Abstract
The human lens nucleus is formed in utero, and from birth onwards, there appears to be no significant turnover of intracellular proteins or membrane components. Since, in adults, this region also lacks active enzymes, it offers the opportunity to examine the intrinsic stability of macromolecules under physiological conditions. Fifty seven human lenses, ranging in age from 12 to 82 years, were dissected into nucleus and cortex, and the nuclear lipids analyzed by electrospray ionization tandem mass spectrometry. In the first four decades of life, glycerophospholipids (with the exception of lysophosphatidylethanolamines) declined rapidly, such that by age 40, their content became negligible. In contrast the level of ceramides and dihydroceramides, which were undetectable prior to age 30, increased approximately 100-fold. The concentration of sphingomyelins and dihydrosphingomyelins remained unchanged over the whole life span. As a consequence of this marked alteration in composition, the properties of fiber cell membranes in the centre of young lenses are likely to be very different from those in older lenses. Interestingly, the identification of age 40 years as a time of transition in the lipid composition of the nucleus coincides with previously reported macroscopic changes in lens properties (e.g., a massive age-related increase in lens stiffness) and related pathologies such as presbyopia. The underlying reasons for the dramatic change in the lipid profile of the human lens with age are not known, but are most likely linked to the stability of some membrane lipids in a physiological environment.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9293-6) contains supplementary material, which is available to authorized users.
Keywords: Presbyopia, Electrospray ionization mass spectrometry, Phospholipid, Ceramide, Cataract, Human lens
Introduction
Many tissues renew their cells with turnover rates that are dependent on the cell type and environment. Erythrocytes are characterized by half-lives of approximately 120 days, whereas epithelial cells, exposed to harsh conditions in the intestine, turn over within 3–6 days (Messaris et al. 2007). In marked contrast, some cells in the human body show very little, or no, turnover during our lifespan. For example, neurons in the adult neocortex are formed perinatally (Bhardwaj et al. 2006; Nowakowski 2006) and muscle cells also do not undergo mitosis (Moss and Leblond 1970). Adipocytes also display a very slow turnover (Meulle et al. 2008; Spalding et al. 2008) and in other tissues, turnover is very low and dependent on age. For example, by age 75, the annual turnover of cardiomyocytes is just 0.45% (Bergmann et al. 2009).
Little is known about the metabolism of such long-lived cells. A key question for human aging is to discover to what extent macromolecules within such cells are subject to resynthesis or repair, and whether such processes are affected by age. If macromolecules are not replaced, over time they can accumulate a range of modifications (Mecocci et al. 1999) that may affect cell, and ultimately tissue, function.
In parts of the human brain it appears that both DNA (Bhardwaj et al. 2006) and protein (Shapira et al. 1988) are very long-lived and, once synthesized around the time of birth, or early childhood, they may not be renewed. Few data are available on the longevity of brain lipids, although circumstantial evidence that a proportion of lipids may also be long-lived has come from analysis of human brain regions. Svennerholm and colleagues documented marked changes in the phospholipid composition of human myelin with age. The proportions of phosphatidylcholine (PC), phosphatidylserine (PS) and sphingomyelin (SM) altered substantially during life (Svennerholm et al. 1978) as did phospholipid fatty acid composition. Total cholesterol, ganglioside and cerebroside levels in human brain regions also altered progressively with age (Svennerholm et al. 1991, 1994), and this may have implications for the functioning of neurons in old age. Brain dolichol levels (Söderberg et al. 1990) as well as those in lung, pancreas and adrenal glands also increase substantially with age (Anders et al. 1989).
Age-related alterations in lipid composition could reflect enzymatic remodeling, or the instability of certain phospholipids. In experimental animals, membrane turnover can be rapid with phospholipid alterations detectable in the brain, heart, liver and kidney cells of rats within 2–10 min of intravenous infusion of lipid precursors (Thies et al. 1994; Shetty et al. 1996). Little is known about the long-term stability of phospholipids under biological conditions. In the absence of enzyme activity, lipid breakdown could occur due to hydrolysis, oxidation (Grether-Beck et al. 2000; Spiteller 2006), or a combination of both. Phospholipids within liposomes are stable for short periods of time at pH 7 (Ho et al. 1987), but are hydrolyzed under more acidic conditions (Ho et al. 1987; Arnhold et al. 2002; Ickenstein et al. 2006).
The lens provides a unique opportunity for investigating the effects of age on macromolecules because of its growth pattern. The lens grows continuously throughout life with newly differentiated fiber cells stacked onto the outside of a pre-existing core. Mature fiber cells in the lens centre have no organelles (Bantseev et al. 1999) and lack significant metabolic activity (Zhu et al. 2010b). Since proteins are present for our lifespan (Lynnerup et al. 2008) and there is also evidence to suggest a lack of lipid turnover (Borchman et al. 1999), the centre of the human lens can be used as a model system to examine the effects of ageing on the long-term stability of lipids within a biological environment.
Age is the number one risk factor for cataract and the changes in the relative amount of lipid with cataract may be an exacerbation of the changes seen with age (Yappert and Borchman 2004; Huang et al. 2005). Data from infrared spectroscopy suggest that lipid oxidation products may increase in the human lens with age (Borchman and Yappert 1998); however, the sensitivity of techniques used for phospholipid analysis has been a limitation in the past. Most studies used whole lenses to examine age-related changes (Broekhuyse 1969; Merchant et al. 1991; Borchman and Yappert 1998; Huang et al. 2005); however, under these conditions, old and more recently synthesized lipids from the outer part of the lens are analyzed together and this makes interpretation difficult. In order to study age-related degradation of lipids, the nuclear region of individual human lenses should be analyzed since this is the part of the lens that is formed in utero. Previous analyses of human lens nuclei showed an increase in the proportion of sphingomyelin and dihydrosphingomyelin (DHSM) relative to glycerophospholipids (GP) with age (Borchman et al. 1994; Yappert et al. 2003). These studies provided the first insight into alterations of the long-lived lipids of the human lens nucleus; however the requirement to pool lipid extracts and the limited number of lenses analyzed reduced the temporal resolution of these findings. Moreover, the underlying cause of this change, for example, an increase in sphingomyelin concentration, decrease in GP concentration or a combination of both, remains to be clearly elucidated. Other lipid classes have also been identified in the human lens, including PSs, lysophosphatidylethanolamines (LPEs) and ceramides (Cers) (Tao and Cotlier 1975; Yappert et al. 2003; Huang et al. 2005; Deeley et al. 2008; Estrada et al. 2010); however, no age-related changes have previously been reported for these lipid classes.
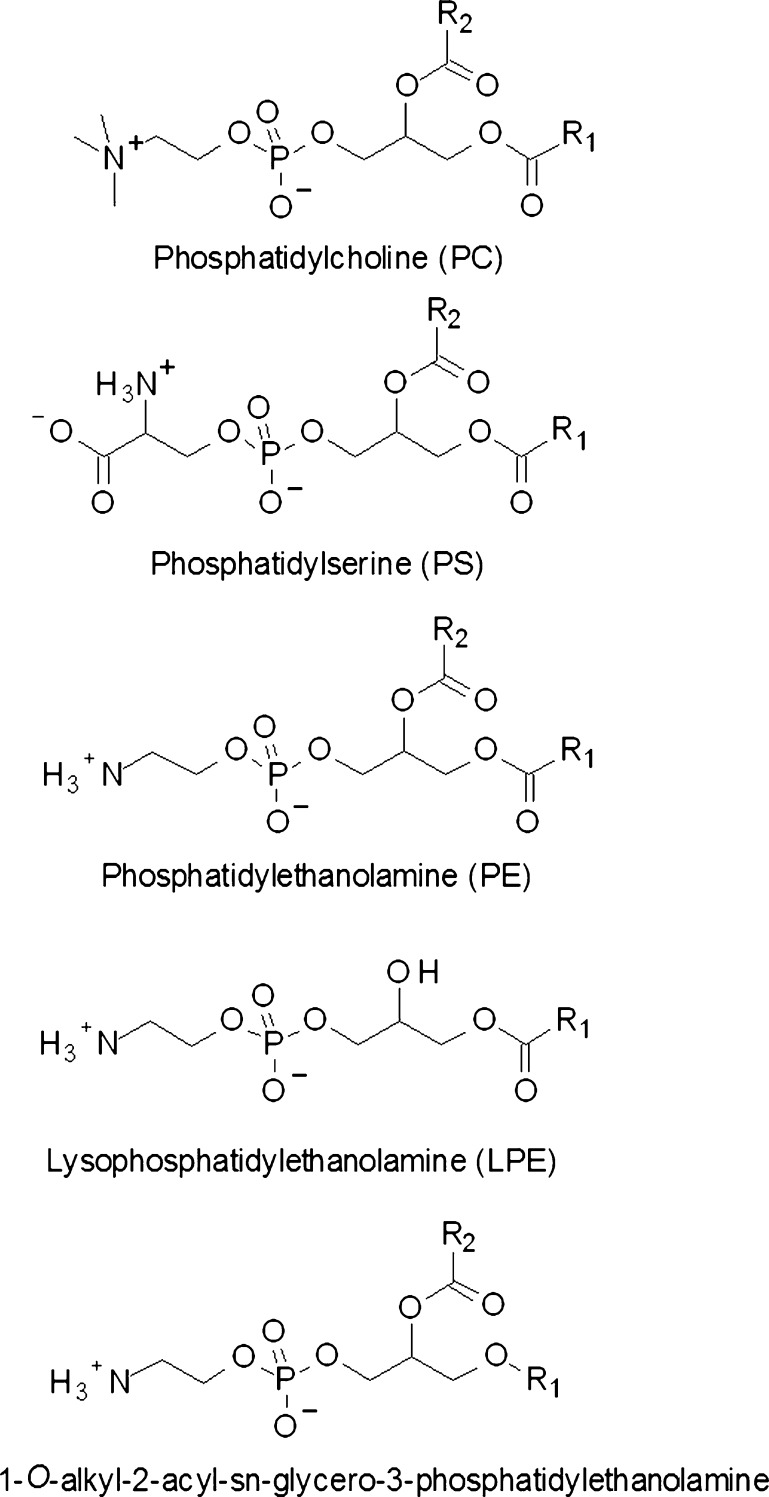
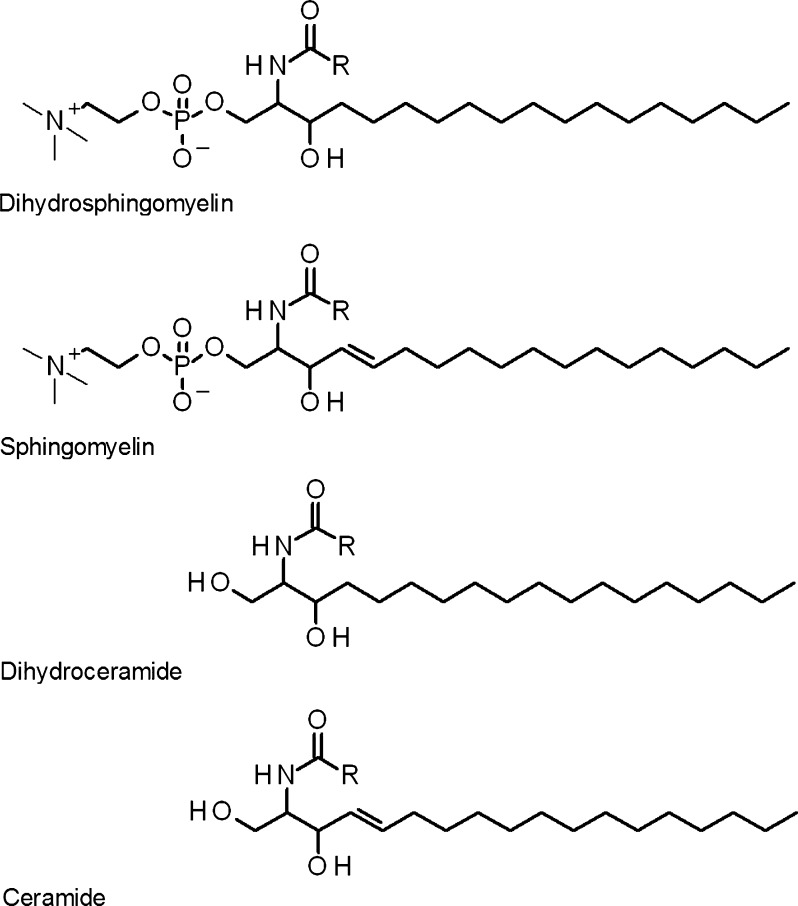
The aim of this study was to characterize alterations to lipids in the centre of the human lens as a function of age. Individual human lenses ranging in age from 12 to 82 years were examined for the levels of PCs, phosphatidylethanolamines (PEs), PSs, LPEs (Fig. 1), SMs, DHSMs, Cers and dihydroceramides (DHCers) (Fig. 2) using electrospray ionization tandem mass spectrometry (ESI-MS).
Fig. 1.
The main glycerophospholipid classes present in the human lens. Lysophosphatidylethanolamines have the same structure as phosphatidylethanolamine except for the absence of a fatty acyl chain on the sn-2 position of the glycerol backbone. 1-O-Alkyl phosphatidylethanolamines have a 1-O-alkyl ether linked fatty acid at the sn-1 position instead of the diester linkage commonly found on glycerophospholipids. 1-O-Alkyl ether linked species are also abundant in phosphatidylserine and lysophosphatidylethanolamine in the human lens. R1, R2 hydrocarbon chain that corresponds to the substituent at the sn-1 and sn-2 positions, respectively
Fig. 2.
The main sphingolipid classes present in the human lens. Dihydrosphingomyelin and sphingomyelin differ based on the presence of a double bond at the trans-4 position of the sphingoid backbone. Dihydroceramide and ceramide differ in an analogous manner. Sphingomyelins have a phosphocholine head group that differentiates them from their related ceramides. R hydrocarbon chains of various length and unsaturation levels
Methods
Materials
All solvents were HPLC grade and purchased from Crown Scientific (Moorebank, Australia). Butylated hydroxytoluene (BHT) was purchased from Sigma-Aldrich (Castle Hill, Australia). Lipid standards were synthesized by Avanti Polar Lipids (Alabaster, USA).
Lenses
Human lenses were collected from eyes donated to the NSW Lions Eye Bank, Sydney Eye Hospital, Sydney. Globe extraction and lens removal occurred between 2 and 6 h after death and the lenses stored at −80°C until required. One 19-week-old fetal lens approximately equivalent to a human lens nucleus was used for comparison (Dilmen et al. 2002). All work was approved by the human research ethics committees at the University of Sydney (#7292) and the University of Wollongong (HE 99/001).
Lipid extraction
Lenses between the ages of 12 and 82 were sectioned into nuclear and cortical regions as described previously (Heys et al. 2008). In brief, lenses were cored using a 4.5 mm trephine, and the ends of the cylinders (approximately 1 mm) were removed to produce the nucleus and remove any cortex or barrier region fiber cells (Heys et al. 2008). Immediately following dissection, nuclear regions were weighed and homogenized in methanol/chloroform (2:1 v/v) containing 0.01% BHT at a solvent/tissue ratio of 20:1. A methanolic internal standard solution was prepared containing PC (19:0/19:0), 95 μM; SM (d18:0/12:0), 84 μM; PE (17:0/17:0), 39 μM; PS (17:0/17:0), 34 μM; LPE (14:0), 39 μM; PG (17:0/17:0), 25 μM; PA (17:0/17:0), 25 μM; Cer (d18:1/10:0), 75 μM and Cer (d18:0/8:0), 75 μM. These concentrations were optimized to reflect the abundances of different lipid classes in the lens and the internal standard solution was added at 1.4 ml g (tissue)−1. Extracts were mixed in a tube rotator overnight at 4°C. Initially, 17 lenses were extracted according to Folch et al. (1957) using an acid wash (1 M H2SO4) instead of aqueous sodium chloride to further enhance the extraction of acidic phospholipids as described previously (Nealon et al. 2008b). During the course of this study, we further optimized our lipid extraction procedure in order to maximize extraction efficiency for a range of animal tissues (in particular to avoid the loss of 1-O-alkenyl ethers) and improve compatibility with ESI-MS. Although the levels of 1-O-alkenyl ethers in the human lens are minimal (Deeley et al. 2009), the subsequent 40 lenses were extracted as described above; however, the sulfuric acid was replaced with aqueous ammonium acetate (0.15 M) (Deeley et al. 2008). No significant differences in human lens phospholipid abundance were observed between the two extraction methods; therefore, all 57 lenses were included in this study. Samples were then stored at −80°C for a maximum of 2 weeks until analysis.
Mass spectrometry
All mass spectra were obtained using a Waters QuattroMicro™ (Waters, Manchester, UK) equipped with a z-spray electrospray ion source. Capillary voltage was set to 3000 V, source temperature 80°C and desolvation temperature 120°C. Cone voltage was set to 50 and 35 V in negative and positive ion modes, respectively. Nitrogen was used as the drying gas at a flow rate of 320 l h−1. Lipid extracts were diluted to approximately 40 μM with the addition of methanol/chloroform (2:1 v/v) and aqueous ammonium acetate (1 M) at 50 μl ml−1. Samples were infused into the electrospray ion source at a flow rate of 10 μl min−1 using the instrument’s on-board syringe pump and lipids quantified as previously described (Deeley et al. 2008). In all precursor ion, neutral loss and product ion scans, argon was used as the collision gas at a pressure of 3 mTorr. For phospholipids, collision energy was set between 22 and 35 eV depending on the scan performed as described elsewhere (Deeley et al. 2008). For ceramides and dihydroceramides, collision energy was set to 35 eV for negative ion mode neutral loss scans of d18:1 (256.3 Da) and d18:0 (258.3 Da) sphingoid bases (Han 2002). For the analysis of LPEs, extracts were diluted to approximately 40 μM with the addition of methanol/chloroform (2:1 v/v) and aqueous sodium acetate (680 mM) at 50 μl ml−1. Neutral loss scans for 43 Da (from [M+Na]+) were performed in positive ion mode with collision energy set at 25 eV. The neutral loss of 43 Da from the ester-linked LPE standard was found to be approximately 58% less efficient than from the 1-O-alkyl ether by comparing the collision-induced dissociation spectra of LPE (14:0) to LPE (18:1e) and LPE (16:0e). Accordingly, a correction factor was applied for the quantification of ester-linked LPEs. We have previously determined the variability of lipid extraction from lenses to be 4% and analytical variability to be 8%.
Statistical analysis
As concentration changes with age were nonlinear for most lipids, generalized additive models (GAM) (Hastie and Tibshirani 1986) were fit to the data, see Figs. 3, 4, 5, 6, 7 and 8. A smooth spline term was used for age and a main effect for the two extraction methods, response was the natural logarithm of lipid concentration. The extraction method showed no significant effect at the 5% level in all cases and hence was omitted in the results shown. The solid line in all figures shows the GAM fit, dashed lines give a 95% confidence band for the fit. All computations were performed using R version 2.10.1 (R Development Core Team 2009) using extension package mgcv (Wood 2004). The degrees of freedom of the spline terms (which control the nonlinearity of the fit) were automatically estimated from the data using cross validation.
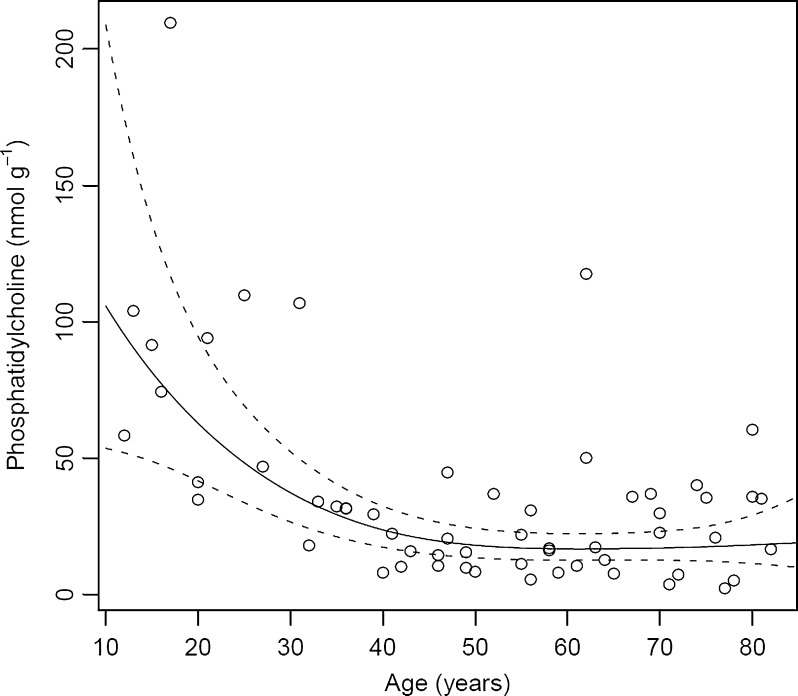
Fig. 3.
Total phosphatidylcholine present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. The solid line is a generalized linear model fit; the dashed lines give a 95% confidence band
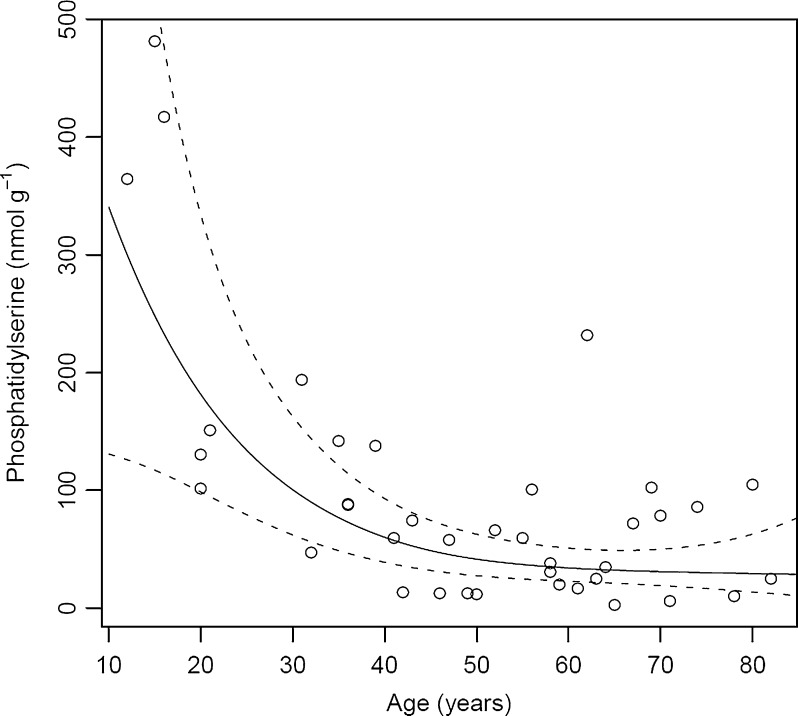
Fig. 4.
Total phosphatidylserine present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. The solid line is a generalized linear model fit; the dashed lines give a 95% confidence band
Fig. 5.
Total phosphatidylethanolamine present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. The solid line is a generalized linear model fit; the dashed lines give a 95% confidence band
Fig. 6.
Total lysophosphatidylethanolamine present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. The solid line is a generalized linear model fit; the dashed lines give a 95% confidence band
Fig. 7.
Total a ceramide and b dihydroceramide present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. The solid line is a generalized linear model fit; the dashed lines give a 95% confidence band
Fig. 8.
Total a sphingomyelin and b dihydrosphingomyelin present in individual human lens nuclei of different ages. All values are expressed as nmol g−1 tissue wet weight. In both cases, a horizontal line fits into the 95% confidence band of the generalized additive model fit; hence, the null hypothesis of no change with age cannot be rejected
Results
Glycerophospholipids
Phosphatidylcholines
Of the GP classes present in the human lens nucleus, phosphatidylcholines were present in the lowest amount. As seen in Fig. 3, total PCs in an adolescent lens nucleus (aged 12–16 years) ranged from 60 to 100 nmol g−1; however, by age 40 total PC levels had decreased to approximately 35 nmol g−1. Aging did not further alter the abundance of total PCs (Fig. 3). All individual PCs demonstrated the same general pattern of decay with age.
Phosphatidylserines
PSs demonstrated a similar trend to that of PCs. Total PS concentration ranged between 360 and 480 nmol g−1 in an adolescent human lens nucleus, and this decreased to ~60 nmol g−1 by middle age and then remained unchanged thereafter (Fig. 4). The major contributors to this 6- to 8-fold decrease in total PS were PS (16:0e/18:1), PS (18:1e/18:1) and PS (18:0e/18:1), which are the most abundant PS molecules in the human lens and account for 75–90% of total PSs. These 1-O-alkyl ethers have recently been identified in the human lens (Deeley et al. 2009). Diester-linked PSs were detected at much lower concentrations (~10–25% of total PSs) and showed a similar pattern of decay with age.
Phosphatidylethanolamines
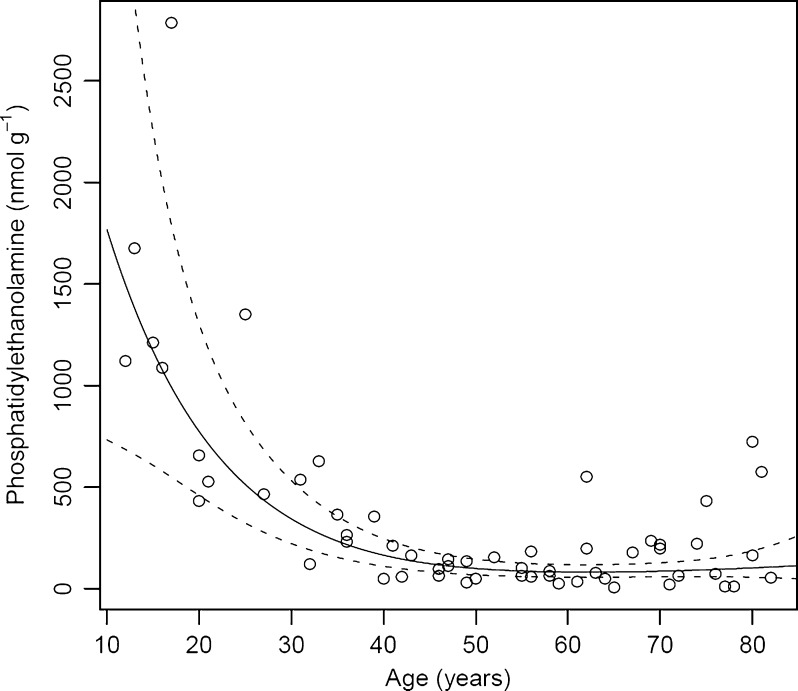
The effect of age on PE concentration is shown in Fig. 5. PEs were the second most abundant GP class in the human lens (see Table 1). Total PEs showed a similar trend to that observed for PCs and PSs. In an adolescent, PE levels ranged between 1,100 and 1,700 nmol g−1, and this decreased until age 40, where the total PE concentration leveled off at approximately 50 nmol g−1. As was found for PS, the major contributors to this 50-fold decrease in total PE concentration over 25 years were due to three 1-O-alkyl PE molecules; PE (16:0e/18:1), PE (18:1e/18:1) and PE (18:0e/18:1) which together comprise ~60% of total PEs. The most abundant of these, PE (18:1e/18:1) decreased from a maximum of 1,400 nmol g−1 in an adolescent to 30 nmol g−1 in a middle-aged lens. Diester-linked PE molecules, present at a much lower concentrations, also diminished with age.
Table 1.
Comparison of the percentage distribution of lipid classes in the fetal, young and old human lens
| Lipid class | Percentage of total lipid (%) | ||
|---|---|---|---|
| Fetal lens (n = 1) | Young lens (10–20 years; n = 4) | Old lens (70–80 years; n = 3) | |
| DHSM | 9.7 | 36 ± 2.5 | 35 ± 0.4 |
| SM | 3.0 | 8.8 ± 0.6 | 9.8 ± 1.7 |
| PC | 50 | 1.2 ± 0.1 | 0.6 ± 0.2 |
| PE | 27 | 19 ± 3.1 | 3.1 ± 1.5 |
| LPE | 0.5 | 29 ± 3.0 | 19 ± 1.3 |
| PS | 10 | 5.7 ± 1.4 | 1.3 ± 0.5 |
| DHCer | 0.3 | 0.2 ± 0.0 | 22 ± 4.0 |
| Cer | 0.3 | 0.1 ± 0.0 | 8.9 ± 1.4 |
DHSM dihydrosphingomyelin, SM sphingomyelin, PC phosphatidylcholine, PE phosphatidylethanolamine, LPE lysophosphatidylethanolamine, PS phosphatidylserine, DHCer dihydroceramide, Cer Ceramide
Lysophosphatidylethanolamines
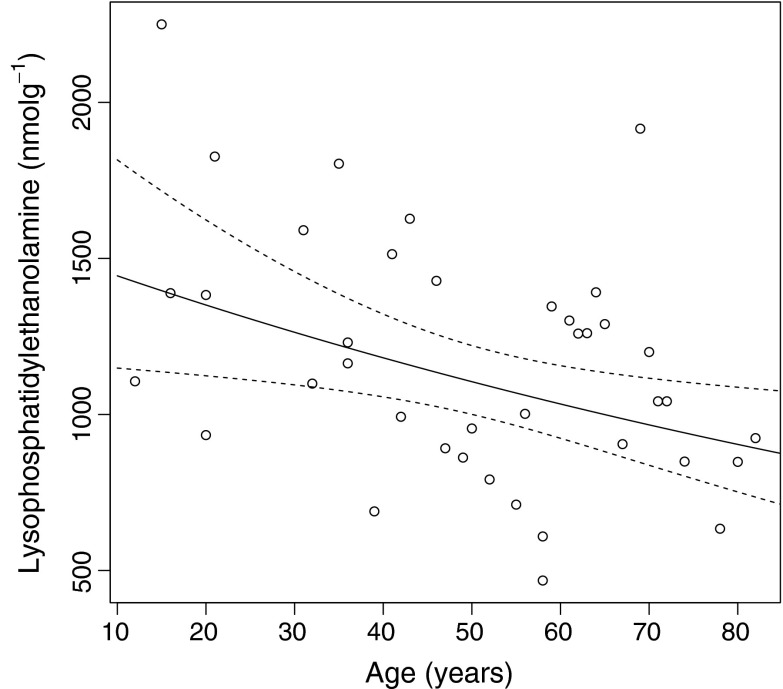
The affect of age on the abundance of LPEs (see Fig. 1 for structure) is shown in Fig. 6. LPE was found to be the most abundant GP class in the human lens nucleus (Table 1). The major contributors to total LPE concentration were 1-O-alkyl ethers, the most abundant being PE (16:0e), PE (18:1e) and PE (18:0e). Structural assignment as alkyl ethers was confirmed by the presence of an m/z 135 ion observed in MS3 spectra (Fig. S1) as previously described (Hsu and Turk 2007). Total LPEs decreased with age but did so in a much more gradual manner than the other GPs and also did not plateau. In order to test that the LPEs were not being created as artifacts of our extraction or analysis procedures we first tested various monophasic and biphasic extraction techniques (see supplementary methods) and found our results to be reproducible for all extractions (Fig. S2). We also separated LPEs from PEs using thin layer chromatography (TLC) and analyzed them directly from the plate using desorption electrospray ionization (see supplementary methods). During this analysis LPEs (Rf ~0.17) were well resolved from PEs (Rf ~0.5) and no LPEs were detected when the PE spot was analyzed (Fig. S3). These experiments confirm that LPEs were not being produced during the electrospray process.
Sphingolipids
Ceramides and dihydroceramides
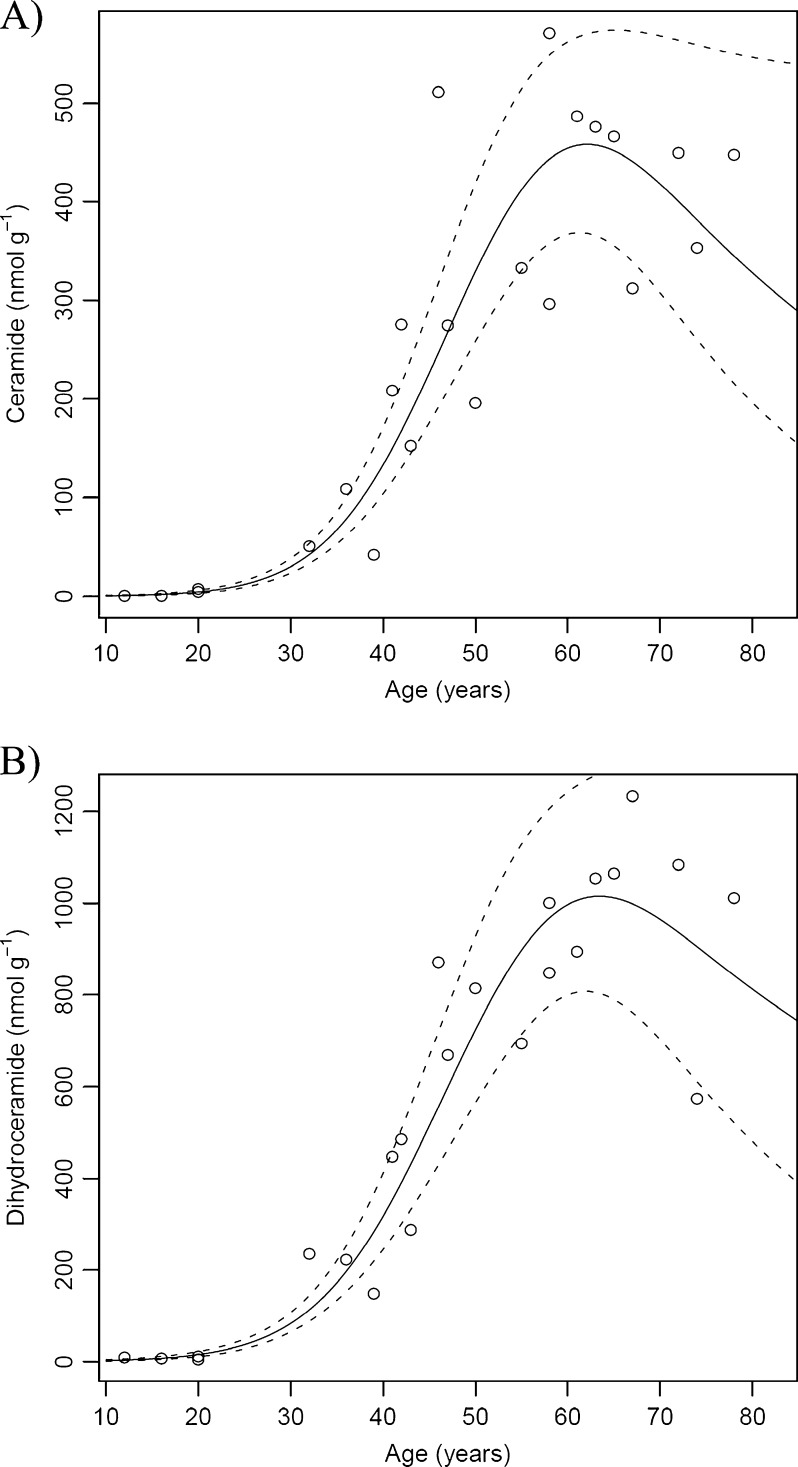
The relationship between Cer and DHCer concentrations and age are shown in Fig. 7a and b, respectively. The trend for both Cer and DHCer was in marked contrast to that observed for the GPs. During adolescence, Cers could not be detected and only trace quantities of DHCers were found in the lens nucleus. Total DHCer increased more than 20-fold from adolescence to age 40 (from ~10 to ~230 nmol g−1) and continued to increase until around age 60, where levels reached approximately 1,200 nmol g−1. At this time the Cer concentration reached 500 nmol g−1. Overall, in less than 50 years, DHCer levels increased more than 110-fold, and Cer levels approximately 80-fold.
Sphingomyelin and dihydrosphingomyelin
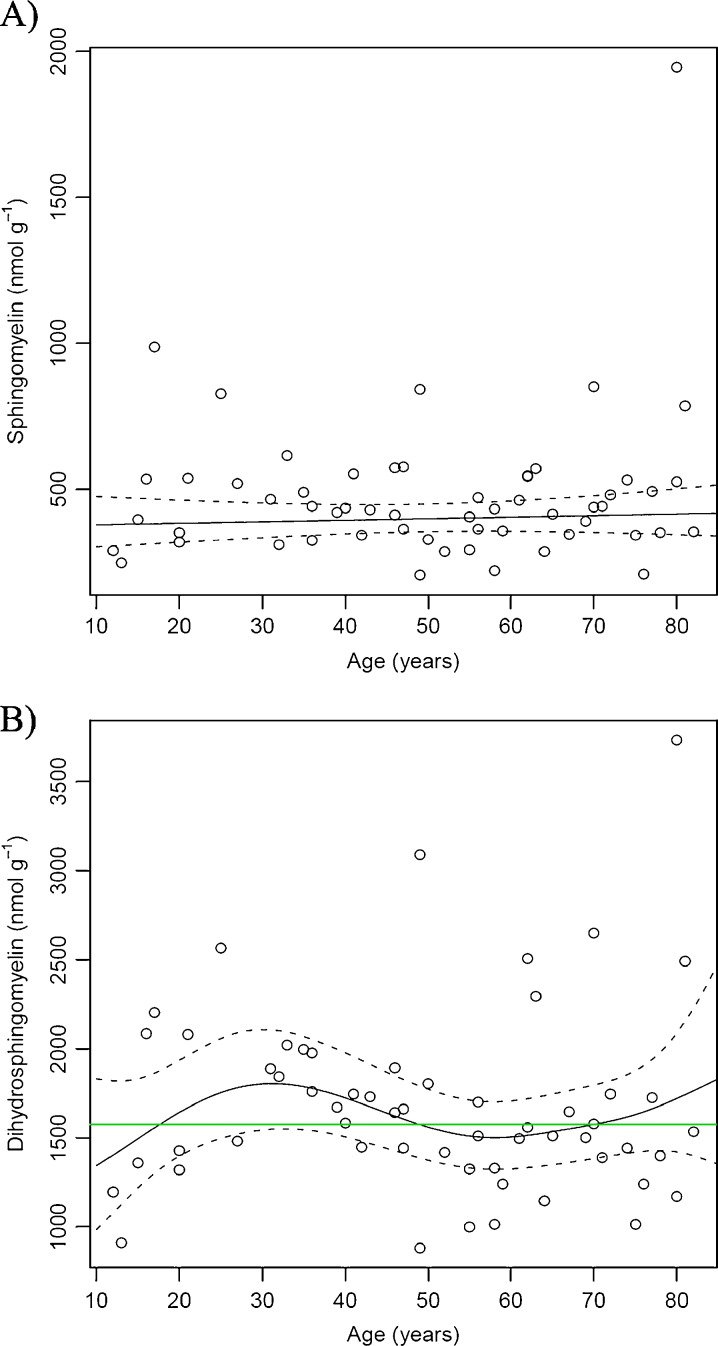
SM and DHSM represented between 8–10% and 35–36% of the total lipids measured in the nucleus, respectively (Table 1). The relationship between total SM and DHSM and age is shown in Fig. 8. DHSM levels were on average 4-fold higher than those of SM (~1,550 vs. 400 nmol g−1). No significant age-dependent effects were observed on the concentration of total SM (Fig. 8a) or total DHSM (Fig. 8b). Broekhuyse (1969), utilizing TLC, reported an increase in the absolute amount of SM (presumably including DHSM) in the whole lens with age. We have recently reported a large increase in DHSM with age in the barrier region of lens (Deeley et al. 2010). Given that the barrier region was not analyzed in the current study, this may explain the differences observed between the nucleus and whole lens.
Fetal lens
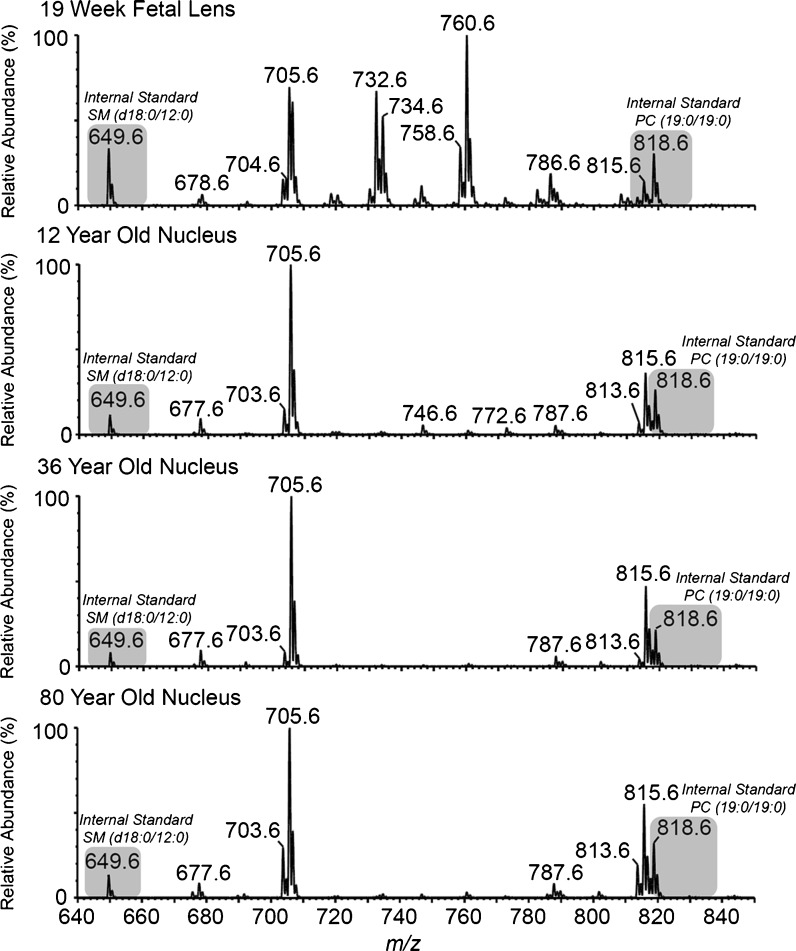
The adult lens nucleus analyzed in this study corresponds to the size of the fetal lens (Dilmen et al. 2002). It was of interest therefore to discover if during lens development, significant changes occurred to phospholipids. The lipid composition of one 19-week-old fetal lens was analyzed. The distribution of lipids across various head groups was very different by comparison to either young or old lens nuclei (Table 1). In post-partum lenses regardless of age, LPE was the major GP class measured (Table 1); however, in a fetal lens, PC was found to be the major lipid class, representing 50% of lipid. In the fetal lens, LPE contributed a mere 0.5% to the total lipid content. The composition of individual lipids in the fetal lens was also very different from that of an adult lens. As an illustration of the marked differences, the major phosphatidylcholines present in the fetal lens were PC (16:0/18:1) (m/z 760.6) and PC (16:0/16:1) (m/z 732.6) (Fig. 9), each of which was almost non-existent in young or old adult nuclei.
Fig. 9.
Precursor ion scan (m/z 184.1) for the phosphocholine head group, displaying dihydrosphingomyelin, sphingomyelin and phosphatidylcholine molecules in a 19-week-old fetus, 12-year-old, 36-year-old and 80-year-old human lens nucleus. Internal standards are present at m/z 649.6 and 818.6 (SM (d18:0/12:0) and PC (19:0/19:0), respectively)
Discussion
This work has demonstrated that the phospholipid composition of fiber cell membranes from the lens nuclei of older individuals is very different from that of young people. In the first four decades of life, a progressive decrease in the content of GPs was observed. PCs, PEs and PSs all diminished to very low levels, which then remained constant. A steep rise in the levels of both ceramides and dihydroceramides was also apparent after age 30. By contrast the concentration of SMs and DHSMs remained relatively constant throughout life.
At present, we do not know the reasons for these pronounced changes. In rodents, diet appears unable to alter the lipid composition of the lens centre (Nealon et al. 2008a). In vitro experiments also indicate that fatty acids taken up by the rat lens are not incorporated into fiber cell phospholipids (Nealon et al. 2011). Taken together this suggests that once phospholipids have been incorporated into mature fiber cells there may be little, or no, turnover. The pronounced changes with age are unlikely to be due to the activity of lens enzymes. Although phospholipase activity has been observed in the whole lens, even at 60 years of age (Kamei 1996), several separate studies indicate that metabolic pathways are absent from the centre of adult human lenses (Dovrat and Gershon 1981; Dovrat et al. 1984; Scharf et al. 1987; Zhu et al. 2010b). Fiber cells in the lens nucleus lack organelles and, since there is no protein turnover (Lynnerup et al. 2008), it is likely that the enzymes which were active in the centre of young lenses have been denatured due to decades of exposure to body temperature. For these reasons, the decrease in GPs in the first 40 years most likely reflects non-enzymatic processes, such as oxidation and/or hydrolysis. Indeed, GPs are more susceptible to oxidation and degradation than sphingolipids (Oborina and Yappert 2003) and products of lipid oxidation have been shown to increase with age (Yappert et al. 1992; Borchman and Yappert 1998). Hydrolysis of GPs within vesicles is promoted by acidic conditions but the rates are generally lower at neutral pH (Ho et al. 1987). No studies have examined very long (decades) exposure to conditions that are found in a biological environment.
The dramatic decrease in GPs was not associated with a concomitant increase in lysophospholipids. In fact, LPEs were found to be major contributors to the lipidome of the human lens nucleus, accounting for approximately 29% of the measured lipidome in young lenses and 19% in old lenses (Table 1). Since they are abundant in young lenses and display a slow age-dependent decline, they may be formed in young lenses by phospholipase A2 as described for other tissues (Fuchs and Schiller 2009) and then suffer a slow decomposition with age. A recent re-evaluation of human lens lipids (Estrada et al. 2010), revealed that lipids previously identified as phosphatidylglycerols during 31P NMR experiments (Borchman et al. 1994; Yappert et al. 2003; Rujoi et al. 2003) are actually LPEs and therefore significant contributors to the nuclear lipid pool. With this revaluation in mind the current findings are in reasonable agreement with previous data by Yappert and colleagues (2003), particularly in older lenses. Also in agreement with the current findings, direct analysis of lens slices by desorption electrospray ionization mass spectrometry (DESI-MS) suggests a greater abundance of LPE (18:1e) than PE (18:1e/18:1) in a 41-year-old nucleus (Ellis et al. 2010). Interestingly, Ellis and co-workers also report the relative abundance of LPE to be slightly higher in the barrier/inner cortex region of the human lens than in the nucleus. It should be noted, however, that DESI-MS data obtained directly from a lens slice may not be a true representation of lipid abundance within the total lens region and quantitative analysis of LPE in lens regions other than the nucleus are yet to be performed. The current data differ from previous studies, however, in that LPE levels were found to be comparable with DHSM levels. In two previous studies, where phospholipids from the human lens nucleus were quantified by phosphorous assays of scrapings from TLC plates, the ratio of LPE to total SM (DHSM+SM) was found to be 0.1 (Cotlier et al. 1978) and 0.23–0.3 (Zigman et al. 1984) compared to 0.64 in young lenses and 0.42 in old lenses in the current study. While the Cotlier data was obtained from various water, urea and guanidine soluble and insoluble fractions and is therefore not directly comparable, our data suggests approximately twice the relative LPE concentration than that observed by Zigman and co-workers. These differences may be a result of the enhanced sensitivity and specificity provided by the ESI-MS technique used in the current study compared to traditional TLC and phosphorous assays.
The structural homology between the major PEs and PSs in the lens suggests a precursor–product relationship. In both classes, the major components were 1-O-alkyl ethers and the fatty acid compositions of the most abundant PE species (16:0e/18:1), (18:1e/18:1) and (18:0e/18:1) correlated with that of the major PS moieties (Deeley et al. 2009). A pathway involving decarboxylation of PS is well known (Borkenhagen et al. 1961; Kanfer and Kennedy 1964; Satre and Kennedy 1978) and PE can be converted to PSs by PS synthase 2 (Saito et al. 1998).
The marked rise in the amount of ceramides after middle age is in agreement with previous data obtained (for a small sample size) using imaging mass spectrometry (Deeley et al. 2010) although the reason for this trend is currently unknown. The fatty acid composition of ceramides was predominantly 16:0 and 24:1 and, in this regard, they mirror the lens SMs and DHSMs. One obvious possibility is that they are being formed by hydrolysis of sphingomyelins in the lens. As noted above, it is not likely that active sphingomyelinases remain in the centre of the human lens after more than 40 years, so other factors are probably responsible. Another possibility is that ceramides arise in the lens from exposure to UVA light, since singlet oxygen, derived from UVA irradiation, is able to generate ceramides from sphingomyelin liposomes (Grether-Beck et al. 2000). In separate model experiments, it has been demonstrated that hydrolysis of SMs and DHSMs to the corresponding ceramides can occur by exposure to elevated temperature (Truscott and Zhu 2010). Nevertheless, the levels of nuclear SMs and DHSMs did not diminish significantly with age, and the ceramides were of comparable magnitude. Therefore, other factors such as the hydrolysis of ceramide phosphates or glycosides, which have been reported in the lens (Ogiso et al. 1993, 1994, 1998; Estrada et al. 2010) may be responsible for the age-dependent increase in the concentration of the ceramides. A comparable dramatic elevation in the concentration of ceramides has been found in the white matter of brains from people with cognitive impairment (Han et al. 2002) where it is thought that they may be derived from hydrolysis of abundant ceramide conjugates such as sulfatides and gangliosides.
The consequences of such marked alterations in the lipid composition of the lens fiber cells may well be profound for the function of the lens. Aging of the human lens is associated with presbyopia (Glasser and Campbell 1998; Glasser et al. 2001; Truscott 2009) and cataract (Harding 2002; Truscott 2005). In the case of age-related nuclear cataract, it has been proposed that the development of a barrier to diffusion of metabolites between the lens cortex and nucleus at middle age may be a prerequisite (Sweeney and Truscott 1998; Moffat et al. 1999). Recent data suggest that the reason for the onset of the barrier is due to the binding of crystallins to fiber cell membranes (Friedrich and Truscott 2009) and in this way the membrane pores are occluded. In vitro studies suggest that α-crystallin binding to human lens lipids increases with age (Grami et al. 2005) and this may be responsible for altering the physical properties of the fiber cell membranes (Zhu et al. 2010a). On the basis of the data in this manuscript, it could be proposed that the binding of proteins after middle age may be traced to a marked change in the lipid composition of the cell membranes. A membrane rich in ceramides, but depleted of GPs, such as found in the centre of older lenses, may be a more receptive surface for binding of crystallins.
Another remarkable finding was that the lipids isolated from fetal lenses were very different from those found in post-natal lenses. Indeed, the lipid profile of a fetal lens was more typical of that from other tissues of the body. Since the lens grows by addition of cells to a lens core (nucleus) that was present at birth, this finding suggests major remodeling of lens fiber cell membranes occurs around the time of birth, or shortly afterwards. The youngest lens examined was that of a 12-year-old and in this case the lipid profile was more typical of adult lenses.
In summary, this study has produced several novel findings: (i) during the first four decades of life there is a dramatic reduction in the level of GPs (PE, PS and PC) in the human lens nucleus, (ii) LPE is the most abundant GP class in the human lens nucleus and displays a relatively slow reduction in concentration with age, (iii) a striking increase in nuclear ceramide and dihydrocermide concentration occurs between the ages of 30 and 60, (iv) the previously reported increase in the relative proportion of sphingomyelin in the lens nucleus with age is solely the result of a reduction in GP, and (v) the lipid profile of the fetal lens is very different from that of the postnatal lens nucleus. In addition, the increased temporal resolution of the current study allowed us to identify a key transition point (at approximately 40 years of age) where GP content becomes negligible and ceramide and dihydroceramide levels increase rapidly. Interestingly, this corresponds to the age where a rapid increase in lens stiffness and presbyopia become evident (Heys et al. 2004).
It is unknown if the major changes in composition characterized for human lens membranes in this study will also be reflected in other human tissues which contain cells that are long-lived. Examples of long-lived cells include brain (Bhardwaj et al. 2006), heart (Bergmann et al. 2009) and adipose tissue (Meulle et al. 2008; Spalding et al. 2008). In the case of brain, it is clear that major changes to lipids occur with age (Svennerholm et al. 1978, 1991, 1994). Knowledge of the long-term stability of phospholipids under biological conditions documented here may provide a guide as to the types of changes expected in the membranes of other old cells.
Electronic supplementary material
(DOC 121 kb)
Acknowledgements
J.R.N. and S.R.E. are supported by an Australian Postgraduate Award. S.J.B and T.W.M. acknowledge the financial support of the University of Wollongong and the Australian Research Council. S.J.B acknowledges funding through the ARC Centre of Excellence for Free radical Chemistry and Biotechnology. R.J.W.T is a National Health and Medical Research Council Fellow and acknowledges the financial support of the National Health and Medical Research Council and National Institutes of Health (USA).
Contributor Information
Roger J. W. Truscott, Phone: +61-2-42213503, FAX: +61-2-42214287, Email: rjwt@eye.usyd.edu.au
Todd W. Mitchell, Phone: +61-2-42215443, FAX: +61-2-42215945, Email: toddm@uow.edu.au
References
- Anders K, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- Arnhold J, Osipov AN, Spalteholz H, Panasenko OM, Schiller J. Formation of lysophospholipids from unsaturated phosphatidylcholines under the influence of hypochlorous acid. Biochimica et Biophysica Acta. 2002;1572(1):91–100. doi: 10.1016/S0304-4165(02)00271-4. [DOI] [PubMed] [Google Scholar]
- Bantseev V, Herbert KL, Trevithick JR, Sivak JG. Mitochondria of rat lenses: distribution near and at the sutures. Curr Eye Res. 1999;19(6):506–516. doi: 10.1076/ceyr.19.6.506.5279. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Yappert MC. Age-related lipid oxidation in human lenses. Investig Ophthalmol Vis Sci. 1998;39(6):1053–1058. [PubMed] [Google Scholar]
- Borchman D, Byrdwell WC, Yappert MC. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Investig Ophthalmol Vis Sci. 1994;35(11):3938–3942. [PubMed] [Google Scholar]
- Borchman D, Tang D, Yappert MC. Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum membranes. Biospectroscopy. 1999;5(3):151–167. doi: 10.1002/(SICI)1520-6343(1999)5:3<151::AID-BSPY5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Borkenhagen LF, Kennedy EP, Fielding L. Enzymatic formation and decarboxylation of phosphatidylserine. J Biol Chem. 1961;236:28–30. [Google Scholar]
- Broekhuyse R. Phospholipids in tissues of eye 3. Composition and metabolism of phospholipids in human lens in relation to age and cataract formation. Biochim Biophys Acta. 1969;187(3):354. doi: 10.1016/0005-2760(69)90009-5. [DOI] [PubMed] [Google Scholar]
- Cotlier E, Obara Y, Toftness B. Cholesterol and phospholipids in protein fractions of human lens and senile cataract. Biochim Biophys Acta. 1978;530(2):267–278. doi: 10.1016/0005-2760(78)90012-7. [DOI] [PubMed] [Google Scholar]
- Deeley JM, Mitchell TW, Xiaojia W, Korth J, Nealon JR, Blanksby SJ, Truscott RJ. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta. 2008;1781(6–7):288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Deeley JM, Thomas MC, Truscott RJW, Mitchell TW, Blanksby SJ. Identification of abundant alkyl ether glycerophospholipids in the human lens by tandem mass spectrometry techniques. Anal Chem. 2009;81(5):1920–1930. doi: 10.1021/ac802395d. [DOI] [PubMed] [Google Scholar]
- Deeley JM, Hankin JA, Friedrich MG, Murphy RC, Truscott RJW, Mitchell TW, Blanksby SJ. Sphingolipid distribution changes with age in the human lens. J Lipid Res. 2010;51:2753–2760. doi: 10.1194/jlr.M007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2009) A language and environment for statistical computing. Vienna, Austria
- Dilmen G, Köktener A, Turhan NÖ, Tez S. Growth of the fetal lens and orbit. Int J Gynecol Obstet. 2002;76(3):267–271. doi: 10.1016/S0020-7292(01)00581-1. [DOI] [PubMed] [Google Scholar]
- Dovrat A, Gershon D. Rat lens superoxide dismutase and glucose-6-phosphate dehydrogenase: studies on the catalytic activity and the fate of enzyme antigen as a function of age. Exp Eye Res. 1981;33(6):651–661. doi: 10.1016/S0014-4835(81)80105-4. [DOI] [PubMed] [Google Scholar]
- Dovrat A, Scharf J, Gershon D. Glyceraldehyde 3-phosphate dehydrogenase activity in rat and human lenses and the fate of enzyme molecules in the aging lens. Mech Ageing Dev. 1984;28(2–3):187–191. doi: 10.1016/0047-6374(84)90019-8. [DOI] [PubMed] [Google Scholar]
- Ellis SR, Wu C, Deeley JM, Zhu X, Truscott RJW, in het Panhuis M, Cooks RG, Mitchell TW, Blanksby SJ. Imaging of human lens lipids by desorption electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2010;21:2095–2104. doi: 10.1016/j.jasms.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Estrada R, Puppato A, Borchman D, Yappert MC. Reevaluation of the phospholipid composition in membranes of adult human lenses by 31P NMR and MALDI MS. Biochim Biophys Acta. 2010;1798(3):303–311. doi: 10.1016/j.bbamem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Friedrich MG, Truscott RJW. Membrane association of proteins in the aging human lens: profound changes take place in the fifth decade of life. Investig Ophthalmol Vis Sci. 2009;50(10):4786–4793. doi: 10.1167/iovs.09-3588. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Schiller J. Lysophospholipids: their generation, physiological role and detection. Are they important disease markers? Mini-Rev Med Chem. 2009;9(3):368–378. doi: 10.2174/1389557510909030368. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vis Res. 1998;38(2):209–229. doi: 10.1016/S0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Glasser A, Croft MA, Kaufman PL. Aging of the human crystalline lens and presbyopia. Int Ophthalmol Clin. 2001;41(2):1–15. doi: 10.1097/00004397-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Grami V, Marrero Y, Huang L, Tang D, Yappert MC, Borchman D. Alpha-crystallin binding in vitro to lipids from clear human lenses. Exp Eye Res. 2005;81(2):138–146. doi: 10.1016/j.exer.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Grether-Beck S, Bonizzi G, Schmitt-Brenden H, Felsner I, Timmer A, Sies H, Johnson JP, Piette J, Krutmann J. Non-enzymatic triggering of the ceramide signalling cascade by solar UVA radiation. EMBO J. 2000;19(21):5793–5800. doi: 10.1093/emboj/19.21.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. Characterisation and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;302(2):199–212. doi: 10.1006/abio.2001.5536. [DOI] [PubMed] [Google Scholar]
- Han X, David MH, Daniel WM, Jr, John K, John CM. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82(4):809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1(3):465–479. doi: 10.1016/S1568-1637(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalised additive models. Stat Sci. 1986;1(3):297–310. doi: 10.1214/ss/1177013604. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Free and bound water in normal and cataractous human lenses. Investig Ophthalmol Vis Sci. 2008;49(5):1991–1997. doi: 10.1167/iovs.07-1151. [DOI] [PubMed] [Google Scholar]
- Ho RJY, Schmetz M, Deamer DW. Nonenzymatic hydrolysis of phosphatidylcholine prepared as liposomes and mixed micelles. Lipids. 1987;22:156–158. doi: 10.1007/BF02537295. [DOI] [Google Scholar]
- Hsu FF, Turk J. Differentiation of 1-O-alk-1′-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2007;18(11):2065–2073. doi: 10.1016/j.jasms.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Grami V, Marrero Y, Tang D, Yappert MC, Rasi V, Borchman D. Human lens phospholipid changes with age and cataract. Investig Ophthalmol Vis Sci. 2005;46(5):1682–1689. doi: 10.1167/iovs.04-1155. [DOI] [PubMed] [Google Scholar]
- Ickenstein LM, Sandström MC, Mayer LD, Edwards K. Effects of phospholipid hydrolysis on the aggregate structure in DPPC/DSPE-PEG2000 liposome preparations after gel to liquid crystalline phase transition. Biochim Biophys Acta. 2006;1758(2):171–180. doi: 10.1016/j.bbamem.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kamei A. Properties of partially purified esterase in human crystalline lens and variation in its enzyme activity during aging and with advance of senile cataract. Biol Pharm Bull. 1996;19(9):1223–1226. doi: 10.1248/bpb.19.1223. [DOI] [PubMed] [Google Scholar]
- Kanfer J, Kennedy EP. Metabolism and function of bacterial lipids: II. Biosynthesis of phospholipids in Escherischia coli. J Biol Chem. 1964;239:1720–1726. [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE. 2008;3(1):e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Fanó G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26(3–4):303–308. doi: 10.1016/S0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Lass JH, Meneses P, Greiner JV, Glonek T. Human crystalline lens phospholipid analysis with age. Invest Ophthalmol Vis Sci. 1991;32(3):549–555. [PubMed] [Google Scholar]
- Messaris E, Kekis P, Memos N, Chatzigianni E, Menenakos E, Leandros E, Konstadoulakis MM. Sepsis: prognostic role of apoptosis regulators in gastrointestinal cells. World J Surg. 2007;31(4):787–794. doi: 10.1007/s00268-005-0742-1. [DOI] [PubMed] [Google Scholar]
- Meulle A, Salles B, Daviaud D, Valet P, Muller C (2008) Positive regulation of DNA double strand break repair activity during differentiation of long life span cells: the example of adipogenesis. Plos One 3(10) [DOI] [PMC free article] [PubMed]
- Moffat BA, Landman KA, Truscott RJW, Sweeney MHJ, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69(6):663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44(2):459–461. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon JR, Blanksby SJ, Abbott SK, Hulbert AJ, Mitchell TW, Truscott RJW. Phospholipid composition of the rat lens is independent of diet. Exp Eye Res. 2008;87(6):502–514. doi: 10.1016/j.exer.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Nealon JR, Blanksby SJ, Mitchell TW, Else PL. Systematic differences in membrane acyl composition associated with varying body mass in mammals occur in all phospholipid classes: an analysis of kidney and brain. J Exp Biol. 2008;211(Pt 19):3195–3204. doi: 10.1242/jeb.019968. [DOI] [PubMed] [Google Scholar]
- Nealon JR, Blanksby SJ, Donaldson PJ, Truscott RJW, Mitchell TW (2011) Fatty acid uptake and incorporation into phospholipids in the rat lens. Investig Ophthalmol Vis Sci 52(2):804–809 [DOI] [PMC free article] [PubMed]
- Nowakowski RS. Stable neuron numbers from cradle to grave. Proc Natl Acad Sci U S A. 2006;103(33):12219–12220. doi: 10.1073/pnas.0605605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oborina EM, Yappert MC. Effect of sphingomyelin versus dipalmitoylphosphatidylcholine on the extent of lipid oxidation. Chem Phys Lipids. 2003;123(2):223–232. doi: 10.1016/S0009-3084(03)00003-3. [DOI] [PubMed] [Google Scholar]
- Ogiso M, Irie A, Kubo H, Komoto M, Matsuno T, Koide Y, Hoshi M. Characterisation of neutral glycosphingolipids in human cataractous lens. J Biol Chem. 1993;268:13242–13247. [PubMed] [Google Scholar]
- Ogiso M, Okinaga T, Komoto M, Nishiyama I, Hoshi M. Comparative-study of glycosphingolipid composition in mammalian lenses. Exp Eye Res. 1994;59(6):653–663. doi: 10.1006/exer.1994.1151. [DOI] [PubMed] [Google Scholar]
- Ogiso M, Shogomori H, Hoshi M. Localization of LewisX, sialyl-LewisX and alpha-galactosyl epitopes on glycosphingolipids in lens tissues. Glycobiology. 1998;8(1):95–105. doi: 10.1093/glycob/8.1.95. [DOI] [PubMed] [Google Scholar]
- Rujoi M, Jin J, Borchman D, Tang D, Yappert MC. Isolation and lipid characterisation of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Investig Ophthalmol Vis Sci. 2003;44(4):1634–1642. doi: 10.1167/iovs.02-0786. [DOI] [PubMed] [Google Scholar]
- Saito K, Nishijima M, Kuge O. Genetic evidence that phosphatidylserine synthase II catalyzes the conversion of phosphatidylethanolamine to phosphatidylserine in Chinese hamster ovary cells. J Biol Chem. 1998;273:17199–17205. doi: 10.1074/jbc.273.27.17199. [DOI] [PubMed] [Google Scholar]
- Satre M, Kennedy EP. Identification of bound pyruvate essential for the activity of phosphatidylserine decarboxylase of Escherichia coli. J Biol Chem. 1978;253:479–483. [PubMed] [Google Scholar]
- Scharf J, Dovrat A, Gershon D. Defective superoxide-dimutase molecules accumulate with age in human lenses. Graefe's Arch Clin Exp Ophthalmol. 1987;225(2):133–136. doi: 10.1007/BF02160345. [DOI] [PubMed] [Google Scholar]
- Shapira R, Wilkinson KD, Shapira G. Racemization of individual aspartate residues in human myelin basic protein. J Neurochem. 1988;50(2):649–654. doi: 10.1111/j.1471-4159.1988.tb02960.x. [DOI] [PubMed] [Google Scholar]
- Shetty HU, Quentin RS, Kazushige W, Stanley IR. Identification of two molecular species of rat brain phosphatidylcholine that rapidly incorporate and turn over arachidonic acid in vivo. J Neurochem. 1996;67(4):1702–1710. doi: 10.1046/j.1471-4159.1996.67041702.x. [DOI] [PubMed] [Google Scholar]
- Söderberg M, Edlund C, Kristensson K, Dallner G. Lipid compositions of different regions of the human brain during aging. J Neurochem. 1990;54(2):415–423. doi: 10.1111/j.1471-4159.1990.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Biol Med. 2006;41(3):362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Vanier MT, Jungbjer B. Changes in fatty acid composition of human brain myelin lipids during maturation. J Neurochem. 1978;30(6):1383–1390. doi: 10.1111/j.1471-4159.1978.tb10470.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Boström K, Helander CG, Jungbjer B. Membrane lipids in the aging human brain. J Neurochem. 1991;56(6):2051–2059. doi: 10.1111/j.1471-4159.1991.tb03466.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Boström K, Jungbjer B, Olsson L. Membrane lipids of adult human brain:lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J Neurochem. 1994;63(5):1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- Sweeney MHJ, Truscott RJW. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67(5):587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Tao RVP, Cotlier E. Ceramides of human normal and cataractous lens. Biochim Biophys Acta. 1975;409:329–341. doi: 10.1016/0005-2760(75)90029-6. [DOI] [PubMed] [Google Scholar]
- Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Physiol Regul Integr Comp Physiol. 1994;267(5):R1273–R1279. doi: 10.1152/ajpregu.1994.267.5.R1273. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Presbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition. Exp Eye Res. 2009;88(2):241–247. doi: 10.1016/j.exer.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Zhu X. Presbyopia and cataract: a question of heat and time. Prog Retinal Eye Res. 2010;29(6):487–499. doi: 10.1016/j.preteyeres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. doi: 10.1198/016214504000000980. [DOI] [Google Scholar]
- Yappert MC, Borchman D. Sphingolipids in human lens membranes: an update on their composition and possible biological implications. Chem Phys Lipids. 2004;129(1):1–20. doi: 10.1016/j.chemphyslip.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yappert MC, Lal S, Borchman D. Age dependence and distribution of green and blue fluorophores in human lens homogenates. Investig Ophthalmol Vis Sci. 1992;33(13):3555–3560. [PubMed] [Google Scholar]
- Yappert MC, Rujoi M, Borchman D, Vorobyov I, Estrada R. Glycero- versus sphingo-phospholipids: correlations with human and non-human mammalian lens growth. Exp Eye Res. 2003;76(6):725–734. doi: 10.1016/S0014-4835(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gaus K, Lu Y, Magenau A, Truscott RJW, Mitchell TW. Alpha- and beta-crystallins modulate the head group order of human lens membranes during aging. Investig Ophthalmol Vis Sci. 2010;51(10):5162–5167. doi: 10.1167/iovs.09-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Korlimbinis A, Truscott RJ. Age-dependent denaturation of enzymes in the human body. Rejuven Res. 2010;13(5):1–8. doi: 10.1089/rej.2009.1009. [DOI] [PubMed] [Google Scholar]
- Zigman S, Paxhia T, Marinetti G, Girsch S. Lipids of human lens fiber cell membranes. Curr Eye Res. 1984;3(7):887–896. doi: 10.3109/02713688409167206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 121 kb)