Abstract
Predicting the prognosis of comatose, post-cardiac-arrest patients is a complex problem in clinical practice. There are several established methods to foretell neurological outcome; however, further prognostic markers are needed. HSP70 (HSPA1A), which increases rapidly in response to severe stress (among others after ischemic or hypoxic events), is a biomarker of cell damage in the ischemic brain and spinal cord. We hypothesized that HSP70 might be a reliable predictor of mortality in post-cardiac-arrest patients. The aim of this study was to analyze the role of extracellular HSP70 in the systemic inflammatory response over time, as well as the predictive value in cardiac arrest patients. Here, we show that the elevation of HSP70 levels in resuscitated patients and their persistence is an independent predictor of 30-day mortality after a cardiac arrest. Forty-six cardiac arrest patients were successfully cooled to 32–34 °C for 24 h, and followed up for 30 days. Twenty-four patients (52.2 %) were alive by the end of follow-up, and 22 patients (47.8 %) died. Forty-six patients with stable cardiovascular disease served as controls. Extracellular HSP70 (measured by ELISA in blood samples) was elevated in all resuscitated patients (1.31 [0.76–2.73] and 1.70 [1.20–2.37] ng/ml for survivors and non-survivors, respectively), compared with the controls (0.59 [0.44–0.83] ng/ml). HSP70 level decreased significantly in survivors, but persisted in non-survivors, and predicted 30-day mortality regardless of age, sex, complications, and the APACHE II score. Extracellular HSP70 could prove useful for estimating prognosis in comatose post-cardiac-arrest patients.
Keywords: HSP70, HSPA1A, Cardiac arrest, Hypoxia–ischemia, Inflammation, Mild hypothermia
Introduction
Within the heat shock protein (Hsp) family, HSP70 (HSPA1A) is a structurally and functionally conserved protein in evolution. It is ubiquitous in all organisms, from archaebacteria and plants to humans (Daugaard et al. 2007). HSP70 plays multiple roles in cellular homeostasis. Its level increases rapidly in response to various types of severe stress, as a protection against a subsequent, near-lethal, ischemic or hypoxic event (Hecker and McGarvey 2011). Besides, it is involved in the folding of proteins, their transport between cellular compartments, and in the breakdown of the irreversibly damaged ones. Previously, human heat shock proteins were regarded as obligate intracellular molecules that essentially contribute to survival by acting as molecular chaperones. Subsequently, HSP70 has been detected also in the circulation, in the extracellular space, and its presence has been demonstrated in the serum of healthy individuals (Pockley et al. 1998). HSP70 could be passively released from necrotic cells, and actively excreted by a non-classical secretory pathway. The mechanisms of HSP70 release have been extensively reviewed recently (Asea 2007).
Additionally, extracellular HSP70 was considered among the intercellular signalling regulators of inflammation (Henderson and Pockley 2010). Extracellular HSP70 is involved in antigen presentation, the immune response, and in signalling the pattern of danger within the extracellular space. Elevated HSP70 in the serum or tissues appears to be a nonspecific indicator of organ ischemia or dysfunction. HSP70 is valuable as an immediate, secreted biomarker of critical, ongoing cellular ischemia in brain and spinal cord ischemia models (Hecker and McGarvey 2011). Our work group demonstrated previously that serum HSP70 levels are associated with disease severity in chronic heart failure patients, but did not find correlation between HSP70 and CRP, TNF-alpha or IL-6 (Gombos et al. 2008).
Cardiac arrest causes generalized ischemia/hypoxia, and subsequent resuscitation inflicts reperfusion injury. This process initiates a complex series of events, known as the post-cardiac arrest syndrome. Although all the organs are damaged by hypoxia, cerebral injury occurs first, because the ischemia tolerance and metabolic reserve of the brain are minimal, and hence its functions are greatly dependent on blood flow. Ischemia and reperfusion injury cause intense stress in the brain by multiple pathways, including oxidative stress, microvascular injury, excitotoxicity, blood–brain barrier dysfunction, postischemic inflammation initiated by neuronal, glial and endothelial cell death, or apoptosis. Reliable biomarkers of brain ischemia and of reperfusion injury are the S100 protein B (S100B), and the neuron-specific enolase (NSE); a strong correlation exists between these markers and in the prognosis of post-cardiac-arrest patients (Shinozaki et al. 2009). Recently, Hecker et al. reviewed the potential of HSP70 as a biomarker for the rapid detection of brain and spinal cord ischemia (Hecker and McGarvey 2011). Elevated serum HSP70 levels of patients with severe traumatic brain injury predicted death within 20 h after injury (da Rocha et al. 2005). Based on the foregoing, we hypothesized that HSP70 might prove not only a reliable biomarker of neuronal damage, but also a good surrogate of endothelial-cell activation and death, as well as of inflammatory reaction in post-cardiac-arrest patients. We further assumed that as an integrative marker of stress, HSP70 could be an independent predictor of mortality in these critically ill subjects. Accordingly, the aim of the present study was to describe the HSP70 response in post-cardiac-arrest patients undergoing mild hypothermia treatment and to analyze its association with overall survival and the levels of biomarkers of endothelial-cell activation, acute-phase reaction, and inflammation.
Materials and methods
Patient population
We performed a prospective, observational study of 46 consecutive comatose patients successfully resuscitated after an out-of-hospital or an in-hospital cardiac arrest. The study was carried out in compliance with the Helsinki Declaration. The study protocol was approved by the local Institutional Review Board (TUKEB), and written informed consent was obtained from the closest relative. All patients aged 18 years or older were eligible for inclusion, if they met the following criteria: comatose (Glasgow Coma Scale score ≤6) after the return of spontaneous circulation, and undergoing coronary angiography, or a percutaneous coronary intervention (PCI). Pregnant women were excluded along with the patients who received thrombolytic therapy, or needed at least double vasopressor support due to refractory cardiogenic shock.
Control population
A total of 46 control patients were selected for HSP70 measurement. All control patients suffered from stable cardiovascular disease. The samples of control patients were collected in the framework of another study being conducted at the Heart Center, Semmelweis University, and a subset (n = 46) was selected (according to age and gender) for HSP70 measurement. We selected controls of matching gender and age (±2 years) before measuring HSP70. The control patients had no prior cardiac arrest, and did not receive hypothermia treatment. Venous blood samples were obtained from the control subjects. Blood samples (native and EDTA) were centrifuged within 2 h, for 15 min, at 2,000×g and 25 °C; the samples were stored at −80 °C until analysis.
Management of patient population
All patients were admitted to the Intensive Care Unit of Heart Center, Semmelweis University (Budapest, Hungary) between 2009 and 2011. Coronary angiography and, when necessary, a PCI was performed before admission to the ICU. All patients were managed according to the locally adopted intensive care protocol. All patients were cooled to 32–34 °C by the rapid infusion of 30 ml/kg body weight cold (4 °C) Ringer lactate solution, followed by external cooling using a water-circulating blanket (Blanketroll III; Cincinnati Subzero Medical Division, Cincinnati, OH, USA). Body temperature was monitored continuously with an esophageal temperature probe (401CSZ: esophageal thermoprobe; Cincinnati Subzero), and maintained at 32–34 °C for 24 h, followed by re-warming at a rate of 0.25–0.33 °C/h to normothermia (defined as core temperature ≥37 °C).
Data collection and blood sampling of patient population
Demographic, pre-hospital and admission data were collected and then, clinical data were recorded continuously. APACHE II (Acute Physiology and Chronic Health Evaluation II) (Knaus et al. 1985), and SAPS II (Simplified Acute Physiology Score II) (Le Gall et al. 1993) severity scores were calculated retrospectively, based on clinical data from the initial 24 h. We recorded all complications during the in-hospital period, and categorized them as follows: infection (n = 12 pneumonia, n = 9 bronchitis, n = 1 pyelonephritis), sepsis (according to the American College of Chest Physicians and the Society of Critical Care Medicine; Bone et al. 1992), ischemia (n = 3 extremities, n = 2 mesenterial, n = 1 combined), and bleeding (n = 4 puncture-related, n = 3 pharyngeal, n = 2 gastrointestinal, n = 1 resuscitation-associated). The Cerebral Performance Categories scale was administered at discharge, as well as survival data were recorded at discharge and at 30 days. On admission to the Intensive Care Unit (0 h), as well as 6 and 24 h later, blood samples were drawn from the arterial catheter. All patients received antithrombotic and anticoagulant therapy at 0, 6, and 24 h; contrast media was used only during angiography. Blood samples (native and EDTA) were centrifuged within 2 h, for 15 min, at 2,000×g and 25 °C; the samples were stored at −80 °C until analysis.
Measurement of biomarkers
We measured the levels of HSP70 in the sera with the DuoSet IC ELISA Development System assay from R&D Systems (Minneapolis, MN, USA; Cat. No. DYC1663E), with a minor modification. We use the term ‘HSP70’ as a synonym for HSPA1A under the new nomenclature (Kampinga et al. 2009). TNF-alpha1, sICAM-11,S100B2, and big Endothelin3 were measured using commercial enzyme-linked immunosorbent assays according to the manufacturer’s instructions (R&D Systems (1); Biovendor Modřice, Czech Republic (2), and Biomedica Wien, Austria (3), respectively). von Willebrand factor (vWF) was measured by in-house ELISA, using rabbit-anti-vWF antibodies (unlabeled and HRP-labeled’ DakoCytomation, Glostrup, Denmark). HSP70, TNF-alpha, sICAM-1 and S100B were measured in serum, while vWF was measured in EDTA plasma. CRP and total protein were determined by Roche Integra 800.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA), and SPSS v13.0 (SPSS Inc., Chicago, IL, USA). Data are presented as median, with 25th and 75th percentiles and range in the box-and-whisker plots, because of their non-normal distribution. Changes over time were analyzed with the repeated-measures test (Friedman ANOVA), and Dunn’s post hoc test. Spearman’s rank correlation coefficients were calculated to estimate the relationship of HSP70 and other variables. Continuous variables were compared between the two groups by Mann–Whitney’s U-test, whereas categorical variables with Pearson’s chi-square test. Survival was plotted according to the Kaplan–Meier method, and differences in survival between the groups were compared with log-rank tests. Univariate Cox proportional hazard regressions were calculated to predict mortality. Thereafter, HSP70 was fitted to multivariate Cox regression models, to assess the effect on survival after adjustment for age, sex, APACHE II score, complications, and all other biomarkers. The results of the Cox regression models are presented as hazard ratios standardized for the 1 SD increase of the predictors, complete with the corresponding 95 % CI, Wald chi-square, and p values of the likelihood ratio tests. Missing data were infrequent and were substituted with mean values of the appropriate group. The power of the relationship between HSP70 level and mortality was sufficient (p = 0.9, alpha = 0.05), as regards the log-rank test, and (p = 0.8, alpha = 0.05) the comparison of the mean HSP70 levels between survivors and non-survivors. Two-tailed p values were calculated, and the significance level was set at p < 0.050.
Results
Baseline characteristics of the patient population
A total of 46 comatose patients were enrolled after a cardiac arrest. Twenty-two of them died within the first 30 days (non-survivors). The demographic data, clinical characteristics, and comparisons of the survivors and non-survivors are shown in Table 1. The median and interquartile range of age in survivors and non-survivors were 57 (47–66) and 66 (61–69) years (p = 0.0141), respectively. Both groups (survivors and non-survivors) were predominated by males and characterized by a higher out-of-hospital cardiac arrest rate. The coronarography and clinical investigations showed acute coronary occlusion in 39 cases and dilated cardiomyopathy with malignant arrhythmias in seven cases in the background of cardiac arrest with similar distribution in survivors (n = 20 coronary occlusion and n = 4 dilated cardiomyopathy) and non-survivors (n = 19 coronary occlusion and n = 3 dilated cardiomyopathy). The cooling method was effective in survivors and non-survivors because the target temperature (32–34 °C) was achieved and maintained for 24 h in both groups. There was no difference in the median of body temperature at baseline, at 6 and 24 h in survivors and non-survivors. Apart from age, severity scores, and complications, the baseline characteristics of the two groups were similar (Table 1). The rates of complications such as bleeding (36.4 % vs. 8.3 %), and ischemia (27.3 % vs. 0 %) (mesenteric and extremities) were significant higher among non-survivors than in survivors. The infection rate was similar in survivors and non-survivors: 54 % vs. 41 % (p = 0.3945), respectively. Sepsis developed in nine patients: three cases in survivors and six cases among non-survivors.
Table 1.
Demographic data, and baseline clinical characteristics of the 46 post-cardiac-arrest patients, including the survivors, and non-survivors
| Parameters | All patients | Survivors | Non-survivors | p valuea |
|---|---|---|---|---|
| No. of patients | 46b | 24 | 22 | |
| Age (years), median (IQR) | 64 (56–69)c | 57 (47–66) | 66 (6–-69) | 0.0141 |
| Males/females | 38/8 | 20/4 | 18/4 | 1.0000 |
| OHCA/IHCA | 36/10 | 17/7 | 19/3 | 0.2894 |
| Body mass index (kg/m2), median (IQR) | 27.8 (24.6–31.0) | 27.8 (26.0–29.4) | 25.9 (24.2–31.1) | 0.7193 |
| Simplified Acute Physiology Score II, median (IQR) | 61 (55–69) | 56 (51–70) | 64 (60–68) | 0.0293 |
| Acute Physiology and Chronic Health Evaluation II, median (IQR) | 29 (25–32) | 27 (25–29) | 32 (28–34) | 0.0115 |
| Time from collapse until the start of ALS/BLS (min), median (IQR) | 1 (0–6) | 1 (0–5) | 1 (0–9) | 0.6268 |
| Time from the collapse until the ROSC (min), median (IQR) | 21 (15–30) | 20 (15–30) | 28 (18–30) | 0.2548 |
| Total duration of CPR (min), median (IQR) | 20 (12–28) | 19 (12–22) | 21 (13–30) | 0.4926 |
| No. of patients requiring further support in another ICU | 16 | 5 | 11 | 0.0626 |
| EF (%), median (IQR) | 35 (28–45) | 36 (31–47) | 31 (25–40) | 0.1632 |
| Body temperature at baseline (°C), median (IQR) | 36.4 (35.8–36.6) | 36.5 (36.1–36.6) | 36.3 (35.3–36.6) | 0.4697 |
| Body temperature at 6 h (°C), median (IQR) | 32.7 (32.0–33.0) | 32.8 (32.5–33.0) | 32.0 (32.0–33.9) | 0.1093 |
| Body temperature at 24 h (°C), median (IQR) | 32.5 (32.1–33.0) | 32.7 (32.4–33.0) | 32.3 (32.1–32.8) | 0.1143 |
| Minimum temperature during the initial 24 h (°C), median (IQR) | 31.9 (31.6–2.6) | 32.2 (31.7–32.7) | 31.8 (31.6–32.0) | 0.0490 |
| No. of patients requiring catecholamine therapy | 29 | 10 | 19 | 0.0972 |
| No. of patients requiring combined catecholamine therapy | 16 | 5 | 11 | 0.1159 |
| No. of patients with cardiogenic shock requiring IABP therapy | 19 | 6 | 13 | 0.0349 |
| Total, 24-h urine output (ml), median (IQR) | 3,410 (2,450–4,130) | 3,510 (3,185–4,610) | 2,950 (1,900–4,070) | 0.0513 |
| Sepsis, no. of cases | 9 | 3 | 6 | 0.2757 |
| Infection as a complication, no. of cases | 22 | 13 | 9 | 0.3945 |
| Bleeding as a complication, no. of cases | 10 | 2 | 8 | 0.0323 |
| Ischemic complications, no. of cases | 6 | 0 | 6 | 0.0080 |
| S100B >200 pg/ml during the initial 24 h, N | 11 | 2 | 9 | 0.0149 |
| Delayed hypothermia (time from collapse until the start of hypothermia (h), median (IQR) | 3.7 (2.7–4.5) | 3.6 (2.5–4.5) | 4.1 (3.0–4.5) | 0.2941 |
| CPC I or II at discharge | 19 | 17 | 2 | 0.0039 |
IQR interquartile range, OHCA out-of-hospital cardiac arrest, IHCA in-hospital cardiac arrest, ALS advance life support, BLS basic life support, ROSC return of spontaneous circulation, EF ejection fraction, CPC Cerebral Performance Categories Scale, S100B biochemical marker of brain cell damage
aMann–Whitney test (continuous variables) or Pearson’s chi-square test (categorical variables) between survivors and non-survivors
bNumber of cases
cMedian values with interquartile ranges
Baseline characteristics of the control population
Forty-six controls with a stable cardiovascular disease were selected for HSP70 measurement. We selected controls of matching gender and age (±2 years) before measuring HSP70. The median, and the interquartile range of age were 63 (54–68) years; 38 males and eight females were enrolled (in numbers equal to that of the study group). The ejection fraction of the control subjects was 58.5 (44.3–65.0) (median, 25th and 75th percentiles). The control patients had no prior cardiac arrest and did not receive hypothermia treatment.
Correction considering hemodilution
Total protein level in the serum decreased permanently in post-cardiac-arrest patients during the initial 24 h, due to excessive volume replacement. The medians and interquartile ranges of total protein concentrations were 60.3 (53.6–63.5), 55.3 (49.7–60.9) and 54.5 (48.3–58.2) g/l, at 0, 6 and 24 h, respectively. Therefore, the changes in total protein values were used to normalize all biomarker concentrations to adjust for the influence of the hemodilution caused by excessive volume expansion.
HSP70 and other biomarker levels in post-cardiac-arrest patients
On admission, HSP70 levels were higher in survivors and non-survivors on admission (1.31 [0.76–2.73] and 1.70 [1.20–2.37] ng/ml) than in controls (0.59 [0.44–0.83] ng/ml), p < 0.001, Mann–Whitney test; Fig. 1), but there was no difference between HSP70 levels in survivors and non-survivors on admission (p > 0.05, Mann–Whitney test). Having performed the Friedman’s ANOVA test, we noticed a statistically significant trend of decrease in HSP70 concentrations in the survivors at 6 h 0.70 (0.38–1.78) ng/ml, and at 24 h 0.56 (0.16–0.71) ng/ml in comparison to baseline values. However, this decline was not observed in the non-survivors either at 6 h 1.70 (0.65–2.70) ng/ml, or at 24 h 1.54 (0.79–1.72) ng/ml (Fig. 1). At 24 h, HSP70 levels were similar in the survivors and in the control group (p > 0.05, Mann–Whitney test), whereas a significant elevation was observed in non-survivors (as compared to controls) (p < 0.001, Mann–Whitney test).
Fig. 1.
HSP70 (HSPA1A) levels in the 46 control patients (Control) and the changes of HSP70 concentrations over time (at 0, 6 and 24 h) in the 24 survivors (white boxes), and in the 22 non-survivors (gray boxes), after a cardiac arrest. Median, 25th and 75th percentiles (boxes), minimum and maximum (whiskers) values are depicted. The p values of Friedman’s ANOVA and Dunn’s post-hoc test (*p < 0.05, ***p < 0.001, vs. 0 h) are indicated. HSP70 levels in post cardiac patient were significantly higher on admission (survivors and non-survivors) than in control group, Mann–Whitney’s U-test, p < 0.001)
The associations of HSP70 levels with inflammatory and endothelial cell activation markers and severity scores
Additional biomarkers of inflammation (CRP, TNF-alpha), endothelial cell activation (big Endothelin-1, sICAM-1, vWF), and neuronal damage (S100B) were studied. The most prominent differences in biomarker concentrations between non-survivors and survivors were observed at 24 h (Table 2). An increase of ET by 1.9-fold, a slight elevation of vWF, as well as 1.6-fold higher CRP and TNF-alpha were observed in non-survivors; however, sICAM-1 and S100B showed no significant differences. We analyzed the biological association of HSP70 levels at 24 h after cardiac arrest by performing a correlation analysis. Weak, significant positive correlations (all p < 0.05) between HSP70 and ET (r = 0.3330), vWF (r = 0.3887), sICAM-1 (r = 0.2942), CRP (r = 0.2923), TNF-alpha (r = 0.3722) were observed at 24 h (Table 3) in all patients. We did not detect an association between HSP70 levels and APACHE II (r = 0.2734, p = 0.0660), or SAPS II (r = 0.2061, p = 0.1694) scores.
Table 2.
Levels of HSP70 (HSPA1A) and of other biomarkers measured 24 h after the initiation of therapeutic hypothermia in post-cardiac-arrest patients (all patients, and subsets created according to 30-day survival)
| Parameters | All patients (n = 46) | Survivors (n = 24) | Non-survivors (n = 22) | p valuea |
|---|---|---|---|---|
| HSP70 (ng/ml) | 0.74 (0.33–1.66)b | 0.56 (0.16–0.71) | 1.54 (0.79–1.72) | <0.001 |
| ET (fmol/ml) | 1.58 (1.16–2.63) | 1.23 (0.99–1.67) | 2.35 (1.49–3.07) | 0.0011 |
| vWF (μg/ml) | 664.6 (477.7–859.3) | 624.3 (433.4–804.6) | 768.0 (557.7–1159.7) | 0.0411 |
| sICAM-1 (ng/ml) | 311.8 (221.1–368.1) | 292.5 (211.3–332.7) | 368.1 (227.6–444.8) | 0.1511 |
| CRP (μg/ml) | 87.0 (49.7–129.4) | 77.0 (36.9–87.0) | 126.0 (95.9–141.9) | 0.0018 |
| TNF-alpha (pg/ml) | 4.73 (2.11–7.62) | 3.80 (1.50–5.23) | 5.93 (4.66–7.62) | 0.0205 |
| S100B (pg/ml) | 3.7 (1.1–216.5) | 2.7 (1.1–75.5) | 8.5 (1.1–420.1) | 0.1441 |
ET Big Endothelin, vWF von Willebrand factor, sICAM-1 soluble intercellular adhesion molecule, CRP acute-phase protein C-reactive protein, TNF-alpha pro-inflammatory cytokine tumor necrosis factor-alpha, S100B the marker of brain damage, S100 protein B
aMann–Whitney test between survivors and non-survivors
bMedian values and interquartile ranges
Table 3.
Biological correlation of HSP70 (HSPA1A) levels measured 24 h after the initiation of therapeutic hypothermia in post-cardiac-arrest patients
| Parameters | Spearman’s rank-order correlationsa | |
|---|---|---|
| r | p value | |
| ET | 0.3330 | 0.0237 |
| vWF | 0.3887 | 0.0076 |
| sICAM-1 | 0.2942 | 0.0472 |
| CRP | 0.2923 | 0.0487 |
| TNF-alpha | 0.3722 | 0.0109 |
| S100B | 0.2881 | 0.0522 |
For abbreviations, see Table 2
aSpearman’s rank-order correlation coefficients (r) with p values are presented
Survival data
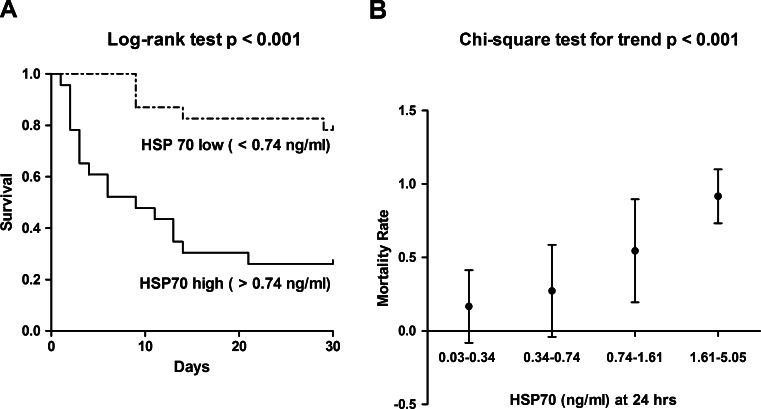
The 30-day survival rate was 0.522 in the whole study population (n = 46). At discharge (mean 8.2 days after the admission), 41.3 % (n = 19) of the patients had a favorable neurological outcome (Cerebral Performance Categories I or II), and 17 of them were alive at the follow-up visit on day 30. Kaplan–Meier survival analysis was done to describe mortality over time in the subsets of patients stratified according to median HSP70 level (0.74 ng/ml; Fig. 2a). In our study, higher HSP70 levels were associated with an increased mortality (log-rank test, p < 0.001; Fig. 2a) during the 30-day follow-up period. Additionally, we observed a linear trend in 30-day mortality rate, and HSP70 levels. As presented in Fig. 2b, the mortality rates increased gradually in the subsets with elevated HSP70 levels at 24 h (four subsets were created according to the 25th, median and 75th percentiles of HSP70, chi-square test for trend, p < 0.001; Fig. 2b).
Fig. 2.
a Kaplan–Meier survival plots in the two patient subsets stratified according to median HSP70 (HSPA1A) levels at 24 h after a cardiac arrest. The p value of log-rank test is indicated. b Mortality rates (95 % CI) in the four subsets of post-cardiac-arrest patients stratified according to 24-h HSP70 (HSPA1A) levels (25th percentile, median, and 75th percentile). The p value obtained by chi-square test for trend is indicated (chi-square = 15.15, degrees of freedom = 1)
Besides HSP70 levels, age, severity scores, complications (Table 1), and some of the biomarkers (Table 2) were associated with 30-day mortality in our patients. Therefore, multivariable Cox proportional hazards regression analysis was done to test the independence of the predictive power of HSP70. In the univariable model (Model 1, Table 4), HSP70 (measured at 24 h and considered as a continuous, 1 SD standardized parameter) significantly predicted the 30-day mortality (hazard ratio 1.926, 95 % CI 1.405–2.638 for 1 SD increase; Table 4). In the multivariable Cox analysis, the prediction of 30-day mortality by HSP70 was adjusted for age, and sex (Model 2, Table 4), severity (APACHE II score, Model 3, Table 4), and presence of complications (Model 4, Table 4.). It remained an independent, significant predictor of mortality in all models. Furthermore, adjusting these models for the biomarkers ET, vWF, CRP, TNF-alpha, S100B and sICAM-1, yielded similar results (data not shown).
Table 4.
Thirty-day mortality in post-cardiac-arrest patients, as predicted by the increased HSP70 (HSPA1A) concentrations measured 24 h after the initiation of therapeutic hypothermia
| Modelsa | HRb | 95 % CI | Chi-square | p value |
|---|---|---|---|---|
| Model 1 | 1.926 | 1.405–2.638 | 12.796 | <0.001 |
| Model 2 | 2.153 | 1.520–3.050 | 15.067 | <0.001 |
| Model 3 | 1.978 | 1.419–2.759 | 12.413 | <0.001 |
| Model 4 | 2.043 | 1.412–2.956 | 12.954 | <0.001 |
aModel 1: univariable Cox regression analysis of HSP70; Model 2: multivariable Cox regression analysis of HSP70 adjusted for age and sex; Model 3: multivariable Cox regression analysis of HSP70 adjusted for APACHE II score; Model 4: multivariable Cox regression analysis of HSP70 adjusted for presence of complications
bHR hazard ratio; HRs for variables shown as standardized hazard ratios (HR per 1 SD increase of the variable) with 95 % CI; Wald chi-square and p values of likelihood ratio tests are presented
Discussion
The biochemical markers measured in blood samples are expected to serve as good predictors of clinical outcomes in comatose cardiac arrest patients after successful resuscitation. Moreover, they might prove even more straightforward in everyday clinical practice than neuroimaging or electrophysiological testing (Shinozaki et al. 2009). In the present study, we have analyzed for the first time HSP70 as a predictor of all-cause mortality in comatose, resuscitated patients, who have undergone hypothermia treatment.
HSP70 increases rapidly in response to various types of severe stress, as a protection against ischemic or hypoxic events. The possible role of HSP70 as a biomarker of cellular damage in ischemia of the brain and spinal cord has been reviewed recently (Hecker and McGarvey 2011). HSP70 is particularly valuable as an immediate, secreted biomarker in cellular ischemia. In line with those observations, we found in our study high HSP70 levels in post-cardiac-arrest patients on admission (Fig. 1). This elevation was most likely linked to necrotic cell death caused by severe hypoxia and ischemia, followed by reperfusion, and serve as danger signal. Indicating a complex inflammatory and stress response (known as the post-cardiac arrest syndrome), HSP70 levels showed associations with the biomarkers of the acute-phase reaction, inflammation, and endothelial-cell activation, namely, ET, vWF, sICAM-1, CRP, TNF-alpha (Table 3). However, we did not find significant correlations between HSP70 and APACHE II or SAPS II scores, i.e., two correlates of clinical severity. In agreement with previous observations (Adrie et al. 2002), we also found higher CRP, and S100B levels in non-survivors than in survivors, indicating that the severity of cellular damage and/or host response to this damage is detrimental, as regards survival. Considering these associations between the biomarkers and mortality, we performed Cox regression analysis to investigate the potential influence of the covariates. We established the persistently high level of extracellular HSP70 during the initial 24 h of care as an independent prognostic marker in post-cardiac-arrest patients (Table 4). HSP70, when added to baseline predictive model (age, gender, severity and complications of post-cardiac arrest syndrome), showed significant and independent improvement of the predictive model. This result may indicate that HSP70 as a danger signal is good additive marker of stress.
A large body of evidence from the literature shows that extracellular HSP70 is important as a useful biomarker in different clinical situations. Elevated HSP70 was demonstrated in the HELLP syndrome, and HSP70 levels were associated with an increased risk of several pregnancy complications (Molvarec et al. 2010). Furthermore, increased HSP70 levels were found in patients with advanced, chronic heart failure, and were correlated with the markers of heart function (Gombos et al. 2008). Additionally, the role of HSP70 in the progression of vascular calcification was also demonstrated: HSP70 levels correlated with the severity of atherosclerosis in patients with carotid artery disease and chronic lower limb ischemia (Krepuska et al. 2011). Increased levels of HSP70 were significantly associated with risk and disease severity in acute coronary syndrome (Zhang et al. 2010). HSP70 was rapidly released into the circulation after myocardial infarction and the HSP70 levels correlated with the levels of troponin T and creatine kinase MB (Dybdahl et al. 2005).The present study is similar, because in the background of cardiac arrest acute myocardial infarction was established in 85 % of patients, confirming that HSP70 is rapidly elevated after myocardial injury. Finally, elevated serum HSP70 levels in patients with severe traumatic brain injury were shown to predict poor outcomes (da Rocha et al. 2005). These studies aggregately confirm that extracellular HSP70 is detectable in various forms of stress, and might gain wide acceptance in predicting the prognosis or determining risk status in the diseases mentioned above. In this study, we have shown that HSP70 is suitable to predict prognosis also in a post-cardiac-arrest population.
Conclusions
The persistently high level of extracellular HSP70 during the initial 24 h of therapeutic hypothermia is an independent prognostic marker of mortality in post-cardiac-arrest patients. HSP70 levels were associated with multiple biomarkers of the acute-phase reaction, inflammation, and endothelial-cell activation, indicating the presence of a complex stress response in these patients.
Several limitations may apply to our study. Since therapeutic hypothermia is a guideline-based part of standard care, including a normothermic patient-control was not possible. Therefore, our study could not be designed to answer the question, why the high HSP70 level persists in a group of resuscitated people, and why is it associated with worse outcome. Our observations are limited by the small number of subjects; however, the statistical power of group estimations was sufficiently high in the case of HSP70 level and mortality (p = 0.9, at alpha = 0.05).
Acknowledgments
The study was supported by grants from the National Development Agency of Hungary (Semmelweis Híd Projekt, TÁMOP-4.2.2-08/1/KMR-2008-0004; Semmelweis Egyetem Magiszter Program, TÁMOP-4.2.2/B-10/1-2010-0013), and the ‘János Bolyai’ Research Scholarship of the Hungarian Academy of Sciences (GSz). The authors are grateful to Gábor Gőbl, MD, Ilona Orphanides, MD, and Mária Rotyis, MD (National Ambulance Service), for contributing the prehospital data, to László Cervenák, MSc, PhD, and Veronika Makó, MSc, PhD for their advice on vWF determination.
Conflict of interest
The authors have not disclosed any potential conflicts of interest.
References
- Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.CIR.0000023891.80661.AD. [DOI] [PubMed] [Google Scholar]
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32(3):579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101 (6):1644–1655 [DOI] [PubMed]
- da Rocha AB, Zanoni C, de Freitas GR, Andre C, Himelfarb S, Schneider RF, Grivicich I, Borges L, Schwartsmann G, Kaufmann M, Regner A. Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma. 2005;22(9):966–977. doi: 10.1089/neu.2005.22.966. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos T, Forhecz Z, Pozsonyi Z, Janoskuti L, Prohaszka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperones. 2008;13(2):199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker JG, McGarvey M. Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones. 2011;16(2):119–131. doi: 10.1007/s12192-010-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Pockley AG. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol. 2010;88(3):445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Krepuska M, Szeberin Z, Sotonyi P, Sarkadi H, Fehervari M, Apor A, Rimely E, Prohaszka Z, Acsady G. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16(3):257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Tamasi L, Losonczy G, Madach K, Prohaszka Z, Rigo J., Jr Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperones. 2010;15(3):237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27(6):367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Hattori N, Shimada T, Hirasawa H. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13(4):R121. doi: 10.1186/cc7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, Hu FB, Tanguay RM, Wu T. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15(5):675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]