Abstract
No proven pharmacological therapies to delay or reverse age-related diastolic dysfunction exist. We hypothesized that late-life low-dose (non-blood-pressure-lowering) angiotensin-converting enzyme inhibition vs. angiotensin II receptor blockade would be equally efficacious at mitigating diastolic dysfunction in the senescent Fischer 344 × Brown Norway rat. Enalapril (10 mg/kg/day; n = 9) initiated at 24 months of age and continued for 6 months, increased myocardial relaxation (e'), reduced Doppler-derived indices of filling pressure (E/e'), favorably lowered the ratio of phospholamban–SERCA2 and reduced oxidative stress markers, Rac1 and nitrotyrosine, in aged hearts. Treatment with losartan (15 mg/kg/day; n = 9) similarly mitigated signs of cardiac oxidative stress, but impairments in diastolic function persisted when compared with untreated rats (n = 7). Our findings favor the idea that the lusitropic benefit of low-dose angiotensin-converting enzyme inhibitor initiated late in life may be related to an antioxidant-mediated modulation of SERCA2, resulting in improved relaxation rather than via overt effects on cardiac structure or blood pressure.
Keywords: Angiotensin-converting enzyme inhibitor, Angiotensin II receptor blocker, Diastolic dysfunction, Oxidative stress, SERCA2, Tissue Doppler
Introduction
Cardiac aging is associated with increases in left ventricular (LV) chamber stiffness and reductions in myocardial relaxation, leading to diastolic dysfunction. Impairments in diastolic function represent a significant public health concern, particularly given the growing number of older persons in industrialized nations (Anderson and Hussey 2000) and the increased prevalence of heart failure with preserved ejection, or diastolic heart failure, among those older than 65 years of age (Bhatia et al. 2006; Kitzman et al. 2001). The only preventive measures showing promise in halting or postponing the subtle degenerative processes of aging that contribute to diastolic dysfunction are caloric restriction (Meyer et al. 2006; Riordan et al. 2008) and physical exercise (Brenner et al. 2001; Groban et al. 2008; Belardinelli et al. 1995; Arbab-Zadeh et al. 2004; Gielen et al. 2010). Currently, there are no proven pharmacological therapies to delay or reverse age-related diastolic dysfunction, independent of medically managing underlying comorbidities such as hypertension, obesity, coronary atherosclerosis, and diabetes, which, in some cases, may be the root cause (Shah et al. 2011; Willens et al. 2005; Okin et al. 2004; Meredith and Ostergren 2006; Hunt et al. 2008; Beckett et al. 2008). This is particularly relevant given that not all older individuals benefit from or are capable of participating in physical exercise, nor is weight loss necessarily recommended for individuals 65 years and older. However, low-dose angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blockers (ARB) may be equally as effective as these more traditional behavioral approaches.
ACE-Is and ARBs are rational pharmacological choices given the central role that the renin–angiotensin system (RAS) has in the development of cardiovascular and target organ damage. Blockade of the RAS has become standard therapy for the management of systolic heart failure (Hunt et al. 2008; Granger et al. 2003; McMurray et al. 2003) and is beneficial in older patients with diastolic dysfunction (Warner et al. 1999; Little et al. 2004; Cleland et al. 2006). In addition, growing evidence suggests that aging is associated with changes in local cardiac RAS activation that are independent of changes in the circulating RAS. For example, gene expression of angiotensinogen, angiotensin-converting enzyme (ACE), and the angiotensin type 1 (AT1) and AT2 receptor are increased in hearts of senescent rats, whereas these same components are unchanged or reduced in the circulation of the aged rodent (Heymes et al. 1994, 1998). Furthermore, increases in cardiac angiotensin II (Ang II) and aldosterone levels in aged rats are associated with LV remodeling (Lacolley et al. 2001). Specifically, Ang II promotes myocyte hypertrophy, increases myocardial collagen synthesis, and is mitogenic to neonatal cardiac fibroblasts (Sadoshima and Izumo 1993; Schorb et al. 1993; Sun et al. 2004). Locally produced Ang II also regulates de novo aldosterone production, which contributes to interstitial fibrosis (Delcayre et al. 2000) and functional alterations in the aged heart (Dostal and Baker 1999).
The pharmacologic inhibition of the RAS, with either an ACE-I or ARB, has been shown to counteract the structural consequences of cardiac aging. Rats treated from weaning through adulthood with the ACE-I, enalapril, or the ARB, losartan, failed to show the expected reduction in myocyte number and increase in cardiac collagen deposition that normally occur during their lifespan (Basso et al. 2007). Similarly, treatment with the ACE-I perindopril for 1 year reduced left ventricular mass and interstitial collagen accumulation in both hypertensive and normotensive adult rats (Michel et al. 1988). Even short-term angiotensin II type 1 receptor inhibition has been shown to be beneficial in reversing or retarding age-related cardiac structural remodeling. Specifically, 12 weeks of low-dose candesartan reduced left ventricular hypertrophy by nearly 50% in a group of pre-senescent rats compared with untreated littermates (Saupe et al. 2003). Still, it is not known whether these improvements in cardiac architecture abrogate age-related impairments in myocardial relaxation and ventricular compliance. Accordingly, in the present study, we sought to determine the relative effectiveness of late-life administration of a low-dose (non-blood-pressure-lowering) ACE-I vs. ARB on the structural and functional abnormalities of normal cardiac aging in the Fischer 344 × Brown Norway (F344BN) rat using echocardiography, blood pressure, and heart rate monitoring. Furthermore, we assessed local cardiac changes in Ang II and Ang-1-7 as well as other cardiac calcium regulatory proteins and oxidative stress markers known to modulate LV relaxation, stiffness, and collagen deposition: SERCA2, phospholamban, RAC1, and nitrotyrosine. We hypothesized that the two strategies of RAS blockade would be similarly efficacious at mitigating diastolic dysfunction.
Methods
Animal model
For all studies, we used male F344BN rats purchased from the National Institute on Aging Colony at Harlan Industries (Indianapolis, IN). The F344BN rat strain has been extensively used for aging studies given that they demonstrate age-related changes in cardiac structure (e.g., reductions in myocyte number, increases in myocyte size, and interstitial collagen deposition) resembling those occurring in humans (Wang et al. 2010; Hacker et al. 2006). Animals were received at 22 months of age and housed individually on a 12-h light/dark cycle in a specific pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. From 22 to 24 months of age, animals were allowed to acclimate to their housing conditions. All experimental protocols were approved by the University of Florida’s Animal Care and Use Committee.
Experimental protocol
At 24 months of age, rats were randomly assigned to receive either enalapril (EN, 10 mg/kg/day; n = 9), losartan, (LOS, 15 mg/kg/day; n = 9) or vehicle (VEH; n = 7) for 6 months. The doses were chosen to provide nominal effects on blood pressure. Drug delivery was accomplished by compounding the various treatments into bacon-flavored food tablets (Bio-Serv, Frenchtown, NJ). Vehicle-containing food tablets were identical to those delivering enalapril or losartan, except that the drug was omitted. Cardiac structure and function was determined by echocardiography at the end of 5.5 months of treatment, at 29.5 months of age. At 30 months of age, rats were sacrificed by rapid decapitation. Whole blood was collected and processed for subsequent determination of angiotensin peptides and serum ACE activity. The thorax was quickly opened, and the heart was rapidly removed and weighed with one half being submerged in 10% formalin and the other frozen in liquid nitrogen, and then stored at −80°C for angiotensin peptide and immunoblot analyses.
In a separate cohort of rats (n = 4/group), tail-cuff blood pressures and heart rate (Hatteras Instruments, Cary, NC) were measured. Approximately 1 week prior to being killed, animals were adapted to the apparatus by being placed in restrainers and experiencing the tail-cuff procedure for 30 min/day over 3 days. Animals were then tested for 30 min/day, during which movement-free signals were averaged for that day. Overall, animals were tested for 3 days to ensure stability within each rat.
Biochemical analyses of angiotensin peptides and ACE activity
Immediately following decapitation, trunk blood was collected in pre-chilled tubes containing peptidase inhibitors (25 mmol/l EDTA, 0.44 mmol/l 1,2-orthophenanthroline monohydrate, 1 mmol/l sodium para-chloro-mercuribenzoate, and 3 μmol/l of the rat renin inhibitor, WFML-1) as described by Ferrario et al. (2005). These inhibitors were used to prevent Ang I generation, Ang I to Ang II conversion, and Ang II and Ang-(1–7) degradation. After 30 min on ice, blood cells were isolated by centrifugation at 3,000×g for 20 min, and aliquots of either plasma or serum were stored at −80°C until use. Left ventricular tissues (n = 7–9 per group) were rapidly collected and snap-frozen in liquid N2 and stored at −80°C for later assay. Angiotensin peptides were extracted from the plasma and tissue samples using C18 Sep-Pak columns (Waters, Milford, MA), and the eluate was analyzed by radioimmunoassay for Ang II and Ang-(1–7) as described by Ferrario et al. (2005). The minimum detectable limits of the Ang II and Ang-(1–7) assays were 0.8 and 2.5 pg/ml, respectively. The intra- and interassay coefficients of variability were 12% and 22% for Ang II and 8% and 20% for Ang-(1–7), respectively.
Serum ACE activity was determined by incubation of the sample with radio-labeled 3H-Hip-Gly (pH 8.0) for 1 h at 37°C. The intra-assay variation was 3.9%, and the interassay variation averaged 5.9%.
Determination of LV structure and function by echocardiography
For the echocardiogram, rats (n = 7–9 per group) were anesthetized with an isoflurane (1.5%)/oxygen mixture by nose cone during spontaneous ventilation. Anesthetized, spontaneously breathing animals were placed in a shallow left lateral decubitus position, with electrocardiographic adhesive electrodes applied to the paws. The left hemithorax was shaved and prepped with acoustic coupling gel to increase probe contact. Animals were secured to a warming table to maintain normothermia. Using a commercially available sector scanner equipped with a 12 MHz phased-array transducer (Envisor, Philips Medical Systems, Andover, MA), images were obtained at 100 mm/s sweep speed and recorded on a digital storage optical disk for off-line analysis. Diastolic posterior wall thickness (PWTed) and LV end-diastolic and end-systolic dimensions (LVDD, LVSD) were measured from midpapillary, short-axis images obtained by M-mode echocardiography. The percentage of LV fractional shortening (%FS), an index of global systolic function, was calculated as ((LVDD-LVSD)/LVDD) × 100. Relative wall thickness was calculated as (2 × PWTed)/LVDD. Mitral inflow measurements of early filling velocities (Emax), deceleration slope (Edecslope), and time (Edectime) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Due to relatively high heart rates and fusion of the early and late Doppler profiles, only the early transmitral filling velocity was measured. Pulsed-Doppler tissue imaging to assess septal mitral annular descent (e') was also obtained from the four-chamber view. The ratio of early transmitral filling-to-mitral annular descent, or E/e', was used as an index of filling pressure. All measured and calculated systolic and diastolic indices are represented as the average of at least five consecutive cardiac cycles to minimize beat-to-beat variability.
Histological determination of cardiac collagen deposition
Vertical, long-axis sections of the formalin-fixed heart were taken through the left ventricle. Specimens were dehydrated with ethanol and embedded into paraffin blocks. Following microtome sectioning, 4-μm tissue sections were stained with Verhoeff-van Gieson for assessment of interstitial and perivascular elastin (black stain) and collagen (pink stain) fibers. The sections were examined under bright field using a Leica DM4000B microscope system (Bannockburn, IL). Bright field photomicrographs were captured with a Leica DFC digital camera and processed using Leica Application Suite software. Adobe Photoshop Creative Suite 3 (Adobe Inc., San Jose, CA) was used to determine the ratios of collagen positive stained pixels in each photomicrograph (four randomized quadrant field images per rat, magnified ×200) by an observer masked to the treatment protocol.
Protein expression by Western Blot analysis of SERCA2, phospholamban, Rac1, 3-nitrotyrosine
Sarcoplasmic reticulum membranes and LV tissue homogenates were prepared as described previously (Groban et al. 2008). Briefly, samples were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Immunoblots were probed for the key calcium regulatory proteins, anti-sarcoplasmic endoplasmic reticulum calcium ATPase 2 (SERCA2) (1:1000 dilution; Abcam, Cambridge, MA) and anti-phospholamban (PLB) (1:5000 dilution; Abcam, Cambridge, MA), and oxidative stress markers, including antibodies for 3-nitrotyrosine (1:100 dilution; Abcam, Cambridge, MA), and the NADPH oxidase subunit, Rac1 (1:500 dilution; Cytoskeleton Inc., Denver, CO). To normalize the variability of protein loading, the antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000 dilution; Cell Signaling, Danvers, MA) was probed onto the stripped membranes corresponding to the aforementioned proteins (Western Blot Recycling Kit: Alpha Diagnostic International, Inc., San Antonio, TX). The bands were scanned and digitized (MCDI image analysis software; Imaging Research Inc., Ontario, Canada). Each band was normalized to its own GAPDH and expressed in arbitrary units. The PLB-to-SERCA2 ratio was used as a measure of SERCA2 inhibition.
Immunohistochemical analysis of Rac1
Immunohistochemistry was used to identify and localize Rac1 in the myocardial tissue. Formalin-fixed and paraffin-embedded tissue sections were deparaffinized, exposed to 3% hydrogen peroxide to block endogenous peroxidase activity, and subjected to antigen retrieval via immersion in citric acid (pH 6.0, 0.01 mol/L) at 95°C for 15 min followed by slow cooling to 60°C . After treatment with the blocking serum, the sections were incubated with monoclonal antibody against the Nox2 NADPH oxidase subunit, Rac1 (Cytoskeleton Inc., Denver, CO) overnight at 4°C, rinsed with phosphate-buffered saline, and incubated with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA) for 3 h at 4°C . Antibody binding was detected with the Vectastain ABC Elite avidin/biotin/peroxidase kit (Vector Laboratories, Burlingame, CA) for 30 min at room temperature, followed by incubation with the peroxidase substrate solution, diaminobenzidine. The tissue sections were counterstained with hematoxylin, dehydrated, mounted, and observed under light microscopy with a ×400 objective.
Statistical analysis
All data analyses were performed using Graph Pad Prism 5.01 software (GraphPad Software, La Jolla, CA). Data were analyzed using one-factor analysis of variance (ANOVA) and Dunnett’s post test. If homogeneity or normality was not satisfactory (e.g., PLB/SERCA2, plasma Ang II) a non-parametric one-way ANOVA (Kruskal–Wallis) was performed on the ranked measurements followed by Dunn’s multiple sample comparisons. All data are expressed as mean ± SEM. A p value of < 0.05 was considered statistically significant.
Results
Effect of low-dose losartan and enalapril on physical characteristics and resting systolic blood pressure and heart rate
In comparison with control animals, body weight, left ventricular weight, whole heart weight, and heart weight index, calculated as whole heart weight divided by body weight (milligrams per kilogram), were not affected by enalapril or losartan treatment (globally referred to as low-dose RAS blockade; Table 1). In addition, low-dose RAS blockade did not significantly lower systolic blood pressure (EN, 144 ± 3; LOS, 141 ± 9 mmHg; VEH, 163 ± 5 mmHg, p > 0.05 or heart rate (EN, 341 ± 6; LOS, 334 ± 15; VEH, 344 ± 6 beats/min, p > 0.05). These data suggest that any observed effects of low-dose RAS blockade on cardiac remodeling are independent of hemodynamic properties of these compounds.
Table 1.
Physical characteristics
| Vehicle (n = 7) | Enalapril (n = 9) | Losartan (n = 9) | |
|---|---|---|---|
| Body weight (g) | 503 ± 17 | 508 ± 11 | 536 ± 16 |
| Heart weight (mg) | 1.34 ± 0.08 | 1.30 ± 0.05 | 1.35 ± 0.06 |
| LV weight (mg) | 1.06 ± 0.03 | 1.00 ± 0.03 | 1.03 ± 0.04 |
| Heart index (mg/kg) | 2.67 ± 0.15 | 2.56 ± 0.07 | 2.51 ± 0.05 |
LV left ventricular
Effect of chronic losartan and enalapril on RAS components in plasma and heart
In order to determine the efficacy of low-dose RAS blockade and the potential role of circulating and local RAS components in the development of age-related diastolic dysfunction, plasma, and tissue concentrations of Ang II and Ang-1-7 and serum ACE activity were determined (Table 2). Indeed, a close link between an increased cardiac RAS and left ventricular remodeling has been implicated in the aging cardiac phenotype (Lakatta 2003; Stein et al. 2010; Ito et al. 2007). In confirmation of treatment, circulating Ang II levels were significantly increased in losartan-treated rats compared with enalapril- and vehicle-treated rats (p < 0.01). Moreover, serum ACE activity was significantly reduced in enalapril-treated rats compared with the other groups (p < 0.05). Plasma Ang-(1–7) levels were not affected by either RAS-I. There was also a tendency for higher Ang II levels in hearts of control animals vs. enalapril- and losartan-treated animals (p = 0.12), but this difference did not achieve statistical significance. In contrast to our original prediction, cardiac Ang-(1–7) was significantly higher in vehicle-treated control rats (p < 0.001), possibly indicating a compensatory mechanism against age-related oxidative stress.
Table 2.
Blood and cardiac biochemistry
| Vehicle (n = 7) | Enalapril (n = 9) | Losartan (n = 9) | |
|---|---|---|---|
| Plasma Ang II (pg/ml) | 17 ± 2 | 16 ± 2 | 28 ± 3* |
| Plasma Ang 1–7 (pg/ml) | 47 ± 8 | 60 ± 17 | 49 ± 10 |
| Serum ACE (nmol/ml/min) | 88 ± 4 | 33 ± 3** | 84 ± 2 |
| Cardiac Ang II (pg/mg) | 5.3 ± 0.5 | 4.0 ± 0.7 | 3.6 ± 0.3 |
| Cardiac Ang 1–7 (pg/mg) | 5.0 ± 0.6 | 2.0 ± 0.4*** | 2.6 ± 0.4**** |
Ang II angiotensin II, Ang 1–7 angiotensin 1–7, ACE angiotensin-converting enzyme
*p < 0.01 vs. vehicle, enalapril **p < 0.001 vs. vehicle, losartan; ***p < 0.001 vs. vehicle; ****p < 0.01 vs. vehicle
Effect of chronic losartan and enalapril on echocardiographic-derived indices of cardiac structure and function
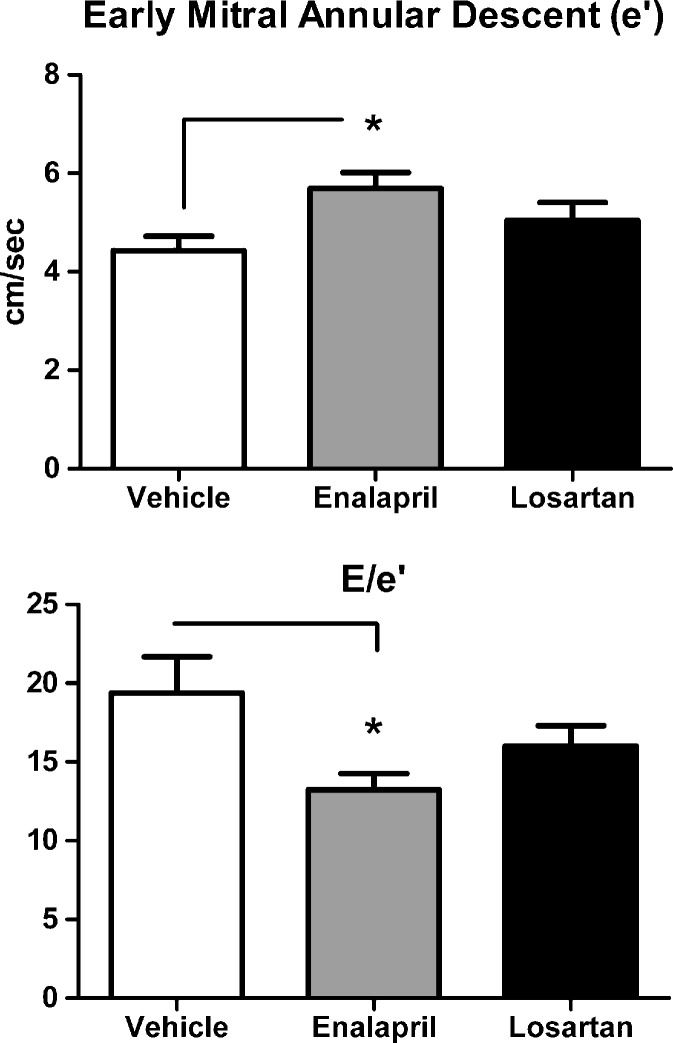
The effects of ACE inhibition and angiotensin receptor blockade on echocardiographic-derived variables of left ventricular geometry and systolic function are summarized in Table 3. Losartan-treated animals had significantly lower PWTs than their vehicle-treated counterparts (p < 0.05), whereas PWTs among enalapril-treated rats were not different from vehicle. Pharmacologic treatment with enalapril and losartan had no overt effect on M-mode measurements of LV chamber dimensions, relative wall thickness or systolic function, as signified by the absence of changes in percent FS. Even though assessment of diastolic function using conventional transmitral Doppler showed no affect of treatment with either the ACE-I or ARB (Table 4), tissue Doppler showed an improvement in early mitral annular descent (e'), a measure of LV relaxation, and reduced filling pressures (E/e', Fig. 1b) in enalapril-treated rats vs. age-matched vehicle-treated controls (Fig. 1; both p’s < 0.05). Doppler imaging (TDI) measures myocardial motion throughout the cardiac cycle, rather than conventional Doppler which uses blood flow velocities to extrapolate physiologic parameters.
Table 3.
M-mode measurements of LV geometry and systolic function
| Vehicle (n = 7) | Enalapril (n = 9) | Losartan (n = 9) | |
|---|---|---|---|
| LVDd (cm) | 0.50 ± 0.03 | 0.48 ± 0.03 | 0.53 ± 0.02 |
| LVSd (cm) | 0.84 ± 0.03 | 0.82 ± 0.03 | 0.86 ± 0.03 |
| PWTd (cm) | 0.190 ± 0.006 | 0.178 ± 0.006 | 0.167 ± 0.004* |
| Relative wall thickness | 0.45 ± 0.02 | 0.44 ± 0.03 | 0.40 ± 0.02 |
| Fractional shortening (%) | 40 ± 1.0 | 42 ± 2.1 | 38 ± 1.2 |
LVDd left ventricular end-diastolic diameter, LVSd left ventricular end-systolic diameter, PWTd posterior wall thickness and diastole
*p < 0.04 vs. vehicle
Table 4.
Heart rate and conventional Doppler measurements of diastolic function
| Vehicle (n = 7) | Enalapril (n = 9) | Losartan (n = 9) | |
|---|---|---|---|
| Heart rate (b/min) | 320 ± 6 | 324 ± 4 | 324 ± 7 |
| Isovolemic relaxation time (s) | 0.023 ± 0.001 | 0.025 ± 0.001 | 0.024 ± 0.001 |
| Velocity of early mitral filling (cm/s) | 81 ± 4 | 78 ± 6 | 79 ± 4 |
| Duration of early mitral filling (s) | 0.044 ± 0.002 | 0.042 ± 0.002 | 0.044 ± 0.003 |
| Deceleration slopes of early filling (cm/s2) | 20.0 ± 2.2 | 19.5 ± 1.0 | 19.5 ± 1.0 |
Fig. 1.
a The tissue Doppler measure of diastolic function, mitral annular velocity (e'), was increased by enalapril, but not losartan, when compared with age-matched vehicle-treated rats. b The Doppler surrogate to left ventricular filling pressure, early transmitral filling-to-mitral annular velocity (E/e'), was reduced by enalapril, but not losartan, when compared with age-matched vehicle-treated rats. Data represent mean ± SEM; *p < 0.05
Moreover, tissue Doppler imaging minimizes the effects of loading conditions and, thus, is a more reliable indicator of diastolic function than conventional Doppler (Groban et al. 2010).
Effect of chronic losartan and enalapril treatment on cardiac calcium regulatory proteins and oxidative stress markers
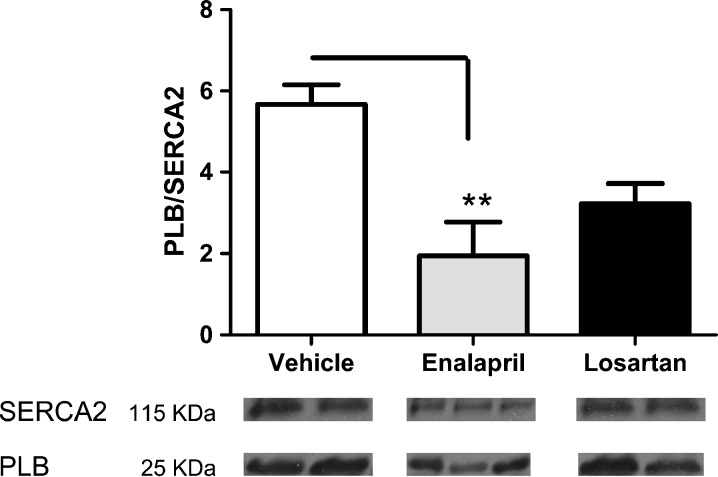
The increase in myocardial relaxation in enalapril-treated rats coincided with an increase in SERCA2 expression, the key protein involved in cytosolic Ca2+ reuptake into the sarcoplasmic reticulum, compared with vehicle- and losartan-treated rats (all p’s < 0.05). While phospholamban, the endogenous inhibitor of SERCA2, was not affected by either treatment, the PLB/SERCA2 ratio was significantly lower in the enalapril group vs. vehicle (p < 0.05; Fig. 2).
Fig. 2.
a The dephosphorylated phospholamban (PLB)/sarco-endoplasmic Ca2+ adenosine triphosphatase (SERCA2) ratio, determined from the cardiac homogenate, was lower in the enalapril-treated rats when compared with vehicle-treated rats. Given that dephosphorylated PLB blocks SERCA2, the lower PLB/SERCA2 ratio indicates improved SERCA2 functioning by enalapril. Values are means ± SEM. **p < 0.01 b Representative immunoblots of SERCA2 and PLB from each group are displayed
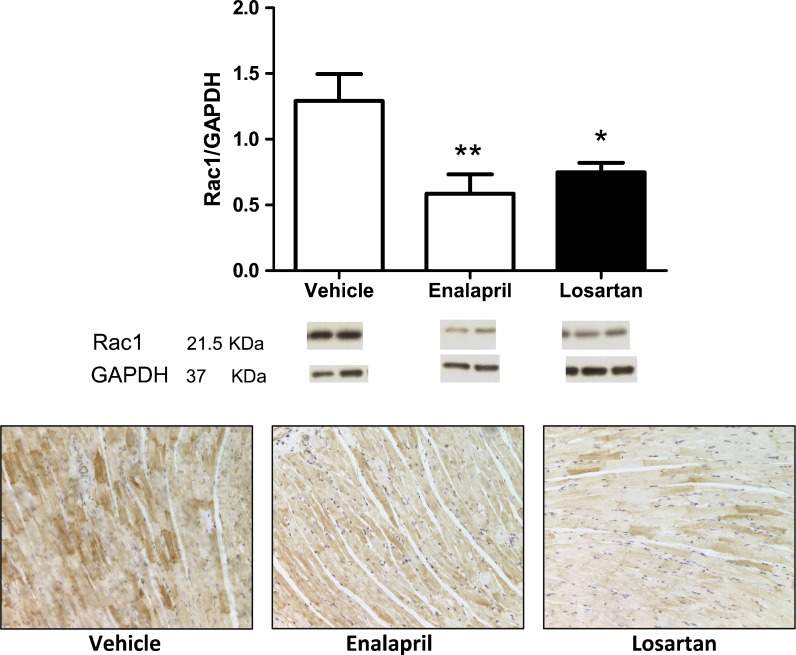
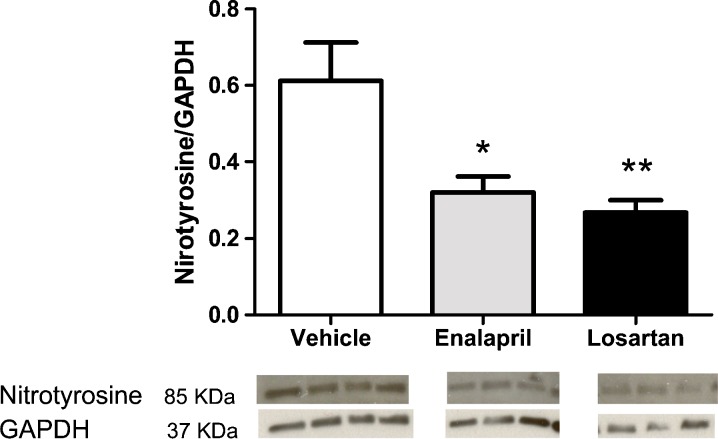
A close link between age-related oxidative stress and a disruption of membrane proteins and/or alterations in the extracellular structure of the heart leading to remodeling has been demonstrated (Wang et al. 2010). Because the NADPH oxidases are primary suppliers of ROS leading to oxidative stress (Cai et al. 2003), the level of the regulatory subunit of the Nox2 NADPH oxidase isoform, Rac1 was evaluated by immunoblot and immunohistochemical techniques. Protein expression of Rac1 was significantly decreased in enalapril- and losartan-treated rats (p = 0.008; Fig. 3). The level of nitrotyrosine, another marker of oxidative/nitrative stress, was also suppressed in enalapril-treated (p < 0.05) and losartan-treated rats (p < 0.01; Fig. 4).
Fig. 3.
a Protein levels of the Nox2 NADPH oxidase subunit, Rac1, were elevated in the senescent hearts from the vehicle-treated rats when compared with rats on RAS blockade. Values are means ± SEM. *p < 0.05 vs. vehicle; **p < 0.01 vs. vehicle. Representative immunoblots of Rac1 and the loading control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from each group are displayed below. b: Protein expression levels of Rac1 in left ventricular tissues from the three groups also shows enhanced staining within the myocytes of the vehicle-treated group vs. losartan- and enalapril-treated groups
Fig. 4.
a Protein expression levels of nitrotyrosine, another marker of oxidative stress, were increased in the senescent hearts from the vehicle-treated rats when compared with enalapril- and losartan-treated rats. Values are means ± SEM. *p < 0.05 vs. vehicle; **p < 0.01 vs. vehicle. b Representative immunoblots of nitrotyrosine and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from each group are displayed below
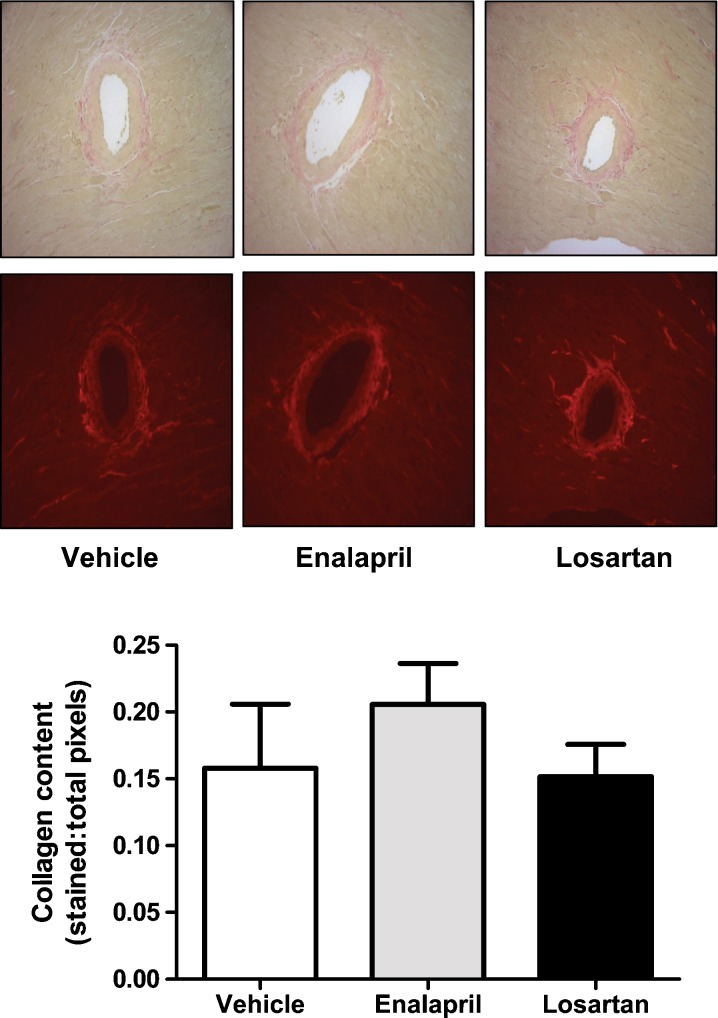
Effect of chronic losartan and enalapril on cardiac collagen deposition
Perivascular fibrosis, as represented by the average grayscale intensity of Verhoeff-van Gieson staining (for collagen), was nominal in control animals and was not altered by treatment with either enalapril or losartan (Fig. 5). Similarly, interstitial fibrosis was not different among groups (data not shown).
Fig. 5.
a Representative Verhoeff-van Gieson staining of perivascular collagen of hearts from each group shown in bright field. b Quantification of perivascular collagen deposition shows no significant differences among groups. Data represent mean ± SEM
Discussion
The major finding of the present study is that there are differential effects of low-dose enalapril and losartan treatment on tissue Doppler indices of diastolic function when administered chronically, late in life to F344BN male rats. This effect was independent of changes in hemodynamic characteristics of these compounds given that we observed no overt changes in either blood pressure or heart rate compared with vehicle-treated controls. Circulating Ang II increased with losartan and ACE activity decreased with enalapril, even though plasma levels of Ang-1-7 levels were not affected by treatment. In contrast, cardiac Ang-1-7 levels were lowest in RAS-I-treated rats whereas tissue Ang II levels were not different from vehicle controls. This highlights the fact that circulating levels of ANG peptides are potentially independent of the local cardiac milieu. Furthermore, tissue Doppler measures of diastolic function, mitral annular velocity (e'), were increased and a surrogate to left ventricular filling pressure, early transmitral filling-to-mitral annular velocity (E/e'), was reduced by enalapril. Enalapril also enhanced the expression of a key cardiac calcium regulatory protein known to modulate LV relaxation, SERCA2, and favorably lowered the ratio of phospholamban/SERCA2. These effects were not observed with losartan treatment. However, both enalapril and losartan mitigated markers of oxidative stress including RAC1 and nitrotyrosine; effects that are known to be modulated by Ang II levels (Wang et al. 2010). ACE-I or ARB treatment has been shown to retard the adverse left ventricular remodeling instigated by aging, hypertension, and heart failure (Basso et al. 2007; Ito et al. 2007; Chrysant 2008; Greenberg et al. 1995). While late-life treatment with losartan reduced PWT in the aged F344BNF rat when compared with vehicle, relative wall thicknesses were not substantially affected by either RAS inhibitor in this study. Indeed, PWT and its relation to chamber size is a well-recognized measure of hypertrophy (Foppa et al. 2005). Taken together with the lack of an affect of enalapril or losartan on cardiac collagen deposition, these findings suggest that attenuation of age-related structural remodeling has minimal impact on the functional response to low-dose RAS blockade in old F344BN male rats. Specifically, our findings favor the idea that the lusitropic benefit of low-dose, chronic ACE inhibition initiated late in life may be related to an antioxidant-mediated modulation of the calcium regulatory protein, SERCA2, resulting in improved relaxation rather than via overt effects on cardiac structure or blood pressure.
In this study, a single dose of each drug was chosen, aiming to have minimal effects on systolic blood pressure in both treatment groups (Strawn et al. 1999; Heller et al. 2005; Ferder et al. 2002; González Bosc et al. 2000; Harding et al. 1993). Although the difference in mean systolic blood pressures between enalapril and losartan-treated rats was only 3 mmHg, there was about a 20 mmHg difference in systolic blood pressure when rats treated with RAS blockade (mean SBP, 142 ± 4 mmHg) were compared with their counterparts receiving vehicle (162 ± 5 mmHg). Certainly, even modest blood-pressure-lowering actions can benefit diastolic function (Solomon et al. 2010; Solomon et al. 2007). However, despite the same reductions in blood pressure in the losartan-treated rats, diastolic function was not improved to a similar extent to the respective improvements in enalapril-treated animals. This is in agreement with the results reported in mice chronically treated with enalapril from weaning vs. treatment with propranolol, nifedipine, or hydrochlorothiazide (Ferder et al. 2002). Ferder et al. (2002) showed that despite similar blood-pressure-lowering effects among antihypertensive agents, only enalapril-treated mice were protected from age-related organ damage, and they had a longer life span. Taken together with the findings from experimental models of hypertension that have shown attenuations in cardiac hypertrophy, cardiac dysfunction and fibrosis with ACE-Is and ARBs at doses that were independent of significant blood-pressure-lowering effects (Sen 1983; Gupta et al. 2005), it is reasonable to suspect that the mitigation of diastolic dysfunction in the enalapril-treated rats was due to something other than the modest reduction in age-related systolic hypertension.
Aging is accompanied by both a decrease and impaired functioning of SERCA2 (Xu and Narayanan 1998), which is a major promoter of impaired myocardial relaxation (Groban 2005). Diastolic relaxation occurs when calcium is removed from troponin C by the activity of SERCA. Decreased expression of SERCA2, or an increase in its inhibitory regulator, phospholamban, results in reduced reuptake of intracellular Ca2+ into the sarcoplasmic reticulum, subsequently leading to an increase in cytosolic calcium load, and impaired relaxation. It has also been shown that angiotensin II (Ang II) decreases SERCA2 gene expression in ventricular cardiomyocytes and that this effect is attenuated by treatment with an ARB or ACE-I (Ju et al. 1996; Reinicke et al. 1995; Holtz et al. 1992). Furthermore, in experimental models of heart failure and hypertension, ACE-I and ARBs improve SERCA2 content, limit oxidative stress, and improve diastolic function (Shao et al. 2005; Flesch et al. 1997). While our data in the enalapril-treated rats corroborate these reports, a similar increase in SERCA2 and improvement in lusitropic function was not observed in losartan-treated rats.
Indeed, a key mechanism involved in cardiac aging and age-related cardiovascular diseases is enhanced oxidative stress (Sohal 2002; Ungvári et al. 2005; Kakarla et al. 2010). Increases in oxidative stress have been associated with age-related declines in heart function (Sohal 2002). The protein SERCA2 is one prominent target of oxidation and tyrosine nitration-induced damage of aging (Klebl et al. 1998; Knyushko et al. 2005). Knyushko et al. (2005) attributed the functional reduction in SERCA2 activity they found in the senescent Fischer 344 rat heart to increased nitrotyrosine modifications of multiple tyrosines within the cardiac SERCA2 protein. Nitration in the senescent heart was found to increase by more than two nitrotyrosines per Ca2+ ATPase molecule, and this was associated with an increase in intracellular calcium with the aged myocytes. Indeed, increases in intracellular calcium lead to reductions in the efficiency of myocyte relaxation. Nitration of select tyrosines on the SERCA2 isoform was not determined in the present study. However, the increased cardiac accumulation of nitrotyrosine, a marker of oxidative/nitrative stress, in the untreated aged rats versus those on RAS blockade suggest that a functional decline in SERCA2 might have partially contributed to the impaired relaxation in this group. Even though nitrotyrosine expression was lessened by losartan in the present study, this did not correspond with an improvement in mitral annular descent (e'), or relaxation, in these animals. It could be that an increase in SERCA2 content and a decrease in presumed oxidative/nitrative damage to SERCA2 are required to favorably affect diastolic function in the F344BN rat, as demonstrated in the enalapril-treated animals.
Attenuation of age-related oxidative stress by RAS blockers may also be due to modulation of myocardial NADPH oxidases (Griendling and FitzGerald 2003; Griendling et al. 2000). Emerging evidence demonstrates that NADPH oxidase-derived ROS are important in cardiac aging, which can be regulated by the RAS (Ito et al. 2007; Wang et al. 2010). Although we do not have direct evidence of cardiac oxidative stress in our aged animals, we found reduced levels of the NADPH oxidase subunit, Rac1, in enalapril- and losartan-treated rats. In agreement with a suspected attenuation of oxidative stress by the RAS blockers, we found an increase in the glutathione/glutathione disulfide ratio (GSH/GSSG), a marker of oxidative stress, and a reduction in protein carbonyl formation, an indicator of oxidative damage, in visceral fat rendered from the same losartan- and enalapril-treated rats compared with their age-matched counterparts receiving vehicle alone (unpublished data).
The disparate findings between enalapril and losartan treatments might be due to differences in potency of RAS blockade, as others have shown similar effects on heart function when losartan was administered at two- to threefold higher doses than enalapril (Wang et al. 2004; Guo et al. 2008). In this study, a single dose was chosen to have minimal effects on systolic blood pressure in the “normative” aging F344BN rat. Another reason for the dissimilar findings between treatments might be due to the multiple actions of ACE-Is, which not only block Ang II production but also alter the levels of other peptides such as bradykinin (Linz et al. 1995). Although we observed an increase in plasma Ang II in losartan-treated but not enalapril-treated rats, local Ang II concentrations were not significantly altered, further suggesting that the effects we observed with enalapril were independent of changes in angiotensin peptides.
In contrast, local Ang II has been implicated in age-related increases in cardiac fibrosis (Groban et al. 2006), and ACE-Is and ARBs attenuate structural remodeling during cardiac aging, a potent risk factor for diastolic dysfunction (Basso et al. 2007; Michel et al. 1988; Saupe et al. 2003). However, in the present study, enalapril improved diastolic function without alterations in structural remodeling perhaps due to insufficient dosing or to the late in life treatment. It is interesting to note, however, that both treatments lowered cardiac Ang-1-7, a peptide shown to attenuate the development of heart failure in a rat model of myocardial infarction (Loot et al. 2002) and blunt Ang II-induced cardiac hypertrophy (Mercure et al. 2008). Whether the higher cardiac Ang-1-7 in untreated BNF344 rats demonstrated in this study, and previously (Groban et al. 2008), represents part of a compensatory mechanism against oxidative stress of normal, “unprovoked” aging, remains speculative.
The results in this study from the late-life RAS blockade indicate that chronic enalapril treatment, as opposed to losartan, improved diastolic function independent of blood-pressure-lowering effects. The lusitropic benefit of enalapril was associated with increases in the cardiac calcium regulatory protein, SERCA2, and reductions in markers of oxidative and nitrosative stress. Cardiac collagen deposition did not appear to have a prominent role in influencing the diastolic functional phenotype of the aged F344BN in this study. It remains to be determined whether commencement of treatment with low, non-depressor doses of enalapril or losartan, even earlier in the lifespan of the F344BN might be more effective at preventing the onset of the degenerative processes that influence diastolic function.
Clinical perspective
Older patients with preclinical or asymptomatic diastolic dysfunction have an increased risk of developing diastolic heart failure (Redfield et al. 2003; Achong et al. 2009), even though traditional cardiovascular risks often are absent. An increasing body of evidence suggests that oxidative stress and an activated RAS contribute to the senescent cardiac phenotype. In view of the still-persisting uncertainty about how to handle and limit the progression of diastolic dysfunction, aggressive control of traditional risk factors including the modulation of age-related oxidative stress, is needed. Whether and to what degree the intriguing effects of low-dose ACE inhibition with enalapril in improving ventricular lusitropy in the “normal” aging BNF344 rat may translate into benefits for older patients without overt comorbidities, such as hypertension, diabetes, and obesity, remains to be tested clinically.
Contributor Information
Leanne Groban, Phone: +1-336-7164498, FAX: +1-336-7168190, Email: lgroban@wfubmc.edu.
Frederico S. M. Machado, Email: fredsmm@yahoo.com.br
Christy S. Carter, Email: ccarter@aging.ufl.edu
References
- Achong N, Wahi S, Marwick TH. Evolution and outcome of diastolic dysfunction. Heart. 2009;95:813–818. doi: 10.1136/hrt.2008.159020. [DOI] [PubMed] [Google Scholar]
- Anderson GP, Hussey PS. Population aging: a comparison among industrialized countries. Health Aff (Millwood) 2000;19:191–203. doi: 10.1377/hlthaff.19.3.191. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A, Dijk E, Prasad A, Pu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:Hl351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Duinitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Berman N, Ginzton L, Purcaro A. Exercise training improves left ventricular diastolic filling in patients with dilated cardiomyopathy. Clinical and prognostic implications. Circulation. 1995;91:2775–2784. doi: 10.1161/01.CIR.91.11.2775. [DOI] [PubMed] [Google Scholar]
- Bhatia RS, Tu JV, Lee DS, Austin PC, Pang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104:22l–226l. doi: 10.1161/01.CIR.104.2.221. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Chrysant SG. Angiotensin II receptor blockers in the treatment of the cardiovascular disease continuum. Clin Ther. 2008;30:2181–2190. doi: 10.1016/j.clinthera.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, Investigators PEP-CHF. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- Delcayre C, Silvestre JS, Gamier A, Oubenaissa A, Cailmail S, Tatara E, Swynghedauw B, Robert V. Cardiac aldosterone production and ventricular remodeling. Kidney Int. 2000;57:1346–1351. doi: 10.1046/j.1523-1755.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Baker KM. The cardiac renin–angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. doi: 10.1161/01.RES.85.7.643. [DOI] [PubMed] [Google Scholar]
- Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Curr Opin Pharmacol. 2002;2:189–194. doi: 10.1016/S1471-4892(02)00139-X. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher FE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Flesch H, Schiffer F, Zolk O, Pinto Y, Stasch JP, Knorr A, Ettelbrock S, Bohin M. Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca(2+)-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens. 1997;15:1001–1009. doi: 10.1097/00004872-199715090-00011. [DOI] [PubMed] [Google Scholar]
- Foppa M, Duncan BB, Rohde L. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovascular Ultrasound. 2005;3:1–13. doi: 10.1186/1476-7120-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:l221–l1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- González Bosc L, Kurnjek ML, Muller A, Basso N. Effect of chronic angiotensin II inhibition on the cardiovascular system of the normal rat. Am J Hypertens. 2000;l3:l301–l1307. doi: 10.1016/s0895-7061(00)01209-7. [DOI] [PubMed] [Google Scholar]
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM—Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–2581. doi: 10.1161/01.CIR.91.10.2573. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- Groban L. Diastolic dysfunction in the older heart. J Cardiothorac Vasc Anesth. 2005;l9:228–236. doi: 10.1053/j.jvca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63:911–920. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- Groban L, Sanders DM, Houle TT, Antonio BL, Ntuen EC, Zvara DA, Kon ND, Kincaid EH. Prognostic value of tissue Doppler-derived E/e' on early morbid events after cardiac surgery. Echocardiography. 2010;27:13l–138l. doi: 10.1111/j.1540-8175.2009.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang J, Elimban V, Dhalla NS. Both enalapril and losartan attenuate sarcolemrnal Na+−K+−ATPase remodeling in failing rat heart due to myocardial infarction. Can J Physiol Pharmacol. 2008;86:139–147. doi: 10.1139/Y08-006. [DOI] [PubMed] [Google Scholar]
- Gupta S, Young D, Sen S. Inhibition of NF–kappaB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H20–H29. doi: 10.1152/ajpheart.00082.2005. [DOI] [PubMed] [Google Scholar]
- Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290:H304–H311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- Harding P, Stonier C, Aber GM. Dose-dependent effects of angiotensin converting enzyme (ACE) inhibitors on glomerular prostanoid production by normotensive rats. Br J Pharmacol. 1993;108:327–330. doi: 10.1111/j.1476-5381.1993.tb12804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J, Trebicka J, Shiozawa T, Schepke M, Neef M, Hennenberg M, Sauerbruch T. Vascular, hemodynamic and renal effects of low-dose losartan in rats with secondary biliary cirrhosis. Liver Int. 2005;25:657–666. doi: 10.1111/j.1478-3231.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Heymes C, Swynghedauw B, Chevalier B (1994) Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation 90:1328–1333 [DOI] [PubMed]
- Heymes C, Silvestre JS, Liorens-Cortes C, Chevalier B, Marotte F, Levy BI, Swynghedauw B, Samuel JL (1998) Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology 139:2579–2587 [DOI] [PubMed]
- Holtz J, Studer R, Reinecke H, Just H, Drexler H. Modulation of myocardial sarcoplasmic reticulum Ca(++)-ATPase in cardiac hypertrophy by angiotensin converting enzyme? Basic Res Cardiol. 1992;87(Suppl 2):191–204. doi: 10.1007/978-3-642-72477-0_17. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin NH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, American college of cardiology Foundation; American Heart Association 2009 Focused update incorporated into the Acc/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2008;53:el-e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Ito N, Ohishi N, Yamamoto K, Tatara Y, Shiota A, Hayashi N, Komai N, Yanagitani Y, Rakugi H, Ogihara T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens. 2007;20:792–799. doi: 10.1016/j.amjhyper.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Ju H, Scammel-La Fleur T, Dixon TM. Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol. 1996;28:1119–1128. doi: 10.1006/jmcc.1996.0103. [DOI] [PubMed] [Google Scholar]
- Kakarla SF, Fannin JC, Keshavarzian S, Katta A, Paturi S, Nalabotu SK, Wu M, Rice FM, Manzoor K, Walker EM, Jr, Blough ER. Chronic acetaminophen attenuates age-associated increases in cardiac ROS and apoptosis in the Fischer Brown Norway rat. Basic Res Cardiol. 2010;105:535–544. doi: 10.1007/s00395-010-0094-3. [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman N, Enright PL, Cardiovascular Health Study Research Group Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/S0002-9149(00)01393-X. [DOI] [PubMed] [Google Scholar]
- Klebl BM, Ayoub AT, Pette D. Protein oxidation, tyrosine nitration, and inactivation of sarcoplasmic reticulum Ca2+−ATPase in low-frequency stimulated rabbit muscle. FEBS Lett. 1998;422:381–384. doi: 10.1016/S0014-5793(98)00053-2. [DOI] [PubMed] [Google Scholar]
- Knyushko TV, Sharov VS, Williams TD, Schöneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol. 2001;37:662–667. doi: 10.1016/S0735-1097(00)01129-3. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Linz W, Wiemer G, Gohlke P, Unger T, Schölkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- Little WC, Wesley-Farrington DJ, Hoyle J, Brucks S, Robertson S, Kitzman DW, Cheng CP. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:l548–l1550. doi: 10.1161/01.CIR.0000013847.07035.B9. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(l-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- Meredith PA, Ostergren J. From hypertension to heart failure—are there better primary prevention strategies? J Renin Angiotensin Aldosterone Syst. 2006;7:64–73. doi: 10.3317/jraas.2006.012. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Pontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Michel JB, Salzmann JL, Cerol ML, Dussaule JC, Azizi N, Corman B, Camilleri JP, Corvol P. Myocardial effect of converting enzyme inhibition in hypertensive and normotensive rats. Am J Med. 1988;84:12–21. doi: 10.1016/0002-9343(88)90200-8. [DOI] [PubMed] [Google Scholar]
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris FE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahldf B, LIFE Study Investigators Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- Reinicke H, Studer R, Zierhut W, Drexler H. Down regulation of sarcoplasmic reticulum (SR) Ca2+ ATPase mRNA by angiotensin II in adult rat cardiomyocytes. Circulation. 1995;92(8 Suppl 1):I283. [Google Scholar]
- Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, Villareal DT, Fontana L, Holloszy JO, Kovács SJ. The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function. Am J Physiol Heart Circ Physiol. 2008;294:H1174–H1182. doi: 10.1152/ajpheart.01236.2007. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.RES.73.3.413. [DOI] [PubMed] [Google Scholar]
- Saupe KW, Sobol SC, Koh SG, Apstein CS. Effects of AT1 receptor block begun late in life on normal cardiac aging in rats. J Cardiovasc Pharmacol. 2003;42:573–580. doi: 10.1097/00005344-200310000-00017. [DOI] [PubMed] [Google Scholar]
- Schorb W, Booz GW, Dostal DE, Conrad FM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.RES.72.6.1245. [DOI] [PubMed] [Google Scholar]
- Sen S. Regression of cardiac hypertrophy. Experimental animal model. Am J Med. 1983;75:87–93. doi: 10.1016/0002-9343(83)90124-9. [DOI] [PubMed] [Google Scholar]
- Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, Kimball TR. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011;54:722–730. doi: 10.1007/s00125-010-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Ren B, Elimban V, Tappia PS, Takeda N, Dhalla NS. Modification of sarcolemmal Na+−K+−ATPase and Na+/Ca2+ exchanger expression in heart failure by blockade of renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2005;288:H2637–H2646. doi: 10.1152/ajpheart.01304.2004. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/S0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP, Valsartan In Diastolic Dysfunction (VALIDD) Investigators Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, Lacourciere Y, Lee J, Seifu Y, Hilkert RJ, Rocha R, Pitt B, Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55:241–248. doi: 10.1161/HYPERTENSIONAHA.109.138529. [DOI] [PubMed] [Google Scholar]
- Stein M, Boulaksil M, Jansen JA, Herold E, Noorman N, Joles JA, van Veen TA, Houtman NJ, Engelen MA, Hauer RN, de Bakker JM, van Rijen HV. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol. 2010;299:H310–H321. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- Strawn WB, Gallagher PB, Tallant EA, Ganten D, Ferrario CM. Angiotensin II AT1-receptor blockade inhibits monocyte activation and adherence in transgenic (mRen2)27 rats. J Cardiovasc Pharmacol. 1999;33:341–351. doi: 10.1097/00005344-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Bedigian MP, Robinson AD, Weber KT. Tissue angiotensin II in the regulation of inflammatory and fibrogenic components of repair in the rat heart. J Lab Clin Med. 2004;143:41–51. doi: 10.1016/j.lab.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Ungvári Z, Gupte SA, Recchia FA, Bátkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo X, Dhalla NS. Modification of myosin protein and gene expression in failing hearts due to myocardial infarction by enalapril or losartan. Biochim Biophys Acta. 2004;1690:177–184. doi: 10.1016/j.bbadis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JG, Jr, Metzger DC, Kitzman DW, Wesley DJ, Little WC. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33:1567–1572. doi: 10.1016/S0735-1097(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillon JC, Lowery NH. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:l521–l1524. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+−cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;275:H2087–H2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]