Abstract
Human progeroid syndromes and premature aging mouse models present as segmental, accelerated aging because some tissues and not others are affected. Skeletal muscle is detrimentally changed by normal aging but whether it is an affected tissue in progeria has not been resolved. We hypothesized that mice which mimic Hutchinson–Gilford progeria syndrome would exhibit age-related alterations of skeletal muscle. Zmpste24−/− and Zmpste24+/+ littermates were assessed for skeletal muscle functions, histo-morphological characteristics, and ankle joint mechanics. Twenty-four-hour active time, ambulation, grip strength, and whole body tension were evaluated as markers of neuromuscular performance, each of which was at least 33% lower in Zmpste24−/− mice compared with littermates (p < 0.06). Contractile capacity of the posterior leg muscles were not affected in Zmpste24−/− mice, but muscles of the anterior leg were 30–90% weaker than those of Zmpste24+/+ mice (p < 0.01). Leg muscles were 32–47% smaller in the Zmpste24−/− mice and contained ~60% greater collagen relative to littermates (p < 0.01). Soleus and extensor digitorum longus muscles of Zmpste24−/− mice had excessive myonuclei and altered fiber size distributions but, otherwise, appeared normal. Ankle range of motion was 70% lower and plantar- and dorsiflexion passive torques were nearly 3-fold greater in Zmpste24−/− than Zmpste24+/+ mice (p ≤ 0.01). The combined factors of muscle atrophy, collagen accumulation, and perturbed joint mechanics likely contributed to poor neuromuscular performance and selective muscle weakness displayed by Zmpste24−/−mice. In summary, these characteristics are similar to those of aged mice indicating accelerated aging of skeletal muscle in progeria.
Keywords: Aging, Lamin, Range of motion, Sarcopenia, Strength
Introduction
Hutchinson–Gilford progeria syndrome (HGPS) is a rare and lethal childhood disease affecting approximately one in four million live births. HGPS is caused by a mutation of exon 11 in the LMNA gene, encoding for lamin A (De Sandre-Giovannoli et al. 2003; Eriksson et al. 2003). Lamin A is a structural protein that functions as a scaffold for the nucleus. Mutations in this protein result in structural and mechanical abnormalities of nuclei, accumulation of prelamin A (i.e., progerin) and early senescence and apoptosis of affected cells (Capell and Collins 2006; Lammerding et al. 2004). As a result, children with HGPS show symptoms of slow growth and premature aging, including alopecia, osteoporosis, vascular disease, and decreased joint mobility that are evident by the age of 3 years (Gordon et al. 2007; Hennekam 2006; Merideth et al. 2008). These children have a lifespan to mid-teenage years and commonly die of myocardial infarctions or stroke.
An incompletely understood feature of HGPS and other progeroid syndromes is that some but not all tissues are affected. Indeed, HGPS is referred to as a disease of segmental accelerated aging (Martin 1990). Halaschek-Wiener and Brooks-Wilson have nicely annotated common age-related diseases that are present and those that are absent in HGPS (Halaschek-Wiener and Brooks-Wilson 2007). The authors have proposed reasons for the presence and absence of those diseases based on the premise that tissues which require stem cells for continuous growth or repair degenerate in individuals with HGPS due to stem cell exhaustion. The age-related disease of skeletal muscle is sarcopenia and it has not been categorized as being present or absent in HGPS. Sarcopenia is characterized by muscle atrophy and weakness and is highly associated with functional impairment and immobility of the elderly (Janssen et al. 2002). Determining whether or not skeletal muscle is an affected tissue in HGPS is critical based not only on clinical importance but also on the idea that stem cells and nuclear abnormalities are fundamentally involved in the segmentation of affected tissues in HGPS.
Reasons to support the idea that skeletal muscle might not be affected in HGPS include the following. First, skeletal muscle is a post-mitotic tissue composed of multinucleated cells, collectively called muscle fibers. Thus, demands for abnormal nuclei to divide are minimal, and severely impaired nuclei could, theoretically, have compensation from neighboring nuclei to avoid apoptosis of the entire fiber. Second, part of age-induced sarcopenia is caused by denervation (Roos et al. 1997) and although peripheral neuropathy can be present in laminopathies such as Charcot–Marie–Tooth syndrome (Stewart et al. 2007), diseases of the nervous system are not common in HGPS (Halaschek-Wiener and Brooks-Wilson 2007). Third, even though muscle strength has not been documented for children with HGPS, one case study states that muscle biopsies from these children appear normal (Macnamara et al. 1970). Finally, Meredith and et al. report that “…our patients with HGPS were surprisingly active and mobile” and that muscle volume was appropriately proportioned to body size (Merideth et al. 2008). These factors are suggestive of skeletal muscle not being an affected tissue in HGPS.
Alternatively, there are also reasons to believe that skeletal muscle could be affected in HGPS. First and foremost, some mutations in the LMNA gene cause muscular dystrophy, for example Emery–Dreifuss and Limb–Girdle muscular dystrophies (Bonne et al. 1999; Muchir et al. 2000), with similar dystrophic pathologies evident in murine models (Grattan et al. 2005). Satellite cells, the stem-like cells in skeletal muscle that are important during growth and repair after injury, have been implicated in these muscle diseases (Frock et al. 2006). If satellite cells are affected in HGPS, the proposal of stem cell exhaustion is relevant to skeletal muscle in this disease as well. Second, it has been hypothesized that age-related muscle atrophy and weakness result from an impaired ability of muscle to repair itself following contraction-induced injuries (Faulkner et al. 1995). Here again, if HGPS satellite cells are not able to facilitate the subtle but continuous repair of muscle, premature aging would occur in a fashion similar to what is hypothesized in normal aging. Third, it has been suggested that the impaired nuclear integrity due to LMNA mutations are particularly problematic for tissues under high mechanical stress, such as skeletal muscle during contraction (Lammerding et al. 2004). Lastly, the case study of an HGPS patient which included analysis of a post-mortem skeletal muscle biopsy reported, “… the muscle was replaced by fibrous strands and showed wasting” (Franklyn 1976), and a review on the musculoskeletal system of HGPS patients states that muscle atrophy is present (Hamer et al. 1988). Together these points indicate that skeletal muscle and its major function, contraction and production of force, could be detrimentally affected in HGPS.
Several mouse models have been developed to study accelerated aging. One such mouse model has a deletion of zinc metalloproteinase (Zmpste24) required for processing of prelamin A to lamin A. Thus, this mouse accumulates prelamin A and has many features common to HGPS (Bergo et al. 2002; Leung et al. 2001). Like HGPS, these mice have not been critically evaluated for skeletal muscle involvement. One report states that deltoid and quadriceps muscles of Zmpste24−/− mice contained abnormally small round, dystrophic fibers (Pendas et al. 2002), while another states that no fiber abnormalities could be identified in skeletal muscles throughout the limb and trunk of Zmpste24−/− mice (Bergo et al. 2002). Contractile function of skeletal muscle has not been investigated in these mice but there are indications that it might be perturbed. For example, by 8 weeks of age there is a significant inability of Zmpste24−/− mice to hang from a grid and the mice develop a slow, hobbling gait and dragging of the hindlimbs (Bergo et al. 2002).
The goal of this project was to determine the extent to which skeletal muscles of Zmpste24−/− mice were affected. We hypothesized that hindlimb muscles of Zmpste24−/− mice would display some key characteristics of age-induced sarcopenia including muscle atrophy and weakness which would be associated with decrements in physical activity and performance. Arthropathy is another common feature in progeria (Hamer et al. 1988), and if present in the Zmpste24−/− mice could contribute to poor neuromuscular performances. Therefore, we also investigated alterations in ankle joint mechanics of Zmpste24−/− mice.
Materials and methods
Animals
Mice were bred from a colony at the University of Minnesota. Breeder pairs originated from the laboratory of Dr. Stephen Young (Leung et al. 2001). All mice were genotyped by PCR analysis of DNA isolated from a tail snip, with previously detailed primers (Fong et al. 2004). Mixed gender Zmpste24+/+ and Zmpste24−/− mice (n = 25 each) were used for this study. Mice were group housed by gender on a 12-h light–dark cycle with chow and water provided ad libitum. Body weights were recorded weekly, starting at 4 weeks of age. During the study, nine Zmpste24−/− mice died; data from these mice were not included. All protocols and animal care procedures were approved by the University of Minnesota Institutional Animal Care.
Study design
Mice were assessed every 2.5 to 5 weeks for physical activity and ankle joint mechanics beginning at 5 weeks of age until 20 weeks of age. Performance via grip strength and whole body tension was assessed at 15 or 20 weeks of age. Also at 15 or 20 weeks of age, soleus and extensor digitorum longus (EDL) muscles were dissected and analyzed ex vivo for contractile functions. A subset of 20-week-old mice also underwent in vivo contractile testing of the left leg. Following the contractility analyses, hindlimb muscles and strips of diaphragm muscle were weighed, snap frozen in liquid nitrogen, and stored at −80°C or were frozen in optimal cutting temperature medium for histological analyses. While still under anesthesia, mice were euthanized by exsanguination.
Physical activity
Twenty-four-hour cage activity was assessed using open field activity chambers (Med Associates Inc., St. Albans, VT) (Greising et al. 2011). Briefly, chambers contain infrared arrays in the x-, y-, and z-axes with two sets of beams in the x-direction, one being elevated to 2.5 or 5.4 cm above the bottom of the cage for Zmpste24−/− and Zmpste24+/+ mice, respectively (to account for their body size differences). Activity Monitor version 6.01 software was used to collect data with a box size set to “2” (3.2 cm2) which is used to calculate movement, delay, and filter the data. Activity measurements included distance traveled, jumping, rearing and stereotypic counts, and total active time during a 24-h period.
Performance testing
Performance was assessed via grip strength and whole body tension (Call et al. 2010). For each trial of grip strength, mice grasped a bar that was attached to a force transducer (Grip Strength Meter; Columbus Instruments, Columbus, OH). For whole body tension, mice were attached to a force transducer (BIOPAC Systems, Inc., Goleta, CA) by suture attached to their tail and were then induced to move forward by tail pinches over a 90-s period. Performance was calculated for each test by averaging the top 5 peak forces recorded. Both tests were conducted by the same investigator and data are reported as force normalized to body mass.
Ankle joint mechanics
Mice were anesthetized with a cocktail of fentanyl citrate (10 mg/kg), droperidol (0.2 mg/kg), and diazepam (5 mg/kg). Passive torque about the left ankle joint was assessed by stabilizing the knee and placing the foot in a plate attached to a servomotor (300B-LR, Aurora Scientific, Aurora, Ontario, Canada) (Baltgalvis et al. 2009; Garlich et al. 2010). The servomotor rotated the ankle to four angles of dorsiflexion (5°, 10°, 15°, and 20°) and four angles of plantarflexion (5°, 10°, 15°, and 20°) and torque was measured at each angle. Range of motion (ROM) about the left ankle joint was measured while the mouse was stabilized in a position in which the hip and knee were both at 90o of flexion (Garlich et al. 2010). The ankle joint was moved into end-range dorsi- and plantarflexion, and images were acquired at those positions and then analyzed using ImageJ software (Frederick, MD) (Garlich et al. 2010).
Skeletal muscle contractility in vivo
At 20 weeks of age, immediately following testing of joint mechanics and while still anesthetized, mice underwent in vivo contractile testing of the left leg (Baltgalvis et al. 2009; Garlich et al. 2010). First, maximal isometric torque of the anterior leg muscle group was measured by subdermal stimulation of the common peroneal nerve. Second, maximal isometric torque of the posterior leg muscle group (soleus, plantaris, and gastrocnemius muscles) was measured by stimulation of the sciatic nerve, with the common peroneal nerve severed. Stimulation was 200-ms total duration consisting of 0.5-ms square-wave pulses at 300 Hz (models S48 and SIU5, respectively, Grass Technologies, West Warwick, RI). The voltage was adjusted from 3.0 to 9.0 V until maximal isometric torques were achieved on the 300B-LR servomotor.
Skeletal muscle contractility ex vivo
A subset of mice at 15 and 20 weeks of age underwent testing of isolated soleus and EDL muscles (Moran et al. 2005; Moran et al. 2006). Contractile characteristics tested included; peak twitch force (Pt), time to peak twitch, one half twitch relaxation time (1/2 RT), maximal isometric tetanic force (Po), rates of force development and relaxation (+dP/dt and −dP/dt), and active and passive stiffness.
Serum creatine kinase activity
Blood was collected from anesthetized mice at 20 weeks of age from the retrooribital sinus. Blood was allowed to clot and serum was separated and stored at −80°C until analysis. Creatine kinase activity was analyzed on Vitros CK/CPK slides (Ortho Clinical Vitros DT60 II Dry Slides) and run on a spectrophotometer (Ortho Clinical Vitros DT II System). Creatine kinase activity is reported as units/liter (U/L).
Histo-morphological analyses
Serial sections from the mid-belly of muscles frozen in OCT compound were cut to 10-μm thickness. Cross-sectional area of ~200 fibers per soleus, and EDL muscle were measured from hematoxylin and eosin-stained sections (Landisch et al. 2008). Capillarity of those muscles were assessed by the periodic acid-Schiff reaction (Josephson 1993). The next three sections of each muscle were analyzed for fiber type via myosin heavy chain isoform I, IIa, or IIb (Landisch et al. 2008). If a fiber did not react to either the myosin heavy chain I, IIa, or IIb antibody, it was classified as a IIx fiber. The final serial sections were used to assess the abundance of nuclei in relationship to the ~200 fibers analyzed. For this, nuclei were labeled using DAPI and a dystrophin antibody (1:20 dilution of NCL-DYS1; Novocastra Laboratories, Newcastle upon Tyne) was used to define the sarcolemma; nuclei were counted as myonuclei per fiber, central nuclei per fiber, or extracellular-related nuclei per total area (Landisch et al. 2008; Moran et al. 2005). Care was taken not to count nuclei if they appeared to be in a vessel. A single investigator who was blinded to genotype conducted each type of histological analysis. Additional 6-μm EDL and soleus muscle sections were examined for the localization of emerin at the nuclear rim using antibodies for emerin (1:25 dilution of ab14208; Abcam, Cambridge MA), dystrophin (1:20 dilution of NCL-DYS1; Novocastra Laboratories, Newcastle upon Tyne), and DAPI.
Hydroxyproline content of frozen EDL, tibialis anterior, soleus, and gastrocnemius muscles were measured biochemically to asses total collagen content (Garlich et al. 2010; Reddy and Enwemeka 1996). Western blot analyses for lamin A/C and prelamin A were carried out on EDL and soleus muscles to assess accumulation of prelamin A. Each frozen muscle was homogenized on ice in 25 mM Tris HCL, 150 mM NaCl, 1% igepal CA-630, 1% sodium deoxycholate, and 0.1% SDS; 5 μL of benzonase nuclease was added to each sample. Approximately, 0.5 μg of protein from each sample was loaded on a 4–12% Bis-Tris gel. Following electrophoresis, proteins were transferred to nitrocellulose membranes and probed using 1:500 dilutions of a Lamin A/C antibody (sc-6215; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and Histone H3 antibody (ab61251; Abcam, Cambridge, MA). Blots were analyzed for the ratio of prelamin A to histone H3 and comparisons made between EDL and soleus muscles of Zmpste24−/− mice.
Statistical analysis
Zmpste24+/+ mice at 15 and 20 weeks of age were not significantly different as determined by Student’s t tests (p ≥ 0.088) on any measured parameter and therefore these data were collapsed to represent a single control group. Likewise, Zmpste24−/− mice were not significantly different at 15 and 20 weeks of age (p ≥ 0.087), and these data were collapsed. Data were analyzed by Student’s t test to determine differences between genotypes for in vivo joint mechanics, and ex vivo contractility measurements, creatine kinase activity, muscle masses, collagen content, and histological analyses. A Student’s t test was also used to compare prelamin A accumulation between EDL and soleus muscles. Chi-square tests were used to analyze the frequency distribution of EDL and soleus muscle fiber cross-sectional area. Longitudinal data were analyzed by two-way repeated measures ANOVA to determine differences between genotypes over time for body mass, physical activity, ROM, and passive torque. Statistical analyses were conducted using SigmaStat version 3.5 (Systat Software Inc; Point Richmond, CA). Significance was accepted at the α < 0.05 level. Data are presented as mean ± standard error.
Results
Body mass and neuromuscular performance
Body mass
Zmpste24−/− mice had halted growth by 5 weeks of age and had lower body mass relative to Zmpste24+/+ littermates through 20 weeks of age. At 20 weeks of age, the difference in body mass between Zmpste24−/− and Zmpste24+/+ littermates was >50% (12.9 ± 0.7 g vs. 28.1 ± 1.2 g; p < 0.001).
Physical activity
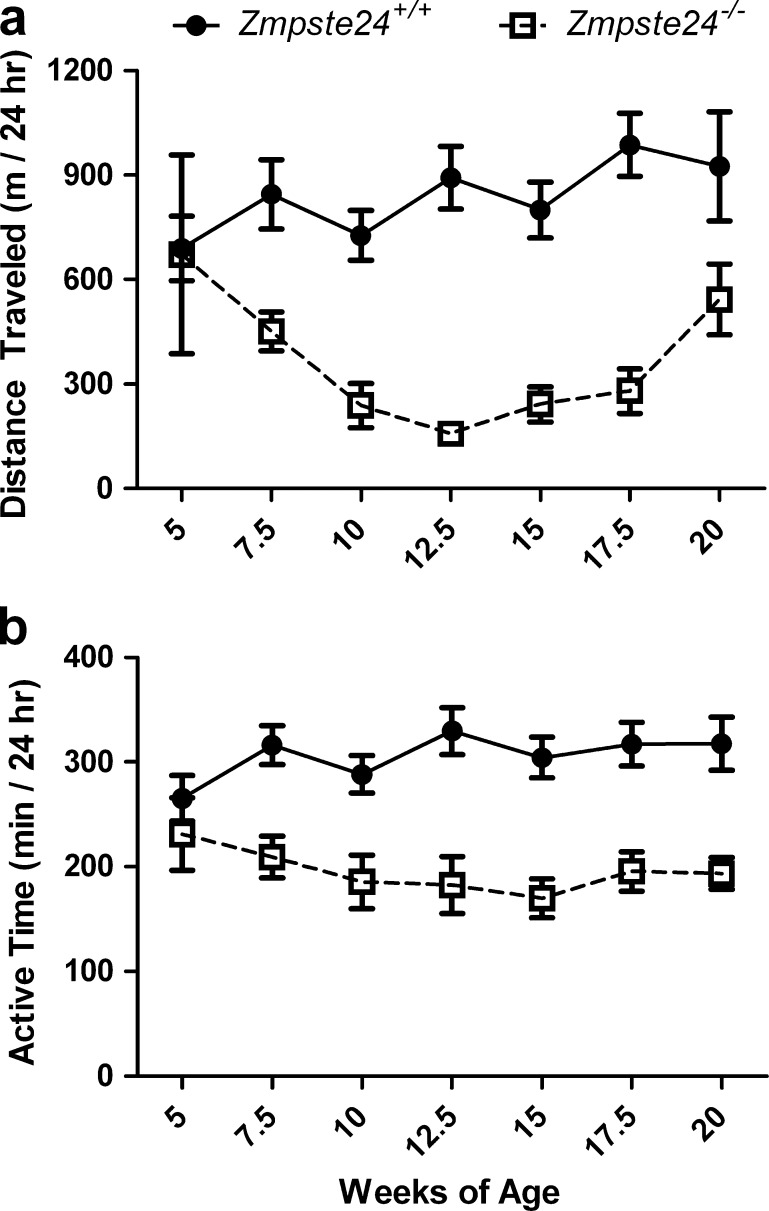
Zmpste24−/− mice were less active than were their Zmpste24+/+ littermates (p < 0.001; Fig. 1). Over the 15-week study period, Zmpste24−/− mice averaged 53% less distance traveled and spent ~37% less time being active, the latter equating to almost a 2-h difference per day. Jumping and stereotypic activities were also ~70% less in the Zmpste24−/− mice (p ≤ 0.011). There was no difference in hindlimb rearing between the groups (p = 0.231).
Fig. 1.
Daily cage activities of Zmpste24+/+ and Zmpste24−/− mice from 5 to 20 weeks of age. a Ambulation distance traveled in the cage per 24 h. b Time spent being active in the cage per 24 h. Data are presented as means ± SE; n = 3 to 13 per genotype per time point
Grip strength and whole body tension
Grip strength was impaired in the Zmpste24−/− mice (2.4 ± 0.4 g/g body mass vs. 3.6 ± 0.3 g/g for Zmpste24+/+ littermates; p = 0.038). Notably, most Zmpste24−/− mice had difficulty grasping the bar to perform the grip strength test which likely confounded these results. Whole body tension, corrected for the smaller size of Zmpste24−/− mice, showed a trend for these mice to have poor performance as Zmpste24−/− mice pulled 7.3 ± 0.9 g/g body mass vs. 9.9 ± 1.0 g/g for Zmpste24+/+ mice (p = 0.056).
Ankle joint mechanics
Range of motion
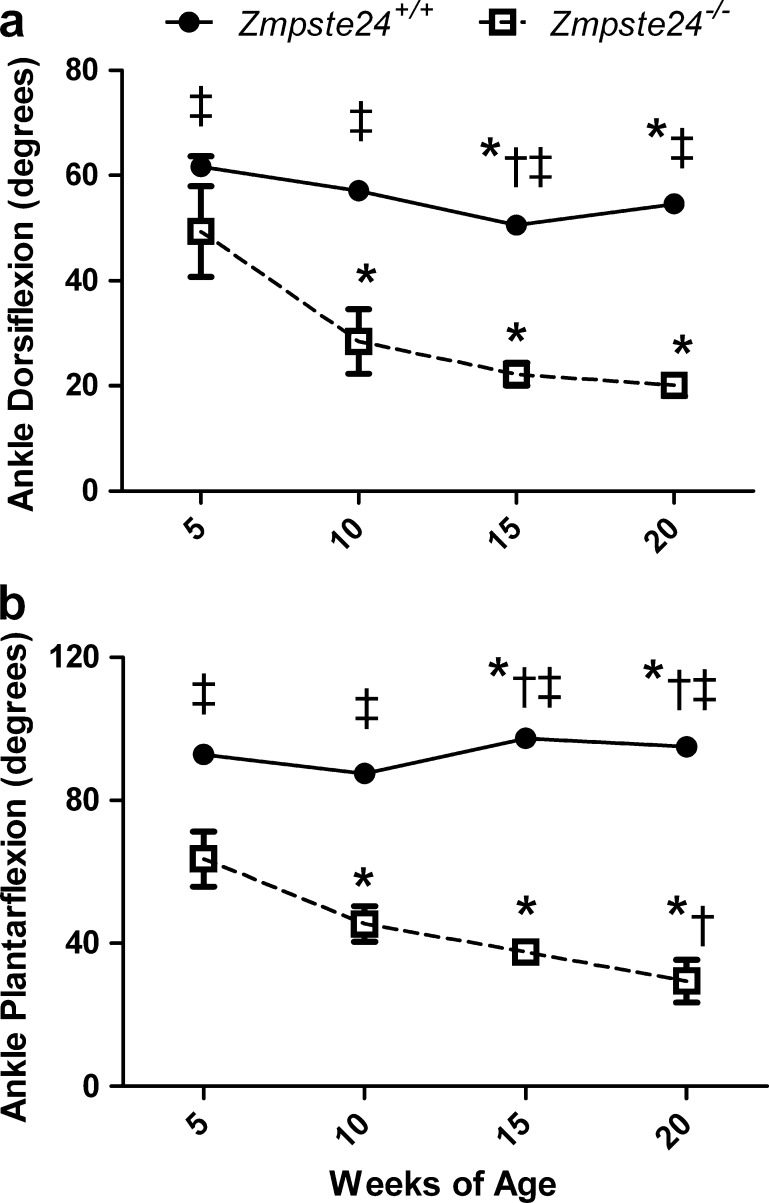
Ankle joint ROM changed with age, and did so differently between Zmpste24−/− and Zmpste24+/+ mice for both dorsi- and plantarflexion (interactions p < 0.001). At each age tested, Zmpste24−/− mice had 20–70% less dorsi- and plantarflexion than Zmpste24+/+ littermates (Fig. 2). Zmpste24−/− mice had progressively decreasing dorsiflexion ROM as they aged, with those at 10, 15, and 20 weeks being less than at 5 weeks of age (Fig. 2). Plantarflexion ROM also decreased with age in the Zmpste24−/− mice, as opposed to a slight increase with age in the Zmpste24+/+ mice. Overall, at 20 weeks of age, Zmpste24−/− mice were functioning with 71% less ROM about the ankle than were Zmpste24+/+ littermates (44 ± 4o vs. 150 ± 2o; p < 0.001).
Fig. 2.
Range of motion about the ankle joint in Zmpste24+/+ and Zmpste24−/− mice from 5 to 20 weeks of age. Data are presented as means ± SE; n = 3 to 13 per genotype per time point. For many data points, the error bars are within the symbols representing the means. Asterisk, within genotype, significantly different than 5 weeks of age. Dagger, within genotype, significantly different than 10 weeks of age. Double dagger, significantly different than Zmpste24−/− mice at corresponding age
Passive torque
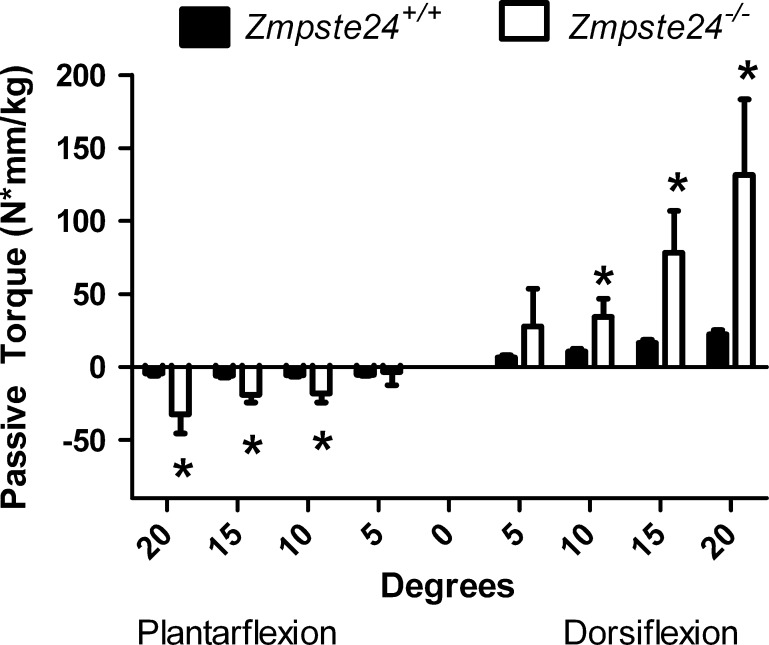
Passive torque about the ankle represents the force applied to the foot that is required to rotate the ankle when the lower limb muscles are inactive. Differences at some degrees of dorsi- and plantarflexion were significant between Zmpste24−/− and Zmpste24+/+ mice at the ages of 5, 10, and 15 weeks (data not shown), but the greatest differences were detected at 20 weeks of age at which time Zmpste24−/− mice had greater passive torques at 10, 15, and 20o of both plantar- and dorsiflexion compared with Zmpste24+/+ littermates (p ≤ 0.004; Fig. 3). These exceptionally high passive torques, particularly those required to move the foot into dorsiflexion, exemplify a stiff joint.
Fig. 3.
Passive torque about the ankle joint in Zmpste24+/+ and Zmpste24−/− mice aged 20 weeks. Data are normalized to body mass and presented as means ± SE; n = 3 to 13 per genotype
Skeletal muscle contractility
In vivo muscle strength
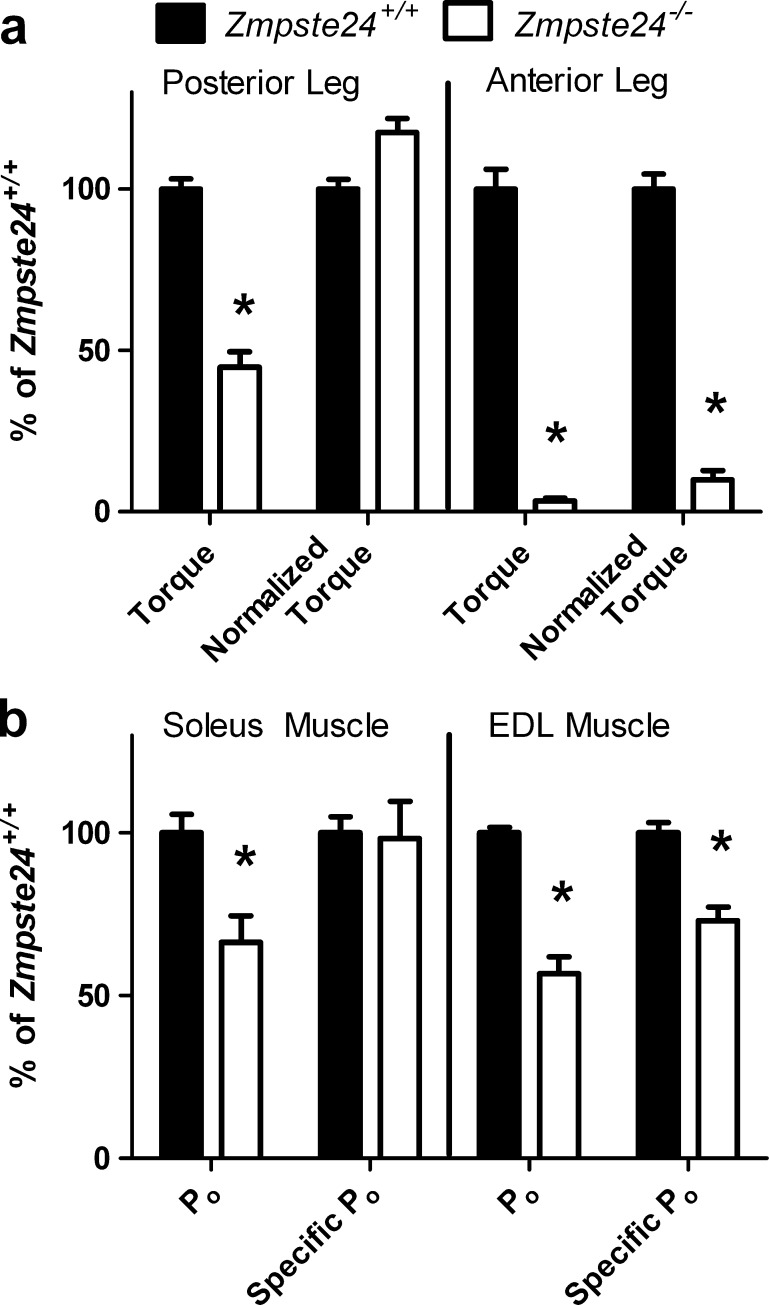
The posterior leg muscles (soleus, plantaris, and gastrocnemius muscles) of Zmpste24−/− mice were weaker than those of Zmpste24+/+ mice (p < 0.001; Fig. 4a, left; 6.6 ± 0.7 vs. 14.6 ± 0.5 N · mm of torque, respectively). However, when torque was normalized to the small posterior muscle masses of the Zmpste24−/− mice, strength was not lower than that of Zmpste24+/+ mice (122.7 ± 4.5 vs. 104.3 ± 3.3 kN · mm−1 kg−1 muscle, respectively; p = 0.091).
Fig. 4.
Force-generating capacities of leg muscles from Zmpste24+/+and Zmpste24−/− mice from 15 to 20 weeks of age. a Maximal isometric torque of muscle groups of the leg. b Maximal isometric force (Po) of isolated leg muscles. Normalized torque is maximal torque relative to the sum of respective muscle masses. Specific Po is maximal isometric tetanic force relative to physiological cross-sectional area of the muscle. Data are shown relative to Zmpste24+/+mice and presented as means ± SE; n = 3 to 14 per genotype. Asterisk, significantly different than Zmpste24+/+mice
Strength of the anterior leg muscles (extensor hallucis longus, EDL and tibialis anterior muscles) of Zmpste24−/− mice was more severely affected than was strength of the posterior leg muscles (Fig. 4a, right). Maximal torque production of the anterior leg muscles of Zmpste24−/− mice averaged 0.1 ± 0.02 vs. 6.5 ± 0.2 N · mm for Zmpste24+/+ mice (p < 0.001). This difference could not be explained by smaller muscles because torque normalized to the mass of the anterior muscles was still extremely low in the Zmpste24−/− mice (5.0 ± 1.5 vs. 50.5 ± 2.3 kN · mm−1 kg−1 muscle for Zmpste24+/+ mice; p < 0.001).
Ex vivo muscle strength
The absolute maximal Po generated by isolated soleus muscles from Zmpste24−/− mice was 34% less than that from than Zmpste24+/+ littermates (109.7 ± 13.4 vs. 165.4 ± 9.4 mN, respectively; p = 0.002; Fig. 4b, left). However, when normalized to cross-sectional area of the muscles (specific Po) there was no difference between groups with an overall average 13.0 ± 0.7 N/cm2 of force (p = 0.879; Fig. 4). On the contrary, both Po and specific Po of EDL muscles were ~40% lower in Zmpste24−/− than Zmpste24+/+ mice (p < 0.001; Fig. 4b, right; 203.6 ± 18.7 vs. 359.1 ± 6.1 mN and 14.4 ± 0.9 vs. 19.8 ± 0.6 N/cm2, respectively). These results follow the same pattern as the in vivo strength analyses, that is, the muscles on the anterior leg (tibialis anterior and EDL muscles) were much more severely affected than were the opposing posterior leg muscles (gastrocnemius and soleus muscles).
Additional contractile characteristics of soleus and EDL muscles further demonstrated the impact of accelerated aging on skeletal muscle. Peak twitch force and active stiffness are parameters related to force generation and EDL but not soleus muscles from the Zmpste24−/− mice showed weakness (Table 1), consistent with the Po results. The muscles’ ability to relax after contraction was consistently diminished in Zmpste24−/− mice as indicated by longer twitch one-half relaxation times and slower tetanic rates of relaxation in both soleus and EDL muscles relative to those of Zmpste24+/+ mice (Table 1). Passive stiffness, indicative of muscles’ elastic properties and resistance to lengthening while not contracting, was also high in both soleus and EDL muscles of Zmpste24−/− mice.
Table 1.
Ex vivo contractile properties of soleus and EDL muscles of Zmpste24+/+ and Zmpste24−/− mice
| Zmpste24+/+ | Zmpste24−/− | p value | |
|---|---|---|---|
| Soleus muscle (n) | (13) | (10) | |
| Pt (mN) | 26.4 ± 1.5 | 24.7 ± 2.7 | 0.507 |
| TPT (ms) | 35.0 ± 1.0 | 43.1 ± 3.8 | 0.026 |
| 1/2 RT (ms) | 4.0 ± 0.1 | 5.7 ± 0.8 | 0.022 |
| +dP/dt (N/s) | 1.7 ± 0.1 | 1.5 ± 0.2 | 0.510 |
| −dP/dt (N/s) | 3.3 ± 0.3 | 1.6 ± 0.2 | 0.002 |
| Active stiffness (N/m) | 269.3 ± 15.1 | 241.6 ± 22.7 | 0.301 |
| Passive stiffness (N/m) | 11.5 ± 0.4 | 16.5 ± 1.4 | <0.001 |
| EDL muscle (n) | (11) | (11) | |
| Pt (mN) | 69.5 ± 2.1 | 35.4 ± 2.6 | <0.001 |
| TPT (ms) | 19.3 ± 0.3 | 18.2 ± 1.5 | 0.475 |
| 1/2 RT (ms) | 1.8 ± 0.1 | 2.2 ± 0.1 | <0.001 |
| +dP/dt (N/s) | 7.3 ± 0.2 | 4.2 ± 0.4 | <0.001 |
| −dP/dt (N/s) | 21.4 ± 0.5 | 7.9 ± 1.3 | <0.001 |
| Active stiffness (N/m) | 433.6 ± 14.2 | 299.3 ± 22.2 | <0.001 |
| Passive stiffness (N/m) | 12.2 ± 0.5 | 18.2 ± 1.0 | <0.001 |
Mean ± SE
Pt peak twitch force, TPT time to peak twitch force, 1/2 RT one half relaxation time, +dP/dt maximal rate of tectanic force development, −dP/dt maximal rate of relaxation
Serum creatine kinase activity
Creatine kinase in the serum is a common marker of skeletal muscle injury and high creatine kinase values are regularly used as a diagnostic tool for muscular dystrophies. There was not a significant difference in serum creatine kinase activity between 20-week-old Zmpste24−/− and Zmpste24+/+ littermates (3,404 ± 761 vs. 2,003 ± 415 UL, respectively; p = 0.150).
Skeletal muscle histo-morphometry
Muscle masses
All leg muscles of Zmpste24−/− mice were smaller than those of Zmpste24+/+ littermates, weighing on average 42% less (p < 0.001). When the substantial difference in body masses were accounted for by normalizing muscle mass to body mass, the tibialis anterior, soleus, and gastrocnemius muscles were not different between groups (Table 2). EDL muscle to body mass was relatively greater in Zmpste24−/− mice (Table 2).
Table 2.
Masses and content of leg muscles of Zmpste24+/+ and Zmpste24−/− mice
| Zmpste24+/+ | Zmpste24−/− | p value | |
|---|---|---|---|
| Muscle mass (mg/g) (n) | (25) | (16) | |
| Extensor digitorum longus | 0.38 ± 0.02 | 0.47 ± 0.02 | 0.003 |
| Tibialis anterior | 1.74 ± 0.09 | 1.82 ± 0.13 | 0.588 |
| Soleus | 0.33 ± 0.03 | 0.36 ± 0.01 | 0.322 |
| Gastrocnemius | 5.25 ± 0.28 | 5.15 ± 0.13 | 0.772 |
| Collagen content (μg/mg) (n) | (8–13) | (3–13) | |
| Extensor digitorum longus | 10.7 ± 2.3 | 17.2 ± 2.0 | 0.005 |
| Tibialis anterior | 17.4 ± 0.5 | 27.4 ± 2.5 | <0.001 |
| Soleus | 17.8 ± 3.2 | 30.6 ± 2.5 | <0.001 |
| Gastrocnemius | 19.2 ± 2.3 | 27.8 ± 1.2 | <0.001 |
Mean ± SE. Muscle masses normalized to body mass. Collagen contents normalized to muscle masses
Collagen content
Collagen content of the leg muscles was measured because passive stiffness of both EDL and soleus muscles was high in Zmpste24−/− mice. Total collagen content of the EDL, tibialis anterior, soleus, and gastrocnemius muscles was 45–72% greater in Zmpste24−/− compared with Zmpste24+/+ mice (Table 2).
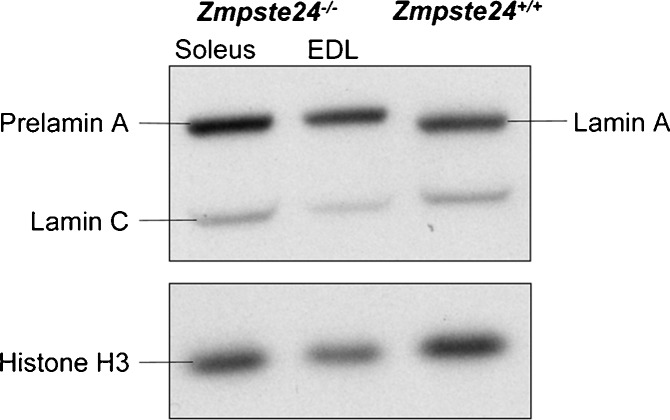
Prelamin A content
The content of prelamin A was measured in soleus and EDL muscles to determine if the contractile differences between those muscles in the Zmpste24−/− mice were associated with the accumulation of prelamin A (Fig. 5). There was no difference in the ratio of prelamin A to histone H3 between soleus and EDL muscles of Zmpste24−/− mice (0.85 ± 0.2 vs. 0.91 ± 0.1, respectively; p = 0.804).
Fig. 5.
Representative blots of prelamin A expression (~73 kDa), relative to histone H3 (15 kDa), in soleus and EDL muscles of Zmpste24−/− mice. Prelamin A is only distinguishable in the Zmpste24−/− muscles, while Zmpste24+/+ muscles have lamin A (69 kDa) and lamin C (62 kDa). The complete analysis was conducted on 12 independent samples; six soleus and six EDL muscles, half from Zmpste24−/− and half from Zmpste24+/+ mice
Histological characteristics of soleus muscles
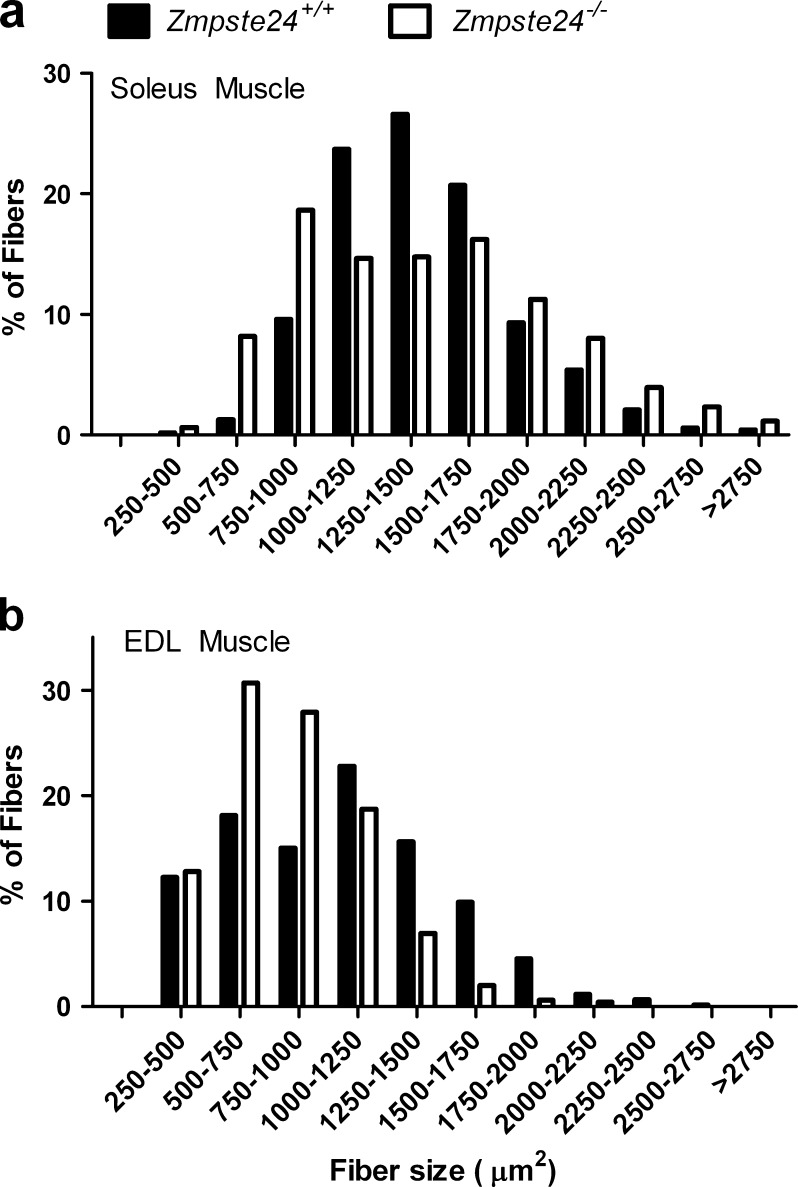
The mean cross-sectional area of all soleus muscle fibers was not different between Zmpste24−/− and Zmpste24+/+ mice (1,431 ± 74 vs. 1,418 ± 156 μm2, respectively; p = 0.946). Despite this, there was a significantly different distribution of fiber sizes with soleus muscles from Zmpste24−/− mice having a broadened distribution with more small and large fibers compared with those of Zmpste24+/+ littermates (p < 0.001; Fig. 6a). Soleus muscles from Zmpste24−/− mice contained the same percentages of myosin heavy chain type I, IIa, and IIx fibers as those from Zmpste24+/+ littermates (Table 3). Capillarity of soleus muscle was not affected by the lack of Zmpste24 (Table 3). Muscles were analyzed for nuclear content because on initial observations of hematoxylin and eosin-stained sections, there were noticeably more nuclei (e.g., Fig. 7). There were no differences between Zmpste24−/− and Zmpste24+/+ mice in the percent of soleus muscle fibers with centrally located nuclei or nuclei in the extracellular space, but there were significantly more myonuclei in soleus muscles of Zmpste24−/− mice. Other than the abundance of myonuclei, there were no other obvious signs of histopathology in soleus muscles of these mice (Fig. 7).
Fig. 6.
Fiber cross-sectional area distribution of soleus (a) and EDL (b) muscles from Zmpste24+/+and Zmpste24−/− mice aged 20 weeks. Data are presented as means; n = 4 to 6 per genotype per muscle
Table 3.
Histological characteristics of soleus and EDL muscles of Zmpste24+/+ and Zmpste24−/− mice
| Zmpste24+/+ | Zmpste24−/− | p value | |
|---|---|---|---|
| Soleus muscle (n) | (5) | (5) | |
| % Type I | 38.3 ± 2.6 | 33.7 ± 2.1 | 0.203 |
| % Type IIa | 59.3 ± 3.0 | 63.4 ± 2.0 | 0.277 |
| % Type IIx | 2.2 ± 0.6 | 3.1 ± 1.4 | 0.559 |
| Capillaries per fiber | 3.3 ± 0.1 | 3.4 ± 0.1 | 0.775 |
| % Central nuclei | 0.72 ± 0.4 | 0.76 ± 0.5 | 0.961 |
| Myonuclei per fiber | 2.7 ± 0.2 | 3.3 ± 0.2 | 0.023 |
| Extracellular nuclei per area (mm2) | 2.1 ± 0.5 | 2.1 ± 0.4 | 0.889 |
| EDL muscle (n) | (4) | (5) | |
| % Type I | 2.0 ± 0.5 | 4.1 ± 0.8 | 0.079 |
| % Type IIa | 12.3 ± 1.8 | 22.2 ± 1.8 | 0.007 |
| % Type IIx | 23.0 ± 2.2 | 25.8 ± 2.8 | 0.467 |
| % Type IIb | 62.5 ± 3.3 | 48.0 ± 2.9 | 0.013 |
| Capillaries per fiber | 2.2 ± 0.3 | 2.2 ± 0.2 | 0.683 |
| % Central nuclei | 1.3 ± 0.2 | 2.3 ± 0.4 | 0.078 |
| Myonuclei per fiber | 1.9 ± 0.2 | 2.4 ± 0.1 | 0.004 |
| Extracellular nuclei per area (mm2) | 4.7 ± 2.1 | 6.2 ± 3.2 | 0.511 |
Mean ± SE. Fiber types based on myosin heavy chain immunohistochemistry and expressed as percentages of total number of fibers. No soleus muscle fibers expressed myosin heavy chain IIb.% Central nuclei, percent of fibers that had central nuclei
Fig. 7.
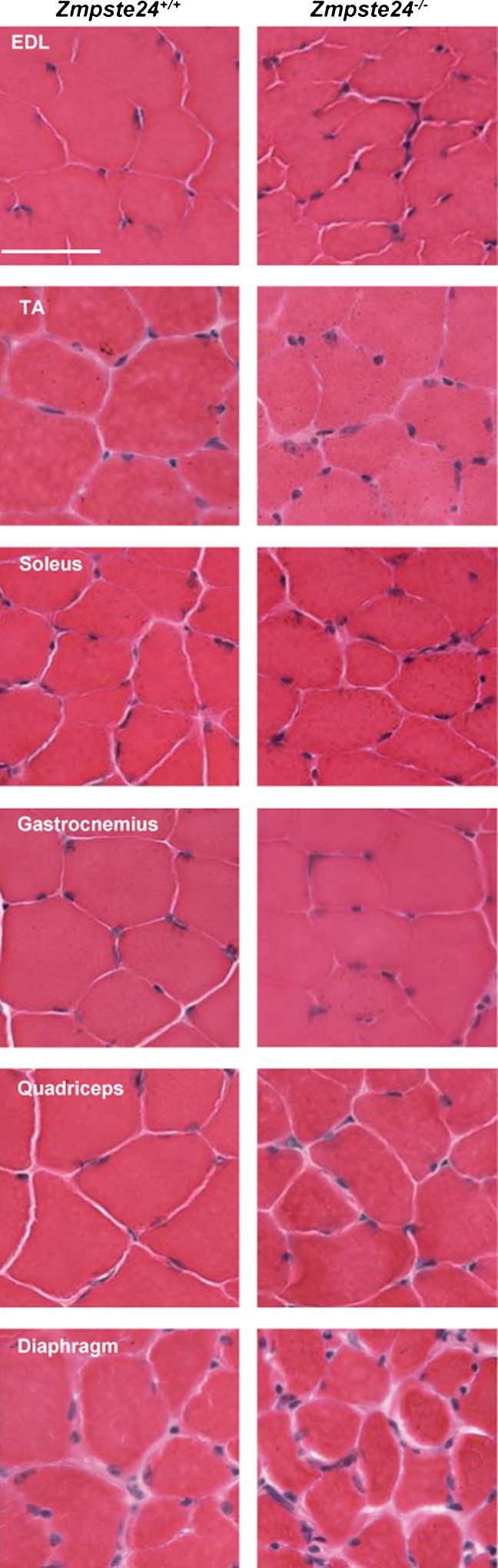
Representative cross-sections of hematoxylin and eosin-stained EDL, tibialis anterior (TA), soleus, gastrocnemius, quadriceps, and diaphragm muscles from Zmpste24+/+and Zmpste24−/− mice. Scale bar is 50 μm
Histological characteristics of EDL muscles
Relative to the minor histological differences between soleus muscles of Zmpste24−/− and Zmpste24+/+ mice, EDL muscles were slightly more affected. EDL muscle fibers of Zmpste24−/− mice were small relative to those of littermates as illustrated by the significant leftward shift in the distribution of fiber cross-sectional areas (p < 0.001; Fig. 6b) and a trend for the overall mean fiber cross-sectional area to be less (837 ± 38 vs. 1,004 ± 99 μm2 for Zmpste24−/− and Zmpste24+/+ mice, respectively; p = 0.071). There was a difference in fiber-type composition in EDL muscles with those from Zmpste24−/− mice having relatively more Type IIa and less Type IIb fibers (Table 3), indicating a fiber-type shift toward a myosin heavy chain isoform that is typically associated with fast-twitch kinetics and both oxidative and glycolytic energetics. Similar to soleus muscles, there were no differences between mice in EDL muscle capillarity, central nuclei, or nuclei in the extracellular space, but EDL muscle fibers of Zmpste24−/− mice contained significantly more myonuclei than those of Zmpste24+/+ mice (Table 3). Aside from the myonuclei, no other histological differences were observed (Fig. 7).
Additional histological characteristics
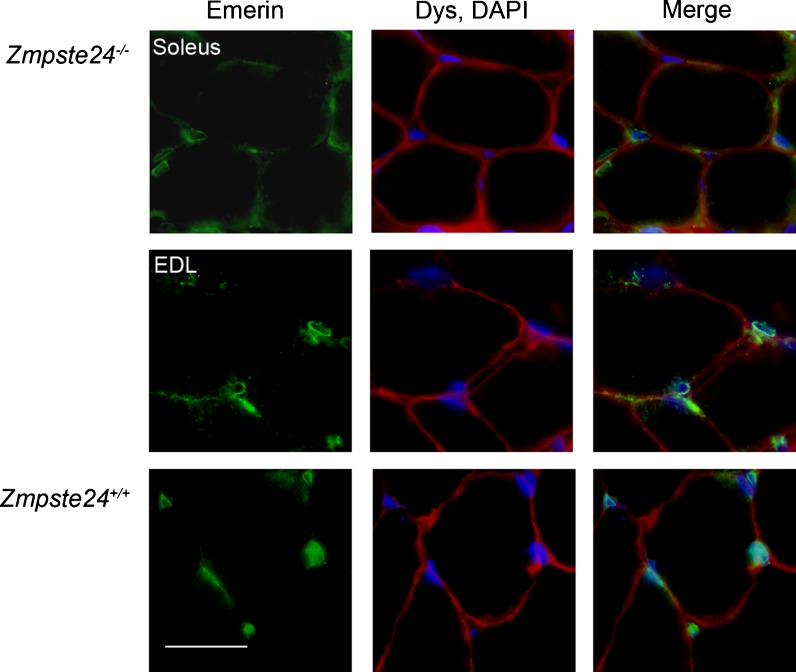
Tibialis anterior, gastrocnemius, quadriceps, and diaphragm muscles from a subset of mice were subjectively evaluated using H&E staining. Soleus and EDL muscles were also evaluated for the localization of emerin to the nuclear rim. All muscles of Zmpste24−/− mice that were evaluated appeared normal and healthy. For example, there were no signs of dystrophic features such as central nuclei, degenerating fibers, fibrosis, or mislocalization of emerin (see Figs. 7 and 8). Both Zmpste24−/− and Zmpste24+/+ mice had emerin present at the nuclei (Fig. 8).
Fig. 8.
Representative cross-sections of immunofluorescently stained EDL and soleus muscles from Zmpste24+/+and Zmpste24−/− mice. Muscles were stained with antibodies against emerin (green), dystrophin (Dys, red), and DAPI (blue). Scale bar is 25 μm
Discussion
Using a variety of tests for assessing the function of skeletal muscle, we show that Zmpste24−/− mice have impaired neuromuscular performance and selective muscle weakness relative to their Zmpste24+/+ littermates. These physiological characteristics are similar to those of aged mice and indicate that some characteristics of sarcopenia are present in this progeroid mouse model. In mice that are aged normally and in the Zmpste24−/− mouse model of accelerated aging, force generation as well as rates of muscle contraction are impaired (Brooks and Faulkner 1988; Moran et al. 2005), as are parameters of neuromuscular performance (Carlson et al. 2010; Ingram 2000). Collectively, these results suggest that skeletal muscle is an affected tissue in HGPS.
This is the first report of perturbations in skeletal muscle contractility in a mouse model of HGPS. Because the primary function of skeletal muscle is to contract and generate force, direct measures of contractility are required to substantiate the involvement of skeletal muscle in progeria. We found that force generation by leg muscles was 30–95% lower in 15- and 20-week-old Zmpste24−/− than Zmpste24+/+ mice indicating a general muscle weakness. The weakness exhibited by isolated soleus muscle and the posterior leg muscles in vivo were completely explained by the small size of those muscles, which in turn were proportional to the small size of the mice. A similar situation appears to exist in HGPS patients as proportional scaling of skeletal muscle to body size has been reported (Gordon et al. 2007; Hamer et al. 1988; Merideth et al. 2008).
In contrast, muscle size could not account for the exceptionally low contractile capacities of isolated EDL muscle and of the anterior leg muscles in vivo. All skeletal muscles that were assessed for prelamin A showed that the protein was present in those from Zmpste24−/− mice and not from Zmpste24+/+ mice, but accumulation of prelamin A was similar between EDL and soleus muscles and, thus, could not explain why anterior but not posterior leg muscles were weak. Bergo et al. described a severe effacement of the anterior tibial tuberosity, the origin of the tibialis anterior muscle, in Zmpste24−/− mice and suggested that lower extremity weakness contributed to the bone abnormality (Bergo et al. 2002). It is conceivable that the reverse situation occurs, that is, the tibial bone effacement contributes to anterior leg muscle weakness because the tibialis anterior muscle, the largest of the anterior leg muscles, is not able to transmit force to the bone properly. Zmpste24−/− mice exhibit tibial bone loss as a result of low osteoblasts and osteoclasts relative to bone volume (Rivas et al. 2009). Likewise, bone abnormalities are a common feature in HGPS (Gordon et al. 2007; Hamer et al. 1988; Merideth et al. 2008).
An alternative possibility is that muscle and bone abnormalities co-evolve during the development of progeria. In addition to the selective contractile deficits we detected, we also noted several anatomical abnormalities in the leg musculature of Zmpste24−/− mice. In four of these mice, either the EDL or soleus muscle was partially fused with an adjacent muscle (in which cases those muscles were not assessed for contractility). Two Zmpste24−/− mice had EDL muscles that were abnormally deep within the anterior leg compartment, and another mouse did not have distinguishable heads of the gastrocnemius muscle. We speculate that the pathologies found in bone and now in skeletal muscle may co-exist due to these tissues’ common mesenchymal origin. Evidence for the involvement of stem cells in physiological aging and accelerated aging diseases is mounting, particularly for mesenchymal stem cells (Pekovic and Hutchison 2008). For example, Scaffidi and Misteli showed that progerin interfered with the differentiation of mesenchymal stem cells toward fat, bone, and cartilage (Scaffidi and Misteli 2008). The other cellular component important in muscle growth and development is that of satellite cells, and although myogenesis was not investigated in the study by Scaffidi and Misteli, other studies have shown that satellite cell and myoblast differentiation are impacted by prelamin A and lamin A perturbations (Frock et al. 2006; Maraldi et al. 2011). Additional in vivo studies on myogenesis and satellite cell function in Zmpste24−/− mice are needed to substantiate speculations on the involvement of mesenchymal stem cells in HGPS. These types of investigations are also critical to elucidate the involvement of unprocessed lamin A in physiological muscle injury and regeneration.
Despite the anatomical leg muscle abnormalities noted, minimal histopathological manifestations were observed in skeletal muscles of Zmpste24−/− mice. This is in agreement with Bergo et al. who stated that no histological abnormalities of skeletal muscle in these mice could be identified (Bergo et al. 2002), and contrary to another group who reported that Zmpste24−/− mice had dystrophic fibers (Pendas et al. 2002). Our histological evaluations of hindlimb and diaphragm muscles from 15- and 20-week-old Zmpste24−/− mice did not reveal primary indications of muscular dystrophy such as inflammation, fibrosis, necrosis, fibers with centralized nuclei, or mis-localization of emerin at the nuclear rim (Sullivan et al. 1999) (nor elevated serum creatine kinase activity (Emery 2002)). The only histological aberration that we consistently observed was excessive myonuclei in fibers of Zmpste24−/− mice. The cause and consequence of these extra myonuclei are not known but theoretically could relate to nuclear pathology or shortened telomeres that have been associated with progeria (Capell and Collins 2006; Rodriguez and Eriksson 2010). The soleus and EDL muscles in which excessive nuclei were found were from the same Zmpste24−/− mice assessed for contractile function, but because nuclei per se are not involved in the generation of force it is difficult to speculate how excessive nuclei could directly cause muscle weakness.
Fibrosis or fat accumulation were not obvious from the histological evaluations of muscle cross-sections from Zmpste24−/− mice. Increased fat mass sometimes occurs with sarcopenia (Fielding et al. 2011) and is an additional parameter that should be assessed in the muscles of Zmpste24−/− mice in the future. Instead, in this study we chose to assess muscle collagen content because of the high passive stiffness measured in soleus and EDL muscles from Zmpste24−/− mice. These biochemical analyses revealed that total collagen content of all four skeletal muscles assayed was quite high relative to that of muscles from Zmpste24+/+ mice. The surplus of collagen was likely the cause of the high passive stiffness. Similar connective tissue accumulation in heart and vascular smooth muscle cells have been shown in progeria mouse models (Pendas et al. 2002; Varga et al. 2006), as well as in HGPS (Olive et al. 2010), but this is the first report in skeletal muscle. Collectively, these results provide evidence for a connective tissue involvement in muscle (skeletal, smooth, and cardiac) in premature accelerated aging, perhaps indicating prior inflammation in these tissues.
Connective tissue involvement is evident in other age-related diseases in HGPS including alopecia, nail defects, sclerotic skin, dental malformations, and osteoporosis (Halaschek-Wiener and Brooks-Wilson 2007; Merideth et al. 2008). Arthropathy such as joint stiffness is also a common feature of HGPS (Gordon et al. 2007; Merideth et al. 2008), but had not previously been investigated in progeria mouse models. In preliminary investigations on anesthetized Zmpste24−/− mice, it was quite apparent that wrist, ankle, knee, and hip joints were very resistant to movement. This prompted us to quantitate joint mechanics and we selected to evaluate the ankle because it is the joint associated with the skeletal muscles analyzed. We found that the torque required to move the ankle into dorsi- and plantarflexion increased as the Zmpste24−/− mice aged, and in parallel, ROM about the ankle progressively decline from 5 to 20 weeks of age while those of littermates remained constant. Abnormal joint mobility has been reported in HGPS patients with ROM deficiencies of similar magnitude, 58 and 49% decrements in wrist and ankle ROMs, respectively (Merideth et al. 2008), to what we observed in the ankle of Zmpste24−/− mice. It is reasonable to conjecture that these joint problems contributed to the slow gait and hindlimb dragging reported previously (Bergo et al. 2002) and to the reduced cage activities measured in this study. Furthermore, the ability of Zmpste24−/− mice to grasp the bar for the grip strength measurement was clearly impaired in this study and likely contributed to the low grip strength and possibly to the report of reduced hang time (Bergo et al. 2002). It is probable that this arthropathy contributed to reduced physical activity by Zmpste24−/− mice. Disuse is one of the underlying causes of sarcopenia (Fielding et al. 2011) and is likely associated with muscle weakness in these progeria mice as well. In a preliminary study, we attempted to overcome disuse by providing Zmpste24−/− mice running wheels in their cages. The mice ran 1–3 km per 24 h for ~75 days but then running dramatically dropped off and mice stopped running. We speculate that the arthropathy became so severe that mice were not able to run. It is also possible that cardiovascular abnormalities in addition to muscle weakness might have limited the ability of the Zmpste24−/− mice to run. These preliminary results are in contrast to exercise rescuing the aging phenotype in another mouse model of progeria (Safdar et al. 2011).
Overall, our data support the hypothesis that hindlimb muscles of Zmpste24−/− mice have some of the key characteristics of sarcopenia including both muscle atrophy and weakness. We also characterized abnormal joint mechanics, which combined with muscle weakness, likely caused the poor neuromuscular performances by Zmpste24−/− mice. We conclude that skeletal muscle is an affected tissue in this progeroid mouse model and it is probable that it is one of the segmented tissues affected in HGPS as well. The identification of skeletal muscle as an affected tissue in progeria lends support to the contention that stem cells, likely satellite cells in this tissue, are fundamentally involved in the segmentation of affected tissues in accelerated aging diseases.
Acknowledgments
The authors would like to thank Stephen Young (University of California, Los Angeles) for the gift of Zmpste24−/− breeders as well as Greg Cochrane, Trevor Keyler, Allison Kosir, Ron McElmurry, and Brandon Peacock for their contributions to this project. The research was supported by the Progeria Research Foundation (J. Tolar) and National Institute of Health grants K02 -AG036827 (D.A. Lowe) and T32-AR07612 (J.A. Call).
Abbreviations
- EDL
Extensor digitorum longus
- HGPS
Hutchinson–Gilford progeria syndrome
- Po
Maximal isometric tetanic force
- ROM
Range of motion
References
- Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–454. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, Wisner ER, Van Bruggen N, Carano RA, Michaelis S, Griffey SM, Young SG. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci U S A. 2002;99:13049–13054. doi: 10.1073/pnas.192460799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call JA, McKeehen JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve. 2010;42:871–880. doi: 10.1002/mus.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- Carlson CG, Rutter J, Bledsoe C, Singh R, Hoff H, Bruemmer K, Sesti J, Gatti F, Berge J, McCarthy L. A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J Neurosci Methods. 2010;186:226–230. doi: 10.1016/j.jneumeth.2009.11.006. [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Levy N. Lamin a truncation in Hutchinson–Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong LG, Ng JK, Meta M, Cote N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG. Heterozygosity for LMNA deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci U S A. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklyn PP. Progeria in siblings. Clin Radiol. 1976;27:327–333. doi: 10.1016/S0009-9260(76)80082-7. [DOI] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlich MW, Baltgalvis KA, Call JA, Dorsey LL, Lowe DA. Plantarflexion contracture in the mdx mouse. Am J Phys Med Rehabil. 2010;89(12):976–985. doi: 10.1097/PHM.0b013e3181fc7c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, McCarten KM, Giobbie-Hurder A, Machan JT, Campbell SE, Berns SD, Kieran MW. Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics. 2007;120:824–833. doi: 10.1542/peds.2007-1357. [DOI] [PubMed] [Google Scholar]
- Grattan MJ, Kondo C, Thurston J, Alakija P, Burke BJ, Stewart C, Syme D, Giles WR. Skeletal and cardiac muscle defects in a murine model of Emery–Dreifuss muscular dystrophy. Novartis Found Symp. 2005;264:118–133. doi: 10.1002/0470093765.ch9. [DOI] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110:109–115. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Brooks-Wilson A. Progeria of stem cells: stem cell exhaustion in Hutchinson–Gilford progeria syndrome. J Gerontol A Biol Sci Med Sci. 2007;62:3–8. doi: 10.1093/gerona/62.1.3. [DOI] [PubMed] [Google Scholar]
- Hamer L, Kaplan F, Fallon M. The musculoskeletal manifestations of progeria. A literature review. Orthopedics. 1988;11:763–769. doi: 10.3928/0147-7447-19880501-14. [DOI] [PubMed] [Google Scholar]
- Hennekam RC. Hutchinson–Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Josephson RK. Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol. 1993;55:527–546. doi: 10.1146/annurev.ph.55.030193.002523. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve. 2008;38:1290–1303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GK, Schmidt WK, Bergo MO, Gavino B, Wong DH, Tam A, Ashby MN, Michaelis S, Young SG. Biochemical studies of Zmpste24-deficient mice. J Biol Chem. 2001;276:29051–29058. doi: 10.1074/jbc.M102908200. [DOI] [PubMed] [Google Scholar]
- Macnamara BG, Farn KT, Mitra AK, Lloyd JK, Fosbrooke AS. Progeria. Case report with long-term studies of serum lipids. Arch Dis Child. 1970;45:553–560. doi: 10.1136/adc.45.242.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi NM, Capanni C, Del Coco R, Squarzoni S, Columbaro M, Mattioli E, Lattanzi G, Manzoli FA. Muscular laminopathies: role of prelamin A in early steps of muscle differentiation. Adv Enzyme Regul. 2011;51(1):246–256. doi: 10.1016/j.advenzreg.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Martin GM. Segmental and unimodal progeroid syndromes of man. In: Harrison DE, editor. Genetic Effect on Aging Vol II. Caldwell: Telford Press; 1990. pp. 423–520. [Google Scholar]
- Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO, 3rd, Gahl WA, Introne WJ. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40:966–975. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100:548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- Olive M, Harten I, Mitchell R, Beers J, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, Gerhard-Herman M, Machan JT, Kutys R, Virmani R, Collins FS, Wight TN, Nabel EG, Gordon LB. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation With the Vascular Pathology of Aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic V, Hutchison CJ. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J Anat. 2008;213:5–25. doi: 10.1111/j.1469-7580.2008.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, Lopez-Otin C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Rivas D, Li W, Akter R, Henderson JE, Duque G. Accelerated features of age-related bone loss in zmpste24 metalloproteinase-deficient mice. J Gerontol A Biol Sci Med Sci. 2009;64:1015–1024. doi: 10.1093/gerona/glp089. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Eriksson M. Evidence for the involvement of lamins in aging. Curr Aging Sci. 2010;3:81–89. doi: 10.2174/1874609811003020081. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(SICI)1097-4598(199706)20:6<679::AID-MUS4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, Capell BC, Cheng J, Faddah D, Perkins S, Avallone H, San H, Qu X, Ganesh S, Gordon LB, Virmani R, Wight TN, Nabel EG, Collins FS. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson–Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]