Abstract
Recent studies have shown that renin–angiotensin system overactivation is involved in the aging process in several tissues as well as in longevity and aging-related degenerative diseases by increasing oxidative damage and inflammation. We have recently shown that angiotensin II enhances dopaminergic degeneration by increasing levels of reactive oxygen species and neuroinflammation, and that there is an aging-related increase in angiotensin II activity in the substantia nigra in rats, which may constitute a major factor in the increased risk of Parkinson’s disease with aging. The mechanisms involved in the above mentioned effects and particularly a potential angiotensin–mitochondria interaction have not been clarified. The present study revealed that activation of mitochondrial ATP-sensitive potassium channels [mitoK(ATP)] may play a major role in the angiotensin II-induced effects on aging and neurodegeneration. Inhibition of mitoK(ATP) channels with 5-hydroxydecanoic acid inhibited the increase in dopaminergic cell death induced by angiotensin II, as well as the increase in superoxide/superoxide-derived reactive oxygen species levels and the angiotensin II-induced decrease in the mitochondrial inner membrane potential in cultured dopaminergic neurons. The present study provides data for considering brain renin–angiotensin system and mitoK(ATP) channels as potential targets for protective therapy in aging-associated diseases such as Parkinson’s disease.
Keywords: Aging, Neurodegeneration, Neuroinflammation, Renin–angiotensin system, Oxidative stress, Parkinson’s disease
Introduction
Increased activity of local renin–angiotensin system, acting via angiotensin II type 1 receptors (AT1), is thought to be involved cellular senescence and age-related degenerative changes in several tissues (Basso et al. 2005; Min et al. 2009; Mukai et al. 2002). In accordance with this, recent studies with angiotensin II type 1 receptor deficient mice indicate that disruption of this receptor promotes longevity through attenuation of oxidative stress and additional mechanisms (Benigni et al. 2009, 2010; Mattson and Maudsley 2009), and completely protects against the age-related progression of atherosclerosis (Umemoto 2008). It is known that angiotensin II, acting via type 1 receptors, is one of the most important known inducers of inflammation and oxidative stress in several tissues (Mattson and Maudsley 2009; Min et al. 2009; Ruiz-Ortega et al. 2001). Normal aging has been associated with a pro-inflammatory, pro-oxidant state that may favor an exaggerated response to injury and degenerative diseases (Choi et al. 2010; Csiszar et al. 2003; Ungvari et al. 2004). In accordance with this, several recent studies have suggested the potential of inhibition of angiotensin for treatment of age-associated diseases and longevity (Benigni et al. 2009, 2010; Mattson and Maudsley 2009; Nishiyama et al. 2009).
Advancing age itself is one of the most significant risk factors for the development of neurodegenerative diseases such as Parkinson’s disease (PD; Collier et al. 2007; Cruz-Muros et al. 2009; Deng et al. 2006; McCormac et al. 2004). Brain possesses a local renin–angiotensin system (Mckinley et al. 2003; Saavedra 2005), and we have recently observed an aging-related increase in angiotensin activity in the nigra that leads to pro-inflammatory and pro-oxidative changes, which may constitute a major factor in the increased risk of PD with aging (Villar-Cheda et al. 2010b). It is known that neuroinflammation, oxidative stress, and microglial NADPH oxidase activation play a major role in dopaminergic neuron degeneration and PD (Rodriguez-Pallares et al. 2007; Wu et al. 2002, 2003). Furthermore, we have shown that angiotensin II, via type 1 receptors, enhances the dopaminergic degeneration process triggered by low/sublethal doses of dopaminergic neurotoxins by amplifying intraneuronal levels of reactive oxygen species (ROS) and the inflammatory response via activation of microglial NADPH oxidase (Joglar et al. 2009; Rey et al. 2007; Rodriguez-Pallares et al. 2008).
The mechanisms involved in the above mentioned effects of increased angiotensin activity and particularly a potential angiotensin–mitochondria interaction have not been clarified. It is well known that angiotensin II acts via type 1 receptors to release high levels of ROS mainly by activation of the NADPH oxidase (Qin et al. 2004; Seshiah et al. 2002; Touyz et al. 2002), which was also observed in the nigrostriatal system (Joglar et al. 2009; Rey et al. 2007; Rodriguez-Pallares et al. 2008). However, recent studies suggest that angiotensin II may stimulate not only cytosolic but also mitochondrial-ROS generation (de Cavanagh et al. 2007; Zhang et al. 2007). In addition, a number of studies support a critical role for mitochondrial ATP-sensitive potassium channels [mito(KATP)] in modulating intracellular ROS (Mattson and Liu 2003; Costa and Garlid 2008). A cross-talk signaling between the NADPH oxidase and the mitoK(ATP) channels has been shown (Daiber 2010; Kimura et al. 2005a; Zhang et al. 2007). This includes not only that NADPH oxidase modulates mitochondrial superoxide (Doughan et al. 2008; Kimura et al. 2005a) but also that mitochondrial superoxide stimulates extramitochondrial NADPH oxidase activity in a feed-forward fashion (Dikalova et al. 2010; Wosniak et al. 2009). In the present study, we used primary cultures of ventral mesencephalon to investigate the possibility that mitoK(ATP) channels are involved in the enhancing effect of angiotensin II on dopaminergic neuron degeneration and potentially the increased risk and progression of PD with aging.
Methods
Primary mesencephalic cultures
Ventral mesencephalic tissue was dissected from rat embryos of 14 days of gestation (E14). All experiments were carried out in accordance with the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) and approved by the corresponding committee at the University of Santiago de Compostela. The tissue was incubated in 0.1% trypsin (Sigma, St. Louis, MO, USA), 0.05% DNase (Sigma), and DMEM (Invitrogen, Paisley, Scotland, UK) for 20 min at 37°C, and then washed in DNase/DMEM and mechanically dissociated. The resulting cell suspension was centrifuged at 50×g for 5 min, the supernatant was carefully removed, and the pellet resuspended in 0.05% DNase/DMEM to the final volume required. The number of viable cells in the suspension was estimated with acridine orange/ethidium bromide. Cells were plated onto 35-mm culture dishes (Falcon, Becton Dickinson, Franklin Lakes, NJ, USA) previously coated with poly-l-lysine (100 μg/ml; Sigma) and laminin (4 μg/ml; Sigma). The cells were seeded at a density of 1.5 × 105 cells/cm2 and maintained under control conditions [DMEM/HAMS F12/ (1:1) containing 10% fetal bovine serum (FBS; Biochrom KG, Berlin, Germany)]. The cell cultures were maintained in a humidified CO2 incubator (5% CO2; 37°C) for 8 days in vitro (DIV; see below); the entire medium was removed on day 2 and replaced with fresh culture medium.
To obtain neuron-enriched cultures, cytosine-β-d-arabino-furanoside (Ara C; 1 μM; Sigma) was added 48 h after the cells were seeded. The cultures were then treated with the dopaminergic neurotoxins 6-OHDA (6-hydroxydopamine) or MPP+ (1-methyl-4-phenylpyridinium), or 6-OHDA or MPP+ and 5-HD (5-hydroxydecanoic acid, see below). This method can enrich neurons to >85% purity (Michel et al. 1997; Gao et al. 2003).
Treatment of cultures and experimental design
Cultures were exposed on 7 DIV to 6-OHDA alone for 24 h (10 or 30 μM, in 0.02% saline ascorbate; Sigma), or on 4 DIV to MPP+ alone for 4 days (0.25 μM; Sigma), or 6-OHDA or MPP+ and angiotensin II (100 nM; Sigma), or 6-OHDA + angiotensin II + the angiotensin II type 1 receptor antagonist ZD 7155 (1 μM), or 6-OHDA or MPP+ and 5-HD [a specific blocker of mitoK(ATP) channels, Sigma, 10 μM], or 6-OHDA or MPP+ + angiotensin II + 5-HD, or angiotensin II alone, or ZD 7155 alone, or 5-HD alone, in order to study the effect on survival of dopaminergic neurons. 5-HD is the most widely used specific inhibitor of mitoK(ATP) channels, and several studies have shown that 5-HD (10 μM) blocks the mitoK(ATP) channels without any effect on cell membrane K(ATP) channels (Costa and Garlid 2008; Garlid et al. 1997; McCullough et al. 1991; Zhang et al. 2007). Several doses of 5-HD were tested in the present and preliminary experiments (10, 100, 500 μM; Rodriguez-Pallares et al. 2009), and the lowest dose that proved effective (10 μM) was used in most of the experiments to prevent potential non-specific side effects of high doses of 5-HD (Wu et al. 2006). In addition, some cultures were treated with a second K(ATP) channel blocker (glibenclamide, 10 μM, Sigma) to confirm the involvement of K(ATP) channels in AII-induced cell death.
The cells were then washed and processed for immunolabeling as detailed below. Some control cultures and cultures subjected to the above mentioned treatments were also treated with MitoTracker Orange (CMTMR; chloromethyl-tetramethylrosamine methyl ester; Molecular Probes, Eugene, OR, USA) or MitoTracker Green FM (MTGFM; 2-[3-[5,6-dichloro-1,3-bis[[4-(chloromethyl)phenyl]methyl]-1,3-dihydro-2H-benzimidazol-2-ylidene]-1-propen-1-yl]-3-methyl-benzoxazoliumchloride; Molecular Probes; 50 nM) or dihydroethidium (DHE) for 3 h to estimate the mitochondrial inner membrane potential (ΔψM), the mitochondrial mass or levels of superoxide/superoxide-derived ROS, respectively, in dopaminergic neurons or microglial cells, as detailed below. Furthermore, the hyperpolarizing effect of 5-HD was confirmed in live cell cultures with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide; Invitrogen), as described below. Finally, some cultures were pre-treated with the mitochondrial uncoupler CCCP (carbonyl cyanide m-chlorophenylhydrazone; Sigma, 10 μM) to confirm that the increase in CMTMR or JC-1 retention is not due to non-specific effects and is dependent on ΔψM. The protonophore CCCP is often used to dissipate the membrane potential and to define the baseline for the analysis of mitochondrial membrane potential with fluorescent dyes (Gottlieb et al. 2003; Brown et al. 1996).
Immunohistochemistry and double-fluorescence labeling
Cultures were fixed with 4% paraformaldehyde in Dulbecco’s phosphate buffered saline (DPBS; pH 7.4) for 20 min, and then incubated at 4°C with a mouse monoclonal anti-tyrosine hydroxylase (TH; Sigma; 1:30,000) as dopaminergic marker. Neuron-enriched cultures were evaluated by immunocytochemical staining with a mouse monoclonal anti-NeuN (Chemicon; Temecula, CA, USA; 1:2,000) as a neuronal marker, a mouse monoclonal anti-glial fibrillary acidic protein (GFAP; Chemicon; 1:1,000) as an astrocyte marker, and a mouse monoclonal anti-CD11b (anti-complement receptor-3, clone MRC OX42; Serotec, Kidlington, Oxford, UK; 1:1,000) as a marker of resting and reactive microglial cells/macrophages. Cultures were then washed and incubated for 1 h with biotinylated horse anti-mouse antibody (Vector; Burlingame, CA, USA) diluted 1:500, and then incubated for 90 min with avidin–biotin–peroxidase complex (ABC, Vector, 1:500). Finally, the labeling was revealed with 0.04% hydrogen peroxide and 0.05% 3,3′-diaminobenzidine (Sigma) as chromogen (see Rodriguez-Pallares et al. 2008 for details).
Cultures grown on glass coverslips were processed for double-fluorescence labeling for DHE or CMTMR or MTGFM and TH or OX42. For TH or OX42, cultures were incubated overnight at 4°C with primary anti-TH (1:30,000) or anti-OX42 (Serotec, 1:1,000) antibodies. The cultures were rinsed with DPBS, then incubated for 150 min with the secondary antibodies [goat anti-mouse (Chemicon; 1:100) conjugated with fluorescein isothiocyanate (FITC) for TH, or biotinylated horse anti-mouse for OX42 (Vector; 1:500)]. OX42 labeling was visualized by incubation of the cultures with streptavidin conjugated with FITC (Sigma; 1:200) for 30 min. Co-localization of markers (see below for DHE, MTGFM, and CMTMR) was confirmed by confocal laser microscopy (TCS-SP2; Leica, Heidelberg, Germany) and use of a sequential scan method to avoid any possible overlap. In all experiments, the control cultures, in which the primary antibody was omitted, were immunonegative for these markers.
Estimation of ΔψM with CMTMR (MitoTracker Orange) or JC-1, and mitochondrial mass with MTGFM (MitoTracker Green FM)
CMTMR (MitoTracker Orange TM) enters mitochondria of living cells in proportion to the negative charge difference between the cytoplasm and the mitochondrial matrix, and therefore provides an estimation of ΔψM. Lipophilic cations accumulate in the mitochondrial matrix, driven by the ΔψM in accordance with the Nernst equation, which predicts that every 61.5-mV increase in membrane potential causes a ten-fold increase in accumulation of the membrane-permeant cation. CMTMR binds irreversibly to mitochondrial matrix thiols and can be fixed for immunocytochemical localization of proteins in the same cells that have previously been exposed to CMTMR. As a result of the thiol binding, CMTMR fluorescence represents the highest level of negativity difference in the mitochondria during exposure to the dye before fixation (Sugrue et al. 1999; Wadia et al. 1998). MitoTracker Green FM (MTGFM) is a mitochondrion-selective probe that becomes fluorescent in the lipid environment of mitochondria. MTGFM contains a thiol-reactive chloromethyl moiety, resulting in stable peptide and protein conjugates after accumulation in mitochondria and, unlike CMTMR, uptake of this probe is less dependent on ΔψM, thus allowing estimation of mitochondrial mass in both live and fixed cells (Metivier et al. 1998; Poot et al. 1996).
The ΔψM or the mitochondrial mass in fixed cells grown in glass coverslips was estimated as follows (Sugrue et al. 1999; Wadia et al. 1998; Buckman et al. 2001). Three hours after exposure to treatments, the medium in each well was supplemented with 50 nM CMTMR or 50 nM MTGFM and incubated at 37°C 5% CO2 for 15 min or 30 min, respectively. The media was removed and the cells were rinsed with cold DPBS, followed by immediate fixation with 4% paraformaldehyde for 10 min. After fixation, the cells were washed briefly in DPBS and incubated for 20 min with a mouse monoclonal anti-TH (Sigma, 1:6,000) containing normal goat serum and 0.3% Triton X-100 diluted in DPBS-BSA. Cultures were then washed and incubated for 15 min with goat anti-mouse secondary antibody conjugated with FITC for CMTMR or with cyanine 3.18 (Cy3) for MTGFM. The coverslips were mounted onto microscope slides with 1,4-diazabicyclo [2.2.2] octane (DABCO; Sigma).
JC-1 is a cationic carbocyanine dye that accumulates in mitochondria. The dye exists as a monomer at low concentrations and yields green fluorescence. At higher concentrations, the dye forms J-aggregates that exhibit a broad excitation spectrum and an emission maximum at 590 nm. The JC-1 dye is only uptaken up by viable cells and it exhibits a spectral shift from green to red in healthy mitochondria with polarized membranes. The JC-1 assay was performed to estimate the ΔΨM in living cells, according to Zhang et al. (2006) as follows. Cells were plated in 96-well plates at 105 cells per well. After treatment with 5-HD, JC-1 (in DMSO) was added to each well to a final concentration of 3 μM and incubated for 30 min at 37°C, with 5% CO2, in the dark. The medium was removed and the cells washed twice with DPBS. Fluorescence intensity was measured in a multifunctional microplate reader (TECAN Infinite 200, Austria) at excitation 485 nm, emission 525 and 595 nm, for detection of the green and red substrate, respectively. The fluorescence signal represents the average signal of the total cell population. The mitochondrial membrane potential is shown as the ratio between the fluorescence of aggregate (red) and monomer (green) forms of JC-1. This ratio is dependent only on the mitochondrial membrane potential, and not on the number of cells, mitochondrial size, shape, or density.
Detection of intracellular superoxide anion/superoxide-derived ROS with dihydroethidium (DHE): estimation of changes in fluorescence intensity
Treatment with DHE is one of the most frequently used methods for the detection of intracellular superoxide. For years, it was assumed that intracellular DHE is oxidized to ethidium, which bound DNA to form a red fluorescent product. However, recent studies have shown that the fluorescence derived from the reaction between superoxide and DHE is due to accumulation of 2-hydroxyethidium and detects superoxide in the cytoplasm (Dikalova et al. 2010; Fink et al. 2004; Zhao et al. 2005). Cultures grown on glass coverslips were incubated with a fresh working solution containing 5 μM DHE (Sigma) in sterile phosphate buffered saline (PBS; pH 7.4) for 30 min at 37°C. The cultures were washed, then fixed and processed for immunofluorescence against TH or OX42 (see above).
CMTMR, MTGFM, and DHE-derived fluorescence was visualized with a laser scanning confocal microscope (TCS-SP2; Leica, Heidelberg, Germany), equipped with a 63× oil immersion 1.4 numerical aperture (NA) objective. All cells were visualized at the same level of laser intensity, detector sensitivity, and pinhole size in order to ensure that fluorescence intensity could be compared among different coverslips and treatments. The images were saved as 8-bit TIFF files and the fluorescence intensity was evaluated by digital image processing with ImageJ software (NIH, Bethesda). The cytoplasm of dopaminergic or microglial cells was identified by TH or OX42 staining. A minimum of 50 TH-ir or OX42-ir fluorescent cells were counted in random visual fields to assess the intensity of DHE-derived or CMTMR or MTGFM fluorescence in cultures labeled for these markers. All measurements were corrected for background fluorescence.
Cell counting
TH-ir cells were counted in five randomly chosen longitudinal and transverse microscopic fields along the diameter of the culture dish, away from the curved edge. The operator was blind to the treatment condition. The microscopic field was defined by a 0.5 × 0.5 cm reticule (i.e., 1.25 cm2). The average number of TH-positive cells in a control culture dish was 1,628 ± 76. The results from at least three separate experiments were recorded, with a minimum sample size of four dishes per group and per run. The results for each batch were normalized to the control group counts (i.e., expressed as a percentage of the control group counts) to counteract any variability among batches. Statistical differences between groups were tested as described below.
[3H] Dopamine uptake
The culture medium was removed completely and cultures were rinsed twice with 1 ml of uptake buffer (Krebs buffer + 1.8 mM Cl2Ca + 25 mM d-glucose). Cells were then incubated for 30 min at 37°C with 1 ml of uptake buffer (containing 1 mM ascorbic acid and 100 μM pargyline; pH 7.4) in the absence (untreated cultures) or presence of 10 μM 5-HD. Uptake was initiated by addition of 20 nM [3H] dopamine ([2,5,6-3H] dopamine; 1 μCi, 12 Ci/mmol; Amersham Biosciences, GE Healthcare, Buckinghamshire, UK) in 20 μl of Krebs buffer. Non-specific uptake values were defined in the presence of 10 μM GBR 12935, a specific inhibitor of the dopamine transporter. Uptake was stopped after 30 min incubation at 37°C by removal of the incubation mixture and the cells were washed twice with cold Krebs buffer. Cells were lysed with 1 ml of 2 N NaOH for 30 min at room temperature, and the radioactivity incorporated into cells was measured by liquid scintillation spectrometry. Results are expressed as percentages of untreated control culture responses.
Statistical analysis
All data were obtained from at least three independent experiments and were expressed as means ± SEM. Two-group comparisons were analyzed by the Student’s t test and multiple comparisons were analyzed by one-way ANOVA followed by Bonferroni’s post hoc test. The normality of populations and homogeneity of variances were tested before each ANOVA. Differences were considered statistically significant at p <0.05. Statistical analyses were carried out with SigmaStat 3.0 from Jandel Scientific (San Rafael, CA, USA).
Results
MitoK(ATP) channel inhibition blocks the enhancing effect of angiotensin II on dopaminergic neuron degeneration
Cultures treated with very low doses of 6-OHDA (10 μM) showed a low non-significant decrease in the number of TH-ir neurons. However, the loss of dopaminergic neurons was significantly enhanced (loss of around 50% of dopaminergic neurons) by treatment with angiotensin II (100 nM). Treatment with the angiotensin II type 1 receptor antagonist ZD 7155 (6-OHDA + angiotensin + ZD) or the mitoK(ATP) channel inhibitor 5-HD (6-OHDA + angiotensin + 5-HD) blocked the loss of dopaminergic neurons (Fig. 1a).
Fig. 1.
Effects of different treatments on the number of TH-ir cells. Treatment with very low doses of 6-OHDA (10 μM; a) or MPP+ (0.25 μM; b) induced a slight decrease (non-significant and significant, respectively) in the number of TH-ir neurons, which was significantly enhanced by angiotensin II (100 nM). The amplifying effect of angiotensin II was blocked by simultaneous treatment with the angiotensin II type 1 receptor antagonist ZD 7155 or with the mitoK(ATP) channel inhibitor (5-HD 10 μM and 100 μM). No significant effect was observed after treatment with angiotensin II or ZD 7155 or 5-HD alone (c). The data are expressed as percentages of the number of TH-ir cells obtained in the respective control cultures (100%). Data represent means ± SEM. *p < 0.05 compared with control group (untreated cells), #p < 0.05 compared with the group treated with 6-OHDA or MPP+ and angiotensin (one-way ANOVA and Bonferroni post hoc test). AII angiotensin II, TH tyrosine hydroxylase, ZD ZD 7155, 5-HD 5-hydroxydecanoic acid
Cultures treated with low doses of MPP+ (0.25 μM) showed a low although significant decrease (20–25% decrease) in the number of TH-ir neurons. Again, the loss of dopaminergic neurons was significantly enhanced (around 50–55% decrease) by treatment with angiotensin II (100 nM). Treatment with the angiotensin II type 1 receptor antagonist ZD 7155 or the mitoK(ATP) channel inhibitor 5-HD (MPP+ + angiotensin + 5-HD) blocked the enhancing effect of angiotensin II on the loss of dopaminergic neurons (Fig. 1b). No significant loss of dopaminergic neurons was observed after administration of angiotensin II alone or 5-HD alone or ZD 7155 alone (Fig. 1c). These data confirm the results of our previous studies showing that angiotensin II increases the effect of low doses of dopaminergic neurotoxins via type 1 receptors, and also demonstrate that mitoK(ATP) receptor activation is involved in this effect (Fig. 2).
Fig. 2.
Photomicrographs of representative TH-ir cells from different experimental groups: a control culture (a), and cultures treated with a low dose of 6-OHDA (10 μM; b), or 6-OHDA (10 μM) + angiotensin (AII, 100 nM; c), or 6-OHDA + AII + 5-HD (10 μM; d), or AII alone (e), or 5-HD alone (f). AII induced a significant increase in 6-OHDA neurotoxicity. This increase was blocked by treatment with the mitoK(ATP) channel inhibitor 5-HD. AII angiotensin II, 5-HD 5-hydroxydecanoic acid. Scale bar: 100 μm
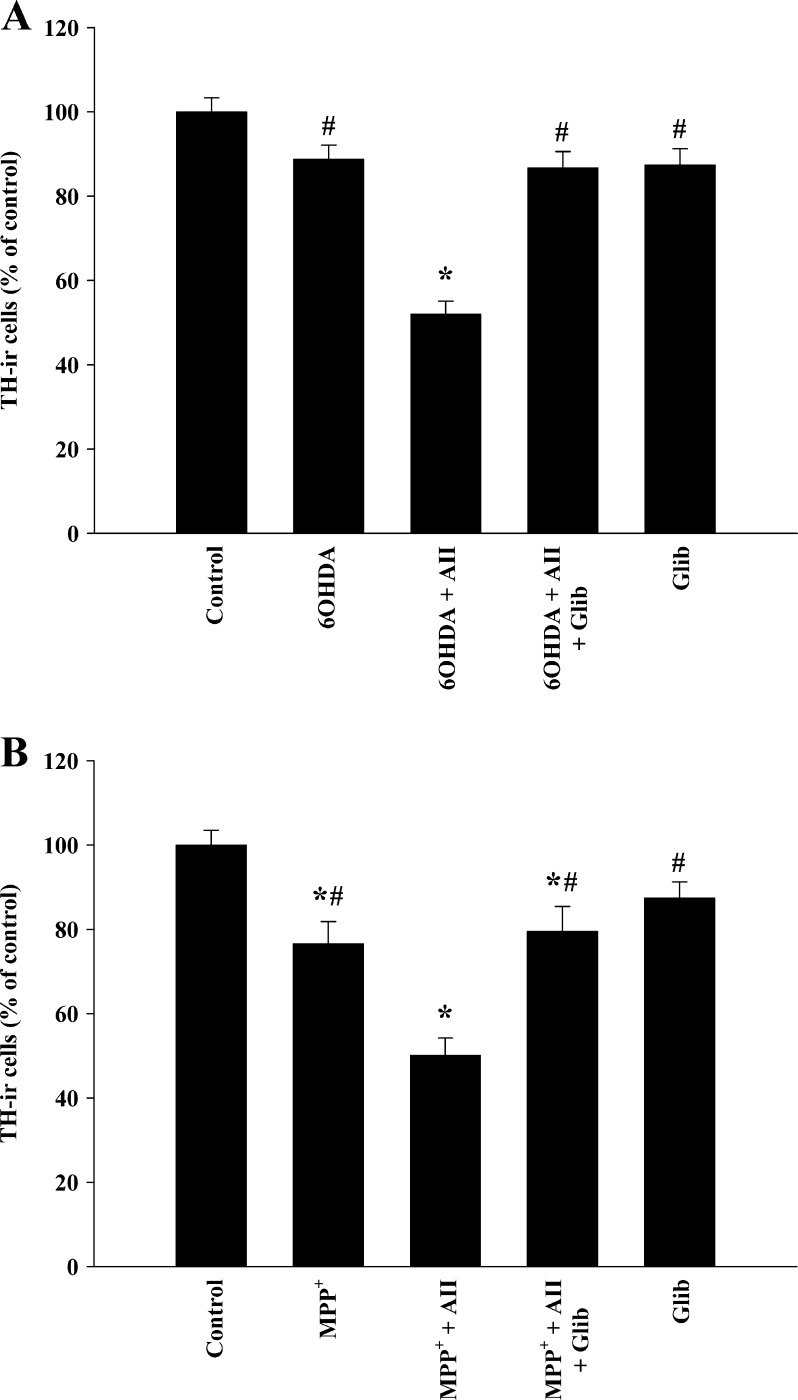
The results observed with 5-HD were confirmed with control cultures treated with the K(ATP) antagonist glibenclamide. Cultures treated with 6-OHDA + angiotensin II or MPP+ + angiotensin II and glibenclamide (10 μM) contained significantly more TH-ir neurons than cultures treated with the neurotoxins and angiotensin alone (Fig. 3).
Fig. 3.
Effects of different treatments on the number of TH-ir cells. Treatment with very low doses of 6-OHDA (10 μM; a) or MPP+ (0.25 μM; b) induced a slight decrease (non-significant and significant, respectively) in the number of TH-ir neurons, which was significantly enhanced by angiotensin II (100 nM). The amplifying effect of angiotensin II was blocked by simultaneous treatment with the K(ATP) channel inhibitor glibenclamide (10 μM), which confirmed the protective effect on dopaminergic degeneration observed with 5-HD. No significant effect was observed after treatment with glibenclamide alone. The data are expressed as percentages of the number of TH-ir cells obtained in the respective control cultures (100%). Data represent means ± SEM. *p < 0.05 compared with control group (untreated cells), #p < 0.05 compared with the group treated with 6-OHDA or MPP+ and angiotensin (one-way ANOVA and Bonferroni post hoc test). AII angiotensin II, TH tyrosine hydroxylase, Glib glibenclamide
[3H] Dopamine uptake assay
The neurotoxins 6-OHDA and MPP+ are accumulated by the dopamine uptake system. It is therefore possible that changes in 6-OHDA- or MPP+-induced neurotoxicity were caused by increased or decreased uptake of the neurotoxin due to 5-HD-induced changes in the dopamine transport activity. In the present study, [3H] dopamine uptake was measured in the absence or presence of 5-HD (10 μM). No significant changes (p > 0.05) in the uptake of [3H] dopamine were observed after treatment with 5-HD (92.9 ± 14.9%) in comparison with non-treated controls (100%).
MitoK(ATP) channel inhibition blocks the enhancing effect of angiotensin II on generation of intracellular superoxide/superoxide-derived ROS in dopaminergic neurons
Detection of superoxide anion and superoxide-derived ROS in cultured cells was achieved by treatment with dihydroethidium (DHE), which is oxidized to 2-hydroxyethidium and fluoresces red. Double labeling for TH and DHE-derived fluorescence revealed that treatment with very low doses of 6-OHDA (10 μM) induced a slight significant increase in the intensity of DHE-derived fluorescence 3 h after treatment. The 6-OHDA-induced increase in DHE-derived fluorescence in dopaminergic neurons was significantly increased by administration of angiotensin II, and decreased to control levels after treatment with the mitoK(ATP) blocker 5-HD (6-OHDA + angiotensin + 5-HD). Administration of angiotensin II alone induced a slight but significant increase in the intensity of DHE-derived fluorescence. Treatment with 5-HD alone induced a small non-significant decrease in the intensity of DHE-derived fluorescence with respect to controls (Figs. 4a and 6a–c).
Fig. 4.
Intracellular levels of superoxide/superoxide-derived ROS estimated by treatment with dihydroethidium (DHE) in dopaminergic neurons (i.e., TH-ir cells). Administration of very low doses of 6-OHDA (10 μM; a) or MPP+ (0.25 μM; b) induced a significant (6-OHDA) or non-significant (MPP+) increase in the intensity of DHE-derived fluorescence. Treatment with 6-OHDA or MPP+ + angiotensin II (AII; 100 nM) induced a significant increase in the intensity of DHE-derived fluorescence, and the increase was blocked by simultaneous administration of the mitoK(ATP) channel inhibitor 5-HD. Treatment with angiotensin II alone induced a significant increase in DHE-derived labeling. No significant effect was observed after treatment with 5-HD alone. Data are means ± SEM. *p < 0.05 compared with control group (untreated cells); #p < 0.05 compared with the group treated with 6-OHDA + AII or MPP+ + AII (one-way ANOVA and Bonferroni’s post hoc test). AII angiotensin II, DA dopaminergic, 5-HD 5-hydroxydecanoic acid
Fig. 6.
Double labeling for TH (green in a, d, g, j; red in m, p, s) and DHE-derived fluorescence (red; a–c), or CMTMR (MitoTracker Orange, red; d–l), or MTG (MitoTracker Green, green; m–u). Cultures treated with 6-OHDA (10 μM) + angiotensin II (AII; 100 nM) displayed intense DHE-derived fluorescence (red) in dopaminergic neurons (b), which was higher than in control cultures (a) or in those cultures treated with 6-OHDA + AII and 5-HD (c). Treatment of cultures with AII (100 nM) alone induced a significant reduction in CMTMR fluorescence (i.e., ΔΨM; red) in dopaminergic neurons (g–i; green) with respect to controls (d–f). The decrease was inhibited by treatment with 5-HD (j–l). However, treatment of cultures with AII (100 nM) alone or AII + 5-HD did not induce significant changes in MTG fluorescence (i.e., mitochondrial mass; green) in dopaminergic neurons (p–u; red) with respect to controls (m–o). Cell nuclei were labeled with Hoechst 33342 (blue) in (o), (r), and (u). AII angiotensin II, MTO MitoTracker Orange, MTG MitoTracker Green, DHE dihydroethidium, TH tyrosine hydroxylase. Scale bar: 75 μm (a–c) and 50 μm (d–u)
Cultures treated with low doses of MPP+ showed similar results. Administration of angiotensin II significantly increased the intensity of DHE-derived fluorescence observed in dopaminergic neurons of control cultures or cultures treated with MPP+ alone, and the increase was blocked by treatment with 5-HD (MPP+ + angiotensin + 5-HD). Administration of angiotensin II alone induced a slight but significant increase in the intensity of DHE-derived fluorescence. Treatment with 5-HD alone induced a small non-significant decrease in the intensity of DHE-derived fluorescence with respect to controls (Fig. 4b).
MitoK(ATP) channel inhibition blocks the angiotensin II-induced decrease in mitochondrial inner membrane potential (ΔψM) in dopaminergic neurons
CMTMR (MitoTracker Orange) was used to estimate ΔψM. CMTMR enters mitochondria in proportion to the negative charge difference between the cytoplasm and the mitochondrial matrix and binds irreversibly to mitochondrial matrix thiols. Double labeling for TH and CMTMR revealed that treatment of cultures with angiotensin II induced a significant reduction in CMTMR fluorescence in dopaminergic neurons, which revealed a decrease in ΔψM in these neurons. Treatment with the mitoK(ATP) blocker 5-HD inhibited the angiotensin II-induced decrease in CMTMR fluorescence (i.e., angiotensin-induced decrease in ΔψM) in dopaminergic neurons (Figs. 5a and 6d–l). Interestingly TH-ir neurons treated with angiotensin + 5-HD or with 5-HD alone showed higher levels of CMTMR fluorescence than TH-ir neurons from untreated cultures. This indicates that 5-HD treatment also blocked the effect of other factors (i.e., in addition to angiotensin II) that act on cultured dopaminergic neurons and decrease their ΔψM (see “Discussion”).
Fig. 5.
a Treatment of cultures with AII induced a significant reduction in CMTMR fluorescence (i.e., ΔΨM) in dopaminergic neurons. Treatment with the angiotensin type 1 receptor antagonist ZD 7155 or the mitoK(ATP) channel blocker 5-HD inhibited the angiotensin II-induced decrease in CMTMR fluorescence. TH-ir neurons treated with AII + 5-HD or 5-HD alone (i.e., without AII) showed higher levels of CMTMR fluorescence than TH-ir neurons in non-treated cultures. This suggests that 5-HD treatment may also block the effect of additional factors that decrease the ΔΨM in cultures. Furthermore, 5-HD was unable to increase CMTMR fluorescence in cells treated with protonophore CCCP, which revealed that the increase in CMTR retention is not due to non-specific effects and is dependent on ΔψM. b MitoTracker Green FM (MTGFM) labeling analysis showed that changes in CMTMR fluorescence were not caused by changes in total mitochondrial mass. c The effects of 5-HD were also confirmed in living cells in mesencephalic cell cultures using the JC-1 assay. 5-HD was also unable to increase J-aggregate fluorescence in cells treated with CCCP. *p < 0.05 compared with control group (untreated cells), #p < 0.05 in comparison with the groups treated with 5-HD + AII or 5-HD alone, $p < 0.05 (one-way ANOVA and Bonferroni’s post hoc test). AII angiotensin II, CCCP carbonyl cyanide m-chlorophenylhydrazone, DA dopaminergic, ZD ZD 7155, 5-HD 5-hydroxydecanoic acid
Increases in CMTMR fluorescence may indicate an increase in ΔψM or an increase in mitochondrial mass. In an attempt to differentiate between these possibilities, we used the probe MTGFM, an indicator of mitochondrial mass, and we observed that MTGFM fluorescence in the dopaminergic neurons remained unchanged 3 h after the corresponding treatment exposure (Figs. 5b and 6m–u). Furthermore, the hyperpolarizing effect of 5-HD was also confirmed in living cells in mesencephalic cell cultures using the JC-1 assay (Fig. 5c). Finally, the negatively charged molecule 5-HD was unable to increase CMTMR or J-aggregate fluorescence in cells treated with protonophore CCCP. This revealed that the increase in CMTMR or JC-1 retention is not due to non-specific effects and is dependent of ΔψM (Fig. 5a, c).
Role of microglial cells: mitoK(ATP) channel inhibition in neuron-enriched cultures
Involvement of microglial cells in the above mentioned effects of angiotensin II and 5-HD was studied in primary mesencephalic cultures in which microglial cells were identified with OX-42, and levels of superoxide/superoxide-derived ROS were estimated with DHE (i.e., double OX42-DHE labeling). Treatment of cultures with 10 μM 6-OHDA alone or angiotensin II alone did not induce a significant increase in the intensity of DHE-derived fluorescence in microglial cells. However, simultaneous treatment with 10 μM 6-OHDA + angiotensin II significantly increased the intensity of DHE-derived fluorescence induced by 6-OHDA alone or angiotensin II alone, which was reduced to control levels by treatment with 5-HD (i.e., 6-OHDA + angiotensin + 5-HD; Fig. 7a).
Fig. 7.
Involvement of microglial cells in the effects of angiotensin II (AII) and 5-HD was studied in primary mesencephalic cultures in which microglial cells were identified with OX-42 (a) and in neuron-enriched cultures (i.e., treated with Ara C; b, c). a Simultaneous treatment with 10 μM 6-OHDA + AII significantly increased the intensity of DHE-derived fluorescence induced by 6-OHDA alone or AII alone in microglial cells, which was reduced to control levels by treatment with 5-HD (i.e., 6-OHDA + AII + 5-HD). In the absence of glia, treatment with very low doses of 6-OHDA (10 μM) or 6-OHDA + AII did not induce significant increase in intracellular levels of superoxide/superoxide-derived ROS in dopaminergic neurons (b), or a significant decrease in the number of TH-ir neurons (c), and 5-HD administration did not induce any significant change. However, higher doses of 6-OHDA (30 μM) induced a significant loss of dopaminergic neurons, which was blocked by 5-HD (c). The data are expressed as percentages of the respective control cultures (100%). Data represent means ± SEM. *p < 0.05 compared with control group, #p < 0.05 compared with group treated with 6-OHDA (30 μM) in (c) (one-way ANOVA and Bonferroni post hoc test). AII angiotensin II, DA dopaminergic, TH tyrosine hydroxylase, 5-HD 5-hydroxydecanoic acid
Neuron-enriched cultures in which glia was eliminated with Ara C were used to confirm the potential role of microglial cells in the observed enhancing effect of angiotensin II and the protective effect of 5-HD on dopaminergic neuron degeneration. In the absence of glia, very low doses of 6-OHDA (10 μM) or 6-OHDA + angiotensin II did not induce a significant increase in levels of superoxide/superoxide-derived ROS (i.e., intensity of DHE-derived fluorescence; Fig. 7b). Moreover, treatment with very low doses of 6-OHDA (10 μM) induced a non-significant decrease in the number of TH-ir neurons in the absence of glia. Interestingly, angiotensin II administration did not induce any additional loss of dopaminergic neurons, and no significant changes were observed after treatment with 5-HD (Fig. 7c). This indicates that glial cell factors (e.g., ROS from microglia) play a major role in the effect of angiotensin II in increasing dopaminergic neuron death. In order to determine if mitoK(ATP) channels in dopaminergic neurons are involved in dopaminergic degeneration induced by higher levels of ROS than those induced by 10 μM 6-OHDA in the absence of glial cells, neuron-enriched cultures were treated with higher doses of 6-OHDA. Doses of 30 μM 6-OHDA induced a significant loss of dopaminergic neurons (decrease of approximately 40%), which was blocked by inhibition of neuronal mitoK(ATP) channels with 5-HD (Fig. 7c).
Discussion
Several studies in different tissues have shown that normal aging is associated with a pro-inflammatory, pro-oxidant state that may favor an exaggerated response to injury and degenerative diseases (Choi et al. 2010; Csiszar et al. 2003; Ungvari et al. 2004) and that increased activity of local angiotensin via angiotensin II type 1 receptors is involved in age-related degenerative diseases (Basso et al. 2005; Mukai et al. 2002). In agreement, we have recently observed an age-related increased angiotensin activity in the nigra that may increase the vulnerability of dopaminergic neurons to additional damage, and the risk of Parkinson’s disease with aging (Villar-Cheda et al. 2010b). Furthermore, the interaction between renin–angiotensin system and dopaminergic system is of particular interest. Several studies have shown an important interaction between dopamine and angiotensin II receptors in peripheral tissues, particularly as regards regulating renal sodium excretion and cardiovascular function (Gildea 2009; Zeng et al. 2006; Khan et al. 2008). Recent evidence suggests that dopaminergic and renin–angiotensin systems directly counterregulate each other in renal cells (Gildea 2009) and also in the nigrostriatal system (Villar-Cheda et al. 2010a), and that abnormal counterregulation between dopamine and angiotensin II plays an important role in degenerative changes (Joglar et al. 2009; Li et al. 2008; Rodriguez-Pallares et al. 2008). Interestingly, several studies have shown that there is an aging-related decrease in dopamine release, which results in a progressive decrease in motor activity (Collier et al. 2007; Gerhardt et al. 2002), and may be responsible for the above mentioned aging-related increase in angiotensin activity (Villar-Cheda et al. 2010a,b).
In cardiovascular and renal tissues, angiotensin II induces oxidative stress and inflammation via activation of the NADPH oxidase (Qin et al. 2004; Seshiah et al. 2002; Touyz et al. 2002). Similarly, we have recently shown that the effect of low/sublethal doses of neurotoxins on dopaminergic cell loss is amplified by angiotensin II via type 1 receptors and protein kinase C, leading to activation of the microglial NADPH oxidase and exacerbation of the glial inflammatory response, which increases ROS released by in microglia and dopaminergic neurons (Joglar et al. 2009; Rey et al. 2007; Rodriguez-Pallares et al. 2008). However, recent studies suggest that angiotensin II may stimulate not only cytosolic (i.e., via NADPH oxidase activation) but also mitochondrial-ROS generation in several tissues (de Cavanagh et al. 2007; Zhang et al. 2007), and numerous studies have shown that mitochondria play a major role in generation of oxidative stress, dopaminergic neuron degeneration, and aging (de Cavanagh et al. 2007; Schapira 2008). This is consistent with recent studies that suggest cross-talk signaling between both the cytosolic NADPH oxidase and mitochondria in several types of cells, and that mitoK(ATP) play a major role in this interaction (Brandes 2005; Daiber 2010; Dikalova et al. 2010; Doughan et al. 2008). Therefore, NADPH oxidase may lead to mitoK(ATP) channel opening, which play a major role in modulating intracellular ROS (Andrukhiv et al. 2006; Costa and Garlid 2008; Mattson and Liu 2003) via regulation of the formation/release of ROS from the mitochondria, and integration of signals from diverse sources (Facundo et al. 2007; Fornazari et al. 2008; Oldenburg et al. 2002). ROS open up mitoK(ATP), and mitochondrial ROS production can be stimulated by the opening of mitochondrial mitoK(ATP) channels (Costa and Garlid 2008; Kimura et al. 2005b; Zhang et al. 2002).
In accordance with the above mentioned studies, the present results reveal that activation of mitoK(ATP) channels may play important role in the effects of angiotensin II on the dopaminergic system via NADPH oxidase activation. Inhibition of mitoK(ATP) channels with 5-HD blocked the increase in dopaminergic cell death induced by angiotensin II, as well as the angiotensin II-induced increase in superoxide/superoxide-derived ROS levels in dopaminergic neurons, and the angiotensin II-induced decrease in mitochondrial inner membrane potential (ΔψM) in dopaminergic neurons. 5-HD is the most widely used specific inhibitor of mitoK(ATP) channels, and several studies have shown that 5-HD (10 μM) blocks the mitoK(ATP) channels without any effect on cell membrane K(ATP) channels (Costa and Garlid 2008; Garlid et al. 1997; McCullough et al. 1991; Zhang et al. 2007). Doses up to 500 mmol/l have been reported to inhibit selectively mitoK(ATP) channels without affecting surface K(ATP) currents (Sato et al. 2000; Hu et al. 1999). However, we did not observe significant differences between the effect of 10 μM 5-HD and 100 μM 5-HD, and high doses of 5-HD may have non-specific side effects independent of any action on mitoK(ATP), as reported for mitoK(ATP) openers, which have been observed to act as channel closers at high concentrations (Garlid 2000; Wu et al 2006). In the present study, we therefore used the lowest dose of 5-HD (10 μM), which proved effective at inhibiting the effects of angiotensin II. Furthermore, it is known that 5-HD does not exert any radical scavenging activity (Kimura et al. 2005a, b), and the results cannot be attributed to a 5-HD-induced decrease in 6-OHDA uptake, as revealed in the present study by the [3H] dopamine uptake assay. In addition, treatment with a second K(ATP) inhibitor (i.e., glibenclamide) confirmed the protective effect observed with 5-HD.
The cross-talk signaling between NADPH oxidase and mitochondria means that angiotensin II stimulation induces NADPH activation, which leads to opening of mitoK(ATP), depolarizes mitochondrial potential, and amplifies ROS generation from mitochondria (Brandes 2005; Kimura et al. 2005a, b; Zhang et al. 2007), but also that mitochondrial ROS have a significant upstream impact on increasing NADPH oxidase activity (Li et al. 2001; Wosniak et al. 2009). Thus, a vicious cycle consisting of mitochondrial and NADPH oxidase-derived ROS formation with each stimulating the other in a positive feedback fashion leads to increased cytoplasmatic ROS levels (Daiber 2010; Dikalova et al. 2010). This has clearly been observed in recent studies in endothelial cells, which showed that inhibition of NADPH activity by apocynin or deletion of NADPH oxidase subunits prevented mitochondrial impairment and attenuated mitochondrial superoxide production via mitoK(ATP) channels (Doughan et al. 2008); in addition, mitochondrial superoxide stimulated extramitochondrial NADPH oxidase activity, which was blocked by SOD2 (manganese-containing mitochondrial superoxide dismutase), the mitochondria-targeted superoxide dismutase mimetic mitoTEMPO or the inhibitor of mitoK(ATP) channels 5-HD (Dikalova et al. 2010).
K(ATP) channels were originally discovered in the heart, although are particularly abundant in the CNS, and occur at highest levels in the substantia nigra and striatum (Busija et al. 2004; Zini et al. 1993). Brain mitochondria contain seven times more mitoK(ATP) channels per milligram of mitochondrial protein than liver or heart (Bajgar et al. 2001; Bednarczyk 2009). It is therefore possible that, as suggested for peripheral tissues (Facundo et al. 2007; Fornazari et al. 2008; Oldenburg et al. 2002), these channels provide in dopaminergic neurons a convergent target that could integrate ROS induced by low levels of neurotoxins (6-OHDA or MPP+) with angiotensin/NADPH oxidase-induced ROS to increase dopaminergic neuron oxidative stress and cell death. It may be speculated that low doses of neurotoxins, or other factors in PD, may generate low levels of ROS by several mechanisms that are insufficient to induce dopaminergic cell death. However, the low levels of ROS, together with those derived from angiotensin-induced NADPH oxidase activation, may act as a trigger to activate mitoK(ATP) channels in the mitochondrial inner membrane, thereby enhancing ROS production and subsequent progression of dopaminergic cell degeneration. This is also supported by our previous studies that observed that the effects of exogenous ROS generated by autooxidation of 6-OHDA are decreased by inhibition of mitoK(ATP) (Rodriguez-Pallares et al. 2009). However, it has also been reported that mitoK(ATP) openers inhibit rotenone-induced microglial activation and neuroinflammation (Zhou et al. 2008). The discrepancies may be related to different experimental conditions. MitoK(ATP) openers have been reported to act as channel closers at high concentrations (Wu et al. 2006). It has also been suggested that opening of mitoK(ATP) may have two distinct consequences, depending on the underlying bioenergetic state (Garlid et al. 2003). When the ΔψM is high, as in normoxic and resting cells, opening of channels causes net K+ influx and matrix alkalinization with a consequent rise in mitochondrial ROS production. When ΔψM is depressed, as occurs during ischemia or treatment with the complex I blocker rotenone, mitoK(ATP) opening may add a parallel K+ conductance that counteracts the decrease in K+ influx and matrix contraction that otherwise occur, and therefore mitoK(ATP) opening may maintain constant volume of the mitochondrial matrix and the intermembrane space (Bajgar et al. 2001; Garlid et al. 2003; Kowaltowski et al. 2001). It is interesting to note that activation of mitoK(ATP) channels and ROS production lead to delayed protection against subsequent intense and potentially lethal insults such as ischemia by activation of adaptive cell responses in several types of cells (i.e., preconditioning; Kis et al. 2003; Oldenburg et al. 2002). Conversely, the present study shows that ROS induced by activation of mitoK(ATP) channels contribute to dopaminergic cell death. This is consistent with the results of previous studies that have shown that dopaminergic neurons function in a “near-compromised” state and are more vulnerable to oxidative stress and to mitochondrial complex I inhibition than other neurons and cell types (Bertarbet et al. 2000; Hirsch et al. 1997; Obeso et al. 2010). Therefore, activation of mitoK(ATP) channels and increased ROS levels, which may be useful for preconditioning and delayed protection in other cell types, may overwhelm the defense mechanisms of dopaminergic neurons. This may also be supported by the hyperpolarizing effect of 5-HD observed in TH-ir neurons treated with angiotensin + 5-HD or with 5-HD alone, which suggests that 5-HD treatment may also block the effect of other factors (i.e., in addition to AII) that act on cultured dopaminergic neurons and decrease their ΔψM. The hyperpolarizing effect of 5-HD may be related to experimental conditions and the cell type, and has been also observed in other studies (Valero et al. 2008). However, this observation is particularly interesting in the case of dopaminergic neurons since a number of previous studies have shown that the dopaminergic neurons have high levels of ROS. A number of factors are thought to be involved, including increased iron content, reduced antioxidant capacity or factors associated with the dopamine synthesized, released, and metabolized in these neurons (Fahn and Cohen 1992; Olanow 1990). The protective defense mechanisms for dopaminergic neurons may be overwhelmed by additional deleterious factors in neurons already subjected to dopamine-derived toxicity thus leading to dopaminergic neuron death (i.e., a “synergistic effect hypothesis”). In the present and previous studies, we suggest that the brain renin–angiotensin system plays a major role in this process since major sources of ROS such as NADPH oxidase and mitochondria are enhanced by renin–angiotensin system activation.
As angiotensin II type 1 receptors and NADPH oxidase have both been observed in dopaminergic neurons and microglial cells (Joglar et al. 2009; Rodriguez-Pallares et al. 2008), it is possible that neurotoxins may interact synergistically with ROS derived from either neuronal or microglial NADPH oxidase activation to activate mitoK(ATP) channels and enhance ROS production. In microglial cells (i.e., OX-42 ir cells), we observed that angiotensin II significantly increased levels of ROS induced by very low doses of neurotoxins (10 μM 6-OHDA), which is inhibited by 5-HD. This suggests a role for microglial mito(KATP) channels in angiotensin-induced amplification of ROS release and microglial activation. In inflammatory cells such as microglia, NADPH oxidase activation leads to ROS production that serves dual functions. Firstly, high concentrations of ROS are released extracellularly to kill invading microorganisms or cells (Babior 1999, 2004). Secondly, low levels of intracellular ROS act as a second messenger in a considerable number of signaling pathways involved in the inflammatory response (Mattson and Maudsley 2009; Qin et al. 2004; Touyz et al. 2002). The present results suggest that mitoK(ATP) play a major role in these mechanisms.
The possible neuronal origin of angiotensin/NADPH oxidase-induced ROS was studied using neuron enriched cultures, in which very low doses of neurotoxin (10 μM 6-OHDA) did not induce a significant loss of dopaminergic neurons, and this effect was not significantly modified by angiotensin II or angiotensin + 5-HD. This suggests that a direct effect of angiotensin II on neuronal NADPH oxidase activation elicits low levels of ROS which, acting together with those derived from 10 μM 6-OHDA, are insufficient to induce significant mitoK(ATP) channel activation and/or dopaminergic degeneration. This is consistent with data showing that in non-inflammatory cells, such as neurons, the NADPH oxidase produces only low rates of ROS for signaling function (Babior 1999, 2004). However, we also demonstrated that neuronal mitoK(ATP) channels are involved in dopaminergic cell death when channels are activated by higher levels of ROS such as those derived from 30 μM 6-OHDA in the present experiments in neuron-enriched cultures (dopaminergic cell death was blocked in the presence of 5-HD), or those derived from activated microglia in primary (i.e., neuron/glia) cultures.
In summary, the data suggest that angiotensin II, via type I receptors, activates microglial NADPH oxidase (i.e., NOX2, phagocytic oxidase, PHOX) that induces mitoK(ATP) opening and increases mitochondrial-derived superoxide, which stimulates extramitochondrial NADPH oxidase activity in a feed-forward fashion and constitutes a vicious cycle leading to enhanced microglial activation and extracellular release of ROS. MitoK(ATP) channels of dopaminergic neurons may respond to high levels of ROS derived from the angiotensin-related microglial activation and increase neuronal NADPH oxidase activity. Furthermore, neuronal mitoK(ATP) channels may provide a convergent target to integrate different sources of neuronal ROS (ROS derived from 6-OHDA or MPP+, or from neuronal NADPH oxidase activated by angiotensin via neuronal type 1 receptors in the present experiments, or from other factors in Parkinson’s disease) and ROS from activated microglia to induce dopaminergic cell death. In conclusion, the present results reveal that mitoK(ATP) channels may play a major role in the enhancing effect of angiotensin II on dopaminergic degeneration triggered by low/sublethal doses of dopaminergic neurotoxins, and possibly in the synergistic interaction of several intra- and/or extraneuronal factors to increase levels of oxidative stress and dopaminergic cell death in PD. This is particularly interesting since aging-related increase in nigral renin–angiotensin system activity may constitute a major factor in the increased risk of PD with aging. Furthermore, the present study provides additional data for considering brain renin–angiotensin system and mitoK(ATP) channels as potential targets for neuroprotection in PD and aging.
Acknowledgments
The authors thank Pilar Aldrey and Iria Novoa for their excellent technical assistance. Funding: Spanish Ministry of Science and Innovation, Institute of Health Carlos III (RD06/0010/0013 and CIBERNED), FEDER, and Galician Government (XUGA).
References
- Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores Arnaiz M, Inserta F. Protective effect of the inhibition of the renin–angiotensin system on aging. Regul Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Bednarczyk P. Potassium channels in brain mitochondria. Acta Biochim Pol. 2009;56:385–392. [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension. 2005;45:847–848. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- Brown PC, Sokolove PM, McCann DJ, Stevens JL, Jones TW. Induction of a permeability transition in rat kidney mitochondria by pentachlorobutadienyl cysteine: a beta-lyase-independent process. Arch Biochem Biophys. 1996;331:225–231. doi: 10.1006/abbi.1996.0302. [DOI] [PubMed] [Google Scholar]
- Buckman JF, Hernandez H, Kress GJ, Votyakova TV, Pal S, Reynolds IJ. MitoTracker labelling in primary neuronal and astrocytic cultures: influence of mitochondrial membrane potential and oxidants. J Neurosci Methods. 2001;104:165–176. doi: 10.1016/S0165-0270(00)00340-X. [DOI] [PubMed] [Google Scholar]
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels—a novel approach to neuroprotection. Brain Res Brain Res Rev. 2004;46:282–294. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Choi DY, Zhang J, Bing G. Aging enhances the neuroinflammatory response and alpha-synuclein nitration in rats. Neurobiol Aging. 2010;31:1649–1653. doi: 10.1016/j.neurobiolaging.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley F, Palfi S, Chu Y, Sortwell C, Collier TJ, Carvey PM. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Muros I, Afonso-Oramas D, Abreu P, Pérez-Delgado MM, Rodríguez M, González-Hernández T. Aging effects on the dopamine transporter expression and compensatory mechanisms. Neurobiol Aging. 2009;30:973–986. doi: 10.1016/j.neurobiolaging.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin–angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine-induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neuroscience. 2006;141:645–661. doi: 10.1016/j.neuroscience.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039–1048. doi: 10.1016/j.freeradbiomed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Fornazari M, de Paula JG, Castilho RF, Kowaltowski AJ. Redox properties of the adenoside triphosphate-sensitive K+ channel in brain mitochondria. J Neurosci Res. 2008;86:1548–1556. doi: 10.1002/jnr.21614. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci. 2003;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid KD. Opening mitochondrial K(ATP) in the heart—what happens, and what does not happen. Basic Res Cardiol. 2000;95:275–279. doi: 10.1007/s003950070046. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.RES.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/S0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens. 2009;18:28–32. doi: 10.1097/MNH.0b013e32831a9e0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. Neuronal vulnerability in Parkinson's disease. J Neural Transm Suppl. 1997;50:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O'Rourke B, Marban E. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol. 1999;55:1000–1005. [PubMed] [Google Scholar]
- Joglar B, Rodriguez-Pallares J, Rodríguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;295:F1110–F1116. doi: 10.1152/ajprenal.90336.2008. [DOI] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin–proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Mitochondrial potassium channels and uncoupling proteins in synaptic plasticity and neuronal cell death. Biochem Biophys Res Commun. 2003;304:539–549. doi: 10.1016/S0006-291X(03)00627-2. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S. Live longer sans the AT1A receptor. Cell Metab. 2009;9:403–405. doi: 10.1016/j.cmet.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA, Delfani K, Irwin I, DeLanney LE, Langston WJ, Janson AM. Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol. 2004;471:387–395. doi: 10.1002/cne.20036. [DOI] [PubMed] [Google Scholar]
- McCullough JR, Normandin DE, Conder ML, Sleph PG, Dzwonczyk S, Grover GJ. Specific block of the anti-ischemic actions of cromakalim by sodium 5-hydroxydecanoate. Circ Res. 1991;69:949–958. doi: 10.1161/01.RES.69.4.949. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FAO, Chai S. The brain renin–angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/S1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells: comparison of seven mitochondrion-specific fluorochromes. Immunol Lett. 1998;61:157–163. doi: 10.1016/S0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Michel PP, Ruberg M, Agid G. Rescue of mesencephalic dopamine neurons by anticancer drug cytosine arabinoside. J Neurochem. 1997;69:1459–1507. doi: 10.1046/j.1471-4159.1997.69041499.x. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Shimokawa H, Higashi M, Morikawa K, Matoba T, Hiroki J, Kunihiro I, Talukder HM, Takeshita A. Inhibition of renin–angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler Thromb Vasc Biol. 2002;22:1445–1450. doi: 10.1161/01.ATV.0000029121.63691.CE. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Matsusaka T, Miyata T. Angiotensin II type 1A receptor deficiency and longevity. Nephrol Dial Transplant. 2009;24:3280–3281. doi: 10.1093/ndt/gfp381. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Oxidation reactions in Parkinson’s disease. Neurology. 1990;40(Suppl 3):32–39. [PubMed] [Google Scholar]
- Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/S0008-6363(02)00439-X. [DOI] [PubMed] [Google Scholar]
- Poot M, Zhang YZ, Kramer JA, Wells KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL, Haugland RP. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996;44:1363–1372. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Rey P, Lopez-Real A, Sanchez-Iglesias S, Muñoz A, Soto-Otero R, Labandeira-Garcia JL. Angiotensin type-1-receptor antagonists reduce 6-hydroxydopamine toxicity for dopaminergic neurons. Neurobiol Aging. 2007;28:555–567. doi: 10.1016/j.neurobiolaging.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Muñoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103:145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Rey P, Parga JA, Muñoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira-Garcia JL. The mitochondrial ATP-sensitive potassium channel blocker 5-hydroxydecanoate inhibits toxicity of 6-hydroxydopamine on dopaminergic neurons. Neurotox Res. 2009;15:82–95. doi: 10.1007/s12640-009-9010-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin–angiotensin system in vascular diseases. Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Sasaki N, Seharaseyon J, O'Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.CIR.101.20.2418. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.RES.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- Sugrue MM, Wang Y, Rideout HJ, Chalmers-Redman RM, Tatton WG. Reduced mitochondrial membrane potential and altered responsiveness of a mitochondrial membrane megachannel in p53-induced senescence. Biochem Biophys Res Commun. 1999;261:123–130. doi: 10.1006/bbrc.1999.0984. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;14:1205–1213. doi: 10.1161/01.RES.0000020404.01971.2F. [DOI] [PubMed] [Google Scholar]
- Umemoto S. Angiotensin II type 1 (AT1) receptor deficiency halts the progression of age-related atherosclerosis in hypercholesterolemia: molecular link between the AT1 receptor and hypercholesterolemia. Hypertens Res. 2008;31:1495–1497. doi: 10.1291/hypres.31.1495. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- Valero RA, Senovilla L, Núñez L, Villalobos C. The role of mitochondrial potential in control of calcium signals involved in cell proliferation. Cell Calcium. 2008;44:259–269. doi: 10.1016/j.ceca.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Rodríguez-Pallares J, Muñoz A, Valenzuela R, Guerra MJ, Baltatu OC, Labandeira-Garcia JL. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson’s disease. Eur J Neurosci. 2010;32:1695–1706. doi: 10.1111/j.1460-9568.2010.07448.x. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Valenzuela R, Rodriguez-Perez AI et al (2010b) Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol Aging. PMID: 20888078 [DOI] [PubMed]
- Wadia JS, Chalmers-Redman RM, Ju WJ, Carlile GW, Phillips JL, Fraser AD, Tatton WG. Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (−)-deprenyl. J Neurosci. 1998;18:932–947. doi: 10.1523/JNEUROSCI.18-03-00932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Teisman P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hu J, Chen YP, Takeo T, Suga S, Dechon J, Liu Q, Yang KC, St John PA, Hu G, Wang H, Wakui M. Iptakalim modulates ATP-sensitive K(+) channels in dopamine neurons from rat substantia nigra pars compacta. J Pharmacol Exp Ther. 2006;319:155–164. doi: 10.1124/jpet.106.106286. [DOI] [PubMed] [Google Scholar]
- Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- Zhang HY, McPherson BC, Liu H, Baman TS, Rock P, Yao Z. H(2)O(2) opens mitochondrial K(ATP) channels and inhibits GABA receptors via protein kinase C-epsilon in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1395–H1403. doi: 10.1152/ajpheart.00683.2001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Prabhakaran K, Borowitz JL, Isom GE. Trimethyltin-induced apoptosis is associated with upregulation of inducible nitric oxide synthase and Bax in a hippocampal cell line. Toxicol Appl Pharmacol. 2006;216:34–43. doi: 10.1016/j.taap.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res. 2007;76:204–212. doi: 10.1016/j.cardiores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yao HH, Wu JY, Ding JH, Sun T, Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12:1559–1570. doi: 10.1111/j.1582-4934.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini S, Tremblay E, Pollard H, Moreau J, Ben-Ari Y. Regional distribution of sulfonylurea receptors in the brain of rodent and primate. Neuroscience. 1993;55:1085–1091. doi: 10.1016/0306-4522(93)90322-7. [DOI] [PubMed] [Google Scholar]