9034-0. [DOI] [PubMed] [Google Scholar]
- Burmeister C, Luërsen K, Heinick A, Hussein A, Domagalski M, Walter RD, Liebau E. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) FASEB J. 2008;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- Che-Mendoza A, Penilla RP, Rodríguez DA. Insecticide resistance and glutathione S-transferases in mosquitoes: a review. Afr J Biotechnol. 2009;8:1386–1397. [Google Scholar]
- Chen L, Hall PR, Zhou XE, Ranson H, Hemingway J, Meehan EJ. Structure of an insect delta-class glutathione S-transferase from a DDT-resistant strain of the malaria vector, Anopheles gambiae. Acta Crystallagr D. 2003;59:2211–2217. doi: 10.1107/S0907444903018493. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Corona M, Robinson GE. Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol. 2006;15:687–701. doi: 10.1111/j.1365-2583.2006.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Ortelli F, Rossiter LC, Hemingway J, Ranson H. The Anopheles gambiae glutathione transferase supergene family: annotation, phylogeny and expression profiles. BMC Genom. 2003;4:35. doi: 10.1186/1471-2164-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Hawkes N, Meredith J, Eggleston P, Hemingway J, Ranson H. Characterisation of the promoters of Epsilon glutathione transferases in the mosquito Anopheles gambiae and their response to oxidative stress. Biochem J. 2005;387:879–888. doi: 10.1042/BJ20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durou C, Poirier L, Amiard JC, Budzinski H, Gnassia-Barelli M, Lemenach K, Peluhet L, Mouneyrac C, Romeo M, Amiard-Triquet C. Biomonitoring in a clean and a multi-contaminated estuary based on biomarkers and chemical analyses in the endobenthic worm Nereis diversicolor. Environ Pollut. 2007;148:445–458. doi: 10.1016/j.envpol.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Enayati AA, Ranson H, Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol Biol. 2005;14:3–8. doi: 10.1111/j.1365-2583.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- Flanagan JU, Smythe ML. Sigma-class glutathione transferases. Drug Metab Rev. 2011;43:194–214. doi: 10.3109/03602532.2011.560157. [DOI] [PubMed] [Google Scholar]
- Frova C. Glutathione transferases in the genomics era: new insights and perspectives. Biomol Eng. 2006;23:149–169. doi: 10.1016/j.bioeng.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family. Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hyun M, Lee J, Lee K, May A, Bohr VA, Ahn B. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res. 2008;36:1380–1389. doi: 10.1093/nar/gkm1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampkotter A, Volkmann TE, de Castro SH, Leiers B, Klotz LO, Johnson TE, Link CD, Henkle-Duhrsen K. Functional analysis of the glutathione S-transferase 3 from Onchocerca volvulus (Ov-GST-3): a parasite GST confers increased resistance to oxidative stress in Caenorhabditis elegans. J Mol Biol. 2003;325:25–37. doi: 10.1016/S0022-2836(02)01174-9. [DOI] [PubMed] [Google Scholar]
- Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem Mol Biol. 2001;31:313–319. doi: 10.1016/S0965-1748(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Kottuparambila S, Shinb W, Brownc MT, Han T. UV-B affects photosynthesis, ROS production and motility of the freshwater flagellate, Euglena agilis Carter. Aquat Toxicol. 2012;122–123:206–213. doi: 10.1016/j.aquatox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Kodrík D. Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress? J Insect Physiol. 2006;52:11–20. doi: 10.1016/j.jinsphys.2005.08.009. [DOI] [PubMed] [Google Scholar]
- LaCourse EJ, Hernandez-Viadel M, Jefferies JR, Svendsen C, Spurgeon DJ, Barrett J, Morgan AJ, Kille P, Brophy PM. Glutathione transferase (GST) as a candidate molecular-based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environ Pollut. 2009;157:2459–2469. doi: 10.1016/j.envpol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Ann Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- Li HM, Buczkowski G, Mittapalli O, Xie J, Wu J, Westerman R, Schemerhorn BJ, Murdock LL, Pittendrigh BR. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol Biol. 2008;17:325–339. doi: 10.1111/j.1365-2583.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- Lim YS, Cha MK, Uhm TB, Park JW, Kim K, Kim IH. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993;192:273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Low WY, Ng HL, Morton CJ, Parker MW, Batterham P, Robin C. Molecular evolution of glutathione S-transferases in the genus Drosophila. Genetics. 2007;177:1363–1375. doi: 10.1534/genetics.107.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumjuan N, Mccarroll L, Prapanthadara LA, Hemingway J, Ranson H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector. Aedes aegypti. Insect Biochem Mol Biol. 2005;35:861–871. doi: 10.1016/j.ibmb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Lumjuan N, Rajatileka S, Changsom D, Wicheer J, Leelapat P, Prapanthadara L, Somboon P, Lycett G, Ranson H. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem Mol Biol. 2011;41:203–209. doi: 10.1016/j.ibmb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Mason G, Provero P, Vaira AM, Accotto GP. Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnol. 2002;2:20. doi: 10.1186/1472-6750-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JY, Zhang CY, Zhu F, Wang XP, Lei CL. Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J Insect Physiol. 2009;55:588–592. doi: 10.1016/j.jinsphys.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Nair PMG, Choi J. Identification characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat Toxicol. 2011;101:550–560. doi: 10.1016/j.aquatox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Nair PMG, Park SY, Choi J. Expression of catalase and glutathione S-transferase genes in Chironomus riparius on exposure to cadmium and nonylphenol. Com Biochem Physiol C. 2011;154:399–408. doi: 10.1016/j.cbpc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Oakley AJ. Glutathione transferases: new functions. Curr Opin Struct Biol. 2005;15:716–723. doi: 10.1016/j.sbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Wang X, Prapanthadara L, Hemingway J, Collins FH. Genetic mapping of two loci affecting DDT resistance in the malaria vector Anopheles gambiae. Insect Mol Biol. 2000;9:499–507. doi: 10.1046/j.1365-2583.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Rossiter L, Ortelli F, Jensen B, Wang X, Roth CW. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem J. 2001;359:295–304. doi: 10.1042/0264-6021:3590295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Jani MK, Vogt RG. An olfactory-specific glutathione-S-transferase in the sphinx moth Manduca sexta. J Exp Biol. 1999;202:1625–1637. doi: 10.1242/jeb.202.12.1625. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV. Analysis of evolution of exon–intron structure of eukaryotic genes. Brief Bioinform. 2005;6:118–134. doi: 10.1093/bib/6.2.118. [DOI] [PubMed] [Google Scholar]
- Sawicki R, Singh SP, Mondal AK, Benes H, Zimniak P. Cloning, expression and biochemical characterization of one Epsilon class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem J. 2003;370:661–669. doi: 10.1042/BJ20021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur J Biochem. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- Suttiprapa S, Loukas A, Laha T, Wongkham S, Kaewkes S, Gaze S, Brindley PJ, Sripa B. Characterization of the antioxidant enzyme, thioredoxin peroxidase, from the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biochem Parasitol. 2008;160:116–122. doi: 10.1016/j.molbiopara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Tu CP. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem. 1994;269:27876–27884. [PubMed] [Google Scholar]
- Tu CP, Akgul B. Drosophila glutathione S-transferases. Methods Enzymol. 2005;401:204–226. doi: 10.1016/S0076-6879(05)01013-X. [DOI] [PubMed] [Google Scholar]
- Umasuthan N, Revathy KS, Lee Y, Whang I, Choi CY, Lee J. A novel molluscan sigma-like glutathione S-transferase from Manila clam, Ruditapes philippinarum: Cloning, characterization and transcriptional profiling. Com Biochem Physiol C. 2012;155:539–550. doi: 10.1016/j.cbpc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J. 2001;357:65–72. doi: 10.1042/0264-6021:3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Shi M, Chen XX. Antimicrobial peptide evolution in the Asiatic honey bee Apis cerana. PLoS One. 2009;4:e4239. doi: 10.1371/journal.pone.0004239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Zhang PB, Banno Y, Fujii H. Identification of a sigma-class glutathione-S-transferase from the silkworm, Bombyx mori. J Appl Entomol. 2006;130:515–522. doi: 10.1111/j.1439-0418.2006.01092.x. [DOI] [Google Scholar]
- Yamamoto K, Fujii H, Aso Y, Banno Y, Koga K. Expression and characterization of a sigma-class glutathione S-transferase of the fall webworm, Hyphantria cunea. Biosci Biotechnol Biochem. 2007;71:553–560. doi: 10.1271/bbb.60592. [DOI] [PubMed] [Google Scholar]
- Yan HR, Meng F, Jia HH, Guo XQ, Xu BH. The identification and oxidative stress response of a zeta class glutathione S-transferase (GSTZ1) gene from Apis cerana cerana. J Insect Physiol. 2012;58:782–791. doi: 10.1016/j.jinsphys.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Yang ML, Zhang JZ, Zhu KY, Xuan T, Liu XJ, Guo YP, Ma EB. Mechanisms of organophosphate resistance in a field population of oriental migratory locust, Locusta migratoria manilensis (Meyen) Arch Insect Biochem Physiol. 2009;71:3–15. doi: 10.1002/arch.20254. [DOI] [PubMed] [Google Scholar]
- Yang J, Wei XM, Xu J, Yang DL, Liu XQ, Yang JM, Fang JH, Hu XK. A sigma-class glutathione S-transferase from Solen grandis that responded to microorganism glycan and organic contaminants. Fish Shellfish Immunol. 2012;32:1198–1204. doi: 10.1016/j.fsi.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Yu QY, Lu C, Li B, Fang SM, Zuo WD, Dai FY, Zhang Z, Xiang ZH. Identification, genomic organization and expression pattern of glutathione S-transferase in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2008;38:1158–1164. doi: 10.1016/j.ibmb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yu XL, Sun RJ, Yan HR, Guo XQ, Xu BH. Characterization of a sigma class glutathione S-transferase gene in the larvae of the honeybee (Apis cerana cerana) on exposure to mercury. Comp Biochem Physiol B. 2012;161:356–364. doi: 10.1016/j.cbpb.2011.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPEG 18 kb)