Abstract
Human diploid fibroblasts (HDFs) exposed to subcytotoxic concentrations of oxidative or stressful agents, such as hydrogen peroxide, tert-butylhydroperoxide, or ethanol, undergo stress-induced premature senescence (SIPS). This condition is characterized by the appearance of replicative senescence biomarkers such as irreversible growth arrest, increase in senescence-associated β-galactosidase (SA β-gal) activity, altered cell morphology, and overexpression of several senescence-associated genes. Copper is an essential trace element known to accumulate with ageing and to be involved in the pathogenesis of some age-related disorders. Past studies using either yeast or human cellular models of ageing provided evidence in favor of the role of intracellular copper as a longevity modulator. In the present study, copper ability to cause the appearance of senescent features in HDFs was assessed. WI-38 fibroblasts exposed to a subcytotoxic concentration of copper sulfate presented inhibition of cell proliferation, cell enlargement, increased SA β-gal activity, and mRNA overexpression of several senescence-associated genes such as p21, apolipoprotein J (ApoJ), fibronectin, transforming growth factor β-1 (TGF β1), insulin growth factor binding protein 3, and heme oxygenase 1. Western blotting results confirmed enhanced intracellular p21, ApoJ, and TGF β1 in copper-treated cells. Thus, similar to other SIPS-inducing agents, HDF exposure to subcytotoxic concentration of copper results in premature senescence. Further studies will unravel molecular mechanisms and the biological meaning of copper-associated senescence and lead to a better understanding of copper-related disorder establishment and progression.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9276-7) contains supplementary material, which is available to authorized users.
Keywords: Cellular senescence, Copper, Metals, Oxidative stress, Human fibroblasts, Ageing
Introduction
Cellular senescence was described more than four decades ago when it was demonstrated that normal cells in culture had a limited ability to proliferate (Hayflick 1965). It was shown that serially cultivated human diploid fibroblasts (HDFs) initially exhibit active cell division, but, after a number of population doublings, dividing cell number decreases. Eventually, they cease dividing, become unresponsive to mitogenic stimuli and enter in a condition termed replicative senescence (RS). In addition, cells in the senescent state exhibit dramatic alterations in structure, mass, and functioning of their subcellular organelles, when compared with proliferating cells. They include an enlarged, flat morphology, increased reactive oxygen species production, lipofuscin accumulation, altered mass and functionality of mitochondria and lysosomes, and enhanced activity of senescence-associated β-galactosidase (SA β-gal; Hwang et al. 2009). Moreover, RS cells present altered expression of several senescence-associated genes (Debacq-Chainiaux et al. 2008).
A senescent phenotype may be achieved earlier, when HDFs in vitro are submitted to subcytotoxic doses of oxidants or other stressful agents such as hydrogen peroxide (Frippiat et al. 2001), tert-butylhydroperoxide (t-BHP), ethanol (Dumont et al. 2002), or ultraviolet B (UVB) radiation (Debacq-Chainiaux et al. 2005). Experimentally, when such agents are used, immediate adaptative responses are expected to occur. In order to circumvent them, the senescent features are assessed only 2 or 3 days after exposure to the last stress. Cells in this condition, termed stress-induced premature senescence (SIPS), remain alive for months and display many features of RS, including the typical senescent phenotype, cell cycle arrest, increased activity of SA β-gal, and gene expression profile alteration (Toussaint et al. 2000). Since SIPS cells are able to mimic many of the processes that occur during RS, they are frequently used to study the mechanisms of cellular ageing.
In biological systems, copper is an essential trace element since it acts as a co-factor of different enzymes such as cytochrome c oxidase, Cu/Zn superoxide dismutase, and others (Gupta and Lutsenko 2009). Apart from this essential role, ionic copper in excess becomes toxic and mediates the generation of the highly reactive hydroxyl radical, able to damage different kinds of biomolecules (Valko et al. 2005).
This dual role implies that copper uptake and utilization is under narrow regulation in order to allow for cell needs and prevent harmful effects. In many cells, most of the copper is taken up through evolutionary conserved copper transporters (CTRs) which include efficient orthologs in humans, hCTR (Zhou and Gitschier 1997), yeasts, yCtr1, and the filamentous fungi Podospora anserina, PaCtr3 (Borghouts et al. 2002). After copper is taken inside liver cells, a Cu-ATPase (ATP7B) transports it across intracellular membranes. These may be vesicles to fuse with the cell membrane and excrete the copper into the bile, or vesicles that may enter the secretory compartment and supply copper as co-factor to the synthesis of the cuproenzymes (Gupta and Lutsenko 2009). Additional copper ions bind metallothionein, but, when in excess, they accumulate in the cytoplasm and cause oxidative damage (Gaetke and Chow 2003). These deleterious effects of copper are not evident early in life but may accumulate as we age. Actually, it is clear that copper is involved in the pathogenesis of age-associated disorders as Alzheimer’s and Parkinson’s disease (Barnham and Bush 2008; Brewer 2010).
The P. anserina ageing model provides interesting information into putative mechanisms on cellular ageing. In contrast to most filamentous fungi, the mycelia of P. anserina wild-type strains attain senescence and may die, after some time of active growth. However, a mutation in the grisea gene results in strains with life extension and delay in senescence. This gene induces the expression of the PaCTR3 permease but, when mutated, leads to its loss of function and decrease in the uptake of copper into the cell (Borghouts et al. 2002). Interestingly, senescence delay observed in the mutant strains may be lost when they are transformed with a constitutively active construct containing PaCtr3-cDNA, and senescence may be postponed when wild-type strains are grown in media added with a copper chelator (Borghouts et al. 2001, 2002). Senescent P. anserina wild-type strains exhibit a PaCTR3 downregulation. However, they evidence an enhanced metallothionein 1 expression, confirming an increase in cytosolic copper, thought to derive from mitochondria (Borghouts et al. 2002), and further adding to copper intervention in senescence.
In senescent HDFs, copper-regulated genes such as heat-shock protein-70 (hspa1a) and metallothionein 2A (mt2a) were found upregulated, indicating that cytosolic copper levels also increase during senescence of HDFs. Thus, the evidence favoring enhanced intracellular copper as longevity modulator, possibly through the generation of ROS, is strong but is much limited regarding cellular senescence (Scheckhuber et al. 2009).
Departing from the hypothesis that the oxidative effect of copper is able to cause cellular senescence, we aimed to verify whether copper exposure of HDFs was able to cause the morphological and molecular alterations typically associated with it. Here, we provide data showing that copper induces biomarkers of senescence similarly to other SIPS agents.
Methods
Cell culture procedures
WI-38 HDFs were purchased from The European Collection of Cell Cultures and were routinely cultivated in 75 cm2 culture flasks containing 15 mL of basal medium Eagle (BME) supplemented with 10% fetal bovine serum (FBS) at 37°C in an atmosphere containing 5% CO2. When confluent, cells were subcultivated as previously described (Hayflick and Moorhead 1961). In slowly growing cultures, the medium was changed every 4 days. To analyze the effect of copper, subconfluent WI-38 cultures at early cumulative population doublings (CPDs ≤ 30) were submitted to 250, 500, 750, or 1,000 μM copper sulfate (CuSO4) in BME containing 10% FBS for 24 h. At the end of the exposure, cells were washed twice with pre-warmed phosphate buffer saline (PBS) and complete medium (BME with 10% FBS) was added. Control cultures at the same early CPDs were incubated with equivalent doses of sodium sulfate (Na2SO4). BME condition represents cells treated with complete medium without sodium or copper sulfate.
Copper cytotoxicity
To assess copper cytotoxicity, cell survival was measured by neutral red assay immediately after cell exposure to several concentrations of copper sulfate (250, 500, 750, and 1,000 μM), as described by Repetto et al. (2008), and compared with controls. Briefly, after copper treatment, the medium was removed, and the cells were incubated with neutral red solution (40 μg/mL) in BME for 3 h at 37°C. The cells were subsequently washed with PBS, and the dye was extracted from the viable cells by their lysis with acetic acid (1% v/v) in 50% (v/v) ethanol. Optical density was then measured at 540 nm (Abs540nm) using a microplate reader (Infinite®200-TECAN). Control cells, treated with sodium sulfate, represented 100% viability.
Cell proliferation assay
To assess the effect of copper treatment on cell proliferation, the sulforhodamine B (SRB) assay was employed (Vichai and Kirtikara 2006). SRB assay was used for cell density determination, based on the measurement of cellular protein content (arbitrary units). For the assay, 10,000 cells per well were plated onto 96-well plates, submitted to the different treatments, and then fixed at different time-points after stress (1, 2, 3, and 4 days after exposure) with 10% trichloroacetic acid (TCA) during 1 h at 4°C. The TCA-fixed cells were stained for 30 min with 0.057% (w/v) SRB in 1% acetic acid solution and then were washed four times with 1% acetic acid. Bound dye was solubilized with 10 mM Tris base solution (pH 10), and the absorbance at 510 nm of each well was recorded using a microplate reader (Infinite®200-TECAN). Cell growth was estimated considering that, for each condition, the respective Abs510nm (day 1) = 1 arbitrary unit of proliferation index.
Senescence-associated β-galactosidase
At 24 h after copper treatment, cells were trypsinized and seeded in six-well culture plates at a density of 20,000 cells per well. Forty-eight hours after plating, the activity of senescence-associated β-galactosidase (SA β-gal) was determined as described by Dimri et al. (1995). The proportion of SA β-gal-positive cells was determined by counting 400 cells per dish under a microscope, using duplicates. The proportions of cells positive for SA β-gal activity are given as percentages of the total number of cells counted.
Real-time PCR analysis
Total RNA was extracted (RNeasy Plus Mini Kit, Qiagen™) from cells 3 days after treatments, in at least three independent cultures. Total RNA (2 μg) was converted in cDNA by reverse transcription reaction. Amplification reaction assays contained 1× SYBR Green Mastermix (Bio-Rad™) and primers (STAB VIDA, Lda.) at optimal concentration. The sequences of gene-specific primers are shown in Table 1. A hot start at 94°C for 3 min was followed by 40 cycles at 94°C for 1 min, 60/65°C during 1 min, and 72°C for 1 min using the iCycler iQ5 real-time polymerase chain reaction (PCR; Bio-Rad™) thermal cycler. The specificity of amplification was checked by performing melting curves and electrophoresis of the amplification products.
Table 1.
Primers used for real-time PCR
| Gene | Sequences (5′ → 3′) | Amplicon size (bp) |
|---|---|---|
| Apolipoprotein J–F | GGA TGA AGG ACC AGT GTG ACA AG | 114 |
| Apolipoprotein J–R | CAG CGA CCT GGA GGG ATT C | |
| TGF-β1–F | AGG GCT ACC ATG CCA ACT TCT | 102 |
| TGF-β1–R | CCG GGT TAT GCT GGT TGT ACA | |
| p21–F | CTG GAG ACT CTC AGG GTC GAA | 123 |
| p21–R | CCA GGA CTG CAG GCT TCC T | |
| IGFBP3–F | CAG AGC ACA GAT ACC CAG AAC TTC | 111 |
| IGFBP3–R | CAC ATT GAG GAA CTT CAG GTG ATT | |
| Fibronectin–F | TGT GGT TGC CTT GCA CGA T | 109 |
| Fibronectin–R | GCT TGT GGG TGT GAC CTG AGT | |
| HO1–F | CCA GCA ACA AGG TGC AAG ATT C | 148 |
| HO1–R | CAC ATG GCA TAA AGC CCT ACA G | |
| TBP–F | TCA AAC CCA GAA TTG TTC TCC TTA T | 122 |
| TBP–R | CCT GAA TCC CTT TAG AAT AGG GTA GA |
Western blot analysis
After treatments, WI-38 cells were washed once with ice-cold PBS and scrapped on ice in a lysis buffer (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 0.1% Triton X-100). Cells were homogenized and sonicated for 5 min. The protein content of these cell extracts was quantified using Bradford assay (Bradford 1976). An equal amount of protein (20 μg/lane) from each cell extract was resolved on SDS-PAGE gels with appropriate polyacrylamide concentrations. Proteins were blotted to a nitrocellulose membrane and, after blocking with 5% non-fat dry milk diluted in Tris buffer saline 0.1 M/0.1% Tween 20 (TBST), were subsequently probed with the specific primary antibodies overnight at 4°C (mouse monoclonal antibody anti-p21, Cell Signaling Technology; rabbit polyclonal antibody anti-fibronectin or anti-TGF β1, Santa Cruz Biotechnology, Inc.; and mouse monoclonal antibody anti-apolipoprotein J, Millipore). After extensive washing with TBST, immunoblots were then incubated with an appropriate peroxidase-conjugated secondary antibody for 1 h at room temperature. After three washes in TBST, immunoblots were detected using the ECL Western Blotting Substrate (Pierce™-Thermo Scientific) and recorded by exposure to an X-ray film. Tubulin was also detected and used as control of protein loading.
Statistical analysis
Student t test was used to compare the means between two different conditions. A p value lower than 0.05 was considered statistically significant.
Results
Copper sulfate effect on cellular viability
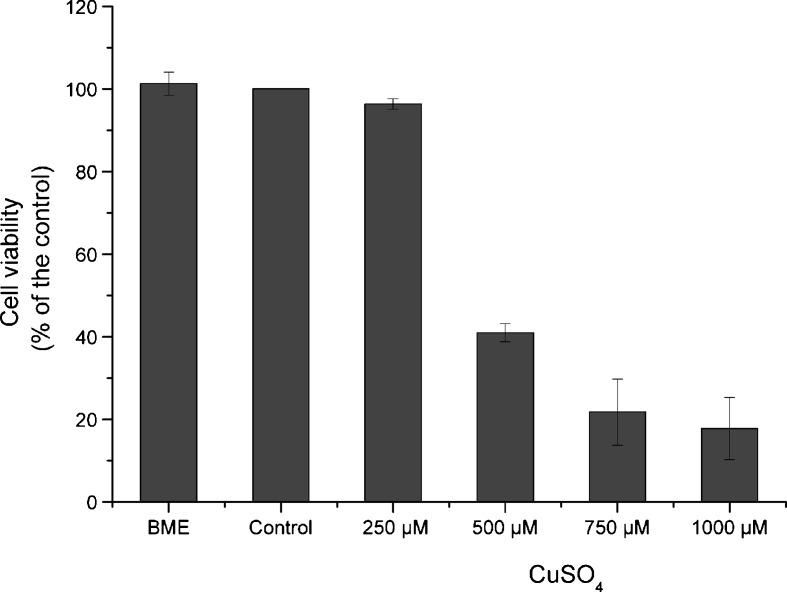
For the determination of the highest dose of copper that could be used without being toxic to WI-38 fibroblasts, several concentrations of copper sulfate were tested. Cells were submitted to 250, 500, 750, and 1,000 μM CuSO4 for 24 h, and the mean viability from three independent experiments was determined for each concentration, assuming that control cells presented 100% viability. Copper cytotoxicity was determined by neutral red assay performed immediately after exposure. As can be seen in Fig. 1, control cells (incubated with the highest dose of sodium sulfate) did not show significant differences in cell viability when compared with BME cells (101.3%). However, cell viability decreased with increasing concentrations of copper sulfate. Cells exposed to 250 μM copper sulfate presented 96.4% of cell viability when compared with controls. This lowest copper sulfate concentration was considered as a subcytotoxic dose, on account that cell exposure to 500, 750, and 1,000 μM resulted in a substantial decrease in cellular viability to 41.0%, 21.8%, and 17.8%, respectively, when compared with controls. The three highest doses of copper sulfate were considered cytotoxic since they yielded cell viabilities lower than 50%. Thus, we decided to emphasize on 250 μM CuSO4 for all the experiments throughout this study. However, in order to evaluate if a higher copper concentration was able to provoke more pronounced senescent effects on cells, the concentration of 500 μM CuSO4 was also tested.
Fig. 1.
Cell viability after exposure to copper sulfate at different concentrations for 24 h. Cell viability decreases with increasing doses of copper sulfate. Control cells, submitted to 1,000 μM sodium sulfate for 24 h, represent 100% viability and did not present significant alteration in viability when compared with cells treated with complete medium (BME). Cell viability was copper sulfate dose-dependent from 250 to 1,000 μM. However, in contrast to 250 μM, a subcytotoxic dose that led to 94.6% of cell viability, cell exposure to the higher doses of copper sulfate, 500, 750, and 1,000 μM, resulted in a decrease in cell viability to 41.0%, 21.8%, and 17.8%, respectively. Data are expressed as mean ± SEM from three independent experiments
Effect of copper on cell morphology and senescence-associated β-galactosidase activity
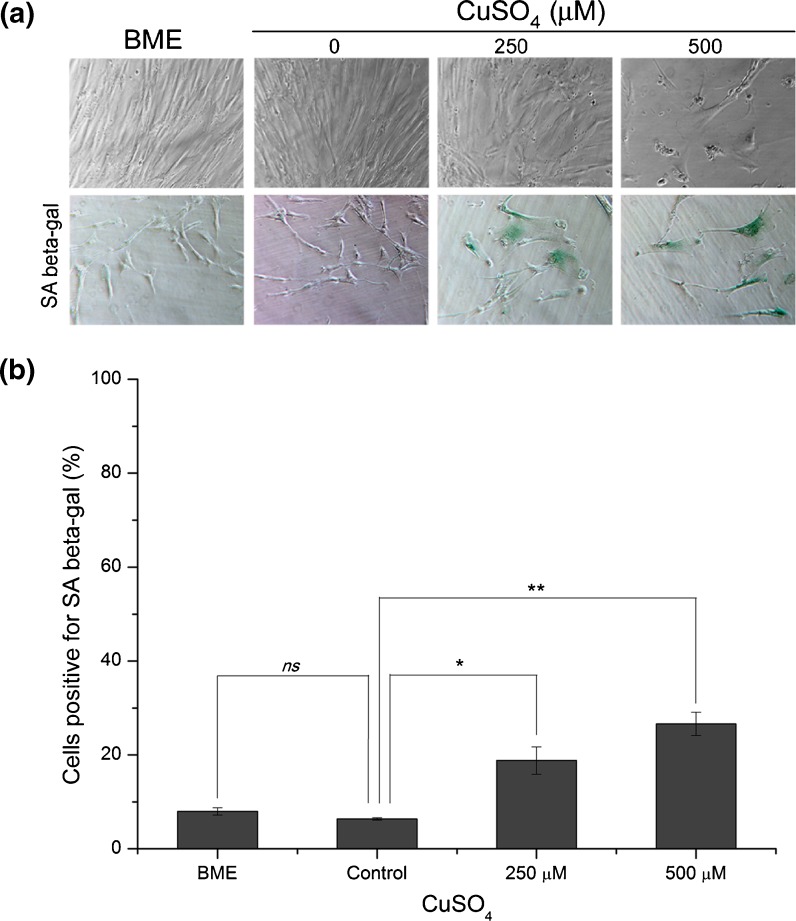
The most evident morphological changes occurring in cellular senescence of fibroblasts are the increase in cell surface area/volume and the alteration of their morphology from small spindle–fusiform to large flat spread (Greenberg et al. 1977; Bayreuther et al. 1988). On the present investigation, cells exposed to 250 or 500 μM copper sulfate presented altered morphological features (Fig. 2a), such as enlarged cell surface as well as stellate outline with thin extensions resembling the typical senescent-like cell morphology. In agreement with the results obtained for cellular viability, cell incubation with 500 μM copper sulfate resulted in a much lower cell density when compared with the other conditions (BME, control, and 250 μM copper), as can be seen in Fig. 2a.
Fig. 2.
Cell morphology and senescence-associated β-galactosidase activity detection on fibroblasts exposed to 250 or 500 μM of copper sulfate. a Wi-38 HDFs exposed to 250 or 500 μM CuSO4 presented enlarged cellular volume and altered shape, resembling the typical senescent phenotype that normally appears on replicatively senescent fibroblasts. b SA β-gal-positive cells increased to 19% and 27% in cells exposed to 250 and 500 μM copper sulfate, respectively, when compared with control cells (6%). Data are expressed as mean ± SEM from three independent experiments. *p < 0.05; **p < 0.01 and ns non-significant, when compared with control
The increased activity of SA β-gal was shown to be a reliable marker of senescence in non-confluent fibroblasts (Dimri et al. 1995) and is commonly used to evidence that. To verify the effects of copper sulfate on SA β-gal activity, 500 μM sodium sulfate (control) and BME (complete medium) were compared, but no significant differences were detected between both groups (p = 0.23). However, as shown in Fig. 2b, the percentage of cells positive for the SA β-gal increased significantly to 19% (p < 0.05) and 27% (p < 0.01) in cells exposed to 250 and 500 μM copper sulfate, respectively, when compared with the 6% of the control. These phenotypic changes are usually taken as indicating cellular senescence (Hwang et al. 2009).
Effect of copper sulfate on cell proliferation
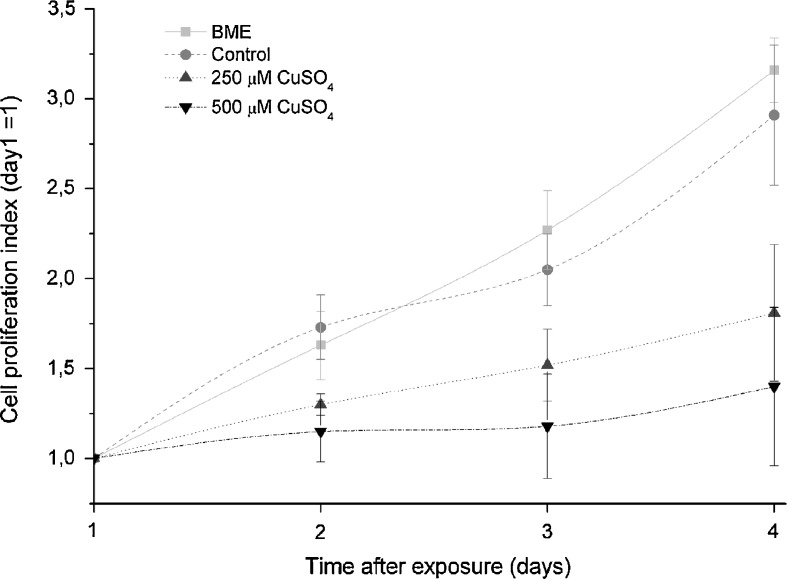
Either replicatively or stress-induced, senescent cells cease proliferating and exhibit cell cycle arrest in G1. To assess the effect of copper in cell proliferation, the total mass of cell proteins was determined in arbitrary units, at different time-points (1, 2, 3, and 4 days) after stress. For each condition, it was assumed that, at day 1, the proliferation index was 1. Fig. 3 shows cell proliferation curves for the different conditions during 4 days. Both BME and control cells proliferated approximately at the same rate, showing an increase in protein content from 1-(day 1) to 3.16- and 2.91-fold (day 4), respectively. Cells treated with 250 or 500 μM copper sulfate presented smaller increases in protein content from 1 (day 1) to 1.81 and 1.40 on day 4, respectively. This lesser increase in protein content observed for cells treated with 250 μM represent a decrease in cell proliferation of about 58% while, for cells exposed to 500 μM, the proliferation was reduced by 79%, when compared with the proliferation rate of control cells.
Fig. 3.
Cell proliferation curves of human diploid fibroblasts exposed to 250 or 500 μM of copper sulfate. Both BME and control cells presented approximately the same proliferation rate during the 4 days after stress. At the fourth day after treatment, cells exposed to 250 or 500 μM CuSO4 showed a decrease in cell proliferation of about 58% and 79%, respectively, when compared with control cells. Data are expressed as mean ± SEM from three independent experiments. *p < 0.05 when compared with the same time-point of the control
Gene expression in fibroblasts exposed to copper sulfate
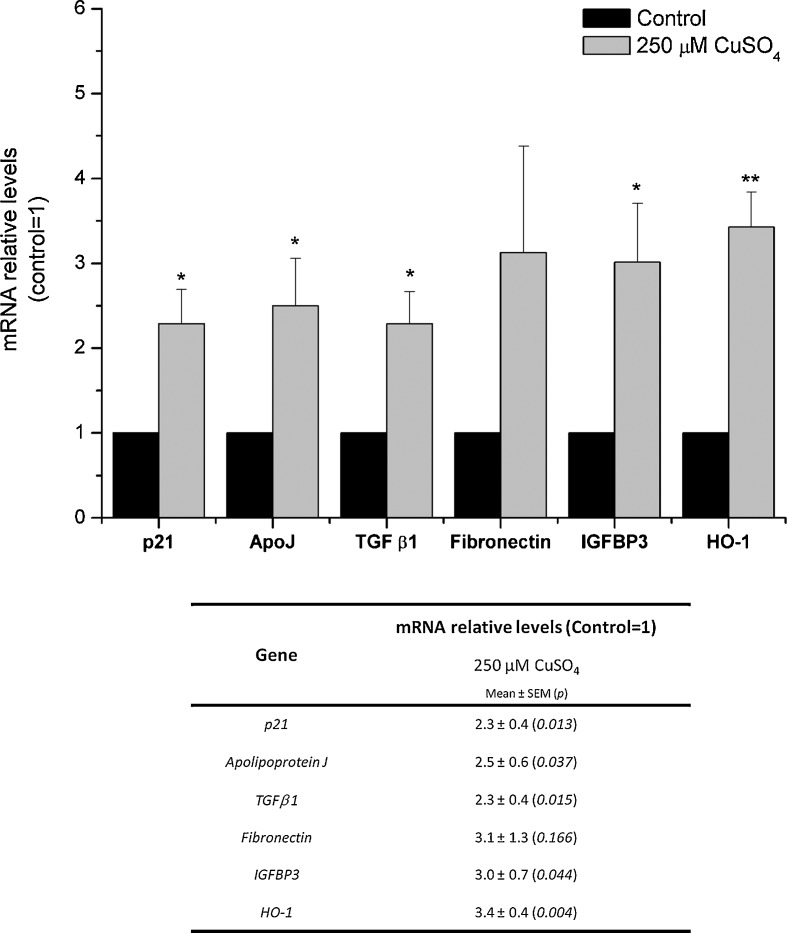
Senescent cells display several typical features which include an alteration in the expression level of several senescence-associated genes (Dumont et al. 2000; Debacq-Chainiaux et al. 2008). The genes encoding cyclin-dependent kinase inhibitor 1A (p21), apolipoprotein J (ApoJ), transforming growth factor beta 1 (TGF β1), fibronectin, insulin growth factor binding protein 3 (IGFBP3), and heme oxygenase-1 (HO-1) are known to be overexpressed both in replicatively, and in prematurely stress-induced, senescent cells. Transcript levels of these genes were quantified by real-time PCR in human fibroblasts 72 h after exposure to copper sulfate, in at least three independent experiments. Results obtained from real-time PCR are depicted in Fig. 4. Cells submitted to 250 μM CuSO4 presented statistically significant increase in mRNA levels of p21, ApoJ, TGF β1, fibronectin, IGFBP3, and HO-1 (respectively 2.3-, 2.5-, 2.3-, 3.1-, 3.0-, and 3.4-fold) when compared with control cells. Cells exposed to 500 μM copper sulfate did not present consistent variations regarding mRNA expression of the selected genes (online resource 1). Such results were interpreted as a consequence of the toxic effects obtained for this concentration.
Fig. 4.
Evaluation of mRNA relative levels of several senescence-associated genes by real-time PCR. Cells exposed to 250 μM CuSO4 presented a statistically significant overexpression of p21, ApoJ, TGF β1, IGFBP3, and HO-1 (respectively 2.3-, 2.5-, 2.3-, 3.0-, and 3.4-fold increase compared with control cells). mRNA levels of fibronectin were also increased in cells treated with copper, although this variation was not statistically significant when compared with control cells. Data are presented as mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 when compared with control
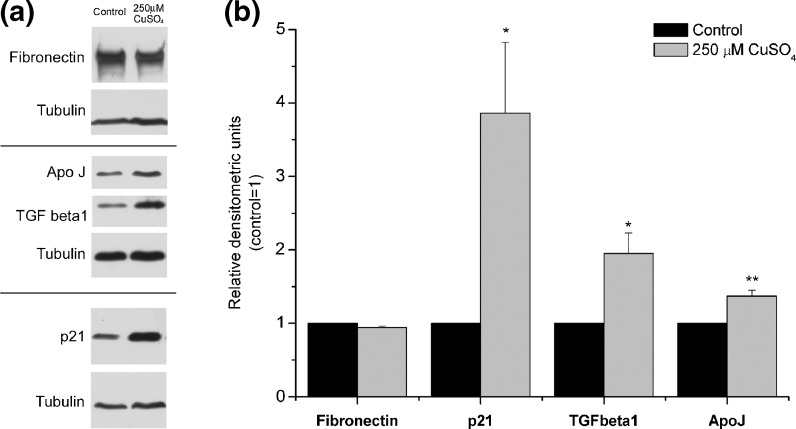
Western blot analysis in fibroblasts exposed to copper sulfate
The protein levels of some of the senescence-associated genes (fibronectin, p21, TGF β1, and ApoJ) were assessed by Western blotting. Fig. 5a shows representative blots of the variations obtained in the content of each protein, and, in Fig. 5b, the relative densitometric means from three independent experiments are plotted. Intracellular levels of fibronectin did not change in WI-38 fibroblasts exposed to 250 μM CuSO4 when compared with control cells. However, the same copper-treated cells presented increased protein levels of p21, TGF β1, and ApoJ (3.7-, 2.0-, and 1.4-fold, respectively) when compared with controls. There were several attempts in order to evaluate the protein levels of IGFBP3. However, the antibody utilized with that purpose was not specific, and, for that reason, we could not conclude about the effect of copper on IGFBP3 protein levels. Once again, incubation of fibroblasts with 500 μM copper sulfate resulted in inconsistent protein variations (online resource 2), most probably due to the cytotoxic effects of such copper concentration.
Fig. 5.
Western blot analysis of fibronectin, p21, ApoJ, and TGF β1 protein levels. a Representative blots for the detection of the different proteins. Tubulin was used as loading control. b The resulting bands were quantified using densitometric analysis of the different signals. Fibronectin intracellular content remained unaltered after exposure to 250 μM CuSO4, comparing with controls. Protein levels of p21, TGF β1, and Apo J were 3.9-, 2-, and 1.4-fold increased, after exposure to 250 μM CuSO4, respectively, when compared with control levels. Data are expressed as mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 when compared with control
Discussion and conclusions
Some agents causing oxidation may be toxic to cells and impose a dramatic limit to their viability. However, their use in subcytotoxic amount induces a change in cell structure and gene expression pattern that directs cells to the condition of senescence. The present study provides evidence that copper has those properties and acts in a way similar to other oxidative agents such as hydrogen peroxide and t-BHP that cause SIPS. In fact, when WI-38 fibroblasts were submitted to copper sulfate for a limited time, they evidenced elevated viability at 250 μM that was strongly reduced at 500 μM and more. The cytotoxic effects of 500 μM copper sulfate were further evidenced in the study of mRNA and protein expression. For this reason, 250 μM was considered subcytotoxic and employed thereafter to verify copper ability to cause SIPS.
Sound data obtained along decades led to the concept that the progressive decline in the proliferative potential followed by growth arrest is the defining feature of the in vitro replicative senescence of fibroblasts (Goldstein 1990; Smith and Pereira Smith 1996) and SIPS (Toussaint et al. 2000). In the present investigation, cell proliferation was found substantially reduced during the 4 days after treatment with 250 μM of CuSO4, when compared with the control cells. These findings are in agreement with previous SIPS studies. In fact, F65 fibroblasts exposed for 2 h to 200 μM H2O2 exhibited a sluggish or absent mitogenic response to several growth stimuli (Chen and Ames 1994). In addition, WI-38 fibroblasts cultivated under mild hyperoxia (von Zglinicki et al. 1995) or submitted to successive stresses under t-BHP treatment (Dumont et al. 2000) showed a drastic decrease in their proliferative capacity, too.
Cell morphology is an important criterion to evaluate the senescence of human fibroblasts in vitro, which usually enlarge, flatten, and loosen spindle morphology and exhibit reduced density. These features were observed in WI-38 fibroblasts 3 days after treatment with copper sulfate, similar to what is seen in other SIPS cellular models (Chen and Ames 1994; Wang et al. 2004). The same stands for SA β-gal activity which was shown to be a reliable marker of senescence in non-confluent cultures of fibroblasts (Dimri et al. 1995) and is used regularly with that purpose. In the present study, WI-38 fibroblast cultures submitted to 250 μM copper sulfate for 24 h showed a higher proportion of cells positive for SA β-gal. This finding parallels other SIPS-inducing conditions such as exposure of cells to successive stresses with t-BHP or ethanol (Debacq-Chainiaux et al. 2008), subcytotoxic levels of hydrogen peroxide (Zdanov et al. 2006), or UVB radiation (Debacq-Chainiaux et al. 2005).
There are several genes whose mRNA levels increase both in replicative and premature senescent HDFs indicating a change in gene expression. Debacq-Chainiaux and colleagues evaluated three different HDF models of senescence and showed that the mRNA levels of senescence-associated genes such as fibronectin, p21, ApoJ, IGFBP3, and TGF β1 were similarly increased in the three cellular models (Debacq-Chainiaux et al. 2008). Those findings prompted us to study their relative transcript levels by real-time PCR, in WI-38 HDFs, 72 h after exposure to copper sulfate.
As already mentioned, the hallmark of cellular senescence is the irreversible arrest of cell division. Senescent cells have been shown to arrest in the G1 phase of cell cycle (Chen et al. 1998) and do not resume proliferation when challenged by physiological mitogens. In mammals, the main regulators of cell cycle progression through G1 phase are heterodimers composed of a cyclin-dependent kinase (CDK4 or CDK6 in this phase) and one member of the D class of cyclins. However, the progression is stalled when the CDK inhibitor p21 exhibits sustained overexpression as occurs in replicative senescence (Stein et al. 1990) and in premature senescence induced by H2O2 (Chen et al. 1998), t-BHP (Dumont et al. 2000), or UVB (Debacq-Chainiaux et al. 2005). The present study, in accordance with the reduced proliferation, provides evidence that p21 transcript levels and protein content are also increased in CuSO4-exposed WI-38 cells.
Fibronectin is an essential extracellular matrix component involved in cell adhesion, cytoskeletal organization, mediation of external mitogenic signals, and wound repair. Fibronectin gene has been found to be overexpressed in senescent pig skin fibroblasts (Martin et al. 1990), in fibroblasts derived from patients with Werner ageing syndrome (LeckaCzernik et al. 1996), and also in several ageing models of human fibroblasts, such as replicative senescence, premature senescence induced by t-BHP or ethanol (Debacq-Chainiaux et al. 2008), and premature senescence induced by UVB radiation (Debacq-Chainiaux et al. 2005). Increased expression of fibronectin was found to positively correlate with cell surface increase observed during senescence of HDFs, suggesting that it may underlie or contribute to replicative senescence morphological changes (Kumazaki et al. 1993). Here, copper-treated WI-38 human fibroblasts presented about threefold increased fibronectin mRNA expression when compared with control cells, although its intracellular protein content, assessed by Western blotting, did not increase after exposure to 250 μM copper sulfate. One possible explanation to this discrepancy is that fibronectin secretion rate might increase with increasing concentrations of copper sulfate, resulting in a faster secretion of fibronectin to the extracellular space, where it usually localizes. This specific point could be elucidated by the quantification of secreted fibronectin in the culture media where cells were grown, but regrettably, it was not evaluated.
Apolipoprotein J, also known as clusterin, is an 80-kDa glycoprotein consisting of two disulfide-linked subunits, alpha and beta, and is constitutively synthesized and secreted by many cell types. ApoJ is mostly recognized as an extracellular chaperone which interacts with many different proteins and inhibits their stress-induced precipitation (Poon et al. 2002) and is involved in numerous physiological processes (Trougakos and Gonos 2006). ApoJ gene is also very sensitive to cellular stressful conditions, especially oxidative stress. ApoJ overexpression was observed in replicatively senescent human fibroblasts and also in prematurely senescent fibroblasts induced by t-BHP, ethanol (Debacq-Chainiaux et al. 2008), or UVB (Debacq-Chainiaux et al. 2005) and is thus considered a faithful biomarker of cellular senescence (Trougakos and Gonos 2006). Its expression was enhanced in the current study too. Due to the varied functional involvement of ApoJ, there is uncertainty regarding its specific intracellular effects. As a wide-range chaperone, a likely explanation is to endow cells with protective survival tools when protein structure may become deranged due to stresses. Yet, another role is to direct damaged proteins for destruction, employing a different mechanism for the same survival purpose. In fact, it was recently reported that ApoJ expression was enhanced in cells grown in excess of copper. Under such conditions, there was evidence for the enhancement of interaction ApoJ/ATP7B copper transporter and its degradation, particularly when mutated (Materia et al. 2011).
TGF β1 is a pleiotropic cytokine involved in many cell functions like cell growth, differentiation, and biosynthesis of extracellular connective tissue. TGF β1 was shown to be overexpressed in replicative, t-BHP-, and ethanol-induced senescence of WI-38 HDFs (Pascal et al. 2005). More specifically, it has already been demonstrated that TGF β1 overexpression is required for the appearance of several biomarkers of cellular senescence, such as the induction of senescent morphogenesis, increased mRNA level of senescence-associated genes, such as ApoJ and fibronectin, and increased senescence-associated β-galactosidase activity, after exposure of HDFs to subcytotoxic stress with H2O2 (Frippiat et al. 2001; Frippiat et al. 2002) or UVB (Debacq-Chainiaux et al. 2005). Similar to other stress-induced premature senescence cellular models, WI-38 HDFs exposed to 250 μM copper sulfate was followed by increased mRNA and protein levels of TGF β1.
TGF β1 is known to induce HO-1 expression in pulmonary epithelial cells (Ning et al. 2002), human renal proximal tubule cells (Hill-Kapturczak et al. 2000), and also in WI-38 HDFs (Pascal et al. 2007). HO-1 expression is transcriptionally activated by agents that generate reactive oxygen species (Soares and Bach 2009), and it has been already shown to be overexpressed in prematurely senescent WI-38 HDFs after exposure to t-BHP and ethanol (Pascal et al. 2007). The present study reports a similar effect in WI-38 fibroblasts with 250 μM copper sulfate.
In addition, TGF β1 is also known to control synthesis and secretion of IGFBP3 by HDFs (Martin and Baxter 1991). IGFBP3 expression is associated with inhibition of cell proliferation and cellular senescence, and, again, its overexpression in RS and SIPS fibroblasts induced by t-BHP, ethanol (Debacq-Chainiaux et al. 2008), or UVB (Debacq-Chainiaux et al. 2005) was described. It has been suggested that growth arrest in premature senescence partly depends on TGF β1 via the overexpression of IGFBP3 (Debacq-Chainiaux et al. 2008). In accordance, fibroblasts exposed to copper sulfate presented increased transcript levels of IGFBP3 in a fashion similar to TGF β1.
The results presented herein show that subcytotoxic concentration of copper sulfate is able to induce senescence features on WI-38 human fibroblasts. Similar to the effects of other well-known SIPS agents, they are observed past adaptative conditions related to immediate gene response and are varied: they include changes in proliferation, in SA β-gal activity, and in gene expression. The coherent pattern of their change gives credit to the inclusion of copper as a SIPS-inducing agent.
It is hoped that further studies addressing the mechanism and biological meaning of copper-associated cell senescence will improve the understanding of the establishment and progression of brain and liver disorders as Alzheimer’s and Wilson’s diseases. In the first, copper not only interacts with amyloid precursor protein through a binding domain but also promotes amyloid β peptide crosslinks and aggregation through metal-mediated oxidative stress (Barnham and Bush 2008). In Wilson’s disease, a deficient intracellular transport of copper results from a structural abnormality of the ATP7B translocator, whose level is regulated by the senescence biomarker ApoJ/clusterin (Materia et al. 2011).
Electronic supplementary material
Effect of 500 μM copper sulfate on mRNA transcript levels of several senescence-associated genes. The incubation of WI-38 fibroblasts with the lower cytotoxic copper concentration (500 μM) resulted in non-significant variations on p21, ApoJ, TGF β1, fibronectin, and HO-1 gene expression, when compared with controls. In addition, IGFBP3 mRNA levels were 1.7-fold increased in 500 μM CuSO4-treated cells, comparing with control cells. When compared with the consistent upregulation of the several senescence-associated genes observed for cells exposed to 250 μM copper, gene expression variations obtained with 500 μM copper sulfate were senseless, suggesting that the transcriptional machinery of these cells were seriously compromised due to the toxic effects of the dose. Data are presented as mean ± SEM from at least three independent experiments. *p < 0.05 when compared with control (JPEG 14 kb)
Western blot analysis of fibronectin, p21, ApoJ, and TGF β1 protein levels in cells submitted to 500 μM copper sulfate. a Representative blots for the detection of the different proteins. Tubulin was used as loading control. b The resulting bands were quantified using densitometric analysis of the different signals. Protein levels of p21 were found significantly increased (3.7-fold) in WI-38 fibroblasts treated with 500 μM copper sulfate for 24h, when compared with controls. Both fibronectin and TGF β1 intracellular protein levels were decreased, and ApoJ protein content did not alter after exposure to the cytotoxic dose of copper, when compared with control cells. These inconsistent protein variations may originate from cellular metabolic alterations reflecting the cytotoxic effects of copper concentration used. Data are expressed as mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 when compared with control (TIFF 9255 kb) (JPEG 22 kb)
Acknowledgments
L. Matos would like to acknowledge “Fundação para a Ciência e Tecnologia” for her PhD grant [SFRH/BD/61820/2009].
Contributor Information
Liliana Matos, Email: lmatos@fcna.up.pt.
Henrique Almeida, Phone: +351-225-513654, FAX: +351-225-513655, Email: almeidah@med.up.pt.
References
- Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s Diseases. Curr Opin Chem Biol. 2008;12(2):222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human-skin fibroblasts invitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988;85(14):5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghouts C, Werner A, Elthon T, Osiewacz HD. Copper-modulated gene expression and senescence in the filamentous fungus Podospora anserina. Mol Cell Biol. 2001;21(2):390–399. doi: 10.1128/MCB.21.2.390-399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghouts C, Scheckhuber CQ, Stephan O, Osiewacz HD. Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PaCtr3 encoding a copper transporter. Int J Biochem Cell Biol. 2002;34(11):1355–1371. doi: 10.1016/S1357-2725(02)00078-X. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Risks of copper and iron toxicity during aging in humans. Chem Res Toxicol. 2010;23(2):319–326. doi: 10.1021/tx900338d. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen-peroxide in human-diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994;91(10):4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G(1) arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Borlon C, Pascal T, Royer V, Eliaers F, Ninane N, Carrard G, Friguet B, de Longueville F, Boffe S, Remacle J, Toussaint O. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta 1 signaling pathway. J Cell Sci. 2005;118:743–758. doi: 10.1242/jcs.01651. [DOI] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Pascal T, Boilan E, Bastin C, Bauwens E, Toussaint O. Screening of senescence-associated genes with specific DNA array reveals the role of IGFBP-3 in premature senescence of human diploid fibroblasts. Free Radic Biol Med. 2008;44(10):1817–1832. doi: 10.1016/j.freeradbiomed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee XH, Basile G, Acosta M, Scott C, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereirasmith O, Peacocke M, Campisi J. A biomarker that identifies senescent human-cells in culture and in aging skin in-vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, Eliaers F, Remacle J, Toussaint O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28(3):361–373. doi: 10.1016/S0891-5849(99)00249-X. [DOI] [PubMed] [Google Scholar]
- Dumont P, Chainiaux F, Eliaers F, Petropoulou C, Remacle J, Koch-Brandt C, Gonos ES, Toussaint O. Overexpression of apolipoprotein J in human fibroblasts protects against cytotoxicity and premature senescence induced by ethanol and tert-butylhydroperoxide. Cell Stress Chaperones. 2002;7(1):23–35. doi: 10.1379/1466-1268(2002)007<0023:OOAJIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276(4):2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Dewelle J, Remacle J, Toussaint O. Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 2002;33(10):1334–1346. doi: 10.1016/S0891-5849(02)01044-4. [DOI] [PubMed] [Google Scholar]
- Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–163. doi: 10.1016/S0300-483X(03)00159-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence—the human fibroblast comes of age. Science. 1990;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Greenberg SB, Grove GL, Cristofalo VJ. Cell-size in aging monolayer-cultures. Vitro. 1977;13(5):297–300. doi: 10.1007/BF02616174. [DOI] [PubMed] [Google Scholar]
- Gupta A, Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem. 2009;1(6):1125–1142. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37(3):614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. Serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem. 2000;275(52):40904–40909. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Yoon G, Kang HT. A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci. 2009;66(15):2503–2524. doi: 10.1007/s00018-009-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during invivo cellular aging of human vascular endothelial-cells and skin fibroblasts. Exp Cell Res. 1993;205(2):396–402. doi: 10.1006/excr.1993.1103. [DOI] [PubMed] [Google Scholar]
- LeckaCzernik B, Moerman EJ, Jones RA, Goldstein S. Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol. 1996;31(1–2):159–174. doi: 10.1016/0531-5565(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Martin JL, Baxter RC. Transforming growth-factor-beta stimulates production of insulin-like growth factor-binding protein-3 by human skin fibroblasts. Endocrinology. 1991;128(3):1425–1433. doi: 10.1210/endo-128-3-1425. [DOI] [PubMed] [Google Scholar]
- Martin M, Elnabout R, Lafuma C, Crechet F, Remy J. Fibronectin and collagen gene-expression during invitro aging of pig skin fibroblasts. Exp Cell Res. 1990;191(1):8–13. doi: 10.1016/0014-4827(90)90028-9. [DOI] [PubMed] [Google Scholar]
- Materia S, Cater MA, Klomp LW, Mercer JF, La Fontaine S. Clusterin (apolipoprotein J), a molecular chaperone that facilitates degradation of the copper-ATPases ATP7A and ATP7B. J Biol Chem. 2011;286(12):10073–10083. doi: 10.1074/jbc.M110.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Song RP, Li CJ, Park E, Mohsenin A, Choi AMK, Choi ME. TGF-beta(1) stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1094–L1102. doi: 10.1152/ajplung.00151.2002. [DOI] [PubMed] [Google Scholar]
- Pascal T, Debacq-Chainiaux F, Chretien A, Bastin C, Dabee AF, Bertholet V, Remacle J, Toussaint O. Comparison of replicative senescence and stress-induced premature senescence combining differential display and low-density DNA arrays. FEBS Lett. 2005;579(17):3651–3659. doi: 10.1016/j.febslet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Pascal T, Debacq-Chainiaux F, Boilan E, Ninane N, Raes M, Toussaint O. Heme oxygenase-1 and interleukin-11 are overexpressed in stress-induced premature senescence of human WI-38 fibroblasts induced by tert-butylhydroperoxide and ethanol. Biogerontology. 2007;8(4):409–422. doi: 10.1007/s10522-007-9084-8. [DOI] [PubMed] [Google Scholar]
- Poon S, Treweek TM, Wilson MR, Easterbrook-Smith SB, Carver JA. Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett. 2002;513(2–3):259–266. doi: 10.1016/S0014-5793(02)02326-8. [DOI] [PubMed] [Google Scholar]
- Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Scheckhuber CQ, Grief J, Boilan E, Luce K, Debacq-Chainiaux F, Rittmeyer C, Gredilla R, Kolbesen BO, Toussaint O, Osiewacz HD (2009) Age-related cellular copper dynamics in the fungal ageing model Podospora anserina and in ageing human fibroblasts. PLoS One 4(3) [DOI] [PMC free article] [PubMed]
- Smith JR, Pereira Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273(5271):63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med. 2009;15(2):50–58. doi: 10.1016/j.molmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Stein GH, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene-product in senescent human fibroblasts. Science. 1990;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40(12):1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts—a model for senescence. Exp Cell Res. 1995;220(1):186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Wang Y, Meng AM, Zhou DH. Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts. Exp Cell Res. 2004;298(1):188–196. doi: 10.1016/j.yexcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Zdanov S, Debacq-Chainiaux F, Remacle J, Toussaint O. Identification of p38(MAPK)-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett. 2006;580(27):6455–6463. doi: 10.1016/j.febslet.2006.10.064. [DOI] [PubMed] [Google Scholar]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A. 1997;94(14):7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of 500 μM copper sulfate on mRNA transcript levels of several senescence-associated genes. The incubation of WI-38 fibroblasts with the lower cytotoxic copper concentration (500 μM) resulted in non-significant variations on p21, ApoJ, TGF β1, fibronectin, and HO-1 gene expression, when compared with controls. In addition, IGFBP3 mRNA levels were 1.7-fold increased in 500 μM CuSO4-treated cells, comparing with control cells. When compared with the consistent upregulation of the several senescence-associated genes observed for cells exposed to 250 μM copper, gene expression variations obtained with 500 μM copper sulfate were senseless, suggesting that the transcriptional machinery of these cells were seriously compromised due to the toxic effects of the dose. Data are presented as mean ± SEM from at least three independent experiments. *p < 0.05 when compared with control (JPEG 14 kb)
Western blot analysis of fibronectin, p21, ApoJ, and TGF β1 protein levels in cells submitted to 500 μM copper sulfate. a Representative blots for the detection of the different proteins. Tubulin was used as loading control. b The resulting bands were quantified using densitometric analysis of the different signals. Protein levels of p21 were found significantly increased (3.7-fold) in WI-38 fibroblasts treated with 500 μM copper sulfate for 24h, when compared with controls. Both fibronectin and TGF β1 intracellular protein levels were decreased, and ApoJ protein content did not alter after exposure to the cytotoxic dose of copper, when compared with control cells. These inconsistent protein variations may originate from cellular metabolic alterations reflecting the cytotoxic effects of copper concentration used. Data are expressed as mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 when compared with control (TIFF 9255 kb) (JPEG 22 kb)